Abstract
Compound-1453 was identified and characterized as a specific inhibitor of bovine viral diarrhea virus (BVDV). The concentration of compound-1453 which results in 50% protection from virus-induced cytopathic effect is ∼2.2 μM, with a therapeutic index of 60, and it is not active against a panel of RNA and DNA viruses. A time-of-addition experiment suggested that compound-1453 targets a stage of the viral life cycle after viral entry. To determine the target of compound-1453, resistant virus was generated. Resistant variants grew efficiently in the presence or absence of 33 μM compound-1453 and exhibited replication efficiency in the presence of compound-1453 approximately 1,000-fold higher than that of the wild-type (wt) virus. Functional mapping and sequence analysis of resistant cDNAs revealed a single amino acid substitution (Glu to Gly) at residue 291 in the NS5B polymerase in all eight independently generated cDNA clones. Recombinant virus containing this single mutation retained the resistance phenotype and a replication efficiency similar to that of the original isolated resistant virus. Since compound-1453 did not inhibit BVDV polymerase activity in vitro (50% inhibitory concentration > 300 μM), we developed a membrane-based assay that consisted of a BVDV RNA replicase complex isolated from virus-infected cells. Compound-1453 inhibited the activity of the wt, but not the drug-resistant, replicase in the membrane assay at concentrations similar to those observed in the viral infection assay. This work presents a novel inhibitor of a viral RNA-dependent RNA replicase.
Bovine viral diarrhea virus (BVDV), a positive-strand RNA virus, is the prototype member of the pestivirus genus, a group of important animal pathogens in the family Flaviviridae (27, 33). The BVDV genome consists of a single-stranded RNA molecule approximately 12.5 kb in length that encodes a single open reading frame flanked by 5′ and 3′ untranslated regions. The 5′ terminus of the genome is not capped; instead, initiation of translation is mediated by an internal ribosomal entry site (30). Translation of the viral genome yields a single polyprotein that is co- and posttranslationally processed by both viral and host proteases (PRs) into structural proteins (Npro, C, Erns, E1, E2, and p7) and nonstructural proteins (NS2, NS3, NS4A, NS4B, NS5A, and NS5B). Although little is known about the mechanism of BVDV RNA replication, it is believed that BVDV uses a strategy similar to that of other positive-strand RNA viruses (33, 47). Upon infection of cells, the genomic RNA serves as an mRNA and is translated to produce the viral nonstructural proteins which are necessary for BVDV replication. Viral RNA replication is initiated by synthesizing a full-length minus-strand RNA complementary to the genomic plus-strand RNA. This minus strand then serves as the preferred template for synthesis of additional plus-strand RNA molecules. Both minus- and plus-strand viral RNAs can be detected at 4 h postinfection (p.i.), with a ratio of plus to minus strands of 2:1 at 4 h p.i. and 10:1 at 12 h p.i. (16). Progeny virus can be detected as early as 8 h p.i. (28).
Flavivirus RNA synthesis is localized to endoplasmic reticular membranes in the perinuclear site of infected cells (25). Little is known about the regulation and components of flavivirus replication complexes during RNA replication. Many efforts, including the use of cell-free systems (1, 3, 8, 9, 53) and subcellular fractionation methods (8, 10), have been made to identify viral and cellular proteins required for viral replication. Although the nonstructural proteins are believed to function in viral RNA replication, little is known about the specific functions of the individual proteins. The N-terminal domain of NS3 contains a serine PR activity responsible for processing the nonstructural proteins at cleavage sites downstream of NS3 (49, 50). NS4A is believed to function as a cofactor for NS3 processing at two of these sites, 4B/5A and 5A/5B (44, 51). The C-terminal domain of NS3 contains both helicase and ATPase activities believed to be essential for RNA replication (43, 46). NS5B is thought to function as the viral RNA polymerase, as it contains conserved motifs found in all positive-strand viral RNA polymerases (21) and it has recently been shown to possess an RNA-dependent RNA polymerase activity (55). The exact function(s) of NS4B and NS5A is unknown. The results of a cross-linking experiment with BVDV-infected cells suggested that NS3, NS4B, and NS5A are associated as components of a multiprotein complex (31). In addition, NS4B has been implicated as an important modulator of BVDV cytopathogenicity, since a mutation in NS4B can attenuate BVDV cytopathogenicity despite NS3 production (31) which correlated invariantly with BVDV cytopathogenicity (14). NS5A has been shown to be a serine phosphoprotein that is tightly associated with one or more cellular kinases (32) as well as to interact with the α subunit of translation elongation factor 1 (20). The finding that defects in BVDV NS5A can be complemented in trans suggests a unique role for NS5A in viral RNA replication (4, 17). Recently, infectious cDNA clones for BVDV have been generated, providing a useful tool to examine the role of individual proteins in the viral RNA replication cycle (4, 26, 45).
In this study, we identified a compound, compound-1453, that selectively inhibited the growth of BVDV and appeared to target a protein involved in viral RNA replication. To determine whether a viral protein is targeted by compound-1453, we have isolated resistant viruses and demonstrated that the causal mutation resides in NS5B, the viral RNA-dependent RNA polymerase (RdRp). In addition, we showed that compound-1453 inhibits BVDV RdRp activity in a membrane-based assay, but not in an in vitro enzymatic assay, indicating that this compound affects the function of the viral RNA replication complex.
MATERIALS AND METHODS
Cell culture, virus, and use of compound.
Cultures of Madin-Darby bovine kidney (MDBK) cells and BVDV (strain NADL) stocks were propagated as previously described (11). To generate first-passage virus stocks from a full-length BVDV cDNA clone or the recombinant cDNA clones, RNA transcripts were synthesized using T7 RNA polymerase in vitro and were transfected (using DMRIE-C transfection reagent [Life Technologies] and following the manufacturer's instructions) into MDBK cells. After 4 days in minimal essential medium (MEM)-5% fetal calf serum (FCS) (heat-inactivated ultra-low-immunoglobulin G FCS), similar cytopathic effects were observed for all virus transfections and the supernatants were harvested. Virus titer was determined by a plaque-forming assay on MDBK cells after 72 to 96 h p.i. Compounds used in this study were synthesized at Bristol-Myers Squibb and were initially dissolved in dimethyl sulfoxide at 50 mg/ml and stored at −20°C.
Multicycle BVDV growth assay.
Cell culture plates (96 well) were seeded with 104 MDBK cells per well in MEM supplemented with 5% FCS. At 24 h later, 500 PFU of BVDV was adsorbed to cells for 1 h and then 100 μl of MEM containing compound, 0.75% dimethyl sulfoxide, and 2% FCS was added. Each dilution was tested in triplicate. Uninfected cells and infected cells without compound were included as controls in each assay plate. After 4 days at 37°C, living cells were stained using MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) (36, 42). The optical density of the wells was read at 540 nm in a microplate reader. Antiviral activity was measured as MTT conversion relative to the differential between compound-added cells and controls. The 50% effective concentration (EC50) is defined as the concentration of compound resulting in 50% protection from virus-induced cytopathic effect, and the 50% cytotoxic concentration (CC50) is defined as the concentration of the compound which kills 50% of the cells in the absence of virus. The therapeutic index (TI) of the compound was then determined according to the equation TI = CC50/EC50.
