Abstract
The V1/V2 and V3 loops are proximal to the CD4 binding site (CD4bs) of human immunodeficiency virus type 1 (HIV-1) gp120 and undergo conformational change upon CD4 receptor engagement by the HIV-1 envelope spike. Nearly all of the reported monoclonal antibodies (MAbs) against the CD4bs exhibit a very limited capacity to neutralize HIV-1. However, one such human MAb, immunoglobulin G1 (IgG1) b12, is uniquely able to neutralize primary isolates across subtypes with considerable potency. The molecular basis for the anti-HIV-1 activity of b12 is not fully understood but is relevant to vaccine design. Here we describe a novel human MAb, 4KG5, whose binding to monomeric gp120 is moderately enhanced by IgG1 b12. In sharp contrast, 4KG5 binding to gp120 is inhibited by soluble CD4 (sCD4) and by all other (n = 14) anti-CD4bs MAbs tested. 4KG5 is unable to recognize gp120 in which either V1, V2, or V3 has been deleted, and MAbs against the V2 or V3 loops inhibit the binding of 4KG5 to gp120. Moreover, 4KG5 is able to inhibit the binding of the CD4-induced MAbs 17b and X5 in the absence of sCD4, whereas 17b and X5 only weakly inhibit the binding of 4KG5 to gp120. Mutagenesis of gp120 provides further evidence of a discontinuous epitope of 4KG5 that is formed by the V1/V2 loop, the V3 loop, and a portion of the bridging sheet (C4). 4KG5 was isolated as a single-chain Fv from a phage display library constructed from the bone marrow of an HIV-1-seropositive subject (FDA2) whose serum neutralizes HIV-1 across subtypes. Despite its source, we observed no significant neutralization with 4KG5 against the autologous (R2) virus and several other strains of HIV-1. The results suggest a model in which antibody access to the CD4bs on the envelope spike of HIV-1 is restricted by the orientation and/or dynamics of the V1/V2 and V3 loops, and b12 avoids these restrictions.
A major frustration in human immunodeficiency virus type 1 (HIV-1) vaccine development is the inability to elicit antibodies (Abs) in animals or humans capable of neutralizing different isolates of HIV-1 (24, 43, 58). Significant titers of potent and broadly HIV-1-neutralizing Abs are not elicited by monomeric envelope protein (15, 22, 71, 83) and are grossly underrepresented in the serum response during natural infection (30, 44). The vast majority of anti-HIV-1 monoclonal Abs (MAbs) elicited by immunization, or during natural infection, have poor or no cross-neutralizing activity and typically bind to determinants that either vary from virus to virus because of mutation or are poorly exposed or completely inaccessible on the surface of infectious virions (47, 58, 60). Until recently, only three human MAbs were identified as having broad and potent HIV-1-neutralizing activity (14): two against gp120, immunoglobulin G (IgG) b12 (4, 65) and 2G12 (67, 70, 82), and one against gp41, 2F5 (52). Additional MAbs, two against gp120, Fab fragment X5 (51) and IgG 447-52D (8, 21), and two against gp41, 4E10 (75, 96) and Fab Z13 (96), have been identified as having cross-isolate HIV-1-neutralizing activity as well. IgG1 b12 belongs to a class of MAbs, termed anti-CD4-binding site (anti-CD4bs) MAbs, which are defined by their ability to inhibit the binding of CD4 to gp120 and vice versa. Anti-CD4bs MAbs also cross-compete with each other to bind gp120. Thus, anti-CD4bs MAbs all have the ability to inhibit the binding of b12 at least to monomeric gp120, yet they do not neutralize primary viruses as broadly and potently as does IgG1 b12.
Recently, the three-dimensional structure of IgG1 b12 was determined (68). The broadness in activity of b12 was related, in part, to its ability to bind to an exceptionally conserved region of gp120 by using its long finger-like third hypervariable loop of the heavy chain to bury a Trp residue in the hydrophobic CD4 pocket (68). Nevertheless, many questions still remain. For example, why do other human CD4bs MAbs with long H3 loops not neutralize primary isolates of HIV-1? What exactly are the molecular contacts between b12 and gp120, and how are the variable loops of gp120 positioned in a complex of gp120 with b12? Because there is no structure available of such a complex, molecular docking has been used to predict the interaction between IgG1 b12 and the gp120 core by using the respective crystal structures (33, 68). Clearly, molecular docking has its limitations. Furthermore, the core gp120 used in structural studies was truncated, deglycosylated, and complexed to soluble CD4 (sCD4) and the 17b MAb Fab fragment (32, 33). The truncated liganded gp120 has important differences from its counterpart on the virion surface, a protomer within a presumed trimer of heterodimers of gp120 and the transmembrane protein gp41 (16, 38, 60, 86). Because of their absence in the core gp120 crystal structure, it remains unclear how the V1/V2 and V3 loops spatially relate to one another and to the CD4 and coreceptor binding sites. The nature of the interaction between the V1/V2 and V3 loops has particular relevance to the virus entry process (5, 7, 26, 28, 35, 39, 40, 76, 89, 90). The engagement of the CD4 receptor with gp120 induces the exposure of the cryptic chemokine receptor binding site on gp120, which overlaps the CD4-induced (CD4i) epitope for MAb 17b (64, 81, 87), as shown in the crystal structure of the gp120 complex (33). Movements in the V1/V2 and V3 loops are also generally believed to accompany CD4 binding (42, 74, 88), and distinct regions of the V3 loop have been implicated in coreceptor recognition (9, 85). Coreceptor engagement probably also occurs in concert with rearrangements in gp41 leading to gp120 dissociation, the insertion of the N-terminal fusion peptide of gp41 into the host membrane, and ultimately to membrane fusion (16). These studies illustrate that gp120 is capable of extensive conformational change initiated by CD4 binding and that these conformational changes likely affect not only the V1/V2 and V3 loops and the bridging sheet but probably also those regions that interact with neighboring gp120s and gp41.
The ability of anti-CD4bs MAbs to neutralize HIV-1 has been associated with their affinity for the trimeric envelope spike on the surface of virions and infected cells, rather than for monomeric gp120 (60). The trimeric envelope complex is metastable, and the structural details of it remain unknown. However, studies on monomeric gp120 are easier and have shown some differences in fine specificity between anti-CD4bs MAbs and CD4 as well as between anti-CD4bs MAbs themselves (the latter differences are generally found to be less significant than the former). For example, attempts have been made to discriminate among anti-CD4bs MAbs with panels of mutants of gp120 (48, 56, 78). The gp120 mutant panel studies have been successful in discriminating among different anti-CD4bs MAbs, but the footprints of b12 and the nonneutralizing anti-CD4bs MAb b6 are overlapping and quite similar (56). Ab competition mapping is another method of discriminating among anti-CD4bs MAbs. In a study by Moore and Sodroski, for example, the murine MAb SC258, directed against a discontinuous epitope in the V2 loop, enhanced the binding of the anti-CD4bs MAb F91 but diminished the binding of b12 (49). The same MAb, however, also inhibited binding of CD4 IgG and had little effect on other anti-CD4bs MAbs.
To our knowledge, there is no known MAb against gp120 whose specificity distinguishes the broadly neutralizing Ab b12 from other nonneutralizing anti-CD4bs MAbs. We describe here a novel human MAb, 4KG5, discovered in the form of a single-chain Fv (scFv), that has a highly complex epitope on gp120 involving the V1, V2, and V3 loops. The binding of 4KG5 to gp120 is inhibited by V2 and V3 loop MAbs, most anti-CD4bs MAbs, and sCD4 but is enhanced by b12. The results, in conjunction with mutagenesis data, suggest that the V1/V2 and V3 loops are close enough to each other and to the CD4bs to be involved in a single epitope. Furthermore, the absence of observed neutralizing activity of 4KG5 together with its loop dependence are consistent with a model in which the positioning of the variable loops relative to the CD4bs on gp120 is different between monomeric and trimeric gp120, which has important implications for vaccine design.
MATERIALS AND METHODS
Materials.
Unless otherwise indicated, the Abs used in this study are IgGs and include the human Abs IgG1 b12 (4); Fabs b12, b6, and b3 (65); Fabs p35, loop2, and DO142 (13); Fab L33 (12); Fab X5 (51); KZ52 (41); Fabs AH48, Ia3, Ia7, FG39, Fbb14, and Fbb21, which are novel and will be described in detail elsewhere (M. B. Zwick, M. Moulard, P. W. H. I. Parren, and D. R. Burton, unpublished data); 2G12 (82), provided by Gabriela Stiegler and Hermann Katinger; 447-52D (20), 694-98D (73), 1008-D, 559-64D, 1027-30D, 670-D (95), 1331A (54), and 654-30D (34), provided by Susan Zolla-Pazner; hNM01 (53), provided by Jason Grebely; F91 (49) and F105 (80), provided by Peter Kwong; 17b and 48d (79), 15e (25), 19b (72) and A32 (46), provided by James Robinson; MAbs obtained from the AIDS Research Reference Reagent Program, including F425-B4e8 (donated by Marshall Posner and Lisa Cavacini); and human IgG against HIV-1 (HIVIG), provided by John Mascola. HIV-1-neutralizing serum from patient FDA2 (59) was prepared from blood drawn on 29 September 1999. The following murine MAbs were also used: 522-149 (49), G3-4 (23), G3-136 (19), G3-299, G3-42, G3-519, G3-537 (77), 1C1 (48), and the rat MAb M91 (11). The polyclonal sheep Ab D7324 was purchased from Cliniqa (Fallbrook, Calif.), and gp120JR-FL was a generous gift from Bill Olson and Paul Maddon (Progenics, Tarrytown, N.Y.). gp120IIIB (ImmunoDiagnostics, Inc.), gp120SF2 (Chiron, Emeryville, Calif.), and plasmids (pPI4) encoding wild-type and loop-deleted constructs of gp140JR-FL were kindly provided by James Binley. The pSVIIIexE7pA− plasmid (97) was a gift from Joe Sodroski. The following reagents were also obtained from the AIDS Research Reference Reagent Program: sCD4 (amino acids 1 to 370; contributed by N. Schuelke), gp120MN, gp120BaL (Division of AIDS), and pNL4-3.Luc.R−E− (contributed by Nathaniel Landau).
Isolation of 4KG5 scFv from a phage display library.
The scFv kappa and lambda libraries from the patient FDA2 were prepared as described previously (1, 2, 96). Briefly, the RNA was isolated from 5 ml of bone marrow (drawn in August 1996) from an HIV-1-seropositive individual (FDA2) with exceptionally broad HIV-1 primary isolate-neutralizing Ab titers (18, 45, 59, 84) and used to prepare libraries of >107 clones by using the phagemid pComb3X as a vector (2). The libraries were subjected to four rounds of affinity selection on 0.5 μg of immobilized gp120JR-FL as described previously (1). The enriched scFv phage pools were screened by enzyme-linked immunosorbent assay (ELISA) to identify positive clones that bound to gp120JR-FL but not to ovalbumin, which served as a negative control.
Production and purification of 4KG5 scFv.
