Abstract
The potential of therapeutic vaccination of animals latently infected with herpes simplex virus type 1 (HSV-1) to enhance protective immunity to the virus and thereby reduce the incidence and severity of recurrent ocular disease was assessed in a mouse model. Mice latently infected with HSV-1 were vaccinated intranasally with a mixture of HSV-1 glycoproteins and recombinant Escherichia coli heat-labile enterotoxin B subunit (rEtxB) as an adjuvant. The systemic immune response induced was characterized by high levels of virus-specific immunoglobulin G1 (IgG1) in serum and very low levels of IgG2a. Mucosal immunity was demonstrated by high levels of IgA in eye and vaginal secretions. Proliferating T cells from lymph nodes of vaccinated animals produced higher levels of interleukin-10 (IL-10) than were produced by such cells from mock-vaccinated animals. This profile suggests that vaccination of latently infected mice modulates the Th1-dominated proinflammatory response usually induced upon infection. After reactivation of latent virus by UV irradiation, vaccinated mice showed reduced viral shedding in tears as well as a reduction in the incidence of recurrent herpetic corneal epithelial disease and stromal disease compared with mock-vaccinated mice. Moreover, vaccinated mice developing recurrent ocular disease showed less severe signs and a quicker recovery rate. Spread of virus to other areas close to the eye, such as the eyelid, was also significantly reduced. Encephalitis occurred in a small percentage (11%) of mock-vaccinated mice, but vaccinated animals were completely protected from such disease. The possible immune mechanisms involved in protection against recurrent ocular herpetic disease in therapeutically vaccinated animals are discussed.
Ocular herpes simplex virus type 1 (HSV-1) infection is the major cause of nontraumatic blindness in developed countries. Initial infection occurs at the corneal epithelium, where, following replication, the virus enters the sensory nerve endings, travels along axons, and becomes latent in the trigeminal ganglion (TG) (14). The virus remains as a lifelong infection in the TG, probably undetected by the immune system. Under certain conditions, which include stress or exposure to UV light, the virus may reactivate, travel back down the nerve, and cause recurrent infection, most often in the cornea (20). The immune mechanisms involved in protection against HSV-1 infections include the recruitment of proinflammatory immune cells. In the case of the eye, these cells may lead to immunopathological disease by infiltrating the stroma, causing opacity and edema of this tissue. In certain cases, the cornea may become highly vascularized and thickened, particularly after repeated recurrent infections, resulting in severe stromal keratitis and visual impairment (29). Current methods of therapy involve the administration of antiviral drugs and corticosteroids, but these are not always effective and may in some cases exacerbate disease (13). Vaccination to prevent primary infection is problematic, since the virus is often acquired very early in life. Therefore, the development of a therapeutic vaccine for individuals with an established latent infection to prevent recurrent ocular disease or significantly decrease its severity is an attractive approach.
While a number of potential vaccine candidates have been shown to provide protection against primary ocular challenge, the efficacy of the few that have been tested in recurrent models of disease has been disappointing. In one study, a virion host shutoff mutant was tested as a live therapeutic vaccine against recurrent infection in the mouse. Although this live vaccine reduced the incidence of virus shedding following reactivation, the incidence of clinical ocular disease was unaffected (34). The use of subunit vaccines incorporating glycoprotein D in mice (16) and rabbits (21) has been similarly disappointing. These difficulties reflect the complex nature of the immune response in HSV-1 infection and the requirement for vaccination to modulate the protective components of immunity while at the same time limiting immunopathology. In this regard, immunohistochemical studies indicate that the initial response to recurrent infection in the eye involves an influx of neutrophils and macrophages together with CD4+ and CD8+ T cells, indicative of a proinflammatory Th1-type response. While this response is involved in viral clearance, it is also likely to drive the pathological damage to the eye that is associated with herpetic keratitis. At later times, the presence of B cells and anti-inflammatory cytokines (interleukin-10 [IL-10]) corresponds with the resolution of ocular disease (23, 27, 28). A successful therapeutic vaccine for ocular HSV-1 disease may, therefore, be one that can modulate the nature of the immune response, providing a higher degree of protection at the mucosal surface of the eye itself while limiting the proinflammatory effects of the virally induced Th1 response.
We have previously shown that intranasal immunization with a mixture of HSV surface glycoproteins in the presence of the recombinant Escherichia coli heat-labile enterotoxin B subunit (rEtxB) as adjuvant provided protection against HSV-1 infection. Following primary ocular challenge, immunized mice showed much reduced corneal disease and only limited spread of virus in the nervous system. The latter was evidenced by a reduction in zosteriform lesions and the incidence of latency in regions of the TG not served by the ophthalmic nerve. Immunized mice were also completely protected against the development of encephalitis, even under challenge conditions, in which the mortality in control, mock-vaccinated animals was as high as 95% (22). The immune response induced by our intranasal vaccine was characterized by the presence of strong secretory IgA responses to HSV-1 antigens in mucosal washings and serum neutralizing antibodies. The dominance of IgG1 in the serum antibody response together with the presence of high levels of IL-4 and IL-10 in lymph node cell cultures from immunized mice suggested that the anti-HSV-1 response was Th2 dominated. However, there was also some gamma interferon (IFN-γ) production in such cultures, indicating that Th1 immunity was also present. We hypothesized that the type of immune response generated to our vaccine may be compatible with modulating immunity in the latently infected animal. In order to test this hypothesis, we have utilized a well-characterized mouse model of recurrent herpetic eye disease (24, 25). Mice infected by corneal scarification in the presence of passive antibodies develop mild epithelial disease in 80 to 100% of cases. In our hands, this leads to the establishment of latency in approximately 93% of animals, as determined by inoculation of medium from 5-day TG explant cultures onto Vero cells (26). A slightly modified version of this model used by another laboratory shows that 80 to 100% of mice had latent virus in the TG following ocular infection (16). Reactivation of virus by exposure to UV light occurs in approximately 60% of mice, as determined by viral shedding, incidence of disease, and cell infiltration (24, 28). We describe here the effects of vaccination on the development of recurrent herpetic ocular disease in this mouse model.