Isolation of compound-1453-resistant BVDV.
Compound-1453-resistant virus was generated by passaging wild-type (wt) BVDV in MDBK cells in the presence of increasing concentrations of compound. Initially virus was grown in the presence of 1.2 μM compound. Subsequently, virus was grown in 3.6, 7.2, and 15 μM compound-1453, with the final passage being in 33 μM compound. Both wt and resistant virus stocks were plaque purified four times in the presence or absence of 33 μM compound and amplified on MDBK monolayers at 37°C.
cDNA cloning and rescue of mutant phenotype.
Full-length infectious BVDV cDNA was generated by in vitro ligation of two BVDV clones, 153E-2 and 192F-1 (S. M. Levine, R. E. Rose, and R. J. Colonno, unpublished data). Plasmid 153E-2 contains the entire BVDV genome except for a deletion of nucleotides (nt) 253 to 1868 flanked by SphI and PinAI sites. 192F-1 contains nt 1 to 3160 of the BVDV genome. 153E-2 was digested with SphI and PinAI and ligated with the gel-purified SphI-to-PinAI fragment from 192F-1. The ligation mixtures were then linearized with XbaI, cleaned up by phenol extraction and ethanol precipitation, and used for in vitro transcription.
To generate compound-1453-resistant BVDV cDNA, total RNA was isolated from MDBK cells infected at a multiplicity of infection (MOI) of 3 with the resistant BVDV, using an RNeasy Total RNA kit (Qiagen). As a control, RNA was isolated in parallel from cells infected with wt BVDV and used for cDNA synthesis. All cDNAs were then cloned into vector 153E-2dlΔ. 153E-2dlΔ contains a 1,906-nt SpeI deletion within the NsiI-XbaI region and is used to eliminate wt background during subcloning. This recombinant plasmid, along with 192F-1, was used to generate infectious RNA transcripts to confirm the resistance phenotype.
To separate clustered mutations, subcloning was performed by replacing the PstI fragment of 153E-2 with the corresponding region of 1453r-3.1, creating 1453r-E>G. The PstI region of 1453r-E>G was sequenced to confirm that only the single A-to-G mutation at nt 11064 was present.
DNA sequence analysis.
The entire DNA sequence of the BVDV cDNA clones 153E2, 153E-2dlΔ, and 192F-1 was determined using an ABI PRISM dye terminator cycle sequencing ready reaction kit and analyzed on an Applied Biosystems 377 automated DNA sequencer. The DNA sequence of the entire region between the NsiI (nt 6330) and XbaI (nt 12602) sites was also determined for eight independent cDNA clones from compound-1453-resistant viral RNA and one clone from the wt BVDV RNA.
Metabolic labeling of RNA by [5-3H]uridine.
To inhibit cellular RNA synthesis, 0.5 μg of actinomycin D (Act. D)/ml was added to BVDV-infected MDBK cells at 7 h p.i. At 9 h p.i., compound-1453 at a final concentration of 33 μM and 5 mCi of [5-3H]uridine (NEN Life Science)/ml were added, and 5 h later the RNA was harvested using an RNeasy Mini kit (Qiagen). RNA amounts were determined by UV absorption, and the results are expressed as specific radioactivity (counts per minute/milligram of RNA).
Membrane preparation and viral RNA replicase assay.
BVDV-infected MDBK cells were suspended in ice-cold hypotonic buffer A (10 mM Tris-HCl, pH 7.4; 10 mM KCl; 1.5 mM MgCl2; 2× PR inhibitor cocktail [Roche]), allowed to swell for 30 min on ice, and then broken by 20 strokes with a Dounce homogenizer. The disrupted cells were pelleted by centrifugation at 1,000 × g for 5 min. The supernatant fraction containing cytoplasmic material and plasma membranes was concentrated by high-speed centrifugation at 200,000 × g for 30 min, resuspended in a small volume (100 μl per 108 cell equivalents) of fresh buffer B (10 mM Tris-HCl, pH 8.0; 10 mM NaCl; 1× PR inhibitor cocktail), and used for an RNA polymerase assay. Replicase reactions were carried out in a total volume of 40 μl in 50 mM Tris-HCl (pH 8.0)-10 mM MgCl2-5 mM dithiothreitol-500 μM each ATP, GTP, and UTP-10 μCi of [α-33P]CTP (3,000 mCi/mmol)-1.5 U of RNasin (Promega)-0.5 μg of Act. D-1 μg of tRNA-5 to 10 μg (total protein) of the membrane preparation. After incubation at 25°C for 3 h, the reaction mixtures were adjusted to 200 μl with water and extracted twice with phenol-chloroform and the RNA products were precipitated in ethanol and analyzed on a 1% native agarose gel (40). For some experiments, nuclease S1 digestion was applied to examine double-stranded RNA products from the polymerase assay. The RNA products were resuspended in 20 μl of S1 digestion buffer (Invitrogen) and then incubated with 5 U of nuclease S1 (Invitrogen) for 30 min at 37°C. After digestion, the nuclease was removed by phenol extraction and the RNAs were precipitated in ethanol and analyzed on the native agarose gel. Radioactivity incorporated into virus-specific RNA was quantitated using ImageQuant software for the PhosphorImager.
Reverse RNase protection assay.
For generation of a protective RNA used in the reverse RNase protection assay, DNA fragments corresponding to the BVDV genome sequences of nt 8895 to 10401 and nt 10401 to 12372 were excised from a BVDV cDNA (192F-1) and subcloned into pBluescript II (Stratagene), where the fragment was flanked by T7 and T3 promoters. These plasmids were then linearized with the proper restriction enzymes and used for in vitro transcription to generate the protective RNAs complementary to the positive or negative sense of BVDV RNA. A gel-isolated radiolabeled RNA product from the RNA polymerase assay was mixed with an excess amount of unlabeled protective RNA (as described above), and the RNA mixture was dried in a savant speed vacuum. The dried RNA mixture was suspended in hybridization buffer (Ambion) and heated to 92°C and then slowly cooled down and maintained at 55°C overnight. The hybridized RNAs were digested by a mixture of RNase A and RNase T1 (Ambion). Fragments were resolved on a 1% agarose gel by electrophoresis.
Indirect immunofluorescence.
Indirect immunofluorescence was performed as described previously (15). Antibodies employed were anti-NS3 monoclonal antibody (MAb) 20.10.6 (1:50 dilution; purchased from Ed J. Dubovi, Cornell University) and rhodamine-conjugated goat anti-mouse antibody (1:100 dilution).
RESULTS
Antiviral activity of compound-1453.