TOP10 Escherichia coli cells (Invitrogen) were transformed with pComb3X bearing the gene encoding the 4KG5 scFv. Bacterial cultures were prepared as described previously for Fab preparation (96). Briefly, 4 liters of super broth (2) containing 50 μg of carbenicillin/ml and 20 mM MgCl2 were inoculated (1:100) with overnight cultures containing the same antibiotics and 20 mM glucose. The flasks were incubated at 37°C while shaking at 250 rpm until an optical density at 600 nm (OD600) of 0.8 was reached, after which time 1 mM isopropyl-β-d-thiogalactopyranoside was added to each culture. These cultures were then incubated for a further 5 to 16 h at 30°C. The cultures were centrifuged at 9,000 × g for 15 min in a Sorvall SLA-3000 rotor at 4°C, and the pellets were resuspended in ∼30 ml of lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole [pH 8.0]) containing fresh phenylmethylsulfonyl fluoride (0.3 mM). The bacteria were lysed by sonication on ice, the bacterial debris was centrifuged at 34,000 × g in a Sorvall SS-34 rotor for 35 min at 4°C, and the centrifuge stopped without braking. The crude scFv supernatant was filtered (0.8-μm pore size, then 0.2-μm pore size) and then slowly rocked for 90 to 120 min at 4°C in a slurry with 5 ml of Ni2+-agarose (Qiagen) that had been preequilibrated with lysis buffer. The slurry was loaded onto a column and washed with 10 volumes of wash buffer (lysis buffer except final concentration of imidazole is 20 mM), and the scFv was eluted with 250 mM imidizole in lysis buffer. The eluate was concentrated by using a Centriprep YM-10 concentrator (Millipore), dialyzed for 16 h against phosphate-buffered saline (PBS) (pH 7.0) at 4°C, sterilized by filtration (0.2-μm pore size), and then stored at −80°C until ready for use.
Construction and production of HIV-1 pseudovirions and env mutants.
The plasmid pSVIIIexE7pAJR-CSF− (76, 97) bears the HIV-1 envelope gene, and if cotransfected into host cells with the luciferase reporter plasmid pNL4-3.Luc.R−E−, single-round infectious clones of HIV-1 pseudovirion are produced, the infectivity of which may be followed by luciferase activity in target cells. To construct analogous R2 and JR-FL pseudovirions, the R2 (62) and JR-FL envelope genes were amplified by PCR with the forward primer DGKpnI (5′-CAGTCTATTATGGGGTACCTGTGTGGAAAGAAGCAACC-3′), containing a KpnI site in the 5′ tail, and the reverse primer JR-FLXhoI (5′-CGCAGACGCAGACTCGAGTTATAGCAAAGCCCTTTCCAAGCC-3′), containing a XhoI site in the 5′ tail. The vector, pSVIIIexE7pA−JR-CSF, was cleaved at unique KpnI and XhoI sites, gel-purified, and ligated with the R2 or JR-FL env gene PCR products, which were similarly prepared. Mutants of gp160JR-CSF for the epitope mapping studies were selected from panels created previously (56, 70). 293T cells grown in Dulbecco's modified Eagle's media (Gibco) supplemented with penicillin, streptomycin, l-glutamine, and fetal bovine serum (10%) were transiently transfected with pSVIIIexE7pA−, carrying env from JR-CSF, R2, JR-FL, or ADA DNA (2 μg) along with pNL4-3.Luc.R−E− (4 μg) by using FuGENE6 transfection reagent (Roche) according to the manufacturer's instructions. Alternatively, plasmid-borne wild-type, mutant, or variable-loop-deleted constructs (66) of gp140JR-FL were used. Mutants T198P, D275V, R298G, and E322K were created in this study with the QuikChange mutagenesis kit by using the plasmid-borne wild-type gp140JR-FL as the template according to the manufacturer's directions. All sequences were verified by DNA sequencing. Plasmid DNAs (2 μg), encoding the various gp140JR-FLconstructs, were used to transiently transfect 293T cells by using the same transfection protocol as for the pseudovirions, except no pNL4-3.Luc.R−E− was added. At 24 h posttransfection, the culture supernatants of all transfected cell cultures were replaced with fresh media and the cultures were incubated for a further 24 h. The supernatants containing pseudoviruses or gp140s were harvested and frozen at −80°C until use in neutralization assays, or they were supplemented with 1% Empigen BB (Calbiochem) and stored at −20°C until further use in ELISAs.
Denaturation and deglycosylation of gp120.
To denature gp120, gp120JR-FL was diluted in 1% sodium dodecyl sulfate and 50 mM dithiothreitol (Sigma) to a concentration of 20 μg/ml. The mixture was boiled for 5 min and then diluted 1:10 in PBS prior to coating ELISA microplate wells (50 μl per well). In one set of deglycosylation experiments, endoglycosidase H (Roche) was added to native gp120JR-FL (1.5 μg) in a 15-μl reaction volume, according to the manufacturer's directions, incubated at 37°C for 16 h, diluted 1:50 in PBS containing 1% bovine serum albumin (BSA) and 0.025% Tween 20, and then used in an ELISA capturing with D7234 Ab, as described below. Alternatively, jack bean mannosidase (Glyko) was added to 1.5 μg of gp120 in a 15-μl reaction volume, according to the manufacturer's directions, incubated at 37°C for 16 h, and then used in an ELISA in a similar fashion. In a second set of deglycosylation experiments, plate-immobilized gp120JR-FL was treated either with sialidase (Glyko) alone at a concentration of 0.1 U/ml in PBS for 2 h at 37°C or with sialidase first and then with 200 U of β-galactosidase (G-5635; Sigma)/ml in 0.1 M phosphate buffer containing 1 mM MgCl2 for 28 h at 37°C.
ELISAs.
Microwells were coated overnight at 4°C with 50 μl of PBS containing gp120JR-FL (2 μg/ml). Wells were washed twice with PBS containing 0.05% Tween 20 and blocked with 3% BSA for 1 h at 37°C. After a single wash, Abs were added to the wells in PBS containing 1% BSA and 0.02% Tween and allowed to incubate at 37°C for 2 h. The wells were washed four to five times, goat anti-human IgG F(ab′)2 alkaline phosphatase (AP) (Pierce) diluted 1:500 in PBS containing 1% BSA was added in the case of human Abs, either India-HIS probe horseradish peroxidase (HRP) conjugate (Pierce) or anti-hemagglutinin peroxidase high-affinity 3F10 (Roche) diluted 1:500 was added in the case of scFv (we later found the 3F10 secondary probe to be preferable because of its higher sensitivity), and the plate was incubated for 40 min at room temperature. The wells were washed five times and developed by adding 50 μl of either AP substrate (for AP conjugate) prepared by adding one tablet of disodium p-nitrophenyl phosphate (Sigma) to 5 ml of AP staining buffer, pH 9.8; 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) solution (for HRP conjugate), prepared by adding 1 μl of H2O2 to 4 ml of ABTS solution (Sigma); or TMB (3,3′,5,5′-tetramethylbenzidine) solution (for HRP conjugate) according to the manufacturer's instructions (Pierce). After ∼30 min, wells containing TMB solution were stopped by adding 50 μl of H2SO4 (2 M), and the OD405 (AP substrate and ABTS) or OD450 (TMB substrate) was read on a microplate reader (Molecular Devices).
Competition ELISAs.
For competition ELISAs, 25 μl of the competing Abs were added to the blocked and washed wells, and this was immediately followed by the addition of 25 μl of the test MAb previously determined to result in an ELISA signal that was ∼75% of the maximum signal without competitor. Biotinylated (BIO) MAbs were detected by using a streptavidin-HRP conjugate (Jackson). The wells were blocked, washed, and probed as described above.
env mutant ELISAs.
For gp160JR-CSF or gp140JR-FL mutants, microwells were coated overnight with sheep Ab against the C-terminal region of gp120 (D7324) at a concentration of 5 μg/ml in PBS and the wells were washed twice and blocked with 3% BSA as above. All gp160 or gp140 mutants, previously normalized in concentration with HIVIG, were diluted in PBS containing 1% BSA and 0.025% Tween 20 and added to the wells. The plate was incubated at 37°C for 2 h prior to washing, and then probing with primary and secondary Abs was carried out as described above. All experiments were performed at least twice, the mutants in which 4KG5 binding to gp120 was diminished were tested against MAb 2G12, and the affinity of 2G12 was not significantly changed.
HIV-1 neutralization assays.
Primary isolates of HIV-1 were assayed for neutralization by using peripheral blood mononuclear cells as target cells and by using the detection of p24 in an ELISA as a reporter assay, as described previously (96) (protocol B). Alternatively, a pseudotype assay was used in which recombinant virions competent for a single round of infection were generated by using the luciferase reporter plasmid pNL4-3.Luc.R−E−, as described previously (97). The degree of virus neutralization was determined by measuring p24 by ELISA or luciferase activity by a luminometer, and the results were reported as a percent reduction of viral infectivity against an Ab-free control.
RESULTS
Identification of 4KG5.
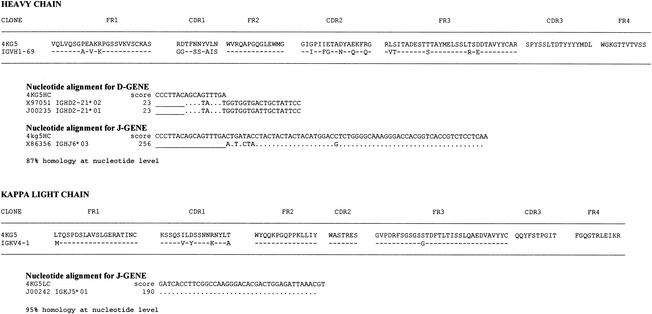
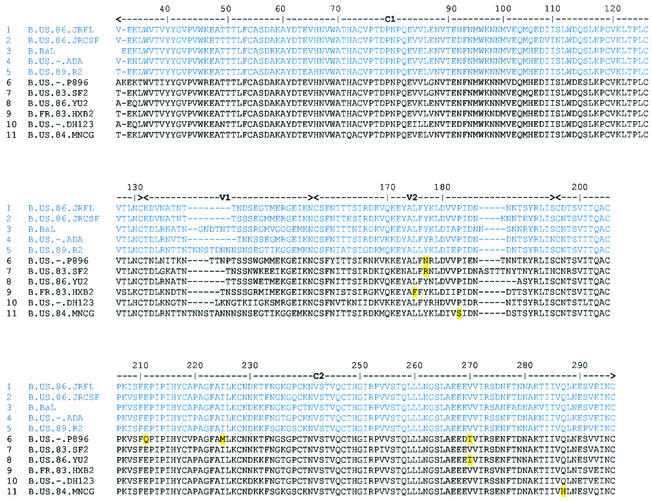
The preparation of both a Fab and an scFv phage display library from the bone marrow of an HIV-1-seropositive individual, FDA2, whose serum exhibits broad HIV-1-neutralizing activity (18, 45, 59, 84) has been described previously (96). Prior to this study, two MAb specificities that derive from these libraries had been reported: Fab Z13 (96), which targets the membrane-proximal external region of HIV-1 gp41, and Fab X5 (51), which targets a CD4i epitope on gp120. In the present study, immobilized gp120JR-FL was used to screen the FDA2 scFv phage library. After four rounds of affinity selection, clones were picked from the enriched phage pools, the phagemid DNA was introduced into non-amber-suppressing (i.e., TOP10) E. coli cells, crude scFv supernatants were tested by ELISA, and the corresponding DNA was sequenced in the variable regions. Several scFvs bound specifically to gp120JR-FL (data not shown), and one, designated 4KG5, was subsequently found by ELISA to bind better to gp120 in the presence of IgG1 b12. The sequences of the heavy and light chain variable regions of 4KG5 revealed that it was a novel specificity, and sequence alignment with the IMGT database (36) webtool (http://www.imgt.cnusc.fr:814/home.html) revealed the closest germ line genes (Fig. 1). Contrary to typical observations with Fabs isolated from our phage display libraries, no somatic variants of 4KG5 were found by DNA sequencing of several individual clones. It is noteworthy that the corresponding Fab library was screened in parallel, but despite multiple panning experiments, also using gp120JR-FL, we were unable to isolate the 4KG5 specificity from the FDA2 Fab library. Since specificities unique to each library type have occasionally been isolated (data not shown), we suggest there is an advantage to screening both types of libraries to achieve broader coverage of the Ab repertoire. We also note here that phage library construction involves scrambling of heavy and light chains, so we cannot be sure that the precise combination of VH and VK found in 4KG5 was present in the repertoire of the FDA2 bone marrow donor.