MATERIALS AND METHODS
Reactivation model.
Female, specific-pathogen-free NIH mice obtained from Harlan Olac, Bicester, United Kingdom, were maintained in the School of Medical Sciences, University of Bristol, Bristol, United Kingdom. At 8 weeks of age, mice were inoculated with HSV in order to establish latent infection in the TG, according to the method described previously (24). Briefly, mice were inoculated intraperitoneally with human serum (Harlan Sera-Lab, Ltd., Loughborough, United Kingdom), containing HSV-1 serum neutralizing antibodies, which was assayed to determine the serum antibody titer required to give 50% virus plaque reduction (50% effective dose [ED50]) (25). Serum was diluted in phosphate-buffered saline (PBS) to give an ED50 of 8,000 (24). After 24 h, mice were anesthetized (100 mg of ketamine per kg of body weight [Parke-Davis, Pontypool, United Kingdom] mixed with 10 mg of xylazine per kg [Bayer, Bury St. Edmunds, United Kingdom]) and infected with 106 PFU of HSV-1 McKrae in a 5-μl drop of medium by ocular scarification of the right cornea with a 26-gauge needle (24). Six weeks after infection, the right eyes of all mice were checked for the presence of any abnormalities, and such mice were discarded. The remaining mice were immunized intranasally three times at 10-day intervals with either 10 μg of HSV-1 glycoproteins, prepared from Vero cells infected with live HSV-1, or mock glycoproteins, prepared from uninfected Vero cells, each mixed with 20 μg of rEtxB to give a final volume of 47 μl (22). Two to 4 weeks after the final immunization, animals were anesthetized and placed with their right eye proptosed below a Hanovia lamp (emitting a peak of 4.02 mJ/cm2 s at 320 nm), and the right corneas and lids were irradiated for 90 s in order to induce reactivation (25).
Measurement of antibody responses.
Individual mice were bled from the tail vein 4 weeks after corneal scarification and 1 week after the final immunization, and the serum was stored at −20°C. Serum from mice infected without passive immunization was collected for use as a positive control. Eye and vaginal washings were collected from mice anesthetized with halothane by pipetting 20 μl of PBS up and down on the surface of each eye 10 times or with 50 μl of PBS pipetted in and out of the vagina 20 times. Samples collected from individual mice over several days were pooled and stored at −20°C.
Sera were analyzed for the presence of HSV-1-specific antibodies, as described previously (7). Briefly, assay plates coated with rabbit anti-HSV-1 (Dako, Ltd., High Wycombe, United Kingdom) were incubated with 1% bovine serum albumin in PBS, followed by HSV-1 antigen, mouse serum, and a rabbit anti-mouse Ig-horseradish peroxidase (HRP) conjugate (Dako Ltd.), with O-phenylenediamine (Sigma) as a substrate.
In order to measure the levels of virus-specific IgA in mucosal fluids, the conjugated secondary antibody was replaced with an HRP-conjugated goat anti-mouse IgA (Sigma). The level of IgG1 and IgG2a in the sera was measured with HRP-conjugated rat anti-mouse IgG1 or IgG2a, respectively. The endpoint titers for individual samples were determined by linear regression analysis.
The presence of neutralizing antibodies in sera of infected mice and mice immunized with HSV-1 or mock glycoproteins was determined by pooling aliquots of serum from individual mice in each group, collected as described above, for use in a plaque reduction assay. The ED50 was calculated by weighted probit analysis (2), and the titers are given as reciprocals.
Assessment of T-cell responses.
Single-cell suspensions of lymphocytes in Hanks' balanced saline solution (HBSS) (Gibco, Paisley, United Kingdom) containing 20 mM HEPES buffer (GIBCO) were prepared from draining lymph nodes, removed from mice either 4 weeks after final immunization or UV irradiation by agitation through wire mesh with a glass rod. Lymphocytes were washed and then cultured at 106 cells per ml in minimal essential medium α (α-MEM), supplemented with 20 mM HEPES, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 4 mM l-glutamine (Gibco), 50 μM 2-mercaptoethanol (Sigma), and 0.5% normal autologous mouse serum in 25-cm2 flasks. Cells were cultured in the presence of UV-inactivated virus (prepared from serum-free supernatant of infected Vero cells) at a predetermined, optimal concentration of 1.5 × 105 PFU/ml, an equivalent dilution of mock virus for assessment of nonviral responses, or medium alone (data not shown). Cultures were incubated at 37°C in a humidified atmosphere of 5% CO2. Aliquots of 100 μl were removed on desired days after initiation of the cultures and placed in triplicate into wells of a 96-well plate for assessment of [3H]thymidine incorporation by standard assay techniques (12).
Additional aliquots of cells were removed for assessment of cytokine levels by a previously described method (3). Briefly, cell samples, set up in triplicate, were cultured overnight at 37°C in a humidified atmosphere of 5% CO2 in capture antibody-coated (rat anti-mouse cytokines) enzyme-linked immunosorbent assay (ELISA) plates, before detection with biotinylated rat anti-mouse cytokines (BD Pharmingen, San Diego, Calif.). Thus, cytokine production by cells over a defined period of culture could be assessed under conditions in which the effect of cytokine lability was minimized. Cytokine levels were calculated by regression analysis against standard curves produced with the appropriate recombinant cytokine (BD Pharmingen, San Diego, Calif.).
Analysis of ocular disease and isolation of virus from eye washings following reactivation of latent virus.