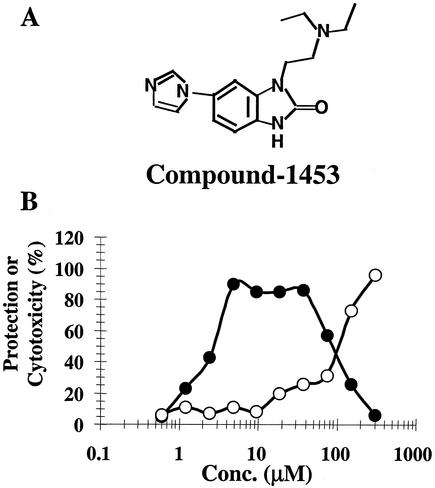
Compound-1453 is a cyclic urea derivative (Fig. 1A) identified as an inhibitor of BVDV replication in a multicycle growth assay (MTT assay) with an EC50 of 1.5 to 4.5 μM and a CC50 of ∼90 to 210 μM, which provides a therapeutic index of ∼60 (Fig. 1B). The activity of compound-1453 against BVDV was also determined using a plaque reduction assay, yielding an EC50 of ∼0.6 μM (results not shown).
FIG. 1.
Inhibition of BVDV replication by compound-1453. (A) Structure of compound-1453. (B) Plates (96-well) of MDBK cells were infected with BVDV (500 PFU/well) or mock infected in the presence of various concentrations of compound-1453; 4 days later, percentages of protection (closed circles) and cytotoxicity (open circles) were calculated through the MTT-based colorimetric assay.
To determine whether compound-1453 is a selective inhibitor of BVDV, experiments were conducted using a panel of different RNA and DNA viruses. Compound-1453 did not exhibit significant inhibitory activity against rhinovirus, influenza virus, respiratory syncytial virus, herpes simplex virus, human cytomegalovirus, or human immunodeficiency virus (HIV) at concentrations inhibitory for BVDV (Table 1), suggesting that compound-1453 is specific for BVDV.
TABLE 1.
Specificity and cytoxicity of compound-1453a
| Virusb | EC50 (μM) | Cell line | CC50 (μM) |
|---|---|---|---|
| BVDV | 1.5-4.5 | MDBK | 90-120 |
| HRV-16 | >333 | HeLa | >330 |
| Flu | >167 | MDBK | >167 |
| RSV | 80 | HEp-2 | 80 |
| HSV | 63 | Vero | 63-140 |
| HCMV | 327 | HFF | NDc |
| HIV | >167 | CEMSS (T cell) | >167 |
Inhibitory effect of compound-1453 on different viruses. Viral titers on the different cell lines are determined by standard viral assays. Known inhibitors are used as assay controls. For example, acyclovir was used as an inhibitor for HSV infection. EC50 and CC50 are defined in Materials and Methods.
HRV-16, rhinovirus; Flu, influenza virus; RSV, respiratory syncytial virus; HSV, herpes simplex virus; HCMV, human cytomegalovirus.
ND, not determined.
Compound-1453 acts at an early stage of the replication cycle.
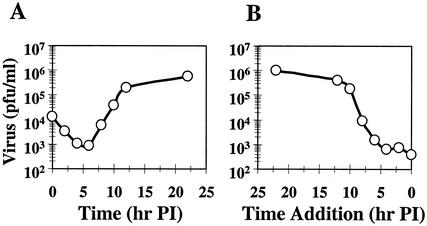
A time-of-addition experiment was performed to determine at what stage virus replication was inhibited by compound-1453. A single-cycle growth curve of BVDV is shown in Fig. 2A. Consistent with published data (16), progeny virus was not detected until 7 h p.i.; a substantial increase in the viral yield was then observed between 7 and 13 h p.i. Figure 2B shows the results from the time-of-addition experiment. Compound was added at different times p.i., with progeny virus harvested at 23 h p.i. and quantified on MDBK cells. Compound-1453 effectively inhibited virus production when it was added at up to 5 h after viral absorption, with a more than 1,000-fold reduction on viral yield observed (Fig. 2B). When the compound was applied after 7 h p.i., the effectiveness of compound-1453 started to decrease. These results indicated that compound-1453 inhibits virus production after entry and prior to the release of progeny virus from the infected cells.
FIG. 2.
Effect of compound-1453 on BVDV growth. (A) One-step growth curve of BVDV. MDBK cells were infected with BVDV at an MOI of 3; at the indicated times (1, 3, 5, 7, 9, 11, 13, or 23 h p.i.), infected cells were harvested and titers of progeny virus were determined by plaque assay. (B) Effect of time of compound-1453 addition on inhibition of BVDV growth. Cells were infected with BVDV at an MOI of 3, and compound-1453 was added at 1, 3, 5, 7, 9, 11, or 13 h p.i. at a final concentration of 33 μM. At 23 h p.i., infected cells were harvested and titers of progeny virus were determined in the absence of compound.
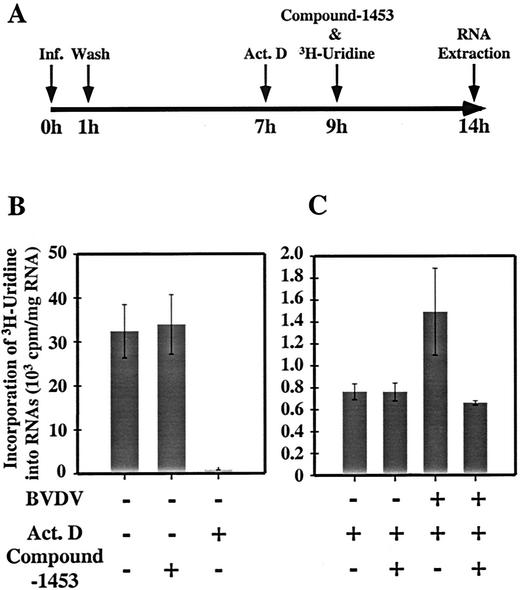
The effect of compound-1453 on viral RNA replication was examined by looking at the incorporation of [3H]uridine into viral RNA in the presence of Act. D, an antibiotic that selectively inhibits DNA-directed RNA synthesis (13, 34). BVDV-infected cells were pretreated with Act. D at 7 h p.i., compound-1453 and [3H]uridine were added at 9 h p.i., and total RNA was isolated at 14 h p.i. for quantification of incorporated [3H]uridine in the RNA (Fig. 3A). In the absence of viral infection, the addition of Act. D, but not compound-1453, reduced the [3H]uridine incorporation (Fig. 3B), indicating that the presence of compound-1453 had no detectable effect on cellular RNA synthesis. In the presence of Act. D, the amount of [3H]uridine incorporated into RNA from the BVDV-infected cell reached a level twofold higher than that from the mock-infected cells, indicating that BVDV RNA synthesis had occurred in the infected cell (Fig. 3C). Addition of compound-1453 decreased the 3H incorporation to a level equal to that for the mock-infected cell (Fig. 3C), indicating that compound-1453 inhibited BVDV RNA synthesis, either directly or indirectly. Consistent with this observation, when examined by Northern blot analysis (results not shown), viral RNA synthesis was completely inhibited even when the compound was added 3 h p.i. Taken together, these results suggest that compound-1453 targets a stage of the replication cycle after viral entry but prior to egress.
