Abstract
A Myc epitope was inserted at residue 283 of herpes simplex virus type 1 (HSV-1) glycoprotein K (gK), a position previously shown not to interfere with gK activity. The Myc-tagged gK localized predominantly to the endoplasmic reticulum, both in uninfected and in HSV-infected cells. gK, coexpressed with the four HSV fusogenic glycoproteins, gD, gB, gH, and gL, inhibited cell-cell fusion. The effect was partially dose dependent and was observed both in baby hamster kidney (BHK) and in Vero cells, indicating that the antifusion activity of gK may be cell line independent. The antifusion activity of gK did not require viral proteins other than the four fusogenic glycoproteins. A syncytial (syn) allele of gK (syn-gK) carrying the A40V substitution present in HSV-1(MP) did not block fusion to the extent seen with the wild-type (wt) gK, indicating that the syn mutation ablated, at least in part, the antifusogenic activity of wt gK. We conclude that gK is part of the mechanism whereby HSV negatively regulates its own fusion activity. Its effect accounts for the notion that cells infected with wt HSV do not fuse with adjacent, uninfected cells into multinucleated giant cells or syncytia. gK may also function to preclude fusion between virion envelope and the virion-encasing vesicles during virus transport to the extracellular compartment, thus preventing nucleocapsid de-envelopment in the cytoplasm.
Herpes simplex virus (HSV) enters cells by fusion of the virion envelope with the plasma membrane, a neutral pH-dependent fusion (55). This event requires the intervention of three fusogenic glycoproteins, gB, gH, and gL (7, 18, 46), and occurs downstream of the interaction of gD with one of the entry receptors (9, 50). While the molecular details of the interaction between gD and its receptors are fairly well known (11-14, 21, 33, 37), the process by which gD, gB, gH, and gL perform fusion remains rather obscure. Little is known also of the molecular mechanisms by which HSV exerts a control over its own fusion activity. A novel paradigm in virus-induced fusion is that fusogenic glycoproteins are primed for fusion and are then maintained in a metastable fusion-inhibited conformation until fusion triggering induces a fusion-active state (23, 51, 54). In the best known examples (influenza virus hemagglutinin, retrovirus, and thick-born encephalitis envelope glycoproteins), priming is controlled by proteolytic cleavage of the glycoproteins, which occurs late in the Golgi apparatus, thus preventing glycoprotein-induced fusion in the early exocytic compartment. The fusion-active conformation is achieved at the time of virus entry into a new cell as a consequence of dramatic refolding of the fusogenic glycoproteins induced by the interaction with the cellular receptor and/or by the acid pH of endosomes.
In the HSV life cycle, at least three steps necessitate a negative control on fusion. First, late in infection, during virus egress, the membranes of the exocytic compartment contain large amounts of fusogenic glycoproteins. Yet, these compartments do not collapse due to fusion of their membranes, and only a fragmentation of Golgi apparatus is observed (8). Inasmuch as HSV glycoproteins do not undergo proteolytic cleavage, a mechanism other than this must exist to negatively control their fusogenic potential. Second, virion fusion with the target cell needs to be timely and tightly regulated. Third, cells infected with wild-type (wt) virus round up, rather than fuse with adjacent uninfected cells, despite the fact that the plasma membranes are highly enriched with fusogenic glycoproteins. Syncytium formation is only seen in cells infected with viruses carrying syncytial (syn) mutations.
Genetic studies have identified loci that carry syncytial mutations. The best characterized are those initially named syn1 and syn3 (47). The prototype syn1 mutant virus is HSV-1(MP), which carries the A40V substitution in the first external (or luminal) domain of gK (3, 15, 24, 42, 43). Several other syn mutations in gK are known (17, 41) that map either at the N-terminal or at a central region (defined as domain I and domain III, respectively, in reference 20). The prototype syn3 mutant is tsB5, mutated in the cytoplasmic tail of gB (6). Additional sites of syn mutations are UL20 and UL24 genes (2, 44, 48).
The objective of this work was to investigate the mechanisms by which HSV enables the fusion of the envelope with the plasma membrane and yet blocks the fusion of infected and uninfected cells. We focused on gK (UL53) (25, 34, 45) because this is the most frequent locus of syn mutations. Several lines of evidence suggest that this glycoprotein may exert control over HSV fusion, although this remains a controversial issue, and gK has even been proposed to be itself a fusogenic protein (26). gK is a polytopic membrane protein (36), whose accumulation does not reach the levels apparent with most of the glycoproteins, as judged by immunofluorescence. Its highly embedded structure in membranes may contribute to its poor immunogenicity and scant reactivity (25). The detection and characterization of gK has therefore been troublesome, there have been uncertainties as to whether gK is a virion protein and whether it reaches the plasma membranes. Overall, gK remains one of the most difficult HSV membrane proteins to study and one not fully characterized. The amino acid sequence predicts a protein with three or four transmembrane domains, depending on the algorithm. The structure of gK, as defined by tag insertions (36), appears to be made of three transmembrane domains (amino acids 125 to 139, 226 to 239, and 311 to 325), which define four hydrophilic domains, I and III located in the luminal or external side and II and IV projecting in the cytoplasm. In a recent study, this topology was not confirmed (19).