Following corneal scarification, eyes were examined on days 1, 3, and 7 with a Zeiss 105L slit lamp microscope (Zeiss, Welwyn Garden City, United Kingdom) for development of epithelial ulcers, stromal disease, and uveitis. The eyes were also checked prior to immunization, and any mice with damaged corneas were removed from the experiment. Similarly, mice were analyzed following UV irradiation on days 1, 2, 4, 6, 10, and 14 for ocular disease as well as for spread of virus to other areas, resulting in ulceration and edema of the eyelid as well as zosteriform herpetic lesions in the skin at sites served by the TG (the snout and lower jaw). The severity of each disease parameter was also scored in those mice with disease (1 = mild disease and 5 = most severe disease). Piloerection, loss of weight, hunched posture, and a significant defect in righting reflex were taken as signs of encephalitis, and such animals were killed by cervical dislocation.
Eye washings were collected on day 0 to check for any spontaneous reactivation, as well as on days 2, 3, 4, 5, and 6, from irradiated eyes by pipetting 20 μl of culture medium onto the surface of the proptosed eye 10 times and transferring the washes to Vero cells for isolation of virus (30).
Statistical analysis.
Significant differences in the immune response and clinical disease score between groups of mice were determined by Student's t test. Incidence data were analyzed with the χ2 test.
RESULTS
Modulation of the anti-HSV-1 antibody response in latently infected mice.
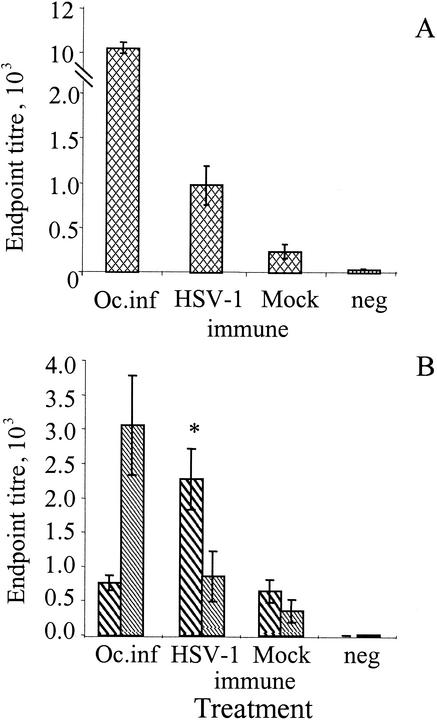
High levels of anti-HSV-1 antibodies were detected in the serum of mice that had been infected with HSV-1 by corneal scarification, with endpoint titers in excess of 1:10,000 (Fig. 1A). Serum from passively immunized mice infected by corneal scarification had low levels of anti-HSV-1 antibodies following immunization with mock glycoproteins (prepared from uninfected Vero cells) mixed with rEtxB as adjuvant. In contrast, immunization with glycoproteins from HSV-1-infected Vero cells mixed with rEtxB dramatically enhanced the levels of anti-HSV-1 antibodies in latently infected animals. The mean endpoint titer in mock-immunized mice was 1:236, compared to 1:971 for the immunized group (P < 0.01).
FIG. 1.
HSV-1-specific serum responses in mice 4 weeks after corneal scarification with HSV-1 McKrae and 1 week following HSV-1 immunization and mock immunization of latently infected mice as determined by ELISA. The serum Ig responses to HSV-1 antigens were analyzed by regression analysis, and the endpoint titers were determined (A). Endpoint titers were also determined for HSV-1-specific serum IgG1 (bold hatched bars) and IgG2a (light hatched bars) in IgG subclass-specific ELISA (B). Mean values with standard error of the mean were calculated from groups of 20 immunized mice and 5 mice following ocular infection (Oc.inf). Sera from naïve mice were included as the negative control (neg). An asterisk shows a significant difference in response between HSV-1-vaccinated and mock-vaccinated mice as judged by Student's t test (P < 0.01). The data presented here are representative of three similar experiments.
Analysis of the serum IgG subclasses following corneal scarification alone showed that IgG2a was the dominant subclass, resulting in an IgG1/IgG2a ratio of 0.25 (Fig. 1B). In contrast, following HSV-1 immunization of latently infected mice, IgG1 was the dominant subclass, with an endpoint titer of 1:2,276 compared with IgG2a at 1:858. The ratio of IgG1 to IgG2a following immunization in the presence of rEtxB was 2.65. This is a considerable enhancement of the IgG1 and IgG2a responses observed in mock-immunized mice with an IgG1 endpoint titer of 1:640 compared with 1:360 for IgG2a, giving an IgG1/IgG2a ratio of 1.78.
Further characterization of HSV-1-specific antibody responses was undertaken, using pooled serum samples, to assess virus neutralization. Serum from infected only mice gave virus neutralization titers of 1:1,083. Latently infected mice immunized with HSV-1 glycoproteins gave an ED50 titer of 1:107, which was in marked contrast to mock-immunized mice, which gave an ED50 titer of 1:7 (data not shown).
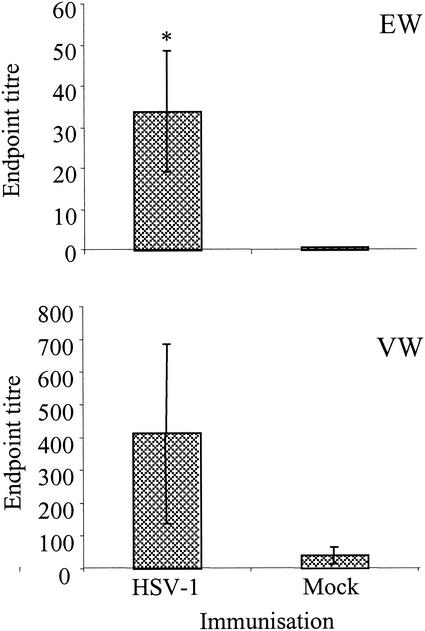
In addition to the stimulation of serum antibodies to HSV-1, immunization of latently infected mice triggered the production of high levels of secretory IgA, particularly at the ocular surface, local to the site of administration, as well as the vagina, a more distant site (Fig. 2). The mean endpoint titer for eye washings from immunized mice, taken 1 week to 10 days following the final immunization, was 1:34. This is a significant increase compared with that of the mock-immunized controls, which gave a mean endpoint titer of 1:0.5 (P < 0.05). The presence of IgA in vaginal washings of immunized mice resulted in a mean endpoint titer of 1:412 compared with 1:40 for the mock-immunized mice. This difference, however, was not significant due to the large variation between individuals within the same group.