FIG. 1.
Deduced amino acid sequences of the heavy and light chain variable regions of 4KG5. Shown also is an alignment with the amino acid sequence of the germ line gene with the highest sequence homology to each variable region, as determined by using the IMGT sequence database (36).
4KG5 binds to a complex epitope on gp120 involving the V1/V2 and V3 loops.
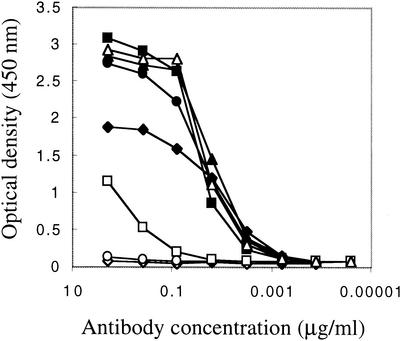
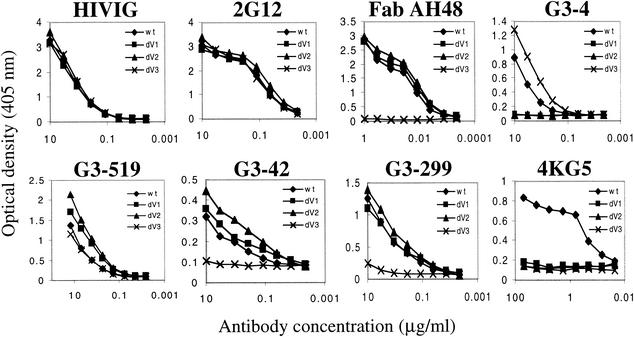
Soluble scFv 4KG5 was produced in E. coli, purified by nickel chromatography via a C-terminal hexahistidine tag (>90% pure by sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis), and used in binding assays. First, 4KG5 was tested for its ability to bind native and denatured forms of gp120JR-FL in comparison to b12, the anti-V3 loop MAb hNM01 (53), and 2G12 (67, 70, 82), which binds to mannose residues on the outer face of gp120. As shown in Fig. 2, the binding of 4KG5 and Fab b12 was completely abolished upon denaturation of gp120 in both cases. By contrast, hNM01 binds equally well to native or denatured gp120, and 2G12 binds but with much lower affinity to denatured gp120, as has been previously noted (82). By Western blotting, we also determined that 4KG5 can bind to gp120JR-FL (data not shown). The binding of 4KG5 to gp120 is highly specific, as confirmed by an absence of reactivity with ovalbumin, gp41, BSA (data not shown), and certain mutants and strains of gp120 (see below). We wished to determine the role, if any, of the variable loops V1, V2, and V3 in the epitope of 4KG5. 4KG5 was tested by ELISA against a small panel of variable loop-deleted mutants of gp140JR-FL in comparison to polyclonal HIVIG, 2G12, an anti-V3 loop Fab (AH48), an anti-V2 loop IgG (G3-4), an anti-C4 IgG (G3-519), and two anti-C4-V3 IgGs (G3-42 and G3-299). The results show that the deletion of the V1, V2, or V3 loop prevents binding of 4KG5 to gp140JR-FL, whereas HIVIG and 2G12 bind with indistinguishable affinity to the ΔV1, ΔV2, and ΔV3 mutants (Fig. 3). To our knowledge, 4KG5 is the only MAb whose binding to gp120 is abolished by deletion of any of the three loops, V1, V2, or V3. Not surprisingly, 4KG5 was also unable to recognize the ΔV1/V2′ or ΔV1/V2* constructs of gp140JR-FL used by Sanders et al. (66). The remaining MAbs behaved as expected: AH48, G3-42, and G3-299 bound all constructs except the V3 loop-deleted gp140, and G3-4 bound full-length and the V3 loop-deleted gp140 but was unable to bind either the ΔV1 or ΔV2 mutant of gp140JR-FL. It is noteworthy that env recognition by the conformation-dependent anti-V2 loop MAb, G3-4, was enhanced by the deletion of V3 and that the anti-C4 and anti-C4-V3 MAbs bound with somewhat enhanced affinity to the V1 and/or V2 loop-deleted variants, suggesting that the V3 loop can act to partially occlude portions of the V1/V2 loop, which in turn might shield the C4-V3 region to MAb access, at least on monomeric gp120.
FIG. 2.
ELISA binding curves of scFv 4KG5 (diamonds), Fab b12 (circles), hNM01 (triangles), and IgG 2G12 (squares) against native (filled symbols) and denatured (open symbols) gp120JR-FL.
FIG. 3.
ELISA binding curves of select anti-gp120 Abs against full-length and loop-deleted constructs of gp140JR-FL. Full-length (diamonds), V1-deleted (squares), V2-deleted (triangles), and V3-deleted (x) gp140JR-FL were captured via the C-terminal region of gp120 with the sheep Ab D7324 and probed with pooled HIVIG, 2G12, Fab AH48, G3-4, G3-519, G3-42, G3-299, and 4KG5. The detection reagents were ABTS for 4KG5 and AP staining solution for all other Abs.
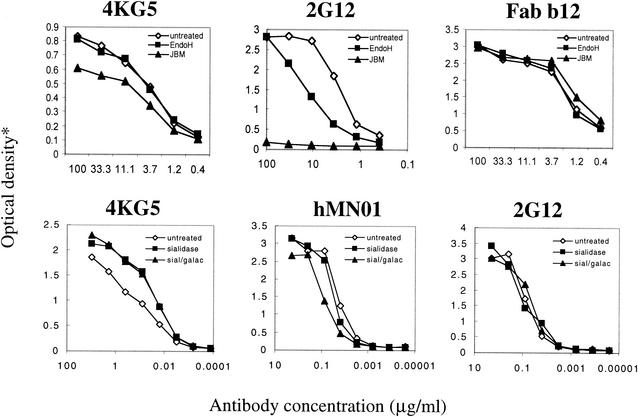
Next, we examined the role of carbohydrate in 4KG5 recognition of gp120. In the first experiment, gp120JR-FL was treated either with endoglycosidase H or jack bean mannosidase and tested for reactivity with 4KG5, Fab b12, and 2G12 (Fig. 4, top panels). Under the conditions used, endoglycosidase H was found to have no effect on 4KG5 activity, but jack bean mannosidase did cause a significant loss in reactivity of 4KG5 to gp120. By contrast, both enzymes considerably diminished 2G12 binding, whereas neither enzyme affected b12 binding. Jack bean mannosidase essentially abolished 2G12 binding, consistent with a previous report (67, 70). In the second experiment, immobilized gp120JR-FL was treated with sialidase alone or sialidase in combination with β-galactosidase and probed with 4KG5, 2G12, and a V3 loop MAb, hNM01 (Fig. 4, bottom panels). Both treatments resulted in a slight enhancement in the binding of 4KG5 to gp120, whereas sialidase alone had little effect on hNM01 binding and in the enzyme combination, hNM01 binding was slightly diminished. Gp120 recognition by 2G12, which depends on mannose residues, was unaffected by sialidase or β-galactosidase (Fig. 4). In summary, 4KG5 binds to a conformational epitope on gp120 that is influenced by carbohydrate and requires the simultaneous display of both the V1/V2 and V3 loops.
FIG. 4.
ELISA binding curves of select MAbs against native and deglycosylated gp120JR-FL. In the top panels, gp120JR-FL was either treated with endoglycosidase H (EndoH, solid squares) or jack bean mannosidase (JBM, solid triangles) or left untreated (open diamonds) overnight at 37°C, then captured via the C-terminal region of gp120 by using the sheep Ab D7324, and probed with 4KG5, 2G12, and Fab b12. In the bottom panels, gp120JR-FL was immobilized in a microplate well and then treated with sialidase for 2 h at 37°C followed by β-galactosidase for 28 h at 37°C (closed triangles) or with sialidase alone (closed squares). Untreated samples (open diamonds) were included as controls. These samples were probed with 4KG5, hNM01, and 2G12. The detection reagent for 4KG5 was ABTS (top panel) or TMB (bottom panel), and AP staining solution was used for the other panels. The OD was read at 450 nm for 4KG5 (bottom panel only) and 405 nm (or all other panels).
Ab competition mapping.
Various Abs were used as competitors at concentrations of 30 μg/ml in an ELISA to determine whether they could inhibit the binding of 4KG5 to gp120JR-FL. Consistent with the suggested role of the V1/V2 and V3 loops in the epitope of 4KG5, MAbs against both the V2 and V3 loops could inhibit 4KG5 binding to gp120 (Table 1). Antibodies to both the crown (e.g., hNM01, loop2, 447-52D, and 19b) and the base (e.g., F425 B4e8) of V3 were able to compete with 4KG5. Interestingly, 14 anti-CD4bs MAbs inhibited the binding of 4KG5 to gp120, whereas b12 actually enhanced 4KG5 binding. In fact, no other Ab that was tested, besides b12, enhanced gp120 recognition by 4KG5. Both of the C4-V3 MAbs, the two C4 MAbs, and sCD4 itself were able to inhibit the binding of 4KG5 to gp120, clearly implicating the C4 region in the epitope of 4KG5. A number of MAbs had little or no effect on 4KG5 binding, including 2G12, C1, and C5 MAbs, CD4i MAbs, and an irrelevant MAb KZ52 (anti-Ebola virus glycoprotein). We note here that the lectin cyanovirin (3) was also a potent inhibitor of 4KG5 binding to gp120. However, we found that cyanovirin was able to inhibit the binding of several anti-HIV-1 MAbs, including IgG1 b12 and V3 loop MAbs, so this result did not yield specific information about the epitope of 4KG5 (data not shown).
TABLE 1.