So far, the properties of gK have been inferred from studies of infected cells. The cell-cell fusion assay developed in recent years provides an opportunity to ask whether gK exerts a direct control on HSV fusion (53). In this assay, cells that express one of the gD receptors, cotransfected with expression plasmids encoding the four glycoproteins gD, gB, gH, and gL, undergo fusion in 24 to 48 h (5, 40). The assay mirrors HSV entry in that it has the same requirements (the four glycoproteins and one of the gD receptors), but the two phenomena are not identical (22). A major advantage of the assay is that it is amenable to quantification.
In this work we coexpressed wt gK with the four HSV glycoproteins (gD, gB, gH, and gL) and observed that gK directly inhibits cell-cell fusion in Vero and BHK cells, without the need of additional viral proteins. We expected that its syn allele might have a reduced capability to block fusion. Indeed, a gK allele carrying the A40V syn substitution present in HSV-1(MP) (43) (hereafter referred to as syn-gK) displayed a reduced ability to block cell-cell fusion.
MATERIALS AND METHODS
Cells and viruses.
BHK, Vero, and 143-tk− cells were grown in Dulbecco's modified Eagle's minimal essential medium containing 5% fetal bovine serum. Unless otherwise indicated, cultures were seeded less than 20 h prior to infection and transfected at about 80% of confluence for cell fusion. R7032 recombinant virus lacking gE and gI was previously described (35).
Antibodies.
Anti-Myc monoclonal antibody (MAb) was purchased from Invitrogen, and anti-Myc polyclonal antibody was from Sigma-Aldrich (Italy). The following antibodies were described previously: MAb-30 (anti-gD) (4), H233 (anti-gB) (39), 52S (anti-gH) (49), VIII-62-65 (anti-gL) (38), MAb to giantin (32), and polyclonal antibody to calnexin (52).
Insertion of Myc tag in gK and VP22.
The Myc tag (10 residues) was inserted in frame at residue 283 of HSV-1(F) gK by a mixed PCR technique. The gK open reading frame was first PCR amplified in two segments carrying the Myc epitope (underlined sequences) at the 3′ or 5′ end with primers 5′-TGACGC CGGGAA TTCATG CTCGCC GTC (EcoRI-fw1) and 5′-CAGATC CTCTTC TGAGAT AAGCTT TTGTTC CTTGTC TGCGTT CTTGGG GGC (rev1) and primers 5′-GAACAA AAGCTT ATCTCA GAAGAG GATCTG GCCGCC GCCCCG GGGCGA TCC (fw2) and 5′-CGGCCT GGGCGG CCGCTC ATACAT CAAACA G (NotI-rev2), respectively. The amplified 5′ and 3′ gK fragments were then mixed together and further amplified with external primers (EcoRI-fw1 and NotI-rev2); the Myc-tagged full-length gK fragment obtained in this way was finally cloned into the EcoRI-NotI sites of the MTS1 vector to give the wtgK-MTS plasmid, which expresses gK sequences under pCMV control. syn-gK (A40V)-MTS plasmid was obtained by site-directed mutagenesis of pwtgK-MTS in bacterial strain BMH 71-18 mutS (Promega) by using primer 5′-GCACCG ATGTAT TTACGT GGTACG CCCCAC CGGTAC CAACAA CGACAC C. T4 polymerase was used in the synthesis step to ensure high fidelity during the polymerization reaction. The construct was sequenced for accuracy. The HSV-1(F) VP22 coding sequence was PCR amplified with primers 5′-GCG CAT CCG ACG CTA GCG TGT TCG (forward) and 5′-TGG GTA CGG AAG CTT ACA CTC GAC GGG C (reverse) and cloned as an NheI-HindIII fragment in the corresponding sites of the pcDNA3.1(−) Myc-HysB vector (Invitrogen), to yield the VP22-Myc plasmid.
Construction of plasmids expressing HSV glycoproteins.
The glycoprotein genes amplified from the HSV-1(F) genome were cloned in the pMTS-1 vector (57), under the control of the cytomegalovirus (CMV) early promoter, as detailed elsewhere (56). This vector is suitable for constitutive expression in mammalian cells. It may also be employed to recombine gK into the baculovirus genome to generate a recombinant baculovirus able to express gK in mammalian cells.
Cell-cell fusion assay.
Subconfluent cultures of BHK or Vero cells, grown on glass coverslips in 24-well plates, were transfected with a DNA mixture containing 20, 40, or 80 ng (as specified in Results) each of the gB, gD, gH, and gL expression plasmids (56), together with 80 ng of pcDNA 3.1(−) Myc-His/Lac vector (Invitrogen), for constitutive expression of β-galactosidase. wt gK-Myc or syn-gK-Myc plasmids were cotransfected at the indicated amounts. The total amount of plasmid DNA transfected per well was kept at 560 ng/well by the addition of empty p-MTS plasmid DNA. Transfections were performed by using Polyfect (Qiagen) according to the manufacturer's instructions. After incubation at 37°C for 24 or 48 h, cells were fixed with 0.2% glutaraldehyde and 0.2% paraformaldehyde in phosphate-buffered saline (PBS). Syncytia were detected by light microscopy observation of β-galactosidase-expressing cells after staining with 5-bromo-4-chloro-3-indolyl-β-galactopyranoside (X-Gal) with a Leitz microscope equipped with a Kodak DC120 digital camera and Kodak Digital Science one-dimensional LE 3.0 software. Blue syncytial areas were quantified by means of the Photoshop Histogram program.
Immunofluorescence.