FIG. 2.
HSV-1-specific IgA in mucosal washings collected from the eye (EW) and vagina (VW) of HSV-1-vaccinated and mock-vaccinated mice as determined by ELISA. Endpoint titers were calculated by regression analysis for individual mice (n = 20), and mean values ± standard errors were determined. An asterisk shows significant difference between HSV-1-vaccinated and mock-vaccinated mice as judged by Student's t test (P < 0.05).
Effects of immunization on anti-HSV-1 T-cell responses in latently infected animals.
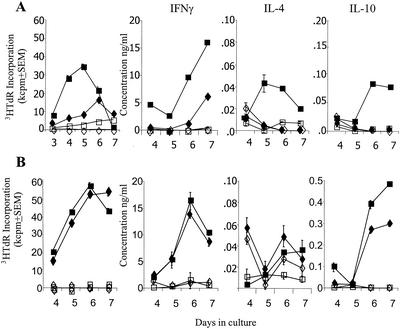
The effect of immunization of latently infected mice on T-cell responses was investigated with lymphocytes isolated from draining lymph nodes either 4 weeks after immunization and prior to reactivation or 4 weeks after subsequent UV-induced reactivation of virus. Cells were cultured in the presence of UV-inactivated HSV-1 or mock antigen, and T-cell proliferation and cytokine production were assessed on days 4 to 7. The results showed strong T-cell proliferative responses to HSV-1 antigens by cells from latently infected, HSV-1-immunized animals (Fig. 3A). A higher and more rapid response indicative of a secondary recall response was observed with cells following reactivation of latent virus in both HSV-1- and mock-immunized animals, with peak incorporation of [3H]thymidine in excess of 50 kcpm on days 6 to 7 (Fig. 3B). The response of these cell cultures to mock antigen was negligible, indicating that proliferation of T cells was specific for viral antigens.
FIG. 3.
Draining lymph node cells from HSV-1-vaccinated (squares) and mock-vaccinated (diamonds) mice prior to reactivation (A) or postreactivation (B), cultured in vitro with HSV-1 (solid symbols) or mock antigen (open symbols), were analyzed for proliferative responses by [3H]thymidine (3HTdR) incorporation and secretion of cytokines IFN-γ, IL-4, and IL-10. Mean values ± standard errors of triplicate cultures are shown. The data presented here are representative of three similar experiments.
Analysis of the cytokines secreted by proliferating T-cell cultures indicated that following immunization, IFN-γ was the main cytokine secreted, with low levels of IL-10 and IL-4 compared to the results for cells from mock-immunized mice and those cultured with mock antigen. Following reactivation, a more rapid IFN-γ response was seen from cells from both HSV-1-immunized and mock-immunized mice in response to HSV-1 antigen, with peak concentrations of IFN-γ observed on day 6 of 16 and 14 ng/ml, respectively. Interestingly, the presence of IL-10 was observed in cells cultured following reactivation of virus, but was significantly enhanced in cell cultures from HSV-1-immunized mice compared to mock-immunized mice, with levels of 0.48 ng/ml on day 7 compared with 0.29 ng/ml, respectively. Increased production of IL-10 in cultures from immunized mice following reactivation was observed in each of three similar experiments. While some IL-4 was detected, the levels observed were very low, making the differences between cultures difficult to distinguish.
Clinical disease and viral shedding following reactivation of latent virus.
Analysis of the eyes of passively immunized mice infected by corneal scarification revealed that 97.5% of mice developed corneal ulcers by day 3. Of these, 50.5% developed some signs of stromal disease and 25% demonstrated uveitis, although none of these developed severe disease symptoms and thus had recovered by day 7 following infection.
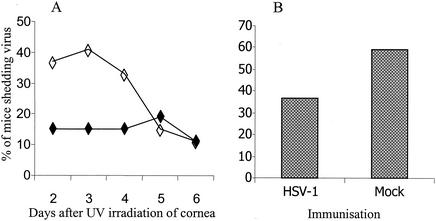
The effect of modulating the immune response to HSV-1 antigens on recurrent herpetic ocular disease was determined following reactivation of latent virus by exposure to UV light. Eye washings were collected immediately prior to UV irradiation to ensure that the presence of virus was not the result of spontaneous shedding; such shedding is rare in this model (21, 22). Indeed, no spontaneous viral shedding was detected in these experiments, indicating that any virus present was most likely due to UV-induced reactivation. The main period for detection of reactivated virus in eye washings taken from mock-immunized mice was represented by days 2, 3, and 4, with a peak incidence on day 3 of 41% (Fig. 4A). In contrast, reactivated virus was only observed in 15% of HSV-1-immunized mice on days 2 to 4, with a peak incidence of 19% occurring on day 5. A significant reduction in the overall incidence of mice shedding virus, over the 10 days following reactivation, was observed between HSV-1-immunized (37%) and mock-immunized (59%) mice (Fig. 4B; χ2 test, P < 0.01).
FIG. 4.
Percentage of HSV-1-vaccinated (solid symbols) and mock-vaccinated (open symbols) mice shedding virus in eye washings days 2 to 6 after UV-induced reactivation of latent virus (A) and total percentage of mice shedding virus on at least 1 day (B). A significant reduction in viral shedding was observed in HSV-1-vaccinated mice (χ2 test, P < 0.01).