FIG. 3.
Effect of compound-1453 on BVDV RNA synthesis. (A) Flowchart of the experiment. (B) Effect of Act. D (0.5 μg/ml) and compound-1453 (33 μM) on cellular RNA synthesis. (C) Effect of compound-1453 on [3H]uridine incorporation into viral RNA. Data represent the mean of three repeats for each sample.
Isolation and characterization of compound-1453-resistant virus.
In an effort to determine the molecular target underlying the antiviral activity, a compound-1453-resistant virus (1453r) was selected. BVDV was propagated for several passages at low multiplicity with increasing concentrations of compound-1453 (from 1.2 to 33 μM) and plaque purified four times in the presence of 33 μM compound. A single-cycle growth experiment was performed to determine the replication ability of 1453r in the presence and absence of compound-1453. Cells were infected with either wt BVDV or 1453r at an MOI of 3 in the presence (33 μM) or absence of compound-1453 for 20 h, and progeny virus was harvested and titrated on MDBK cells without compound. The yield of wt virus was inhibited almost 1,000-fold in the presence of compound, while no apparent reduction was observed with the 1453r virus (Table 2). It should be noted that the plaques formed by 1453r virus in the presence of compound were slightly smaller than those in the absence of compound (results not shown).
TABLE 2.
Growth of 1453r virusa
| Virus | Titer (PFU/ml)
|
Ratio (+cpd-1453)/(−cpd-1453) | |
|---|---|---|---|
| + cpd-1453 | − cpd-1453 | ||
| wt | 2.8 × 103 | 1.7 × 106 | 1.7 × 10−3 |
| 1453r | 1.4 × 106 | 1.4 × 106 | 1.0 |
Cells were infected with either wt or 1453r virus at an MOI of 3 in the presence or absence of compound-1453 (cpd-1453; 33 μM). At 20 h p.i., cells were harvested and the titer of the progeny virus was determined by plaque assay in the absence of compound.
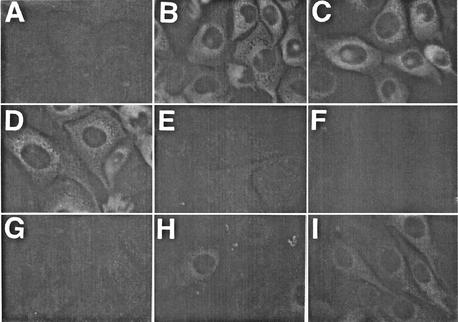
We next investigated the effect of compound-1453 on the expression of BVDV proteins. Cells were infected with either wt or 1453r virus, and at 23 h p.i., indirect immunofluorescence was used to examine the expression of BVDV NS3. In contrast to mock-infected cells (Fig. 4A), strong cytoplasmic staining was detected in wt-infected (Fig. 4B) and 1453r-infected (Fig. 4C) cells when a MAb specific for NS3 was used. Similar NS3 staining was also observed in 1453r-infected cells when compound-1453 was added after absorption (0 h p.i.; Fig. 4D), confirming that 1453r replication was not inhibited by the compound. No apparent BVDV NS3 expression was detected in wt-infected cells when the compound was added at 0, 2, and 5 h p.i. (Fig. 4E, F, and G). A gradual increase in the intensity of NS3 staining and positive-cell numbers was observed when the compound was added at 7 and 9 h p.i. (Fig. 4H and I). This is consistent with the effects on viral yield seen to result from the time-of-addition experiments (Fig. 2B). Since NS3 expression in 1453r-infected cells was not affected by compound-1453, even when compound was added at 0 h p.i., our results clearly demonstrate that the inhibition of NS3 expression in wt-infected cells was not due to cytotoxicity affecting a host cell function.
FIG. 4.
Effect of time of compound-1453 addition on BVDV NS3 protein expression. MDBK cells were mock infected (A) or infected with wt (B, E, F, G, H, and I) or 1453r (C and D) virus at an MOI of 3. Compound-1453 was added at 0 (D and E), 2 (F), 5 (G), 7 (H), and 9 (I) h p.i., and using anti-NS3 MAb 20.10.6, cells were processed for indirect immunofluorescence at 23 h p.i.
Mapping of compound-1453 target.
To determine the target gene of compound-1453 inhibition, sequencing was performed on the region of the BVDV genome encoding nonstructural proteins NS3-NS5B (from the NsiI [nt 6330] site in NS3 to the XbaI [nt 12602] site at the 3′ end of the genome). Eight different cDNA clones were generated from three independently isolated resistant virus isolates, and one was generated from wt BVDV. As shown in Table 3, sequence analysis indicated that although there were several mutations found in each clone, only a single mutation was present in all eight isolates, an A-to-G substitution at nt 11064. This mutation occurred in the region encoding the viral RNA polymerase and changed the amino acid coding sequence from a Glu to a Gly at amino acid 291 of NS5B (amino acid 3560 in the full-length BVDV genome). The R3356K change was also present in six out of eight isolates. However, this substitution is probably not involved in compound-1453 resistance, since there are several wt strains of BVDV that have a lysine at residue 3356.
TABLE 3.
Mapping of compound-1453 resistance
| Amino acida | Amino acid mutation in:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| wt | 1453r-1.1b | 1453r-1.2 | 1453r-1.3 | 1453r-2.1 | 1453r-1.4 | 1453r-1.5 | 1453r-1.6 | 1453r-3.1 | |
| 2726 | Frame shift | ||||||||
| 2772 | Frame shift | ||||||||
| 2858R | K | ||||||||
| 2859N | D | ||||||||
| 3014N | D | ||||||||
| 3016D | G | ||||||||
| 3094P | S | ||||||||
| 3121N | S | ||||||||
| 3180A | T | ||||||||
| 3208D | N | N | |||||||
| 3221V | A | ||||||||
| 3299K | R | ||||||||
| 3317E | K | ||||||||
| 3356R | K | K | K | K | K | K | |||
| 3363R | G | ||||||||
| 3462H | R | ||||||||
| 3510V | I | ||||||||
| 3560E | G | G | G | G | G | G | G | G | |
| 3573N | S | ||||||||
| 3596D | G | ||||||||
| 3599R | G | ||||||||
| 3650T | A | ||||||||
| 3704K | R | ||||||||
| 3716G | V | ||||||||
| 3729L | Q | ||||||||
| 3804E | G | ||||||||
| 3805 | Frame shift | ||||||||
| 3917K | S | ||||||||
| 3927G | V | ||||||||
| 3982T | P | ||||||||
Amino acid position in the BVDV genome.