Subconfluent BHK, I143 tk−, and Vero cells grown on glass coverslips were transfected with the glycoprotein-expressing plasmids. When indicated, cells were infected with R7032 (5 PFU/cell) at 24 h after transfection and fixed with methanol 10 h later. For colocalization experiments with antibodies to giantin (1:100) or to calnexin (1:50), cells were fixed with paraformaldehyde and permeabilized with 0.1% Triton in PBS. Fixed cells were incubated for 1 h at room temperature with anti-glycoprotein MAbs (1:400), followed by anti-mouse fluorescein- or rhodamine-conjugated immunoglobulin G (Sigma and Jackson Laboratory, respectively). Wheat germ agglutinin (WGA)-fluorescein (Sigma-Aldrich) or concanavalin A (ConA)-rhodamine (Sigma-Aldrich) was employed at a 1:150 dilution in PBS.
Radioimmunoprecipitation.
BHK cells grown in T25 flasks, transfected or not with pwtgK-MTS, were metabolically labeled overnight with [35S]methionine-cysteine mixture (50 μCi/ml in Dulbecco's modified Eagle's minimal essential medium without methionine and cysteine; Radiochemical Center, Amersham). Cells were lysed at 36 h after transfection with PBS containing 1% Nonidet P-40, 1% sodium deoxycholate, and 0.1 mM (each) protease inhibitors Nα-p-tosyl-l-lysine chloromethyl ketone (Sigma) and l-1-tosylamide-2-phenyl-methyl-chloromethyl ketone (Sigma). After high-speed centrifugation (100,000 × g for 1 h), clarified supernatants were incubated with anti-Myc antibody (1:100) for 3 h at 4°C. The immune precipitates were collected with protein A-Sepharose beads (Sigma), extensively washed, and loaded in sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis (SDS-12% PAGE) after denaturation in electrophoresis loading buffer for 1 h at 37°C. The fixed gel was soaked for 15 min in Amplify (Radiochemical Center, Amersham) and exposed to a phosphorimager (Bio-Rad) equipped with Molecular Analyst software.
RESULTS
Construction and expression of Myc-tagged gK.
In order to detect gK, a Myc tag was inserted at residue 283, as detailed in Materials and Methods. This position was previously shown to accept a heterologous epitope without modifying gK function (20). The Myc-tagged gK was subsequently cloned in the pMTS vector (57), under the control of the CMV early promoter, generating pwtgK-MTS.
The expression of gK was verified in BHK cells 48 h after transfection with pwtgK-MTS. Cells were labeled with [35S]methionine-cysteine mixture between 24 and 36 h after transfection. gK was immunoprecipitated with anti-Myc MAb, and the proteins were separated by SDS-PAGE. Figure 1 shows that pwtgK-MTS-transfected cells, but not cells transfected with empty vector, expressed a protein with apparent molecular mass of 38 kDa, a molecular mass compatible with that of untagged gK (20).
FIG. 1.
Radioimmunoprecipitation of Myc-tagged gK. BHK cells were transfected with pwtgK-MTS encoding the Myc-tagged gK or left untransfected. Cells were labeled with a mixture of [35S]methionine and cysteine and harvested 36 h after transfection. Anti-Myc antibody was used to immunoprecipitate gK. The immunoprecipitated proteins were separated by SDS-PAGE and visualized by phosphorimager analysis. Left lane, molecular weight (MW) markers; figures on left, molecular weights of markers, in kilodaltons. The arrow points to gK.
gK localizes predominantly to the ER in both uninfected and HSV-infected cells.
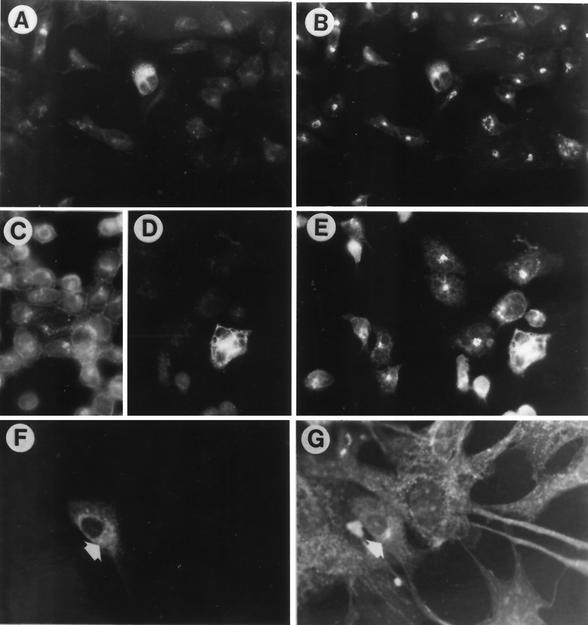
In order to investigate the intracellular localization of gK, Vero, BHK, and 143-tk− cells were transfected with pwtgK-MTS and stained for immunofluorescence at the indicated times. Replicate cultures were superinfected with R7032, an HSV recombinant that does not express gE and gI (35), two Fc-binding proteins that contribute to unspecific antibody binding (30). Cells were stained with the anti-Myc antibody and double-stained with the endoplasmic reticulum (ER) marker calnexin (52) or ConA or with the Golgi marker giantin (32) or WGA. The first lectin reacts with mannose-rich oligosaccharides typical of ER glycoconjugates and is therefore considered an ER marker, whereas the second reacts with complex-type oligosaccharides typical of the Golgi apparatus (see reference 8). Figures 2 and 3 show that in all cells gK distribution was diffused to the cytoplasm and reticulum (Fig. 2A, C, and E and 3A, D, and F), coincided with that of calnexin (Fig. 2B) and ConA (Fig. 2F), and differed somewhat from that of giantin (Fig. 3B) or WGA (Fig. 3G), suggesting a predominant localization at the ER. The same localization was observed in BHK cells, although observation of these cells is somewhat more difficult, due to their long and spindle shape (data not shown). gK localization in R7032-superinfected cells coincided with that of the calnexin and ConA markers (Fig. 2D and F) and also with that of giantin (Fig. 3E), whose distribution appeared to be grossly increased and enlarged in transfected superinfected cells. We note that enlargement and cell type-dependent modifications of the Golgi apparatus following HSV infection have been known for a long time (8). Altogether, the results indicate that Myc-gK had an intracellular distribution essentially similar to that of authentic gK (28), with a preferential colocalization with the ER markers and with the modified Golgi. Occasionally, in R7032-superinfected cells, gK appeared also at plasma membranes (Fig. 2C).