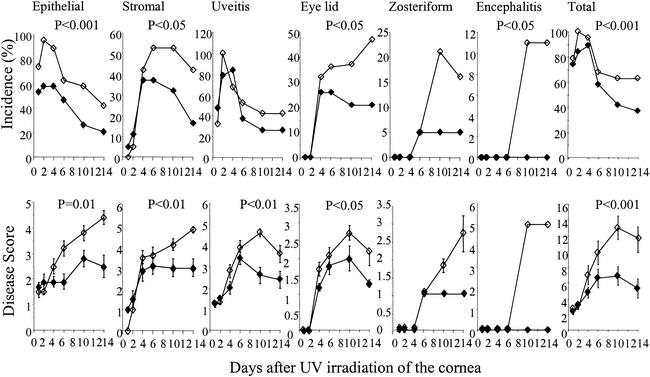
Reactivation of HSV-1 is partly a consequence of corneal damage caused by exposure to UV light resulting in the development of some corneal abnormalities immediately following UV treatment in both groups of mice. Such abnormalities, however, are short term, and only those mice with HSV-1-associated disease continue to show signs of corneal disease and increased severity. Thus, the incidence of epithelial disease in HSV-1-vaccinated mice was significantly reduced over the 14 days analyzed compared with that in the mock-vaccinated mice (χ2 test, P < 0.001), with a maximum of 58% compared with 95%, respectively, on day 2 (Fig. 5). Stromal disease levels were initially similar in both groups of mice, but those vaccinated with HSV-1 peaked at 37% on days 4 to 6 and then declined steadily so that only 16% were affected by day 14. Such disease in mock-immunized mice increased significantly to a peak of 53% by day 6, staying at this level until day 10, before declining to 42% by day 14 (χ2 test, P < 0.05%). No significant difference in the incidence of uveitis between the two groups was observed, with 84% of HSV-1-immunized mice showing signs of uveitis on day 4 compared with 100% for mock-immunized animals on day 2. Lid disease was first observed in both groups on day 4, with 26% of HSV-1-immunized mice affected compared with 32% of mock-immunized mice. The incidence of lid disease in mock-immunized mice continued to increase significantly to a peak of 47% by day 14. In contrast, a significantly lower incidence of lid disease was observed up to day 14 in HSV-1-immunized mice (χ2 test, P < 0.05), with only 21% affected by day 10. Likewise, zosteriform spread was observed in 5% of mice from both groups on day 6, but no further development of this form of the disease was observed in HSV-1-immunized mice. In contrast, in the mock-immunized mice, the incidence of zosteriform spread increased further to 21%. By day 10, 11% of mock-immunized mice developed encephalitis and were killed by cervical dislocation. Taken together, a significant reduction in the incidence of HSV-1-associated disease was observed in HSV-1-immunized versus mock-immunized mice over the days analyzed following reactivation of virus by UV irradiation (χ2 test, P < 0.001).
FIG. 5.
Incidence and severity of the different clinical parameters following reactivation of latent virus in HSV-1-vaccinated (solid symbols) and mock-vaccinated (open symbols) mice. Disease scores run from 0 (no disease) to 5 (very severe disease). Each group started with 20 mice. Disease scores represent mean values of mice with disease in each group ± standard errors. Significant differences between HSV-1- and mock-vaccinated mice were determined by Student's t test.
In addition to this clear difference in disease incidence, analysis of disease severity in the mice with clinical symptoms further highlighted the protective efficacy of intranasal vaccination of latently infected animals. Although similar levels of ocular disease were observed in both groups of mice in the first few days following reactivation of virus, the mice vaccinated with HSV-1 glycoproteins and rEtxB recovered quickly from UV damage, whereas mock-vaccinated animals continued to develop disease and disease severity increased. In particular, a significant reduction in disease severity was observed for epithelial disease (Student's t test, P < 0.01), stromal disease (Student's t test, P < 0.01), uveitis (Student's t test, P < 0.01), and lid disease (Student's t test, P < 0.05). Furthermore, HSV-1-vaccinated mice developed less severe zosteriform lesions, and none showed signs of encephalitis. Thus, there was a significant difference in total incidence and severity of recurrent clinical disease between the two groups (Fig. 5; Student's t test, P < 0.001).
DISCUSSION
In this paper, we show that intranasal vaccination of latently infected mice with a mixture of HSV-1 glycoproteins and EtxB can stimulate protective immunity in a well-characterized mouse model of recurrent ocular herpetic disease. The immune response generated in this way was characterized by increased anti-HSV-1 antibody levels of local IgA and serum IgG, an increased IgG1/IgG2a ratio, and elevated IL-10 production by lymphocytes from local lymph nodes. Vaccination was also associated with a reduction in the percentage of mice shedding virus at the corneal surface, together with reduced incidence of disease induced by UV irradiation. Furthermore, the mice that developed clinical symptoms showed enhanced recovery and limited spread of virus through the nervous system, with only a few vaccinated mice developing lid disease and zosteriform lesions. None showed signs of encephalitis.
Corneal infection in this model results in approximately 90% of mice becoming latently infected with HSV-1 in the ophthalmic region of the TG (16, 26). The presence of latent virus was also shown to correlate well with the development of ocular disease following infection; thus, in this set of experiments, which showed 97.5% of mice developing some signs of ocular disease, the presence of latent virus would be comparable with results of 80 to 100% from previous studies.