Summary of the ability of various anti-HIV-1 antibodies to inhibit the binding of 4KG5 scFv to gp120JR-FL
| Antibody identification | Antibody specificity | Effect of antibody (30 μg/ml) on 4KG5 binding to gp120JR-FLb |
|---|---|---|
| p35 | C1 (d)a | 0 |
| 522-149 | C1 (d) | 0 |
| G3-4 | V2 (d) | I ↓ |
| G3-136 | V2 (d) | I ↓ |
| 19b | V3 loop | I ↓↓↓ |
| 447-52D | V3 loop | I ↓↓↓ |
| hNM01 | V3 loop | I ↓↓ |
| AH48c | V3 loop | I ↓↓↓ |
| loop2 | V3 loop | I ↓↓↓ |
| F425 B4e8 | V3 loop | I ↓↓↓ |
| 694-88D | V3 loop | I ↓↓↓ |
| G3-299 | C4-V3 (d) | I ↓ |
| G3-42 | C4-V3 (d) | I ↓ |
| G3-519 | C4 | I ↓ |
| G3-537 | C4 | I ↓ |
| b12 | CD4bs | E ↑ |
| b6 | CD4bs | I ↓↓↓ |
| b3 | CD4bs | I ↓↓ |
| F91 | CD4bs | I ↓↓ |
| F105 | CD4bs | I ↓↓ |
| 15e | CD4bs | I ↓↓↓ |
| L33 | CD4bs | I ↓↓ |
| 1008-D | CD4bs | I ↓↓ |
| 654-30D | CD4bs | I ↓↓ |
| 559-64D | CD4bs | I ↓↓ |
| 1027-30D | CD4bs | I ↓↓ |
| Ia3c | CD4bs | I ↓↓ |
| Ia7c | CD4bs | I ↓↓ |
| FG39c | CD4bs | I ↓ |
| Fbb14c | CD4bs | 0 |
| sCD4 | true CD4bs | I ↓ |
| A32 | C1-C4 (CD4i) | 0 |
| 17b | CD4i | 0 |
| X5 | CD4i | 0 |
| 48d | CD4i | 0 |
| Fbb21c | CD4i | 0 |
| 2G12 | Carbohydrate (outer face) | 0 |
| 1C1 | C5 | 0 |
| M91 | C5 | 0 |
| 670-D | C5 | 0 |
| 1331A | C5 | 0 |
| D7324 | C5 (polyclonal) | 0 |
| KZ52 | Ebola group | 0 |
| FDA2 | Patient serum (polyclonal) | I ↓↓ |
| HIVIG | Pooled HIV-1+ human IgG (polyclonal) | I ↓↓ |
d, discontinuous (epitope).
4KG5 was used at a concentration previously determined to result in ∼75% maximal binding (∼0.3 μg/ml), and was detected via the hexahistidine tag by using the India-HIS probe reagent (Pierce). 0, 75 to 110% maximal binding (i.e., no effect); I, inhibition; ↓ 50 to 74% maximal binding; ↓↓, 25 to 49% maximal binding; ↓↓↓, <25% maximal binding; E, enhancement (emphasized with bold type); ↑, >130% maximal binding.
Novel human antibody identified in this study.
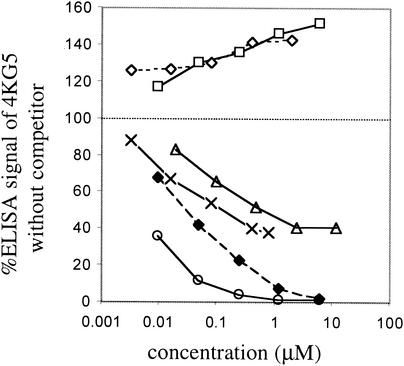
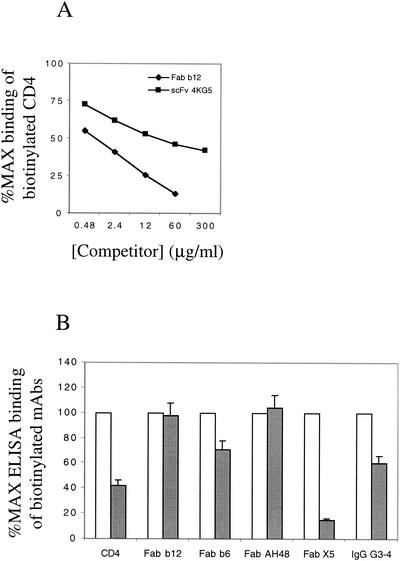
Inhibition curves were generated for Fab and IgG1 b12, Fabs b6 and AH48, IgG G3-4, and sCD4 competing with scFv 4KG5 for binding to gp120. The results indicate that at high concentrations (300 μg/ml), b6 and AH48 were able to completely inhibit 4KG5 from binding gp120JR-FL, whereas sCD4 only inhibited the maximal binding by about 60% and both b12 Fab and IgG enhanced 4KG5 binding by roughly 50% at that concentration (Fig. 5). IgG G3-4 showed incomplete inhibition at a concentration of 120 μg/ml (12 μM). In a reciprocal format, the panel of MAbs was tagged with biotin (BIO) and the ability of 4KG5 to inhibit the binding of these MAbs to gp120 was assessed (Fig. 6). Compared to Fab b12, 4KG5 was relatively inefficient in inhibiting the binding of BIO-sCD4 (Fig. 6A). BIO IgG G3-4 and Fab X5 were significantly inhibited from binding gp120 by 4KG5 (300 μg/ml). In contrast, 4KG5 neither enhanced nor inhibited b12 binding to gp120 (Fig. 6B), even though b12 enhanced 4KG5 binding to gp120 (Fig. 5). Similarly, 4KG5 appeared to have no effect on the binding of Fab AH48 and a limited effect on b6 binding to gp120, despite the use of a concentration of 4KG5 that was 4 logs in excess of those of AH48 and b6 (Fig. 6B). Such nonreciprocal competition in MAb binding to gp120 has been described previously (49).
FIG. 5.
ELISA inhibition curves of 4KG5 with various MAbs against gp120JR-FL. A concentration of 4KG5 previously determined to result in approximately 75% of the maximal signal (∼0.3 μg/ml) was coincubated with various concentrations of b12 Fab (squares), b12 IgG (open diamonds), b6 Fab (circles), sCD4 (triangle), AH48 Fab (filled diamonds), or G3-4 IgG (X), and the level of 4KG5 binding to immobilized gp120JR-FL was measured via the hexahistidine tag of 4KG5 by using the India-HIS probe (Pierce) as a secondary reagent.
FIG. 6.
Effect of 4KG5 as an inhibitor of biotinylated sCD4 and of various MAbs against gp120JR-FL. (A) ELISA inhibition curves of biotinylated sCD4 against gp120JR-FL were determined by using scFv 4KG5 and Fab b12 as inhibitors at various concentrations. (B) ELISA inhibition bar graph showing the relative binding of biotinylated agents in the absence (open bars) or presence (filled bars) of 300 μg of scFv 4KG5/ml. In each case, a concentration of biotinylated agent previously determined to result in approximately 75% of the maximal signal (∼0.03 μg/ml) was used.
Fine epitope mapping of 4KG5 with gp140 and gp160 mutant panels.
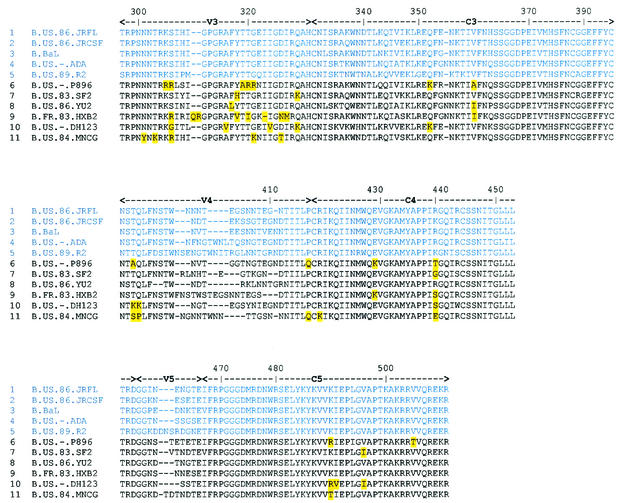
To further explore the specificity of 4KG5, a panel of recombinant HIV-1 envelope proteins was probed with 4KG5 by ELISA. As would be expected from the dependence of 4KG5 on the variable loops, 4KG5 did not exhibit very broad reactivity with the panel, recognizing only 5 of 11 different HIV-1 envelopes, including JR-FL, JR-CSF, BaL, ADA, and R2 (the R2 virus [62] derives from the same individual, FDA2, from whom 4KG5 was cloned). No binding of 4KG5 was observed with envelope proteins from 89.6, SF2, YU2, HxB2, DH123, or MN (data not shown). A sequence alignment was made of gp120s from these strains of HIV-1 and was examined for potential sequence patterns that could explain the ability (or inability) of 4KG5 to bind to gp120 (Fig. 7). Positions in which residues are totally conserved in the 4KG5-positive group but are nonhomologous in the 4KG5-negative group were highlighted at the nonhomologous residue. Highlighted residues were found in the V2, C2, V3, C3, V4, C4, and C5 regions of gp120 and appear to concentrate in the V3 loop and at position 440 in the C4 region (Fig. 7). Given the results of the mutagenesis and MAb competition studies, the inability of 4KG5 to bind to envelope proteins in the 4KG5-negative group is most likely a consequence of differences in V3 and C4, rather than in V1 or V2 or elsewhere in gp120.
FIG. 7.
Amino acid sequence alignment of gp120s to which 4KG5 binds (rows 1 to 5) or does not bind (rows 6 to 11). Highlighted are those residues that are totally conserved in that position in the 4KG5-positive group but are divergent or nonhomologous in the 4KG5-negative group. Such residues may play a role in 4KG5 recognition.
To more-finely map the epitope of 4KG5, a panel of mutants of gp140JR-FL and gp160JR-CSF, each bearing a single substitution were probed with 4KG5 by ELISA (Table 2). A number of mutations reduced the binding of 4KG5 to gp160 by 90% or greater, including F176A (V2), D180A (V2), I184A (V2), T198P (C2, strand β3), R298G (base of V3), P313A (V3 crown), W395A (V4), I423A (C4), and V430A (C4). In each case, MAb 2G12 was used to probe the mutant envelope proteins and the binding affinity was not significantly changed (data not shown). One mutation appeared to slightly enhance the binding of 4KG5 to gp160, K171A (V2). Still other mutations had no significant effect on the ability of 4KG5 to recognize gp160, including K121A (C1, strand β2), D275V (C2, D loop), N302A (V3), E322K (V3), D368A (C3), E429A (C4), K432A (C4), T455A (C4), G473A (C5), and D474A (C5). We make special note of the R298G mutant since this substitution has previously been shown to enhance the binding of CD4, an anti-CD4bs MAb, F105, and anti-V3 loop MAbs to gp120 (90). By contrast, this mutation almost completely abolished 4KG5 binding to gp120. We went on to confirm the results of Wyatt et al. by determining that changing R298 to G either did not affect or enhanced the binding of several anti-CD4bs and anti-V3 loop MAbs. By contrast, however, IgG1 b12 binding to the R298G mutant was ∼10% of wild-type levels, revealing a distinct difference in the way that b12 recognizes gp120 in comparison to other anti-CD4bs antibodies (data not shown). In general, the results of the mutational analysis of 4KG5 recognition of gp160 suggest that 4KG5 binds to a discontinuous epitope involving residues in the V2, V3, and C4 regions.
TABLE 2.