FIG. 2.
Pairs of micrographs of 143-tk− and Vero cells transfected with pwtgK-MTS and double stained with antibody to Myc (A, C, and E), to calnexin (B and D), or ConA (F). Anti-Myc antibody only stained the transfected cells, whereas the antibody to calnexin and the lectin stained all cells. In panels C and D, transfected cells were superinfected with R7032 at 24 h after transfection and fixed 10 h later. Panels A, C, and E show that anti-Myc staining was reticular and diffused to the cytoplasm, with a pattern indistinguishable from that of calnexin or ConA (arrows), in both uninfected and R7032-infected cells. Panels A to D, 143-tk− cells; magnification, ×40. Panels E and F, Vero cells; magnification, ×63.
FIG. 3.
Pairs of micrographs of 143-tk− and Vero cells transfected with pwtgK-MTS and double stained with antibody to Myc (A, D, and F) and to giantin (B and E) or WGA (G). Anti-Myc antibody only stained the transfected cells, whereas the MAb to giantin and WGA stained all cells. In panels C to E, transfected cells were superinfected with R7032 24 h after transfection and fixed 10 h later. In panel C, cells were stained with anti-gD MAb to show that all cells in the culture were indeed infected. Panels A, D, and F show that anti-Myc staining was reticular and diffused to cytoplasm. This pattern overlaps with that of giantin in infected cells (E) but not in the uninfected cells (B and G). Panels A to E, 143-tk− cells; magnification, ×40. Panels F and G, Vero cells; magnification, ×63.
Expression of gK inhibits cell-cell fusion in different cell lines.
In order to investigate whether gK inhibits cell-cell fusion, BHK cells were cotransfected with plasmids encoding gD, gB, gH, and gL, together or not with the wt gK-Myc plasmid. A plasmid carrying a LacZ gene under the CMV promoter (Invitrogen) was included. In this way the transfected cells can be monitored by X-Gal staining, and the cells recruited in syncytia can be quantified from digital micrographs, as detailed in Materials and Methods. As expected, cells recruited in syncytia expressed all the four glycoproteins (Fig. 4). In preliminary experiments, performed with equimolar amounts of each plasmid (80 ng each/well), we observed that the number of cells recruited in syncytia was smaller in the cultures expressing wt gK. Such a condition does not necessarily reflect the steady-state accumulation of equimolar amounts of gK relative to the other glycoproteins, as each glycoprotein is subjected to different posttranscriptional control, intracellular trafficking, and degradation, etc. In particular, gK appears to be poorly expressed, at least by immunofluorescence, relative to the other glycoproteins. Given that cells were already being transfected with the maximum allowable amount of DNA, in order to relatively increase the amount of gK, we reduced the amount of plasmid DNA for the four glycoproteins to 40 or 20 ng per well and increased that of the gK plasmid to 160 ng per well. In all assays, the amount of DNA was made equal by the addition of empty p-MTS-1 vector. The results of the experiment illustrated in Fig. 5 are quantitated in Fig. 6 and 7. It can be seen that gK expression inhibited syncytium formation by about 70% at 48 h. Syncytium formation by the four glycoproteins showed a tendency toward a dose-dependent effect (compare 20 and 40 ng at 48 h, in the absence of gK) and increased over time. To ensure that the inhibitory effect of gK was specific and not due to a toxic effect of the Myc epitope, a Myc-tagged HSV protein, VP22, replaced gK in replicate fusion assays. Myc-VP22 did not significantly affect syncytium formation (Fig. 6).
FIG. 4.
Micrographs of BHK cells cotransfected with the plasmids encoding gB, gD, gH, and gL and stained with MAbs to gB (A), gD (B), gH (C), and gL (D).
FIG. 5.
Digital micrographs of BHK (upper and middle images) or Vero (lower images) cells transfected with the four glycoproteins (gB, gD, gH, and gL) in the absence (left) or presence of wt-gK (middle) or in the presence of syn-gK (right). All cultures were also transfected with a LacZ plasmid. Cells were stained with X-Gal. Middle images show higher magnifications of upper panels. BHK cells received 40 ng (each) of gB, gD, gH, and gL plasmids and 160 ng of gK plasmid. Vero cells received 80 ng (each) of gB, gD, gH, and gL plasmids and 160 ng of gK plasmid.
FIG. 6.