UV irradiation itself damages the corneal epithelium and causes uveitis, events that are thought to be necessary for reactivation of virus in the TG (28). Such damage, however, is short lived, so that only those mice with viral reactivation develop the more severe stromal disease associated with herpes stromal keratitis (HSK). Consequently, in this study, a high percentage of both the immunized and mock-immunized mice developed corneal disease following UV irradiation. However, by day 6, most mice had recovered from UV-induced damage, resulting in a fall in the incidence of corneal disease. This was particularly evident in the case of uveitis, from which only 38% of HSV-1-vaccinated mice were affected following initial damage compared with 55% of mock-vaccinated animals, which had uveitis that continued to increase in severity. Similarly, for both epithelial and stromal disease, the severity of disease in the mock-immunized mice with clinical symptoms continued to increase for the duration of the experiment, while HSV-1-vaccinated mice developed less severe disease. Spread of virus from the initial site of infection to other areas within the same dermatome led to lid disease in 48% of mock-immunized mice, whereas only 25% of HSV-1-immunized mice developed the disease, with reduced severity. The further spread of virus causing zosteriform lesions was only observed in 5% of HSV-1-immunized mice, and the severity of these lesions was also low. In addition, none of the HSV-1-immunized mice developed signs of encephalitis. These results indicate that HSV-1 vaccination results in a reduction in the spread of virus through the nervous system when compared with mock-vaccinated mice. Overall, there was a significant reduction in both the incidence and severity of clinical disease in vaccinated mice following reactivation of latent virus, compared with the level in mock-vaccinated animals. Furthermore, in this model, recurrent herpetic eye disease is usually associated with shedding of virus in the tears (25). Therefore, isolation of virus from eye washings provided an indicator of the presence of reactivating virus. In previous studies, approximately 60% of mice shed virus in the tears following UV irradiation of the cornea (17, 24, 25, 34), which is comparable with the incidence in the mock-immunized mice in the present experiments. In contrast, this incidence was only 37% in HSV-1-immunized mice. A similar reduction in viral shedding was also observed in mice vaccinated with the virion host shutoff (vhs)-defective mutant of HSV-1 (17). At present, it is not clear whether this lower incidence is due to antibody-mediated neutralization of virus at the surface of the eye or a reduction in the number of reactivating events in the TG.
In the present study, the immune response induced by vaccination of latently infected mice was similar to that obtained by vaccination of naïve mice (22). High levels of virus-specific serum Ig, comparable to that obtained following corneal scarification of mice with live HSV-1, were detected by enzyme-linked immunosorbent assay (ELISA) and by neutralization in plaque reduction assays. Of particular importance for protective immunity at the corneal surface was the induction of virus-specific IgA in eye washings, the site of infection and recurrent disease. While some mice also developed high levels of IgA at the more distant mucosal site, the vagina, this was highly variable, which may be a consequence of the transient nature of IgA in mucosal washings. However, the presence of virus-specific IgA at the ocular surface, the site of infection and recurrence, has been shown to play a part in protection against ocular disease and corneal scarring (9). Analysis of the spread of virus in in vitro cultures has shown that virus-specific antibodies prevent spread between epidermal cells by neutralization during transmission across the intercellular gap (19). Similar mechanisms may also limit the spread of virus between epidermal cells and axonal termini in vivo (11). Thus, following reactivation, virus-specific antibody is likely to play an important role in limiting viral shedding from the nerve endings in the cornea and the spread of virus between neighboring corneal cells once they are infected.
Other mechanisms that may be involved in reduced corneal disease include the switch from the Th1-dominated, proinflammatory immune response observed in mice after a primary infection to a more balanced Th1/Th2-type response associated with anti-inflammatory cytokines observed following immunization. This approach contrasts with that aimed at providing protection against vaginal challenge with the closely related HSV-2. In these studies, induction of strong Th1-type responses enhanced viral clearance, resulting in reduced incidence and severity of genital lesions (10). However, following infection of the eye, the persistence of proinflammatory cytokines following viral clearance results in continued influx of Th1 immune cells, leading to opacity and vascularization of this highly specialized, normally transparent organ (6). Thus, the potential to modify the immune response to reactivating virus by immunization with rEtxB toward a more balanced Th1/Th2-type anti-inflammatory response, as observed in previous studies after immunization of naïve mice (22), was considered to be advantageous. It is also possible, however, that the major factor that affects the protection from disease observed in the present studies is the increase in levels of IL-10. This may reflect the activation of a T regulatory population following immunization that is able to suppress the immunopathological processes associated with ocular disease.
Accordingly, a switch in the dominance of IgG1 and IgG2a was observed, with the change in the IgG1/IgG2a ratio from 0.25 following ocular infection to 2.65 following HSV-1 immunization of latently infected animals indicating the potency of rEtxB to modulate the immune response. Interestingly, serum from mock-immunized mice also showed higher levels of IgG1 than IgG2a, resulting in a ratio of 1.78, suggesting that the use of rEtxB alone is capable of influencing the balance of the IgG subclasses. This is consistent with our observations that show rEtxB can, when given alone, modulate Th1 responses to autoantigens mediating protection in animal models of autoimmune disease (18). Several studies have also shown the importance of the anti-inflammatory cytokines IL-4 and IL-10 in the resolution of stromal keratitis (4, 5). The involvement of IL-4 in the resolution of corneal disease, however, is contradictory, the presence of IL-4 also being associated with development of HSK (1) and increased mortality (15). Recent studies have indicated that the mode of action of IL-4 is dependent on the other cell types present, such as CD4+ or CD8+ T cells (8). While we have not yet assessed the effects of immunization on the activation of HSV-specific cytotoxic T lymphocytes, the clear association with increased IL-4 and IL-10 levels suggests that such responses are unlikely to be a key feature of vaccination in this way. Further investigations will be required to determine this. Different roles for IL-4 and IL-10 have also been demonstrated following administration of either IL-4 or IL-10 DNA by different routes prior to ocular infection. The use of IL-4 DNA resulted in a significant switch toward a Th2 subset balance, whereas IL-10 did not (4). The involvement of IL-4 in this study is not clear; the level of IL-4 seen following immunization of these latently infected animals is comparable to that seen with previous studies involving immunization of naïve mice (22). Analysis of the cytokines following reactivation of virus showed similar levels of IL-4 in both HSV-1- and mock-immunized mice. Whereas, although only low levels of IL-10 were observed following immunization, this was greatly enhanced upon reactivation, in excess of that seen in the mock-immunized controls. The induction of IL-10 has been shown to be of primary importance in regulation of inflammatory responses and the resolution of HSK (1, 4, 5). The presence of IL-10 following HSV-1 corneal infection has been reported to be associated with suppression of certain chemokines, such as macrophage inflammatory protein 2 (MIP-2) and MIP-1α, resulting in a reduction in the migration of inflammatory cells to the cornea (31, 35). In addition, IL-10 has been shown to down-regulate expression of proinflammatory cytokines such as IL-2 and IL-6 (32). Further studies are required to determine the mechanism involved in IL-10 secretion. The observation that only local administration of IL-10 was effective led to the conclusion that either corneal epithelial cells or infiltrating immune cells may be able to secrete IL-10 (35). However, the cytokine profile observed here was similar to that following exposure of human monocytes to rEtxB, with high IFN-γ and IL-10 levels (33). This cytokine profile was consistent with that described for a subset of IL-10-secreting lymphocytes that possess regulatory properties (Tr1 cells) and that have been shown to be capable of preventing or treating Th1-mediated autoimmune diseases. This suggests that the ability of EtxB to trigger activation of T-regulatory cells (18) may be an important feature in modulating the immune-mediated damage associated with herpetic ocular disease.