Summary of the effects of amino acid substitutions in gp160JR-CSF or gp140JR-FL on 4KG5 recognition
| Mutanta | Region | % WT bindingb |
|---|---|---|
| E102A | C1 | 65 |
| K121A | C1 (strand β2) | 90 |
| T123A | C1 (strand β2) | 50 |
| K171A | V2 | 200 |
| F176A | V2 | <1 |
| D180A | V2 | 7 |
| I184A | V2 | 2 |
| D185A | V2 | 30 |
| T198Pc | C2 (strand β3) | 1 |
| S199A | C2 (strand β3) | 25 |
| D275Vd | C2 (D-loop) | 100 |
| N276A | C2 (D-loop) | 60 |
| R298Ge | Base of V3 | 0.8 |
| N301A | V3 | 40 |
| N302A | V3 | 85 |
| P313A | V3 crown | <1 |
| E322Kf | V3 | 110 |
| A329K | V3 | 40 |
| H330A | Base of V3 | 35 |
| D368A | C3 | 100 |
| T388A | C3 | 50 |
| W395A | V4 | 10 |
| I423A | C4 | 0.3 |
| E429A | C4 | 100 |
| V430A | C4 | 2 |
| K432A | C4 | 50 |
| T455A | C4 | 150 |
| N461A | V5 | 25 |
| R469A | C5 | 30 |
| G473A | C5 | 100 |
| D474A | C5 | 100 |
Amino acid numbering is relative to HIV-1HxB2, where 1 is the initial methionine (29). All mutants are in a gp160JR-CSF background except mutants T198P, D275V, R298G, and E322K, which are in a gp140JR-FL background.
Apparent affinities were calculated as the antibody concentration at half maximal binding. Apparent affinities relative to wild-type (WT) gp140/gp160 were calculated by using the formula: [(apparent affinity of wild type)/(apparent affinity of mutant)] × 100%. The mutants for which 4KG5 binding to gp120 was diminished were tested against MAb 2G12, and 2G12 binding was not significantly changed (data not shown). Mutants for which the apparent affinity of 4KG5 relative to the wild type was <10% are shown in bold.
A T198P substitution was previously determined to affect the interaction between the V1/V2 and V3 loops (94).
A D275V substitution was found to affect an interaction between the V3 loop and the C2 region of gp120 (10).
An R298G substitution has been found to enhance binding of CD4, the anti-CD4bs MAb F105, and anti-V3 loop MAbs to gp120 (90). We determined that the binding of IgG1 b12 to this R298G mutant is only ∼10% of wild-type levels, whereas the binding of several other anti-CD4bs and anti-V3 loop MAbs is equal to or greater than wild-type levels.
The type of charge on the side chain of the residue at position 322 has been associated with coreceptor usage (11/25 rule) (26).
Neutralization assays with 4KG5.
Neutralization assays with scFv 4KG5 were performed in a single-round infection envelope complementation assay. The inhibitory dose required to reduce infectivity of HIV-1R2, HIV-1ADA, HIV-1JR-CSF, and HIV-1JR-FL by 50% for scFv 4KG5 was greater than 50 μg/ml (data not shown). In a peripheral blood mononuclear cell assay, 4KG5 was also unable to neutralize HIV-1JR-FL or HIV-1BaL (50% inhibitory dose > 80 μg/ml for both), and the presence of 5 μg of 4KG5/ml did not enhance the ability of IgG1 b12 to neutralize either of these primary isolates (data not shown). The T-cell-line-adapted strains HIV-1MN and HIV-1HxB2 were resistant to neutralization by 4KG5, but this was expected, as 4KG5 was unable to recognize envelope from these strains in an ELISA.
DISCUSSION
We have described a novel MAb, 4KG5, that binds to a complex epitope on HIV-1 gp120 involving the V1, V2, and V3 loops as well as the C4 region, defining a new Ab competition group against HIV-1 gp120. It is particularly interesting that, although the 4KG5 epitope overlaps with those of the V2, V3, C4-V3, C4, CD4bs, and CD4i MAbs, it does not overlap with the b12 epitope. Hence, 4KG5 delineates a specific physical boundary on the epitope of b12 that sets it apart from the CD4bs proper and, indeed, from the epitopes of all other anti-CD4bs MAbs tested. Thus, it is becoming increasingly clear that gp120 bears a remarkable number of functionally nonequivalent epitopes overlapping the CD4bs, a property that likely derives, at least in part, from the structural plasticity of gp120, which confounds the task of relating function to the only known crystal structure (i.e., the CD4-17b-gp120 ternary complex) (33). It would be intriguing to observe the physical relationship of 4KG5 to the CD4bs in a complex of 4KG5, b12, and gp120. Indeed, 4KG5 may prove particularly useful in crystallization attempts of full-length gp120 bearing both the V1/V2 and V3 loops, as 4KG5 might stabilize the loops with respect to one another and to C4 in the core.
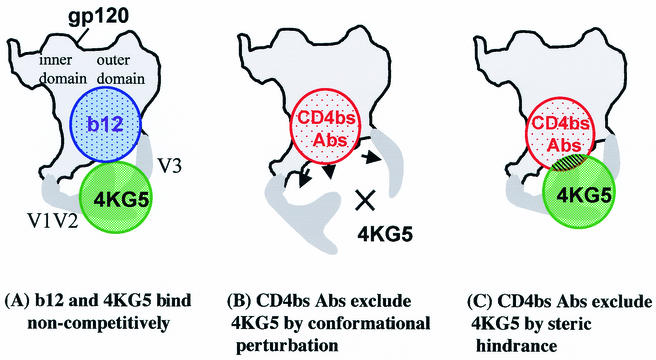
It is remarkable that b12 enhances 4KG5 binding to gp120 yet the C4 region in the bridging sheet seems to be involved in the epitopes of both MAbs. Other MAbs that bind to the C4 region of gp120 such as G3-537 and the CD4i MAbs, 17b and X5, are inhibited by b12 (49). Moreover, mutagenesis analysis shows that several mutations in C4 (e.g., K421A, M426A, and I439A) markedly diminish b12 recognition of gp120 (56). One likely explanation for this apparent discrepancy is that mutations in the bridging sheet, including C4, can have a profound effect on the spatial relationship that exists between the variable loops. Although a crystal structure of gp120 that includes its variable loops is not yet available, we present a cartoon of gp120 with the variable loops included to model 4KG5 binding to gp120 (Fig. 8A). Since there is no residual binding whatsoever of 4KG5 to gp120 upon deletion of V1, V2, or V3, this suggests that most of the contacts 4KG5 makes with gp120 is to these loops, particularly with V3, since V3 loop MAbs appear to be more-potent inhibitors of 4KG5 than V2 loop MAbs. The contribution of C4 to the epitope of 4KG5 may relate more to a stabilization of the loops in a particular arrangement (as discussed) rather than to direct contact with 4KG5, although we cannot rule out some contact between 4KG5 and C4. We also show two possible models for how conventional, nonneutralizing anti-CD4bs MAbs might inhibit 4KG5 binding to gp120. In the first, the conventional anti-CD4bs MAbs induce a conformational change in gp120 that affects the V1/V2 and V3 loops such that 4KG5 can no longer recognize its epitope (Fig. 8B). In the second model, the conventional anti-CD4bs MAbs recognize the same conformational state of gp120 as b12 but sterically hinder 4KG5 from accessing its epitope (Fig. 8C). While it is not possible at present to distinguish between these two mechanisms, or indeed among others not considered, it seems likely that elements of both models may contribute to the inhibition of 4KG5 binding to gp120 by anti-CD4bs MAbs. Because the competition between 4KG5 and sCD4 is not complete, this would suggest that there is sufficient flexibility in gp120 such that both 4KG5 and sCD4 could bind simultaneously to gp120, though each with reduced affinity. A lack of reciprocal inhibition and/or enhancement, as observed in some of the MAb competition experiments (Fig. 5 and 6B), is not itself a novel phenomenon (49). In particular, it has been shown that 17b diminishes the affinity of sCD4 for gp120 by decreasing the on rate and increasing the off rate (93), despite the fact that sCD4 clearly increases the affinity of 17b to gp120.
FIG. 8.
Cartoon models of 4KG5 binding to gp120 in the presence of IgG1 b12 and conventional anti-CD4bs MAbs. (A) A model in which both 4KG5 and b12 bind noncompetitively to gp120. (B) A model in which conventional anti-CD4bs MAbs perturb the conformational state of gp120, including the spatial relationship between the V1/V2 and V3 loops, such that 4KG5 cannot bind to gp120. (C) A model in which conventional anti-CD4bs MAbs recognize a conformational state of gp120 that is similar to that recognized by b12, but in so doing, the anti-CD4bs MAbs sterically hinder 4KG5 from binding to its epitope, which remains intact. Both models B and C may be possible and are not necessarily mutually exclusive. In some cases, inhibition of 4KG5 binding to gp120 is incomplete, such as with CD4, suggesting that simultaneous, albeit weaker, binding of the two molecules to gp120 is possible (data not illustrated).
Zhu et al. proposed a model in which the V1/V2 and V3 loops interact, and this interaction is disrupted by a particular mutation (T198P) in the bridging sheet (94). Our results show that the T198P mutation reduces 4KG5 binding, consistent with the idea that this mutation may disrupt an interaction between V1/V2 and V3. Indeed, there have been reports suggesting that the V1/V2 loops interact with the V3 loop on the HIV-1 envelope spike (5, 7, 28) and that carbohydrate may play a role in this interaction (37, 55, 61). Thus, it is significant that we found a subtle influence of glycans on 4KG5 recognition of gp120 (Fig. 4). There have also been reports suggesting that the V3 loop interacts with the C2 (10) or C4 (6, 48, 50, 90) regions of gp120, and in another study, V3, V2, and the CD4bs were found to be cooperatively involved in binding curdlan sulfate (27).
Much more remains to be learned about the interactions that are made with the V1/V2 and V3 loops. For example, mutations in V2 (e.g., D180A and I184A) have been shown to reduce binding of b12 to gp120 more severely than by simply removing the entire V1/V2 loop (56). It is interesting that these V2 loop mutations also abolish 4KG5 binding to gp120. This raises the question of whether b12 and 4KG5 are in simultaneous contact with certain residues in the V2 loop, perhaps by approaching the loop from opposite sides. Alternatively, and perhaps more likely, 4KG5 may be in contact with the V2 loop and b12 binding is merely modulated by the V2 loop via conformational effects. Still another possibility is that these mutations in the V2 loop diminish binding of both MAbs by conformational changes, although the complete knockout of 4KG5 binding to gp120 by deleting the V1 or the V2 loop suggests at least some contact to these loops. Some mutations in the bridging sheet affect b12 binding (e.g., T198P) (94), whereas others (e.g., I423P) (91) do not. Perhaps most intriguing is our observation that the R298G substitution diminishes b12 binding to gp120 by 90% or greater (Table 2). The R298G substitution does not diminish gp120 recognition by anti-V3 loop MAbs, F105 (90), and other anti-CD4bs MAbs (M. B. Zwick and D. R. Burton, unpublished data); however, interestingly, an R298A substitution greatly diminishes CCR5 binding (85). More investigation into the molecular interactions between the variable loops, both within monomeric gp120 and among protomers in oligomeric gp120, is clearly warranted as they affect access by Abs to the CD4bs, a major target for vaccine design.