Quantification of effect of wt gK and syn-gK on syncytium formation in BHK cells. Digital micrographs of cultures transfected with 20 ng (or 40 ng) (each) of plasmid DNA for the four glycoproteins (gD, gB, gH, and gL), 160 ng of gK plasmid or VP22-Myc plasmid, and 80 ng of LacZ plasmid. Cells were stained with X-Gal 24 or 48 h after transfection. Areas of syncytia were quantified as detailed in Materials and Methods. At least 6 pictures were scored for each sample. The average is expressed in each bar. Thin lines represent standard errors.
FIG. 7.
Quantification of effect of wt gK and syn-gK on syncytium formation in Vero cells. Digital micrographs of cultures transfected with 80 ng (each) of gD, gB, gH, and gL, 160 ng of gK plasmid, and 80 ng of LacZ plasmid. Cells were stained with X-Gal 48 h after transfection. Areas of syncytia were quantified as detailed in Materials and Methods. The average is expressed in the each bar. Thin lines represent standard errors.
BHK cells were the cells of first choice because they are very prone to syncytium formation following transfection of the four HSV glycoproteins (56). In order to ascertain whether the inhibitory effect of gK could be observed in another cell line, the experiment was repeated in Vero cells. These cells have a lower intrinsic propensity to form syncytia. Therefore, each well was transfected with 80 ng of each of the four glycoprotein plasmids and 160 ng of gK plasmid. It can be seen from Fig. 5 and 7 that gK reduced syncytium formation in Vero cells, implying that gK inhibitory activity may be cell line independent. Other cell lines (e.g., Hep-2 and SK-N-SH) could not be investigated because of their idiosyncratic resistance to undergoing fusion in this assay. Attempts to express gK from baculovirus yielded a relatively low number of transduced cells (data not shown). This limited the possibility of investigating whether expression of gK prior to or subsequent to the fusogenic glycoproteins exerted a more drastic reduction on fusion.
Effect of syn-gK on cell-cell fusion.
Cells infected with syncytial strains of HSV give rise to polykaryocytes. Here we wanted to ascertain whether the mechanism by which a syn allele of gK exerts its syncytial phenotype is a loss of fusion-blocking activity. We introduced the A40V syn mutation in the Myc-tagged gK by site-directed mutagenesis. BHK and Vero cells were cotransfected with the syn gK and the four glycoprotein plasmids plus the LacZ plasmid. Syncytia were scored at 24 and 48 h after transfection. It can be seen from Fig. 5 and 6 that in BHK cells syn gK blocked cell-cell fusion at 48 h after transfection to a lower extent than wt gK. A partial reversion was also observed in Vero cells (Fig. 5 and 7), although, as noted above, in these latter cells the ratio of gK plasmid to those of the other glycoproteins was lower than in BHK cells.
DISCUSSION
A Myc-tagged version of HSV gK localized predominantly to the ER in transfected cells. In transfected HSV-superinfected cells, gK maintained the predominant colocalization with ER markers but colocalized also with a Golgi marker, whose distribution appeared to be modified in infected cells, in agreement with previous data on HSV-induced Golgi modifications (8). This gK distribution is in accordance with perinuclear localization reported earlier (28) and provides evidence that the localization of Myc-tagged gK does not substantially differ from that of native gK.
We show here that gK inhibited cell-cell fusion mediated by the four HSV glycoproteins, gD, gB, gH, and gL. The effect was to some extent dose dependent. The highest detectable reduction was about 70%. Complete inhibition of cell fusion was not achieved, reflecting either the need for a viral partner or limitations in the experimental system. Thus, (i) the amounts of gK that we could express relative to the other four glycoproteins appeared to be low, at least as assessed by immunofluorescence. (ii) The temporal pattern of gK expression relative to that of the glycoproteins was not the most appropriate, as gK was probably required at the time the other glycoproteins were being synthesized. Altogether, the results show that gK exerted an anti-cell-cell fusion activity and that this inhibition occurred in the absence of viral proteins other than the four fusogenic glycoproteins and was observed in two cell lines. The results do not preclude the possibility that, in the infected cell, gK interacts with a viral protein not present in the assay, e.g., one of the proteins that are sites of syn mutations, and that in the presence of this putative partner gK may exert its full inhibitory activity. A candidate partner for gK may be the UL20 protein, which appears to affect gK activity both in HSV and in pseudorabies virus (PrV) (16, 19).
The rationale for assaying the effect of a syn allele of gK was to determine whether a correlation exists between the antifusion activity of wt gK and the lack of syncytial phenotype in wt virus-infected cells. It was expected that, if the lack of syncytial phenotype in wt HSV-infected cells is due to the antifusion activity of gK, replacing the wt gK with a syn allele results in the abolition of the block. This is what we observed, even though we only got a partial ablation of the block. The partial effect, as discussed above for wt gK, may be a consequence of inappropriate quantitative and/or temporal patterns of syn-gK expression or reflect the need for an additional viral partner. Nevertheless, the results provide evidence that gK is responsible for, or contributes to, preventing wt virus-infected cells from fusing with adjoining uninfected cells.