In conclusion, we have demonstrated the potential of therapeutic vaccination to protect against recurrent ocular HSV-1 infection. Mucosal administration of viral antigens, together with the use of the potent immunomodulator rEtxB, induced an immune response that protected against corneal damage and blindness. The possible mechanisms involved include a rapid IgA antibody response at the corneal surface, enabling neutralization of virus. The presence of high levels of the IgG1 subclass of HSV-1-specific antibody helped give a more balanced Th1/Th2 and anti-inflammatory response. The induction of IL-10 is involved in the regulation of inflammatory responses, possibly due to the induction of Tr1 cells by rEtxB. Taken together, the modulation of both humoral and cell-mediated immune responses resulted in a significant reduction in the incidence and severity of this immunopathological disease.
Acknowledgments
We thank The Wellcome Trust for providing financial support for this work.
Thanks also go to Carolyn Shimeld for her invaluable help and advice.
REFERENCES
- 1.Babu, J. S., S. Kanangat, and B. T. Rouse. 1995. T cell cytokine mRNA expression during the course of the immunopathologic ocular disease herpetic stromal keratitis. J. Immunol. 154:4822-4829. [PubMed] [Google Scholar]
- 2.Bailey, M., N. A. Williams, A. D. Wilson, and C. R. Stokes. 1992. Probit: weighted probit regression analysis. J. Immunol. Methods 153:261-262. [DOI] [PubMed] [Google Scholar]
- 3.Beech, J. T., T. Bainbridge, and S. J. Thompson. 1997. Incorporation of cells into an ELISA system enhances antigen-driven lymphokine detection. J. Immunol. Methods 205:163-168. [DOI] [PubMed] [Google Scholar]
- 4.Chun, S., M. Daheshia, N. A. Kuklin, and B. T. Rouse. 1998. Modulation of viral immunoinflammatory responses with cytokine DNA administered by different routes. J. Virol. 72:5545-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daheshia, M., N. Kuklin, E. Manickan, S. Chun, and B. T. Rouse. 1998. Immune induction and modulation by topical ocular administration of plasmid DNA encoding antigens and cytokines. Vaccine 16:1103-1110. [DOI] [PubMed] [Google Scholar]
- 6.Deshpande, S. P., M. Zheng, S. Lee, and B. T. Rouse. 2002. Mechanisms of pathogenesis in herpetic immunoinflammatory ocular lesions. Vet. Microbiol. 86:17-26. [DOI] [PubMed] [Google Scholar]
- 7.Erturk, M., T. J. Hill, C. Shimeld, and R. Jennings. 1992. Acute and latent infection of mice immunised with HSV-1 ISCOM vaccine. Arch. Virol. 125:87-101. [DOI] [PubMed] [Google Scholar]
- 8.Ghiasi, H., Y. Osorio, G.-C. Perng, A. B. Nesburn, and S. L. Wechsler. 2001. Recombinant herpes simplex virus type 1 expressing murine interleukin-4 is less virulent than wild-type virus in mice. J. Virol. 75:9029-9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghiasi, H., S. L. Wechsler, S. Cai, A. B. Nesburn, and F. M. Hofman. 1998. The role of neutralizing antibody and T-helper subtypes in protection and pathogenesis of vaccinated mice following ocular HSV-1 challenge. Immunology 95:352-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gyotoku, T., F. Ono, and L. Aurelian. 2002. Development of HSV-specific CD4+ Th1 responses and CD8+ cytotoxic T lymphocytes with antiviral activity by vaccination with the HSV-2 mutant ICP10ΔPK. Vaccine 20:2796-2807. [DOI] [PubMed] [Google Scholar]
- 11.Halford, W. P., L. A. Veress, B. M. Gebhardt, and D. J. Carr. 1997. Innate and acquired immunity to herpes simplex virus type 1. Virology 236:328-337. [DOI] [PubMed] [Google Scholar]
- 12.Harper, H. M., L. Cochrane, and N. A. Williams. 1996. The role of small intestinal antigen-presenting cells in the induction of T-cell reactivity to soluble protein antigens: association between aberrant presentation in the lamina propria and oral tolerance. Immunology 89:449-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hendricks, R. L. 1997. An immunologist's view of herpes simplex keratitis: Thygeson Lecture 1996, presented at the Ocular Microbiology and Immunology Group meeting, October 26, 1996. Cornea 16:503-506. [PubMed] [Google Scholar]
- 14.Hill, T. J. 1987. Ocular pathogenicity of herpes simplex virus. Curr. Eye Res. 6:1-7. [DOI] [PubMed] [Google Scholar]
- 15.Ikemoto, K., R. B. Pollard, T. Fukumoto, M. Morimatsu, and F. Suzuki. 1995. Small amounts of exogenous IL-4 increase the severity of encephalitis induced in mice by the intranasal infection of herpes simplex virus type 1. J. Immunol. 155:1326-1333. [PubMed] [Google Scholar]