The first number denotes the independent viral isolate; the second number denotes the cDNA clone from an individual isolate
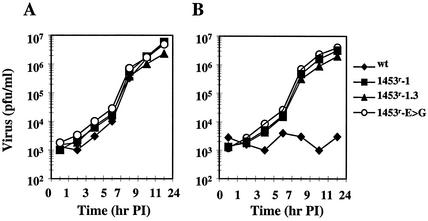
To determine whether the E3560G change was necessary and sufficient to confer resistance to compound-1453, the single mutation was inserted into a BVDV infectious cDNA (Levine et al., unpublished) and infectious virus was generated (designated 1453r-E>G). To determine whether other mutations also contribute to the resistance phenotype, we generated virus from a cDNA containing the entire nonstructural coding region from clone 1.3 (designated 1453r-1.3). Generation of infectious virus was carried out in the absence of compound; therefore, no selective pressure was applied to the virus. To examine replication efficiencies, growth kinetics of wt virus, the original resistant isolate (1453r-1), and recombinant viruses (1453r-1.3 and 1453r-E>G) were compared. As shown in Fig. 5, all three mutant viruses exhibited growth kinetics in the absence of compound-1453 similar to that of the wt virus (Fig. 5A). However, in the presence of compound-1453, replication of the wt virus was completely inhibited and the other three viruses displayed similar replication rates and virus yields in the presence and absence of compound (Fig. 5B). This demonstrates that the E3560>G mutation in NS5B is sufficient and necessary to confer resistance to compound-1453.
FIG. 5.
Effect of compound-1453 on viral growth. A one-step growth curve was performed in MDBK cells. Cells were infected with virus at an MOI of 3 and incubated in the absence (A) or presence (B) of 33 μM compound-1453. Virus samples were collected at the indicated times, and titers were determined by plaque assay.
Compound-1453 inhibits BVDV RNA replicase activity in vitro.
The results described above demonstrated that the E3560>G mutation in the BVDV NS5B polymerase is responsible for the compound-1453-resistant phenotype. To examine this inhibition in more detail, we cloned and expressed the BVDV polymerase from an infectious viral genome. To our surprise, compound-1453 did not inhibit the BVDV polymerase in the in vitro enzyme assay (50% inhibitory concentration > 300 μM), although multiple methods similar to published assay conditions were tried (references 2 and 55 and results not shown). Since compound-1453 was identified from a cell-based tissue culture system, the lack of inhibition in the in vitro enzyme assay could be due simply to the in vitro enzyme assay not mimicking the conformational aspects of BVDV replicase as it exists in infected cells.
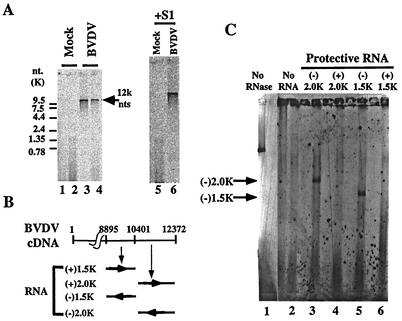
We therefore developed an assay system to measure BVDV replicase activity in the context of an authentic replication complex. It has been reported that the RNA replicase complex of many positive-strand viruses is associated with membrane structures within the cytoplasm of infected cells and that its replicase activity can be measured in the isolated membrane fraction (1, 7, 18, 35, 37, 41, 48). To further investigate the inhibition effect of compound-1453 on the BVDV replicase, we developed a membrane assay to quantify the activity of the BVDV replicase complex. All of the experiments described below were performed in the presence of Act. D. First, as shown in Fig. 6A, a radiolabeled RNA band of ∼12.5 kb (close to the genome size of BVDV) was found in an assay which used membrane isolated from BVDV-infected cells but not from mock-infected cells (Fig. 6, lanes 3 and 4 and lanes 1 and 2, respectively). A radiolabeled RNA of the same size was also present after S1 digestion (Fig. 6A, lane 6). These results suggest that the activity observed here was most likely derived from the BVDV RNA replicase and that the newly synthesized RNA is, at least in part, a double-stranded RNA. To confirm that this was BVDV RNA and to determine the polarity of the synthesized RNA product, an RNase protection experiment was performed (Fig. 6B and C). The radiolabeled 12.5-kb RNA product from the membrane assay was hybridized to each of four BVDV RNA fragments (including two positive- and two negative-sense BVDV RNA transcripts [Fig. 6B]), digested with RNase, and analyzed by gel electrophoresis. Results are summarized in Fig. 6C. No protection was observed when positive-sense protective RNAs were used (Fig. 6C, lanes 4 and 6). However, protected RNA fragments 2 kb and 1.5 kb in size were detected when negative-sense protective RNAs were used (Fig. 6C, lanes 3 and 5), suggesting that this is BVDV-specific RNA and that the majority (if not all) of the RNA synthesized in the membrane assay was of plus-strand polarity. Taken together, these results show that we have isolated membrane-bound BVDV RNA replicase activity.
FIG. 6.
Analysis of RNA products in the membrane-based BVDV polymerase assay. (A) [33P]CTP-labeled RNA products from mock-infected (lanes 1, 2, and 5) or virus-infected (lanes 3, 4, and 6) cell membranes were phenol-chloroform extracted and ethanol precipitated and then either directly loaded (lanes 1 to 4) or treated with S1 nuclease (lanes 5 and 6) before loading on a 1% agarose gel. Positions of the RNA size marker are indicated on the left. A position (12K) indicated by an arrow on the right was extrapolated on the basis of the positions of the RNA size markers. (B) RNA fragments used in a reverse RNase protection assay. + and − refer to positive- and negative-sense polarities of RNA relative to the BVDV genome. Numbers refer to nucleotide positions in the BVDV genome. (C) RNA products after RNase digestion. Lane 1, the RNA product from the membrane assay (no RNase digestion); lane 2, the RNA product from the membrane digested with RNase; lanes 3, 4, 5, and 6, RNA products after hybridization with the protective RNAs indicated in panel B and digestion with RNase. The positions of protective RNA (according to the results of toluidine blue staining) are indicated by arrows.
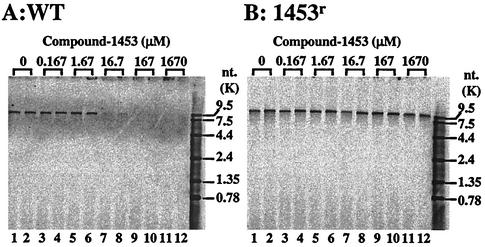
Since the membrane assay reflects BVDV RNA replicase activity, we used it to determine the inhibitory effect of compound-1453 on the BVDV RNA replicase. The production of the 12.5-kb RNA was almost completely inhibited in membranes isolated from wt-infected cells in the presence of 16.7 μM compound-1453 (Fig. 7A, lanes 7 and 8). The 50% inhibitory concentration was calculated to be between 1.67 and 16.7 μM, similar to or slightly higher than that observed in our cell-based MTT assay. As predicted, compound-1453 was unable to inhibit the replicase activity from 1453r-infected cells at a compound concentration up to 167 μM (Fig. 7B, lanes 9 and 10), although slight inhibition was observed at the highest concentration tested (1,670 μM; Fig. 7B, lanes 11 and 12). These results further confirmed that compound-1453 inhibits the activity of the BVDV replicase.