As mentioned in the introduction, several considerations support the hypothesis that the fusogenic potential of HSV glycoproteins is kept under negative control in the exocytic compartment. A role for gK is strongly supported by the phenotype of the cells infected with two mutant viruses, one of which carries a LacZ insertion and inactivation in the gK gene and the other carries a gK gene deletion (27, 29). The first virus was characterized by the accumulation of unenveloped nucleocapsids in the cytoplasm—indicative of fusion between the virion envelope and membranes of vesicles that encase the virions during their transport to the extracellular space (10); the second virus was characterized by accumulation of virions in the cytoplasm. For both viruses, release of virions to the extracellular space was blocked, cumulatively indicating that insertional inactivation or deletion of gK affected fusion events in the exocytic compartment that culminate in virus egress to the extracellular space (27, 29). Consistent with this view is the localization of gK to the ER. Altogether, gK is a candidate to negatively control HSV fusion both in the exocytic compartment and at the cell surface.
In the animal alphaherpesvirus PrV, gM inhibits fusion mediated by the four fusogenic glycoproteins (31). Interestingly, gM is also a polytopic glycoprotein (1, 34). In the PrV system, gK did not inhibit fusion. However, despite the analogies, the cell-cell fusion system mediated by the PrV glycoproteins differs strikingly from that of HSV. Thus, an intact gD is dispensable in PrV but not in HSV fusion (31).
gK may exert its inhibition on cell-cell fusion as a direct consequence of its presence at the cell surface, e.g., it may affect membrane fluidity by virtue of its polytopic nature, or it may affect a cellular protein involved in fusion. Alternatively, its action may be indirect, e.g., through alterations in cell surface expression of one of the fusogenic glycoproteins. The first two hypotheses seem unlikely in view of the observation that gK did not reduce fusion in a heterologous non-HSV system, e.g., fusion mediated by paramyxovirus HN and F (unpublished data). The hypothesis that gK blocks fusion by interaction with one of the fusogenic glycoproteins is tenable in view of the finding that inhibition was observed in a system where gK and the four fusogenic glycoproteins were the only viral gene products.
Current findings identify gK as a key player in the negative control of HSV fusion and show that gK blocks fusion without the requirement for viral proteins other than the fusogenic glycoproteins, even though its full inhibitory activity may be exerted in the presence of additional viral proteins. A gK-mediated block to fusion is exerted at the plasma membrane and, likely, in the ER. This adds to our understanding of the complex molecular events that govern the interaction of infected and adjacent uninfected cells. The location of syn mutations at loci other than gK suggests that HSV gains control of its fusogenic potential by multiple mechanisms, just as it gains control of its own proapoptotic activity of the cell cycle, etc., by a variety of viral gene products.
Acknowledgments
We thank Tony Minson (University of Cambridge), Pat Spear (Northwestern University, Chicago, Ill.), Peter Hauri (University of Zurich), and Ari Helenius (Swiss Federal Institute of Technology, Zurich, Switzerland) for the gift of MAbs to gH, gL, giantin, and calnexin. We thank F. Cocchi for the gift of the plasmid encoding syn-gK and C. Taddei and Luciana Dipietrangelo, both from the University of Bologna, for help with confocal microscopy. We acknowledge Elisabetta Romagnoli for invaluable help with cell cultures.
The study was supported by grants from Cofin-MIUR 2001, 2002, and 2003, FIRB, MIUR-CNR Functional Genomics, and University of Bologna.
REFERENCES
- 1.Baines, J. D., and B. Roizman. 1993. The UL10 gene of herpes simplex virus 1 encodes a novel viral glycoprotein, gM, which is present in the virion and in the plasma membrane of infected cells. J. Virol. 67:1441-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baines, J. D., P. L. Ward, G. Campadelli-Fiume, and B. Roizman. 1991. The UL20 gene of herpes simplex virus 1 encodes a function necessary for viral egress. J. Virol. 65:6414-6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bond, V. C., and S. Person. 1984. Fine structure physical map locations of alterations that affect cell fusion in herpes simplex virus type 1. Virology 132:368-376. [DOI] [PubMed] [Google Scholar]
- 4.Brandimarti, R., T. Huang, B. Roizman, and G. Campadelli-Fiume. 1994. Mapping of herpes simplex virus 1 genes with mutations which overcome host restrictions to infection. Proc. Natl. Acad. Sci. USA 91:5406-5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Browne, H., B. Bruun, and T. Minson. 2001. Plasma membrane requirements for cell fusion induced by herpes simplex virus type 1 glycoproteins gB, gD, gH and gL. J. Gen. Virol. 82:1419-1422. [DOI] [PubMed] [Google Scholar]
- 6.Bzik, D. J., B. A. Fox, N. A. DeLuca, and S. Person. 1984. Nucleotide sequence of a region of the herpes simplex virus type 1 gB glycoprotein gene: mutations affecting rate of virus entry and cell fusion. Virology 137:185-190. [DOI] [PubMed] [Google Scholar]
- 7.Cai, W. H., B. Gu, and S. Person. 1988. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J. Virol. 62:2596-2604. (Erratum, 62:4438.) [DOI] [PMC free article] [PubMed]