The two observations that, with the exception of b12, anti-CD4bs MAbs (i) are not capable of broad neutralization of HIV-1 across subtypes and (ii) inhibit 4KG5 binding to gp120 appear to be more than coincidental. The lack of cross-neutralization by most anti-CD4bs MAbs may result from a suboptimal angle of approach to the CD4bs such that they are sterically hindered by the interaction between the variable loops and/or neighboring protomers, whereas b12 avoids these problems. In a similar way, most anti-CD4bs MAbs obstruct binding of 4KG5 to monomeric gp120, possibly by altering the interaction between V1/V2 and V3, whereas b12 and 4KG5 prefer a similar conformation in the V1/V2-V3 interaction. Indeed, an association of structural changes in the V2 and V3 loops has been made with acquired neutralization resistance in a passaged simian-human immunodeficiency virus (HXBc2P 3.2) (92). A recent study suggests that HIV-1 thwarts Ab-mediated neutralization through conformational heterogeneity in gp120 (entropic masking) (31). In such a scenario, dynamic motion between V1/V2 and V3 could contribute to the inability of 4KG5 and most anti-CD4bs MAbs to bind to and neutralize HIV-1 envelope spikes, whereas b12 is comparatively unaffected by these dynamic movements.
Some individual characteristics of 4KG5 are shared with other anti-gp120 specificities. For example, the C4-V3 MAbs are affected by mutations in V3 and C4 (50) and are inhibited by V3 and C4 MAbs (49). Unlike 4KG5, however, the C4-V3 MAbs compete weakly with b12 (49) and are unaffected by the deletion of the V1 or V2 loop (Fig. 3). MAbs other than 4KG5 also can have differential effects on various anti-CD4bs MAbs. A murine MAb, SC258, directed against a discontinuous epitope in the V2 loop enhanced the binding of F91 to gp120 but reduced the binding of b12 (49). In a similar fashion, a murine MAb G3-1472 that binds to a linear epitope on the V3 loop enhanced the binding of the CD4bs MAb F91 to gp120, whereas IgG b12 binding was reduced (49). Another murine anti-V3 loop MAb, 5G11, was found to enhance the binding of b12 to gp120 but it also enhanced the binding of all other anti-CD4bs MAbs tested (49). 4KG5 even shares certain features with CCR5. Similar to 4KG5, the interaction of CCR5 with gp120 appears to involve interaction or compatibility between the V1/V2 and V3 loops (5, 26, 28, 39, 76). In the absence of sCD4, 4KG5 is able to inhibit the CD4i MAbs, 17b and X5, and certain mutations in or near the bridging sheet diminish binding to 4KG5, 17b (63, 64, 91), X5 (A. F. Labrijn and D. R. Burton, unpublished data), and CCR5 (64, 85, 91). So far, 4KG5 has been shown to bind only gp120s from R5 viruses. Lastly, the third complementarity-determining region of the heavy chain of 4KG5 (CDR H3) is rich in Tyr residues (amino acid sequence, SPYSSLTDTYYYYMDL), which is similar to the N-terminal region of CCR5 that is known to bind to gp120 (amino acid sequence, DYQVSSPIYDINYYTSE); the latter requires tyrosine sulfation for CCR5 reactivity (9).
4KG5 had no significant neutralizing activity against HIV-1 (ADA, R2, JR-CSF, and JR-FL), nor did it potentiate the neutralizing activity of b12. That 4KG5 binds well to gp120 from these strains suggests that the loops are oriented differently on the viral spike than on the monomer, consistent with previous studies indicating that the epitopes available on monomeric gp120 are different from those available on trimeric gp120 (57, 60, 69). This issue emphasizes that full-length monomeric gp120 is unable to fully mimic the antigenicity of trimeric, functionally active virion spikes and argues for the development of vaccine candidates of gp120 that better retain or mimic the native structure found on infectious virus. It is pertinent that neutralizing Abs from monkeys infected with simian-human immunodeficiency virus appeared to bind to a discontinuous epitope involving both V2 and V3 sequences (17). Hence, it would seem that including V1/V2 and V3 loops in a vaccine candidate, if optimally displayed, could themselves also elicit neutralizing Abs, however isolate specific they may be. More importantly, stabilized loops could favor the elicitation of b12-like Abs with broad activity while disfavoring conventional, nonneutralizing anti-CD4bs MAbs.
Acknowledgments
We thank Dawn Slifka, Darren Michaels, Emil Schultz, Jeremy Heath, and Ann Hessell for excellent technical support and James Binley and Ralph Pantophlet for helpful discussions.
We acknowledge support from the GCRC of The Scripps Research Institute (M01 RR00833). We further acknowledge financial support from the Elizabeth Glaser Pediatric AIDS Foundation and the Natural Sciences and Engineering Research Council of Canada (M.B.Z.) and grants from the National Institutes of Health, AI37438 (G.V.Q.), AI40377 (P.W.H.I.P.), and AI33292 (D.R.B.).
REFERENCES
- 1.Barbas, C. F., III, E. Bjorling, F. Chiodi, N. Dunlop, D. Cababa, T. M. Jones, S. L. Zebedee, M. A. Persson, P. L. Nara, E. Norrby, and D. R. Burton. 1992. Recombinant human Fab fragments neutralize human type 1 immunodeficiency virus in vitro. Proc. Natl. Acad. Sci. USA 89:9339-9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbas, C. F., III, D. R. Burton, J. K. Scott, and G. J. Silverman. 2001. Phage display: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 3.Boyd, M. R., K. R. Gustafson, J. B. McMahon, R. H. Shoemaker, B. R. O'Keefe, T. Mori, R. J. Gulakowski, L. Wu, M. I. Rivera, C. M. Laurncot, M. J. Currens, J. H. Cardellina, R. W. Buckheit, P. L. Nara, L. K. Pannell, R. C. Sowder, and L. E. Henderson. 1997. Discovery of Cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob. Agents Chemother. 41:1521-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton, D. R., J. Pyati, R. Koduri, S. J. Sharp, G. B. Thornton, P. W. H. I. Parren, L. S. W. Sawyer, R. M. Hendry, N. Dunlop, P. L. Nara, M. Lamacchia, E. Garratty, E. R. Stiehm, Y. J. Bryson, Y. Cao, J. P. Moore, D. D. Ho, and C. F. Barbas. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024-1027. [DOI] [PubMed] [Google Scholar]
- 5.Carrillo, A., and L. Ratner. 1996. Cooperative effects of the human immunodeficiency virus type 1 envelope variable loops V1 and V3 in mediating infectivity for T cells. J. Virol. 70:1310-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrillo, A., and L. Ratner. 1996. Human immunodeficiency virus type 1 tropism for T-lymphoid cell lines: role of the V3 loop and C4 envelope determinants. J. Virol. 70:1301-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, M., C. Shi, V. Kalia, S. B. Tencza, R. C. Montelaro, and P. Gupta. 2001. HIV gp120 V1/V2 and C2-V3 domains glycoprotein compatibility is required for viral replication. Virus Res. 79:91-101. [DOI] [PubMed] [Google Scholar]
- 8.Conley, A. J., M. K. Gorny, J. A. I. Kessler, L. J. Boots, M. Ossorio-Castro, S. Koenig, D. W. Lineberger, E. A. Emini, C. Williams, and S. Zolla-Pazner. 1994. Neutralization of primary human immunodeficiency virus type 1 isolates by the broadly reactive anti-V3 monoclonal antibody, 447-52D. J. Virol. 68:6994-7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cormier, E. G., and T. Dragic. 2002. The crown and stem of the V3 loop play distinct roles in human immunodeficiency virus type 1 envelope glycoprotein interactions with the CCR5 coreceptor. J. Virol. 76:8953-8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Costa, S., K. S. Slobod, R. G. Webster, S. W. White, and J. L. Hurwitz. 2001. Structural features of HIV envelope defined by antibody escape mutant analysis. AIDS Res. Hum. Retrovir. 17:1205-1209. [DOI] [PubMed] [Google Scholar]
- 11.di Marzo Veronese, F. R., R. Rahman, R. Pal, C. Boyer, J. Romano, V. S. Kalyanaraman, B. C. Nair, R. C. Gallo, and M. G. Sarngadharan. 1992. Delineation of immunoreactive, conserved regions in the external envelope glycoprotein of the human immunodeficiency virus type I. AIDS Res. Hum. Retrovir. 8:1125-1132. [DOI] [PubMed] [Google Scholar]
- 12.Ditzel, H. J., J. M. Binley, J. P. Moore, J. Sodroski, N. Sullivan, L. S. W. Sawyer, R. M. Hendry, W. P. Yang, C. F. Barbas III, and D. R. Burton. 1995. Neutralizing recombinant human antibodies to a conformational V2- and CD4-binding site-sensitive epitope of HIV-1 gp120 isolated by using an epitope-masking procedure. J. Immunol. 154:893-906. [PubMed] [Google Scholar]
- 13.Ditzel, H. J., P. W. H. I. Parren, J. M. Binley, J. Sodroski, J. P. Moore, C. F. Barbas, and D. R. Burton. 1997. Mapping the protein surface of human immunodeficiency virus type 1 gp120 using human monoclonal antibodies from phage display libraries. J. Mol. Biol. 267:684-695. [DOI] [PubMed] [Google Scholar]
- 14.D'Souza, M. P., D. Livnat, J. A. Bradac, S. Bridges, The AIDS Clinical Trials Group Antibody Selection Working Group, and Collaborating Investigators. 1997. Evaluation of monoclonal antibodies to HIV-1 primary isolates by neutralization assays: performance criteria for selecting candidate antibodies for clinical trials. J. Infect. Dis. 175:1056-1062. [DOI] [PubMed] [Google Scholar]
- 15.Earl, P. L., C. C. Broder, R. W. Doms, and B. Moss. 1997. Epitope map of human immunodeficiency virus type 1 gp41 derived from 47 monoclonal antibodies produced by immunization with oligomeric envelope protein. J. Virol. 71:2674-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckert, D. M., and P. S. Kim. 2001. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70:777-810. [DOI] [PubMed] [Google Scholar]
- 17.Etemad-Moghadam, B., G. B. Karlsson, M. Halloran, Y. Sun, D. Schenten, M. Fernandes, N. L. Letvin, and J. Sodroski. 1998. Characterization of simian-human immunodeficiency virus envelope glycoprotein epitopes recognized by neutralizing antibodies from infected monkeys. J. Virol. 72:8437-8445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenyö, E. M., J. Albert, and J. McKeating. 1996. The role of the humoral immune response in HIV infection. AIDS 10(Suppl. A):S97-S106. [DOI] [PubMed] [Google Scholar]
- 19.Fung, M. S. C., C. R. Y. Sun, W. L. Gordon, R.-S. Liou, T. W. Chang, W. N. C. Sun, E. S. Daar, and D. D. Ho. 1992. Identification and characterization of a neutralization site within the second variable region of human immunodeficiency virus type 1 gp120. J. Virol. 66:848-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorny, M. K., A. J. Conley, S. Karwowska, A. Buchbinder, J.-Y. Xu, E. A. Emini, S. Koenig, and S. Zolla-Pazner. 1992. Neutralization of diverse human immunodeficiency virus type 1 variants by an anti-V3 human monoclonal antibody. J. Virol. 66:7538-7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorny, M. K., C. Williams, B. Volsky, K. Revesz, S. Cohen, V. R. Polonis, W. J. Honnen, S. C. Kayman, C. Krachmarov, A. Pinter, and S. Zolla-Pazner. 2002. Human monoclonal antibodies specific for conformation-sensitive epitopes of V3 neutralize human immunodeficiency virus type 1 primary isolates from various clades. J. Virol. 76:9035-9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham, B. S., M. J. McElrath, R. I. Connor, D. H. Schwartz, G. J. Gorse, M. C. Keefer, M. J. Mulligan, T. J. Matthews, S. M. Wolinsky, D. C. Montefiori, S. H. Vermund, J. S. Lambert, L. Corey, R. B. Belshe, R. Dolin, P. F. Wright, B. T. Korber, M. C. Wolff, P. E. Fast, the AIDS Vaccine Evaluation Group, and the Correlates of HIV Immune Protection Group. 1998. Analysis of intercurrent human immunodeficiency virus type 1 infections in phase I and II trials of current AIDS vaccines. J. Infect. Dis. 177:310-319. [DOI] [PubMed] [Google Scholar]
- 23.Ho, D. D., M. S. Fung, Y. Z. Cao, X. L. Li, C. Sun, T. W. Chang, and N. C. Sun. 1991. Another discontinuous epitope on glycoprotein gp120 that is important in human immunodeficiency virus type 1 neutralization is identified by a monoclonal antibody. Proc. Natl. Acad. Sci. USA 88:8949-8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho, D. D., and Y. Huang. 2002. The HIV-1 vaccine race. Cell 110:135-138. [DOI] [PubMed] [Google Scholar]
- 25.Ho, D. D., J. A. McKeating, X. L. Li, T. Moudgil, E. S. Daar, N. C. Sun, and J. E. Robinson. 1991. Conformational epitope on gp120 important in CD4 binding and human immunodeficiency virus type 1 neutralization identified by a human monoclonal antibody. J. Virol. 65:489-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman, N. G., F. Seillier-Moiseiwitsch, J. Ahn, J. M. Walker, and R. Swanstrom. 2002. Variability in the human immunodeficiency virus type 1 gp120 Env protein linked to phenotype-associated changes in the V3 loop. J. Virol. 76:3852-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jagodzinski, P. P., J. Wustner, D. Kmieciak, T. J. Wasik, A. Fertala, A. L. Sieron, M. Takahashi, T. Tsuji, T. Mimura, M. S. Fung, M. K. Gorny, M. Kloczewiak, Y. Kaneko, and D. Kozbor. 1996. Role of the V2, V3, and CD4-binding domains of GP120 in curdlan sulfate neutralization sensitivity of HIV-1 during infection of T lymphocytes. Virology 226:217-227. [DOI] [PubMed] [Google Scholar]
- 28.Koito, A., G. Harrowe, J. A. Levy, and C. Cheng-Mayer. 1994. Functional role of the V1/V2 region of human immunodeficiency virus type 1 envelope glycoprotein gp120 in infection of primary macrophages and soluble CD4 neutralization. J. Virol. 68:2253-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korber, B., B. T. Foley, C. Kuiken, S. K. Pillai, and J. G. Sodroski. 1998. Numbering positions in HIV relative to HXB2CG, p. III-102-III-111. In B. Korber, C. L. Kuiken, B. Foley, B. Hahn, B. Korber, F. McCutchan, J. W. Mellors, and J. Sodroski (ed.), Human retroviruses and AIDS 1998. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, N.M.