- 16.Keadle, T. L., K. A. Laycock, J. K. Miller, K. K. Hook, E. D. Fenoglio, M. Francotte, M. Slaoui, P. M. Stuart, and J. S. Pepose. 1997. Efficacy of a recombinant glycoprotein D subunit vaccine on the development of primary and recurrent ocular infection with herpes simplex virus type 1 in mice. J. Infect. Dis. 176:331-338. [DOI] [PubMed] [Google Scholar]
- 17.Keadle, T. L., L. A. Morrison, J. L. Morris, J. S. Pepose, and P. M. Stuart. 2002. Therapeutic immunization with a virion host shutoff-defective, replication-incompetent herpes simplex virus type 1 strain limits recurrent herpetic ocular infection. J. Virol. 76:3615-3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luross, J. A., T. Heaton, T. R. Hirst, M. J. Day, and N. A. Williams. 2002. Escherichia coli heat-labile enterotoxin B subunit prevents autoimmune arthritis through induction of regulatory CD4+ T cells. Arthritis Rheum. 46:1671-1682. [DOI] [PubMed] [Google Scholar]
- 19.Mikloska, Z., P. P. Sanna, and A. L. Cunningham. 1999. Neutralizing antibodies inhibit axonal spread of herpes simplex virus type 1 to epidermal cells in vitro. J. Virol. 73:5934-5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nash, A. A., and P. Cambouropoulos. 1993. The immune response to herpes simplex virus. Semin. Virol. 4:181-186. [Google Scholar]
- 21.Nesburn, A. B., R. L. Burke, H. Ghiasi, S. M. Slanina, and S. L. Wechsler. 1998. A therapeutic vaccine that reduces recurrent herpes simplex virus type 1 corneal disease. Investig. Ophthalmol. Vis. Sci. 39:1163-1170. [PubMed] [Google Scholar]
- 22.Richards, C. M., A. T. Aman, T. R. Hirst, T. J. Hill, and N. A. Williams. 2001. Protective mucosal immunity to ocular herpes simplex virus type 1 infection in mice by using Escherichia coli heat-labile enterotoxin B subunit as an adjuvant. J. Virol. 75:1664-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimeld, C., J. L. Whiteland, S. M. Nicholls, D. L. Easty, and T. J. Hill. 1996. Immune cell infiltration in corneas of mice with recurrent herpes simplex virus disease. J. Gen. Virol. 77:977-985. [DOI] [PubMed] [Google Scholar]
- 24.Shimeld, C., D. L. Easty, and T. J. Hill. 1999. Reactivation of herpes simplex virus type 1 in the mouse trigeminal ganglion: an in vivo study of virus antigen and cytokines. J. Virol. 73:1767-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimeld, C., T. Hill, B. Blyth, and D. Easty. 1989. An improved model of recurrent herpetic eye disease in mice. Curr. Eye Res. 8:1193-1205. [DOI] [PubMed] [Google Scholar]
- 26.Shimeld, C., T. J Hill, W. A. Blyth, and D. L. Easty. 1990. Passive immunization protects the mouse eye from damage after herpes simplex virus infection by limiting spread of virus in the nervous system. J. Gen. Virol. 71:681-687. [DOI] [PubMed] [Google Scholar]
- 27.Stumpf, T. H., R. Case, C. Shimeld, D. L. Easty, and T. J. Hill. 2002. Primary herpes simplex virus type 1 infection of the eye triggers similar immune responses in the cornea and the skin of the eyelids. J. Gen. Virol. 83:1579-1590. [DOI] [PubMed] [Google Scholar]
- 28.Stumpf, T. H., C. Shimeld, D. L. Easty, and T. J. Hill. 2001. Cytokine production in a murine model of recurrent herpetic stromal keratitis. Investig. Ophthalmol. Vis. Sci. 42:372-378. [PubMed] [Google Scholar]
- 29.Thomas, J., and B. T. Rouse. 1997. Immunopathogenesis of herpetic ocular disease. Immunol. Res. 16:375-386. [DOI] [PubMed] [Google Scholar]
- 30.Tullo, A. B., C. Shimeld, W. A. Blyth, T. J. Hill, and D. L. Easty. 1983. Ocular infection with herpes simplex virus in nonimmune and immune mice. Arch. Ophthalmol. 101:961-964. [DOI] [PubMed] [Google Scholar]
- 31.Tumpey, T. M., H. Cheng, X. T. Yan, J. E. Oakes, and R. N. Lausch. 1998. Chemokine synthesis in the HSV-1-infected cornea and its suppression by interleukin-10. J. Leukoc. Biol. 63:486-492. [DOI] [PubMed] [Google Scholar]
- 32.Tumpey, T. M., V. M. Elner, S. H. Chen, J. E. Oakes, and R. N. Lausch. 1994. Interleukin-10 treatment can suppress stromal keratitis induced by herpes simplex virus type 1. J. Immunol. 153:2258-2265. [PubMed] [Google Scholar]
- 33.Turcanu, V., T. R. Hirst, and N. A. Williams. 2002. Modulation of human monocytes by Escherichia coli heat-labile enterotoxin B-subunit; altered cytokine production and its functional consequences. Immunology 106:316-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker, J., K. A. Laycock, J. S. Pepose, and D. A. Leib. 1998. Postexposure vaccination with a virion host shutoff defective mutant reduces UV-B radiation-induced ocular herpes simplex virus shedding in mice. Vaccine 16:6-8. [DOI] [PubMed] [Google Scholar]
- 35.Yan, X. T., M. Zhuang, J. E. Oakes, and R. N. Lausch. 2001. Autocrine action of IL-10 suppresses proinflammatory mediators and inflammation in the HSV-1-infected cornea. J. Leukoc. Biol. 69:149-157. [PubMed] [Google Scholar]