- 8.Campadelli, G., R. Brandimarti, C. Di Lazzaro, P. L. Ward, B. Roizman, and M. R. Torrisi. 1993. Fragmentation and dispersal of Golgi proteins and redistribution of glycoproteins and glycolipids processed through the Golgi apparatus after infection with herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 90:2798-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campadelli-Fiume, G., F. Cocchi, L. Menotti, and M. Lopez. 2000. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev. Med. Virol. 10:305-319. [DOI] [PubMed] [Google Scholar]
- 10.Campadelli-Fiume, G., F. Farabegoli, S. Di Gaeta, and B. Roizman. 1991. Origin of unenveloped capsids in the cytoplasm of cells infected with herpes simplex virus 1. J. Virol. 65:1589-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carfi, A., S. H. Willis, J. C. Whitbeck, C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and D. C. Wiley. 2001. Herpes simplex virus glycoprotein D bound to the human receptor HveA. Mol. Cell 8:169-179. [DOI] [PubMed] [Google Scholar]
- 12.Cocchi, F., M. Lopez, P. Dubreuil, G. Campadelli-Fiume, and L. Menotti. 2001. Chimeric nectin1-poliovirus receptor molecules identify a nectin1 region functional in herpes simplex virus entry. J. Virol. 75:7987-7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cocchi, F., L. Menotti, P. Mirandola, M. Lopez, and G. Campadelli-Fiume. 1998. The ectodomain of a novel member of the immunoglobulin superfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex viruses 1 and 2 in human cells. J. Virol. 72:9992-10002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connolly, S. A., D. J. Landsburg, A. Carfi, D. C. Wiley, R. J. Eisenberg, and G. H. Cohen. 2002. Structure-based analysis of the herpes simplex virus glycoprotein D binding site present on herpesvirus entry mediator HveA (HVEM). J. Virol. 76:10894-10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Debroy, C., N. Pederson, and S. Person. 1985. Nucleotide sequence of a herpes simplex virus type 1 gene that causes cell fusion. Virology 145:36-48. [DOI] [PubMed] [Google Scholar]
- 16.Dietz, P., B. G. Klupp, W. Fuchs, B. Kollner, E. Weiland, and T. C. Mettenleiter. 2000. Pseudorabies virus glycoprotein K requires the UL20 gene product for processing. J. Virol. 74:5083-5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolter, K. E., R. Ramaswamy, and T. C. Holland. 1994. Syncytial mutations in the herpes simplex virus type 1 gK (UL53) gene occur in two distinct domains. J. Virol. 68:8277-8281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forrester, A., H. Farrell, G. Wilkinson, J. Kaye, N. Davis-Poynter, and T. Minson. 1992. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J. Virol. 66:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster, T. P., X. Alvarez, and K. G. Kousoulas. 2003. Plasma membrane topology of syncytial domains of herpes simplex virus type 1 glycoprotein K (gK): the UL20 protein enables cell surface localization of gK but not gK-mediated cell-to-cell fusion. J. Virol. 77:499-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster, T. P., G. V. Rybachuk, and K. G. Kousoulas. 2001. Glycoprotein K specified by herpes simplex virus type 1 is expressed on virions as a Golgi complex-dependent glycosylated species and functions in virion entry. J. Virol. 75:12431-12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geraghty, R. J., C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618-1620. [DOI] [PubMed] [Google Scholar]
- 22.Harman, A., H. Browne, and T. Minson. 2002. The transmembrane domain and cytoplasmic tail of herpes simplex virus type 1 glycoprotein H play a role in membrane fusion. J. Virol. 76:10708-10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinz, F. X., and S. L. Allison. 2001. The machinery for flavivirus fusion with host cell membranes. Curr. Opin. Microbiol. 4:450-455. [DOI] [PubMed] [Google Scholar]
- 24.Hoggan, M. D., and B. Roizman. 1959. The isolation and properties of a variant of herpes simplex producing multinucleated giant cells in monolayer cultures in the presence of antibody. Am. J. Hyg. 70:208-219. [DOI] [PubMed] [Google Scholar]
- 25.Hutchinson, L., K. Goldsmith, D. Snoddy, H. Ghosh, F. L. Graham, and D. C. Johnson. 1992. Identification and characterization of a novel herpes simplex virus glycoprotein, gK, involved in cell fusion. J. Virol. 66:5603-5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutchinson, L., F. L. Graham, W. Cai, C. Debroy, S. Person, and D. C. Johnson. 1993. Herpes simplex virus (HSV) glycoproteins B and K inhibit cell fusion induced by HSV syncytial mutants. Virology 196:514-531. [DOI] [PubMed] [Google Scholar]
- 27.Hutchinson, L., and D. C. Johnson. 1995. Herpes simplex virus glycoprotein K promotes egress of virus particles. J. Virol. 69:5401-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutchinson, L., C. Roop-Beauchamp, and D. C. Johnson. 1995. Herpes simplex virus glycoprotein K is known to influence fusion of infected cells, yet is not on the cell surface. J. Virol. 69:4556-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jayachandra, S., A. Baghian, and K. G. Kousoulas. 1997. Herpes simplex virus type 1 glycoprotein K is not essential for infectious virus production in actively replicating cells but is required for efficient envelopment and translocation of infectious virions from the cytoplasm to the extracellular space. J. Virol. 71:5012-5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson, D. C., M. C. Frame, M. W. Ligas, A. M. Cross, and N. D. Stow. 1988. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J. Virol. 62:1347-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klupp, B. G., R. Nixdorf, and T. C. Mettenleiter. 2000. Pseudorabies virus glycoprotein M inhibits membrane fusion. J. Virol. 74:6760-6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linstedt, A. D., and H. P. Hauri. 1993. Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kDa. Mol. Biol. Cell 4:679-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez, W. M., and P. G. Spear. 2002. Amino acid substitutions in the V domain of nectin-1 (HveC) that impair entry activity for herpes simplex virus types 1 and 2 but not for pseudorabies virus or bovine herpesvirus 1. J. Virol. 76:7255-7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGeoch, D. J., C. Cunningham, G. McIntyre, and A. Dolan. 1991. Comparative sequence analysis of the long repeat regions and adjoining parts of the long unique regions in the genomes of herpes simplex viruses types 1 and 2. J. Gen. Virol. 72:3057-3075. [DOI] [PubMed] [Google Scholar]