- 30.Kostrikis, L. G., Y. Cao, H. Ngai, J. P. Moore, and D. D. Ho. 1996. Quantitative analysis of serum neutralization of human immunodeficiency virus type 1 from subtypes A, B, C, D, E, F, and I: lack of direct correlation between neutralization serotypes and genetic subtypes and evidence for prevalent serum-dependent infectivity enhancement. J. Virol. 70:445-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwong, P. D., M. L. Doyle, D. J. Casper, C. Cicala, S. A. Leavitt, S. Majeed, T. D. Steenbeke, M. Venturi, I. Chaiken, M. Fung, H. Katinger, P. W. Parren, J. Robinson, D. Van Ryk, L. Wang, D. R. Burton, E. Freire, R. Wyatt, J. Sodroski, W. A. Hendrickson, and J. Arthos. 2002. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature 420:678-682. [DOI] [PubMed] [Google Scholar]
- 32.Kwong, P. D., R. Wyatt, E. Desjardins, J. Robinson, J. S. Culp, B. D. Hellmig, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1999. Probability analysis of variational crystallization and its application to gp120, the exterior envelope glycoprotein of type 1 human immunodeficiency virus (HIV-1). J. Biol. Chem. 274:4115-4123. [DOI] [PubMed] [Google Scholar]
- 33.Kwong, P. D., R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laal, S., S. Burda, M. K. Gorny, S. Karwowska, A. Buchbinder, and S. Zolla-Pazner. 1994. Synergistic neutralization of human immunodeficiency virus type 1 by combinations of human monoclonal antibodies. J. Virol. 68:4001-4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Labrosse, B., C. Treboute, A. Brelot, and M. Alizon. 2001. Cooperation of the V1/V2 and V3 domains of human immunodeficiency virus type 1 gp120 for interaction with the CXCR4 receptor. J. Virol. 75:5457-5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lefranc, M. P. 2001. IMGT, the international ImMunoGeneTics database. Nucleic Acids Res. 29:207-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Losman, B., A. Bolmstedt, K. Schønning, Å. Björndal, C. Westin, E. M. Fenyö, and S. Olofsson. 2001. Protection of neutralization epitopes in the V3 loop of oligomeric human immunodeficiency virus type 1 glycoprotein 120 by N-linked oligosaccharides in the V1 region. AIDS Res. Hum. Retrovir. 17:1067-1076. [DOI] [PubMed] [Google Scholar]
- 38.Lu, M., S. C. Blacklow, and P. S. Kim. 1995. A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat. Struct. Biol. 2:1075-1082. [DOI] [PubMed] [Google Scholar]
- 39.Maeda, Y., M. Foda, S. Matsushita, and S. Harada. 2000. Involvement of both the V2 and V3 regions of the CCR5-tropic human immunodeficiency virus type 1 envelope in reduced sensitivity to macrophage inflammatory protein 1α. J. Virol. 74:1787-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malenbaum, S. E., D. Yang, L. Cavacini, M. Posner, J. Robinson, and C. Cheng-Mayer. 2000. The N-terminal V3 loop glycan modulates the interaction of clade A and B human immunodeficiency virus type 1 envelopes with CD4 and chemokine receptors. J. Virol. 74:11008-11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maruyama, T., P. W. H. I. Parren, A. Sanchez, I. Rensink, L. L. Rodriguez, A. Khan, C. J. Peters, and D. R. Burton. 1999. Recombinant human monoclonal antibodies to Ebola virus. J. Infect. Dis. 179:S235-S239. [DOI] [PubMed] [Google Scholar]
- 42.Mbah, H. A., S. Burda, M. K. Gorny, C. Williams, K. Revesz, S. Zolla-Pazner, and P. N. Nyambi. 2001. Effect of soluble CD4 on exposure of epitopes on primary, intact, native human immunodeficiency virus type 1 virions of different genetic clades. J. Virol. 75:7785-7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McMichael, A., and T. Hanke. 2002. The quest for an AIDS vaccine: is the CD8+ T-cell approach feasible? Nat. Rev. Immunol. 2:283-291. [DOI] [PubMed] [Google Scholar]
- 44.Moog, C., H. J. A. Fleury, I. Pellegrin, A. Kirn, and A. M. Aubertin. 1997. Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type 1-infected individuals. J. Virol. 71:3734-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore, J. P., Y. Cao, J. Leu, L. Qin, B. Korber, and D. D. Ho. 1996. Inter- and intraclade neutralization of human immunodeficiency virus type 1: genetic clades do not correspond to neutralization serotypes but partially correspond to gp120 antigenic serotypes. J. Virol. 70:427-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore, J. P., F. E. McCutchan, S. W. Poon, J. Mascola, J. Liu, Y. Cao, and D. D. Ho. 1994. Exploration of antigenic variation in gp120 from clades A through F of human immunodeficiency virus type 1 by using monoclonal antibodies. J. Virol. 68:8350-8364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore, J. P., P. W. H. I. Parren, and D. R. Burton. 2001. Genetic subtypes, humoral immunity, and human immunodeficiency virus type 1 vaccine development. J. Virol. 75:5721-5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore, J. P., Q. J. Sattentau, R. Wyatt, and J. Sodroski. 1994. Probing the structure of the human immunodeficiency virus surface glycoprotein gp120 with a panel of monoclonal antibodies. J. Virol. 68:469-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore, J. P., and J. Sodroski. 1996. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J. Virol. 70:1863-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore, J. P., M. Thali, B. A. Jameson, F. Vignaux, G. K. Lewis, S. W. Poon, M. Charles, M. S. Fung, B. Sun, P. J. Durda, L. Akerblom, B. Wahren, D. D. Ho, Q. J. Sattentau, and J. Sodroski. 1993. Immunochemical analysis of the gp120 surface glycoprotein of human immunodeficiency virus type 1: Probing the structure of the C4 and V4 domains and the interaction of the C4 domain with the V3 loop. J. Virol. 67:4785-4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moulard, M., S. K. Phogat, Y. Shu, A. F. Labrijn, X. Xiao, J. M. Binley, M. Y. Zhang, I. A. Sidorov, C. C. Broder, J. Robinson, P. W. H. I. Parren, D. R. Burton, and D. S. Dimitrov. 2002. Broadly cross-reactive HIV-1-neutralizing human monoclonal Fab selected for binding to gp120-CD4-CCR5 complexes. Proc. Natl. Acad. Sci. USA 99:6913-6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muster, T., F. Steindl, M. Purtscher, A. Trkola, A. Klima, G. Himmler, F. Rüker, and H. Katinger. 1993. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 67:6642-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakamura, M., M. Terada, H. Sasaki, M. Kamada, and T. Ohno. 2000. Virolysis and in vitro neutralization of HIV-1 by humanized monoclonal antibody hNM-01. Hybridoma 19:427-434. [DOI] [PubMed] [Google Scholar]
- 54.Nyambi, P. N., M. K. Gorny, L. Bastiani, G. van der Groen, C. Williams, and S. Zolla-Pazner. 1998. Mapping of epitopes exposed on intact human immunodeficiency virus type 1 (HIV-1) virions: a new strategy for studying the immunologic relatedness of HIV-1. J. Virol. 72:9384-9391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogert, R. A., M. K. Lee, W. Ross, A. Buckler-White, M. A. Martin, and M. W. Cho. 2001. N-linked glycosylation sites adjacent to and within the V1/V2 and the V3 loops of dualtropic human immunodeficiency virus type 1 isolate DH12 gp120 affect coreceptor usage and cellular tropism. J. Virol. 75:5998-6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pantophlet, R., E. O. Saphire, P. Poignard, P. W. H. I. Parren, I. A. Wilson, and D. R. Burton. 2003. Fine mapping of the interaction of neutralizing and non-neutralizing monoclonal antibodies with the CD4 binding site of human immunodeficiency virus type 1 gp120. J. Virol. 77:642-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parren, P. W. H. I., I. Mondor, D. Naniche, H. J. Ditzel, P. J. Klasse, D. R. Burton, and Q. J. Sattentau. 1998. Neutralization of HIV-1 by antibody to gp120 is determined primarily by occupancy of sites on the virion irrespective of epitope specificity. J. Virol. 72:3512-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parren, P. W. H. I., J. P. Moore, D. R. Burton, and Q. J. Sattentau. 1999. The neutralizing antibody response to HIV-1: viral evasion and escape from humoral immunity. AIDS 13(Suppl. A):S137-S162. [PubMed] [Google Scholar]
- 59.Parren, P. W. H. I., M. Wang, A. Trkola, J. M. Binley, M. Purtscher, H. Katinger, J. P. Moore, and D. R. Burton. 1998. Antibody neutralization-resistant primary isolates of human immunodeficiency virus type 1. J. Virol. 72:10270-10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Poignard, P., E. Ollmann Saphire, P. W. H. I. Parren, and D. R. Burton. 2001. gp120: biologic aspects of structural features. Annu. Rev. Immunol. 19:253-274. [DOI] [PubMed] [Google Scholar]
- 61.Pollakis, G., S. Kang, A. Kliphuis, M. I. Chalaby, J. Goudsmit, and W. A. Paxton. 2001. N-linked glycosylation of the HIV type-1 gp120 envelope glycoprotein as a major determinant of CCR5 and CXCR4 coreceptor utilization. J. Biol. Chem. 276:13433-13441. [DOI] [PubMed] [Google Scholar]
- 62.Quinnan, G. V., Jr., P. F. Zhang, D. W. Fu, M. Dong, H. J. Alter, and International Collaborators. 1999. Expression and characterization of HIV type 1 envelope protein associated with a broadly reactive neutralizing antibody response. AIDS Res. Hum. Retrovir. 15:561-570. [DOI] [PubMed] [Google Scholar]
- 63.Rizzuto, C., and J. Sodroski. 2000. Fine definition of a conserved CCR5-binding region on the human immunodeficiency virus type 1 glycoprotein 120. AIDS Res. Hum. Retrovir. 16:741-749. [DOI] [PubMed] [Google Scholar]