FIG. 7.
Effect of compound-1453 on the membrane-based BVDV wt (A) and 1453r (B) replicase activities. The same amount of protein (5 μg) from each membrane preparation was used for the assay. Each concentration of compound-1453 was run in duplicate as indicated above the autoradiogram. RNA products from these reactions were analyzed by electrophoresis on a 1% agarose gel. Sizes of RNA markers are indicated on the right.
DISCUSSION
In this study, we identified a cyclic urea derivative, compound-1453, as a replicase inhibitor specific to the pestivirus BVDV. Cyclic urea has previously been used as a main structure to design an inhibitor of HIV PR, because it has excellent hydrogen bond acceptor properties (24). Carbonyl oxygen provides a structure similar to water which can accept two hydrogen bonds from the backbone amide hydrogen of HIV PR residues Ile 50 and Ile 50′ and induce the fit of the flaps of the HIV PR dimer over the inhibitor. The rigid, cyclic ring provides a restricted conformation which has a positive entropic effect. Moreover, hydrophobic substituents on the ring provide an optimal stereochemistry and the conformation needed for complementary interaction between cyclic urea and HIV PR and can significantly change the potency of the inhibition (19). These properties might be favorable for compound-1453. Structure-activity relation studies established for this cyclic urea indicated that ketonic oxygen and substituents on the nitrogen of urea are required for antiviral activity (data not shown).
Four pieces of experimental evidence support our conclusion that compound-1453 specifically targets the virus and blocks BVDV replication. First, this inhibitor did not inhibit the panel of RNA and DNA viruses listed in Table 2 as well as failing to inhibit a brome mosaic virus RNA polymerase complex in a similar membrane assay previously described (references 39 and 38 and results not shown). Second, we observed that compound-1453 was active only when it was added at an early time in the virus growth cycle and that it inhibited uridine incorporation into viral RNA. Thirdly, a specific mutation was mapped to the NS5B polymerase of resistant virus and this single E291G mutation in the replicase gene conferred the resistance phenotype. We also observed direct inhibition by compound-1453 of BVDV RNA polymerase activity in a membrane-based assay that included other viral and cellular proteins. Therefore, taking these findings together, we conclude that compound-1453 targets the NS5B replicase and affects the activity of the RNA polymerase complex.
The causal mutation for the resistance phenotype maps to E3560G of the BVDV NS5B replicase. This region of NS5B is highly conserved among the pestiviruses, suggesting that this site plays an important role in the function of the polymerase. In addition to previously described polymerase motifs (29), sequence alignments between BVDV NS5B and other flavivirus replicases have revealed two additional highly conserved motifs (23). One of these motifs, designated nc, consists of the residues KRPRVIQYPEAKTR and includes the glutamate residue involved in compound-1453 activity at position 3560. Mutational analysis of this region abolished RNA synthesis in vitro, suggesting that this sequence is important for replicase activity. Because this motif is rich in lysine and arginine residues, Lai et al. speculate that this region plays a role in interacting with RNA template, primers, or nucleotides (23). However, further studies are required to determine exactly what role this domain has in RNA replication. More recently, structural studies have shown that this region in the hepatitis C virus RNA replicase lies in the finger domain and functions as a nucleotide binding site (6). Recently, Baginski et al. identified an inhibitor of BVDV, VP32947, that also targets the NS5B replicase (2). However, the mutation conferring resistance to this compound involves a phenylalanine-to-serine substitution at residue 224, a region which shows very little conservation between hepatitis C virus and BVDV.
The simplest explanation for the inhibition by compound-1453 of BVDV polymerase activity in a membrane-based assay, but not in the in vitro purified enzyme assay, is that the compound only recognizes the replicase when it is in a complex. Results from the RNase protection experiments suggest that the elongation step of viral RNA synthesis, rather than the entire viral RNA replication cycle, was measured in the BVDV membrane assay, since no newly synthesized minus-strand RNA was detected in the absence of the compound. In general, in comparison to the initiation step, the elongation step is considered to be a relatively simple process during viral RNA replication. Little is known about how the BVDV replicase interacts with other viral proteins, the template RNA, and possible cellular proteins to form a functional complex during the elongation step of RNA synthesis. Although some BVDV replication proteins, such as NS3, were detected in our membrane fraction (results not shown), whether the helicase activity of NS3 is required for the polymerase activity in our assay needs to be further investigated. Our preliminary results suggest that in the membrane assay, the BVDV polymerase is tightly associated with minus-strand RNA templates, since plus-strand RNA synthesis was not affected by the addition of large amounts of excess exogenous RNA, including BVDV replicon RNA, in competition experiments (results not shown). These results indicated that the integrity of the BVDV replication complex might be dependent on its interaction with membranes. The poliovirus replication complex has been shown to be membrane associated, and this association has been postulated to be important for the function of the complex (41). In contrast, the Qβ bacteriophage replication complex is not dependent on membrane association; the complex is held by strong protein-protein interactions (54). It is conceivable that compound-1453 binds to a specific pocket of the BVDV polymerase and inhibits its activity in a replicase complex consisting of other viral and/or cellular proteins. In the in vitro enzyme assay, such a binding pocket of the polymerase for compound-1453 may be folded differently or may not even exist.
Whether other viral replication proteins or cellular proteins are required for BVDV replicase activity in the membrane fraction is not clear. In general, it is believed that replication of positive-strand RNA viruses requires the involvement of host cellular factors (12, 22). It is clear, however, that the BVDV replicase activity we measured in the membrane assay more closely mimics that in the infected cells than that in the in vitro enzyme assay, since similar inhibitory concentrations of compound-1453 were observed in the cell culture and membrane assays. In the presence of a large excess of cellular proteins, the compound could selectively inhibit BVDV polymerase activity of the wt but not the drug-resistant complex, suggesting that the inhibitor is highly specific. A reconstitution experiment with membrane preparations subjected to further fractionation might provide more detailed information on the requirement of other BVDV replication proteins, and compound-1453 would certainly provide a useful tool for examination of their functional requirement(s) for effects on polymerase activity.
Specific inhibitors provide a powerful tool for the study of viral replication and pathogenesis. They can specifically block a certain stage of the viral life cycle and increase our understanding of viral biochemistry and its difference from host cells. In some sense, inhibitors can generate “mutants” without mutations. For many active site inhibitors, such as inhibitors of HIV reverse transcriptase and PR, they can usually be cocrystallized with the enzymes and provide a more detailed structure-activity relation for the study of the rational design of specific inhibitors. In the present work, we provide strong biologic and genetic evidence to support the conclusion that compound-1453 specifically inhibits the BVDV replicase, although we have not demonstrated its exact mechanism of action. Further work with this compound should allow us to determine the precise role of compound-1453 in the inhibition of BVDV RNA synthesis and provide insight into the mechanism of BVDV replication.