- 35.Meignier, B., R. Longnecker, P. Mavromara-Nazos, A. E. Sears, and B. Roizman. 1988. Virulence of and establishment of latency by genetically engineered deletion mutants of herpes simplex virus 1. Virology 162:251-254. [DOI] [PubMed] [Google Scholar]
- 36.Mo, C., and T. C. Holland. 1997. Determination of the transmembrane topology of herpes simplex virus type 1 glycoprotein K. J. Biol. Chem. 272:33305-33311. [DOI] [PubMed] [Google Scholar]
- 37.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427-436. [DOI] [PubMed] [Google Scholar]
- 38.Novotny, M. J., M. L. Parish, and P. G. Spear. 1996. Variability of herpes simplex virus 1 gL and anti-gL antibodies that inhibit cell fusion but not viral infectivity. Virology 221:1-13. [DOI] [PubMed] [Google Scholar]
- 39.Pereira, L., D. Dondero, B. Norrild, and B. Roizman. 1981. Differential immunologic reactivity and processing of glycoproteins gA and gB of herpes simplex virus types 1 and 2 made in Vero and HEp-2 cells. Proc. Natl. Acad. Sci. USA 78:5202-5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pertel, P. E., A. Fridberg, M. L. Parish, and P. G. Spear. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279:313-324. [DOI] [PubMed] [Google Scholar]
- 41.Pertel, P. E., and P. G. Spear. 1997. Partial resistance to gD-mediated interference conferred by mutations affecting herpes simplex virus type 1 gC and gK. J. Virol. 71:8024-8028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pogue-Geile, K. L., G. T. Lee, S. K. Shapira, and P. G. Spear. 1984. Fine mapping of mutations in the fusion-inducing MP strain of herpes simplex virus type 1. Virology 136:100-109. [DOI] [PubMed] [Google Scholar]
- 43.Pogue-Geile, K. L., and P. G. Spear. 1987. The single base pair substitution responsible for the Syn phenotype of herpes simplex virus type 1, strain MP. Virology 157:67-74. [DOI] [PubMed] [Google Scholar]
- 44.Post, L. E., S. Mackem, and B. Roizman. 1981. Regulation of alpha genes of herpes simplex virus: expression of chimeric genes produced by fusion of thymidine kinase with alpha gene promoters. Cell 24:555-565. [DOI] [PubMed] [Google Scholar]
- 45.Ramaswamy, R., and T. C. Holland. 1992. In vitro characterization of the HSV-1 UL53 gene product. Virology 186:579-587. [DOI] [PubMed] [Google Scholar]
- 46.Roop, C., L. Hutchinson, and D. C. Johnson. 1993. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J. Virol. 67:2285-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruyechan, W. T., L. S. Morse, D. M. Knipe, and B. Roizman. 1979. Molecular genetics of herpes simplex virus. II. Mapping of the major viral glycoproteins and of the genetic loci specifying the social behavior of infected cells. J. Virol. 29:677-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanders, P. G., N. M. Wilkie, and A. J. Davison. 1982. Thymidine kinase deletion mutants of herpes simplex virus type 1. J. Gen. Virol. 63:277-295. [DOI] [PubMed] [Google Scholar]
- 49.Showalter, S. D., M. Zweig, and B. Hampar. 1981. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect. Immun. 34:684-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spear, P. G., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 275:1-8. [DOI] [PubMed] [Google Scholar]
- 51.Stiasny, K., S. L. Allison, C. W. Mandl, and F. X. Heinz. 2001. Role of metastability and acidic pH in membrane fusion by tick-borne encephalitis virus. J. Virol. 75:7392-7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tatu, U., and A. Helenius. 1997. Interactions between newly synthesized glycoproteins, calnexin and a network of resident chaperones in the endoplasmic reticulum. J. Cell Biol. 136:555-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turner, A., B. Bruun, T. Minson, and H. Browne. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 72:873-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weissenhorn, W., A. Dessen, L. J. Calder, S. C. Harrison, J. J. Skehel, and D. C. Wiley. 1999. Structural basis for membrane fusion by enveloped viruses. Mol. Membr. Biol. 16:3-9. [DOI] [PubMed] [Google Scholar]
- 55.Wittels, M., and P. G. Spear. 1991. Penetration of cells by herpes simplex virus does not require a low pH-dependent endocytic pathway. Virus Res. 18:271-290. [DOI] [PubMed] [Google Scholar]
- 56.Zhou, G., E. Avitabile, G. Campadelli-Fiume, and B. Roizman. 2003. The domains of glycoprotein d required to block apoptosis induced by herpes simplex virus 1 are largely distinct from those involved in cell-cell fusion and binding to nectin1. J. Virol. 77:3759-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou, G., V. Galvan, G. Campadelli-Fiume, and B. Roizman. 2000. Glycoprotein D or J delivered in trans blocks apoptosis in SK-N-SH cells induced by a herpes simplex virus 1 mutant lacking intact genes expressing both glycoproteins. J. Virol. 74:11782-11791. [DOI] [PMC free article] [PubMed] [Google Scholar]