- 64.Rizzuto, C. D., R. Wyatt, N. Hernandez-Ramos, Y. Sun, P. D. Kwong, W. A. Hendrickson, and J. Sodroski. 1998. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science 280:1949-1953. [DOI] [PubMed] [Google Scholar]
- 65.Roben, P., J. P. Moore, M. Thali, J. Sodroski, C. F. Barbas III, and D. R. Burton. 1994. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J. Virol. 68:4821-4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sanders, R. W., L. Schiffner, A. Master, F. Kajumo, Y. Guo, T. Dragic, J. P. Moore, and J. M. Binley. 2000. Variable-loop-deleted variants of the human immunodeficiency virus type 1 envelope glycoprotein can be stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits. J. Virol. 74:5091-5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sanders, R. W., M. Venturi, L. Schiffner, R. Kalyanaraman, H. Katinger, K. O. Lloyd, P. D. Kwong, and J. P. Moore. 2002. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J. Virol. 76:7293-7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saphire, E. O., P. W. Parren, R. Pantophlet, M. B. Zwick, G. M. Morris, P. M. Rudd, R. A. Dwek, R. L. Stanfield, D. R. Burton, and I. A. Wilson. 2001. Crystal structure of a neutralizing human IgG against HIV-1: a template for vaccine design. Science 293:1155-1159. [DOI] [PubMed] [Google Scholar]
- 69.Sattentau, Q. J., and J. P. Moore. 1995. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J. Exp. Med. 182:185-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scanlan, C. N., R. Pantophlet, M. R. Wormwald, E. O. Saphire, R. Stanfield, I. A. Wilson, H. Katinger, R. A. Dwek, P. M. Rudd, and D. R. Burton. 2002. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of α1→2 mannose residues on the outer face of gp120. J. Virol. 76:7306-7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schønning, K., A. Bolmstedt, J. Novotny, O. S. Lund, S. Olofsson, and J. E. S. Hansen. 1998. Induction of antibodies against epitopes inaccessible on the HIV type 1 envelope oligomer by immunization with recombinant monomeric glycoprotein 120. AIDS Res. Hum. Retrovir. 16:1451-1456. [DOI] [PubMed] [Google Scholar]
- 72.Scott, C. F., Jr., S. Silver, A. T. Profy, S. D. Putney, A. Langlois, K. Weinhold, and J. E. Robinson. 1990. Human monoclonal antibody that recognizes the V3 region of human immunodeficiency virus gp120 and neutralizes the human T-lymphotropic virus type IIIMN strain. Proc. Natl. Acad. Sci. USA 87:8597-8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Skinner, M. A., A. J. Langlois, C. B. McDanal, J. S. McDougal, D. P. Bolognesi, and T. J. Matthews. 1988. Neutralizing antibodies to an immunodominant envelope sequence do not prevent gp120 binding to CD4. J. Virol. 62:4195-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stamatatos, L., and C. Cheng-Mayer. 1995. Structural modulations of the envelope gp120 glycoprotein of human immunodeficiency virus type 1 upon oligomerization and differential V3 loop epitope exposure of isolates displaying distinct tropism upon virion-soluble receptor binding. J. Virol. 69:6191-6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stiegler, G., R. Kunert, M. Purtscher, S. Wolbank, R. Voglauer, F. Steindl, and H. Katinger. 2001. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 17:1757-1765. [DOI] [PubMed] [Google Scholar]
- 76.Sullivan, N., Y. Sun, J. Binley, J. Lee, C. F. Barbas III, P. W. H. I. Parren, D. R. Burton, and J. Sodroski. 1998. Determinants of human immunodeficiency virus type 1 envelope glycoprotein activation by soluble CD4 and monoclonal antibodies. J. Virol. 72:6332-6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun, N. C., D. D. Ho, C. R. Y. Sun, R. S. Liou, W. Gordon, M. S. Fung, X. L. Li, R. C. Ting, T. H. Lee, N. T. Chang, and T. W. Chang. 1989. Generation and characterization of monoclonal antibodies to the putative CD4-binding domain of human immunodeficiency virus type 1 gp120. J. Virol. 63:3579-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thali, M., C. Furman, D. D. Ho, J. Robinson, S. Tilley, A. Pinter, and J. Sodroski. 1992. Discontinuous, conserved neutralization epitopes overlapping the CD4 binding region of the HIV-1 gp120 envelope glycoprotein. J. Virol. 66:5635-5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thali, M., J. P. Moore, C. Furman, M. Charles, D. D. Ho, J. Robinson, and J. Sodroski. 1993. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J. Virol. 67:3978-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thali, M., U. Olshevshy, C. Furman, D. Gabuzda, M. Posner, and J. Sodroski. 1991. Characterization of a discontinuous human immunodeficiency virus type 1 gp120-epitope recognized by a broadly reactive neutralizing human monoclonal antibody. J. Virol. 65:6188-6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Trkola, A., T. Dragic, J. Arthos, J. M. Binley, W. C. Olson, G. P. Allaway, C. Cheng-Mayer, J. Robinson, P. J. Maddon, and J. P. Moore. 1996. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature 384:184-187. [DOI] [PubMed] [Google Scholar]
- 82.Trkola, A., M. Purtscher, T. Muster, C. Ballaun, A. Buchacher, N. Sullivan, K. Srinivasan, J. Sodroski, J. P. Moore, and H. Katinger. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type I. J. Virol. 70:1100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.VanCott, T. C., J. R. Mascola, L. D. Loomis-Price, F. Sinangil, N. Zitomersky, J. McNeil, M. L. Robb, D. L. Birx, and S. Barnett. 1999. Cross-subtype neutralizing antibodies induced in baboons by a subtype E gp120 immunogen based on an R5 primary HIV-1 envelope. J. Virol. 73:4640-4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vujcic, L. K., and G. V. Quinnan, Jr. 1995. Preparation and characterization of human HIV type 1 neutralizing reference sera. AIDS Res. Hum. Retrovir. 11:783-787. [DOI] [PubMed] [Google Scholar]
- 85.Wang, W. K., T. Dudek, Y. J. Zhao, H. G. Brumblay, M. Essex, and T. H. Lee. 1998. CCR5 coreceptor utilization involves a highly conserved arginine residue of HIV type 1 gp120. Proc. Natl. Acad. Sci. USA 95:5740-5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weissenhorn, W., A. Dessen, S. C. Harrison, J. J. Skehel, and D. C. Wiley. 1997. Atomic structure of the ectodomain from HIV-1 gp41. Nature 387:426-430. [DOI] [PubMed] [Google Scholar]
- 87.Wu, L., N. Gerard, R. Wyatt, H. Choe, C. Parolin, N. Ruffing, A. Borsetti, A. Cordoso, E. Desjardin, W. Newman, C. Gerard, and J. Sodroski. 1996. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature 384:179-183. [DOI] [PubMed] [Google Scholar]
- 88.Wyatt, R., J. Moore, M. Accola, E. Desjardin, J. Robinson, and J. Sodroski. 1995. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J. Virol. 69:5723-5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wyatt, R., N. Sullivan, M. Thali, H. Repke, D. Ho, J. Robinson, M. Posner, and J. Sodroski. 1993. Functional and immunologic characterization of human immunodeficiency virus type 1 envelope glycoproteins containing deletions of the major variable regions. J. Virol. 67:4557-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wyatt, R., M. Thali, S. Tilley, A. Pinter, M. Posner, D. Ho, J. Robinson, and J. Sodroski. 1992. Relationship of the human immunodeficiency virus type 1 gp120 third variable loop to a component of the CD4 binding site in the fourth conserved region. J. Virol. 66:6997-7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xiang, S. H., P. D. Kwong, R. Gupta, C. D. Rizzuto, D. J. Casper, R. Wyatt, L. Wang, W. A. Hendrickson, M. L. Doyle, and J. Sodroski. 2002. Mutagenic stabilization and/or disruption of a CD4-bound state reveals distinct conformations of the human immunodeficiency virus type 1 gp120 envelope glycoprotein. J. Virol. 76:9888-9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ye, Y., Z. H. Si, J. P. Moore, and J. Sodroski. 2000. Association of structural changes in the V2 and V3 loops of the gp120 envelope glycoprotein with acquisition of neutralization resistance in a simian-human immunodeficiency virus passaged in vivo. J. Virol. 74:11955-11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang, W., A. P. Godillot, R. Wyatt, J. Sodroski, and I. Chaiken. 2001. Antibody 17b binding at the coreceptor site weakens the kinetics of the interaction of envelope glycoprotein gp120 with CD4. Biochemistry 40:1662-1670. [DOI] [PubMed] [Google Scholar]
- 94.Zhu, C. B., L. Zhu, S. Holz-Smith, T. J. Matthews, and C. H. Chen. 2001. The role of the third beta strand in gp120 conformation and neutralization sensitivity of the HIV-1 primary isolate DH012. Proc. Natl. Acad. Sci. USA 98:15227-15232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zolla-Pazner, S., J. O'Leary, S. Burda, M. K. Gorny, M. Kim, J. Mascola, and F. McCutchan. 1995. Serotyping of primary human immunodeficiency virus type 1 isolates from diverse geographic locations by flow cytometry. J. Virol. 69:3807-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zwick, M. B., A. F. Labrijn, M. Wang, C. Spenlehauer, E. Ollmann Saphire, J. M. Binley, J. P. Moore, G. Stiegler, H. Katinger, D. R. Burton, and P. W. H. I. Parren. 2001. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 75:10892-10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zwick, M. B., M. Wang, P. Poignard, G. Stiegler, H. Katinger, D. R. Burton, and P. W. H. I. Parren. 2001. Neutralization synergy of human immunodeficiency virus type 1 primary isolates by cocktails of broadly neutralizing antibodies. J. Virol. 75:12198-12208. [DOI] [PMC free article] [PubMed] [Google Scholar]