Acknowledgments
We thank Burt Rose for providing infectious BVDV cDNA clones prior to publication.
REFERENCES
- 1.Ali, N., K. D. Tardif, and A. Siddiqui. 2002. Cell-free replication of the hepatitis C virus subgenomic replicon. J. Virol. 76:12001-12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baginski, S. G., D. C. Pevear, M. Seipel, S. C. C. Sun, C. A. Benetatos, S. K. Chunduru, C. M. Rice, and M. S. Collett. 2000. Mechanism of action of a pestivirus antiviral compound. Proc. Natl. Acad. Sci. USA 97:7981-7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartholomeusz, A. I., and P. J. Wright. 1993. Synthesis of dengue virus RNA in vitro: initiation and the involvement of proteins NS3 and NS5. Arch. Virol. 128:111-121. [DOI] [PubMed] [Google Scholar]
- 4.Behrens, S., C. W. Grassmann, H. Thiel, G. Meyers, and N. Tautz. 1998. Characterization of an autonomous subgenomic pestivirus RNA replicon. J. Virol. 72:2364-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumenthal, T., and G. G. Carmichael. 1979. RNA replication: function and structure of QB replicase. Annu. Rev. Biochem. 48:525-548. [DOI] [PubMed] [Google Scholar]
- 6.Bressanelli, S., L. Tomei, F. A. Rey, and R. De Francesco. 2002. Structural analysis of the hepatitis C virus RNA polymerase in complex with ribonucleotides. J. Virol. 76:3482-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, J., and P. Ahlquist. 2000. Brome mosaic virus polymerase-like protein 2a is directed to the endoplasmic reticulum by helicase-like viral protein 1a. J. Virol. 74:4310-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu, P. W., and E. G. Westaway. 1985. Replication strategy of Kunjin virus: evidence for recycling role of replicative form RNA as template in semiconservative and asymmetric replication. Virology 140:68-79. [DOI] [PubMed] [Google Scholar]
- 9.Chu, P. W., and E. G. Westaway. 1987. Characterization of Kunjin virus RNA-dependent RNA polymerase: reinitiation of synthesis in vitro. Virology 157:330-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu, P. W., and E. G. Westaway. 1992. Molecular and ultrastructural analysis of heavy membrane fractions associated with the replication of Kunjin virus RNA. Arch. Virol. 125:177-191. [DOI] [PubMed] [Google Scholar]
- 11.Collett, M. S., R. Larson, C. Gold, D. Strick, E. K. Anderson, and A. F. Furchio. 1988. Molecular cloning and nucleotide sequence of the pestivirus bovine viral diarrhea virus. Virology 165:191-199. [DOI] [PubMed] [Google Scholar]
- 12.Dasgupta, A., P. Zabel, and D. Baltimore. 1980. Dependence of the activity of the poliovirus replicase on a host cell protein. Cell 19:423-429. [DOI] [PubMed] [Google Scholar]
- 13.Dmitrieva, T. M., M. V. Scheglova, and V. I. Agol. 1979. Inhibition of activity of encephalomyocarditis virus-induced RNA polymerase by antibodies against cellular components. Virology 92:271-277. [DOI] [PubMed] [Google Scholar]
- 14.Donis, R. O. 1995. Molecular biology of bovine viral diarrhea virus and its interactions with the host. Vet. Clin. N. Am. Food Anim. Pract. 11:393-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao, M., and D. M. Knipe. 1992. Distal protein sequence can affect the function of a nuclear localization signal. J. Virol. 12:1330-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong, Y., R. Trowbridge, T. B. Macnaughton, E. G. Westaway, A. D. Shannon, and E. J. Gowans. 1996. Characterization of RNA synthesis during a one-step growth curve and of the replication mechanism of bovine viral diarrhoea virus. J. Gen. Virol. 77:2729-2736. [DOI] [PubMed] [Google Scholar]
- 17.Grassmann, C. W., O. Isken, N. Tautz, and S.-E. Behrens. 2001. Genetic analysis of the pestivirus nonstructural coding region: defects in the NS5A unit can be complemented in trans. J. Virol. 75:7791-7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green, K. Y., A. Mory, M. H. Fogg, A. Weisberg, G. Belliot, M. Wagner, T. Mitra, E. Ehrenfeld, C. E. Cameron, and S. V. Sosnovtsev. 2002. Isolation of enzymatically active replication complexes from feline calicivirus-infected cells. J. Virol. 76:8582-8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodge, C. N., P. E. Aldrich, L. T. Bacheler, C.-H. Chang, C. J. Eyermann, S. Garber, M. Grubb, D. A. Jackson, P. K. Jadhav, B. Korant, P. Y. S. Lam, M. B. Maurin, J. L. Meek, M. J. Otto, M. M. Rayner, C. Reid, T. R. Sharpe, L. Shum, D. L. Winslow, and S. Erickson-Viitanen. 1996. Improved cyclic urea inhibitors of the HIV-1 protease: synthesis, potency, resistance profile, human pharmacokinetics and X-ray crystal structure of DMP 450. Chem. Biol. 3:301-314. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, C. M., D. R. Perez, R. French, W. C. Merrick, and R. O. Donis. 2001. The NS5A protein of bovine viral diarrhoea virus interacts with the alpha subunit of translation elongation factor-1. J. Gen. Virol. 82:2935-2943. [DOI] [PubMed] [Google Scholar]
- 21.Kamer, G., and P. Argos. 1984. Primary structural comparison of RNA-dependent polymerases from plant, animal, and bacterial viruses. Nucleic Acids Res. 12:7269-7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai, M. M. C. 1998. Cellular factors in the transcription and replication of viral RNA genomes: a parallel to DNA-dependent RNA transcription. Virology 244:1-12. [DOI] [PubMed] [Google Scholar]
- 23.Lai, V. C. H., C. C. Kao, E. Ferrari, J. Park, A. S. Uss, J. Wright-Minogue, Z. Hong, and J. Y. N. Lau. 1999. Mutational analysis of bovine viral diarrhea virus RNA-dependent RNA polymerase. J. Virol. 73:10129-10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lam, P. Y. S., P. K. Jadhav, C. J. Eyermann, C. N. Hodge, Y. Ru, L. T. Bacheler, J. L. Meek, M. J. Otto, M. M. Rayner, Y. N. Wong, C. Chang, P. C. Weber, D. A. Jackson, T. R. Sharpe, and S. Erickson-Viitanen. 1994. Rational design of potent, bioavailable, nonpeptide cyclic ureas as HIV protease inhibitors. Science 263:380-384. [DOI] [PubMed] [Google Scholar]
- 25.Lubiniecki, A. S., and C. J. Henry. 1974. Autoradiographic localization of RNA synthesis directed by arboviruses in the cytoplasm of infected BHK-21. cells. Proc. Soc. Exp. Biol. Med. 145:1165-1169. [DOI] [PubMed] [Google Scholar]
- 26.Mendez, E., N. Ruggli, M. S. Collett, and C. M. Rice. 1998. Infectious bovine viral diarrhea virus (strain NADL) RNA from stable cDNA clones: a cellular insert determines NS3 production and viral cytopathogenicity. J. Virol. 72:4737-4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyers, G., and H.-J. Thiel. 1996. Molecular characterization of pestiviruses. Adv. Virus Res. 47:53-118. [DOI] [PubMed] [Google Scholar]
- 28.Nuttall, P. A. 1980. Growth characteristics of two strains of bovine virus diarrhoea virus. Arch. Virol. 66:365-369. [DOI] [PubMed] [Google Scholar]
- 29.Poch, O., I. Sauvaget, M. Delarue, and N. Tordo. 1989. Identification of four conserved motifs among the RNA-dependent polymerase encoding elements. EMBO J. 8:3867-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poole, T. L., C. Wang, R. A. Popp, L. N. D. Potgieter, A. Siddiqui, and M. S. Collett. 1995. Pestivirus translation initiation occurs by internal ribosome entry. Virology 206:750-754. [DOI] [PubMed] [Google Scholar]
- 31.Qu, L., L. K. McMullan, and C. M. Rice. 2001. Isolation and characterization of noncytopathic pestivirus mutants reveals a role for nonstructural protein NS4B in viral cytopathogenicity. J. Virol. 75:10651-10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reed, K. E., A. E. Gorbalenya, and C. M. Rice. 1998. The NS5A/NS5 proteins of viruses from three genera of the family Flaviviridae are phosphorylated by associated serine/threonine kinases. J. Virol. 72:6199-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rice, C. M. 1996. p. 931-959. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott-Raven, Philadelphia, Pa.
- 34.Sarris, A. H., E. G. Niles, and E. S. Canellakis. 1977. The mechanism of inhibition of bacteriophage T7 RNA synthesis by acridines, diacridines and actinomycin D. Biochim. Biophys. Acta 474:268-278. [DOI] [PubMed] [Google Scholar]
- 35.Schaad, M. C., P. E. Jensen, and J. C. Carrington. 1997. Formation of plant RNA virus replication complexes on membranes: role of an endoplasmic reticulum-targeted viral protein. EMBO J. 16:4049-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sladowski, D., S. J. Steer, R. H. Clothier, and M. Balls. 1993. An improved MTT assay. J. Immunol. Methods 157:203-207. [DOI] [PubMed] [Google Scholar]
- 37.Strauss, J. H., and E. G. Strauss. 1994. The alphaviruses: gene expression, replication, and evolution. Microbiol. Rev. 58:491-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun, J., and C. C. Kao. 1997. RNA synthesis by the brome mosaic virus RNA-dependent RNA polymerase: transition from initiation to elongation. Virology 233:63-73. [DOI] [PubMed] [Google Scholar]
- 39.Sun, J., S. Adkins, G. Faurote, and C. C. Kao. 1996. Initiation of (−)-strand RNA synthesis catalyzed by the BMV RNA-dependent RNA polymerase: synthesis of oligonucleotides. Virology 226:1-12. [DOI] [PubMed] [Google Scholar]
- 40.Sun, J. H., and C. C. Kao. 1995. Double-stranded RNA is nonspecifically bound by agarose. Anal. Biochem. 230:185-187. [DOI] [PubMed] [Google Scholar]
- 41.Takegami, T., B. L. Semle, C. W. Anderson, and E. Wimmer. 1983. Membrane fractions active in poliovirus RNA replication contain VPg precursor polypeptides. Virology 128:33-47. [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi, H., M. Baba, and S. Shigeta. 1991. An application of tetrazolium (MTT) colorimetric assay for the screening of anti-herpes simplex virus compounds. J. Virol. Methods 33:61-71. [DOI] [PubMed] [Google Scholar]
- 43.Tamura, J. K., P. Warrener, and M. S. Collett. 1993. RNA-stimulated NTPase activity associated with the p80 protein of the pestivirus bovine viral diarrhea virus. Virology 193:1-10. [DOI] [PubMed] [Google Scholar]
- 44.Tautz, N., K. Elbers, D. Stoll, G. Meyers, and H.-J. Thiel. 1997. Serine protease of pestiviruses: determination of cleavage sites. J. Virol. 71:5415-5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vassilev, V. B., M. S. Collett, and R. O. Donis. 1997. Authentic and chimeric full-length genomic cDNA clones of bovine viral diarrhea virus that yield infectious transcripts. J. Virol. 71:471-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warrener, P., and M. S. Collett. 1995. Pestivirus NS3 (p80) protein possesses RNA helicase actvity. J. Virol. 69:1720-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westaway, E. G. 1987. Flavivirus replication strategy. Adv. Virus Res. 33:45-90. [DOI] [PubMed] [Google Scholar]
- 48.Wimmer, E., C. U. Hellen, and X. Cao. 1993. Genetics of poliovirus. Annu. Rev. Genet. 27:353-436. [DOI] [PubMed] [Google Scholar]
- 49.Wiskerchen, M., and M. S. Collett. 1991. Pestivirus gene expression: protein p80 of bovine viral diarrhea virus is a proteinase involved in polyprotein processing. Virology 184:341-350. [DOI] [PubMed] [Google Scholar]
- 50.Wiskerchen, M., S. K. Belzer, and M. S. Collett. 1991. Pestivirus gene expression: the first protein product of the bovine viral diarrhea virus large open reading frame, p20, possesses proteolytic activity. J. Virol. 65:4508-4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu, J., E. Mendez, P. R. Caron, C. Lin, M. A. Murcko, M. S. Collett, and C. M. Rice. 1997. Bovine viral diarrhea virus NS3 serine proteinase: polyprotein cleavage sites, cofactor requirement, and molecular model of an enzyme essential for pestivirus replication. J. Virol. 71:5312-5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xue, W., and H. C. Minocha. 1993. Identification of the cell surface receptor for bovine viral diarrhoea virus by using anto-idiotypic antibodies. J. Gen. Virol. 74:73-79. [DOI] [PubMed] [Google Scholar]
- 53.You, S., and R. Padmanabhan. 1999. A novel in vitro replication system for Dengue virus. Initiation of RNA synthesis at the 3′-end of exogenous viral RNA templates requires 5′- and 3′-terminal complementary sequence motifs of the viral RNA. J. Biol. Chem. 274:33714-33722. [DOI] [PubMed] [Google Scholar]
- 54.Young, R. A., and T. Blumenthal. 1975. Phage Q-beta ribonucleic acid replicase. Subunit relationships determined by intramolecular cross-linking. J. Biol. Chem. 250:1829-1832. [PubMed] [Google Scholar]
- 55.Zhong, W., L. L. Gutshall, and A. M. Del Vecchio. 1998. Identification and characterization of an RNA-dependent RNA polymerase activity within the nonstructural protein 5B region of bovine viral diarrhea virus. J. Virol. 72:9365-9369. [DOI] [PMC free article] [PubMed] [Google Scholar]