Abstract
Infection with human cytomegalovirus (HCMV) results in complex interactions between viral and cellular factors which perturb many cellular functions. HCMV is known to target the cell cycle, cellular transcription, and immunoregulation, and it is believed that this optimizes the cellular environment for viral DNA replication during productive infection or during carriage in the latently infected host. Here, we show that HCMV infection also prevents external signaling to the cell by disrupting the function of TNFRI, the 55-kDa receptor for tumor necrosis factor alpha (TNF-α), one of the receptors for a potent cytokine involved in eliciting a wide spectrum of cellular responses, including antiviral responses. HCMV infection of fully permissive differentiated monocytic cell lines and U373 cells resulted in a reduction in cell surface expression of TNFRI. The reduction appeared to be due to relocalization of TNFRI from the cell surface and was reflected in the elimination of TNF-α-induced Jun kinase activity. Analysis of specific phases of infection suggested that viral early gene products were responsible for this relocalization. However, a mutant HCMV in which all viral gene products known to be involved in down-regulation of major histocompatibility complex (MHC) class I were deleted still resulted in relocalization of TNFRI. Consequently, TNFRI relocalization by HCMV appears to be mediated by a novel viral early function not involved in down-regulation of cell surface MHC class I expression. We suggest that upon infection, HCMV isolates the cell from host-mediated signals, forcing the cell to respond only to virus-specific signals which optimize the cell for virus production and effect proviral responses from bystander cells.
Human cytomegalovirus (HCMV), like all herpesviruses, can establish lifelong persistence after primary infection. In contrast to primary infection which is generally asymptomatic, reactivation of the virus, particularly in the immunocompromised, often results in life-threatening disease (18). During productive infection, HCMV undergoes a regulated cascade of viral gene expression. These phases have been operationally defined as immediate-early (IE), early, and late. IE gene products, the major transcripts of which map to the major IE region of the genome and generate the major IE72 and IE86 families of proteins, play a pivotal role in regulating the expression of early and late viral genes as well as regulating cellular gene expression (45). Early and late viral gene products include viral functions associated with viral DNA replication and virus packaging.
Like other DNA viruses, the ability of HCMV to perturb normal cellular functions is well established. Virus infection can induce changes in cellular gene expression immediately upon binding (7, 43, 49). Similarly, expression of the viral IE and early genes also results in their physical and functional interactions with cellular factors, resulting in perturbation of cellular transcription, cell cycle, and expression of secreted chemokines and cytokines (15, 34). Virus-induced changes in levels of cellular transcription factors have been related to transcriptional activation of viral and cellular genes required during infection, and virus-mediated disruption of cell cycle control is believed to optimize the cellular environment for viral DNA replication.
HCMV infection is known to inhibit killing by cytotoxic T cells by down-regulating cell surface expression of major histocompatibility complex (MHC) class I by a variety of specific mechanisms (3, 5, 47). HCMV infection is also known to regulate cell expression of MHC class II (9, 23). Similarly, other cell surface proteins associated with peptide processing have been shown to be down-regulated during HCMV infection, although the relevance of this is not yet known (36). Fairley et al. have recently shown that HCMV infection also results in down-regulation of epidermal growth factor receptor (13). In this study, we show that HCMV infection also results in the perturbation of the small 55-kDa tumor necrosis factor alpha (TNF-α) receptor (TNFRI), suggesting that down-regulation of cell receptors that mediate a variety of cell signals resulting in the elimination of receptor-mediated cell signaling may be a common occurrence during HCMV infection. Through TNFRI, TNF-α elicits a wide range of biological effects. It is a major mediator of apoptosis as well as inflammation and immunity and has been implicated in a wide range of human diseases (11). Binding of TNF-α to TNFRI activates several signal transduction pathways which ultimately result in the induction of two major transcription factors, NF-κB and c-Jun. These factors play major roles in the control of cell proliferation, differentiation, and programmed cell death (11).
In addition to these effects on cellular functions, TNF-α is known to be able to prevent replication of DNA and RNA viruses in some systems (20). Conversely, TNF-α can also aid virus infection by promoting viral gene expression and productive infection (19). In the case of HCMV, virus infection has been shown to activate TNF-α expression (12, 38). However, TNF-α has been shown to activate or inhibit viral IE gene expression, depending on the differentiation state of monocytic cells, such that in differentiated, permissive cells, the HCMV major IE promoter is strongly inhibited by TNF-α (16, 40). Also, TNF-α has been shown to inhibit virus production in certain HCMV-infected cell types (4, 10). Clearly, TNF-α has profound effects on cells. Consequently, the ability of HCMV to prevent the infected cell from responding to TNF-mediated signals from the host yet at the same time inducing TNF-α expression in order, perhaps, to differentially influence uninfected bystander cells might be a logical strategy for the virus. We believe that such virus-mediated down-regulation of cell surface TNFRI expression prevents the cell from responding to a potent cellular cytokine that might initiate signals that conflict with virus-induced signals required to optimize the cellular milieu for productive infection.
MATERIALS AND METHODS
Cells and viruses.
HFF 5 (primary human fibroblasts) were maintained in Eagle's minimal essential medium (MEM) containing 10% fetal calf serum at 37oC and in 5% CO2. THP1 cells, a myelomonocytic cell line which can be differentiated with phorbol myristate acetate to a macrophage phenotype with subsequent permissiveness for HCMV infection (48), were cultured in RPMI 10 medium at 37°C and in 5% CO2. U373 cells, a glioblastoma cell line fully permissive for HCMV infection, were maintained in Eagle's MEM containing 10% fetal calf serum at 37°C and in 5% CO2. Infection with HCMV (AD169), UV-inactivated virus, or a recombinant AD169 deletion virus missing IRS1 to US9 and US11 (26) have been described previously (44). Green fluorescent protein (GFP)-tagged HCMV was a generous gift of Richard Greaves and has been described previously (35).
To analyze IE expression specifically, cells were pretreated with cycloheximide (50 μg/ml) for 3 h before virus adherence and for 3 h during virus adherence. After this adherence period, virus and cycloheximide were washed off the cells, and infection was allowed to progress for 3 h in the absence of cycloheximide. After this time, actinomycin D (20 μg/ml) was added for a further 14 h. To inhibit viral late gene expression, cells were infected in the continual presence of the viral DNA polymerase inhibitor phosphonoformate (100 μg/ml).
Fluorescence-activated cell sorting (FACS) analysis and immunofluorescence.
For indirect immunofluorescence, approximately 5 × 104 U373 cells per well were seeded on eight-well slides (Nunc) and infected with HCMV at 1 to 5 PFU/cell. Cells were fixed in 4% paraformaldehyde for 15 min and then permeabilized in 1% Triton X-100 in phosphate-buffered saline for 5 min. For dual staining, cells were stained with a mouse monoclonal antibody to TNFRI (R&D Systems) or an immunoglobulin G1 (IgG1) isotype-matched control antibody (R&D Systems) and detected using phycoerythrin (PE)-conjugated sheep anti-mouse immunoglobulins. Cells were then incubated with a fluorescein isothiocyanate (FITC)-conjugated mouse monoclonal antibody against both IE72 and IE86 (anti-IE72/IE86 monoclonal antibody) (Biosys) or a FITC-conjugated IgG1 isotype-matched control antibody (R&D Systems).
For triple staining to codetect trans-Golgi network (TGN), TNFRI, and HCMV IE proteins, GFP-tagged HCMV was used to infect U373 cells. Cells were then fixed and stained with a mouse anti-TNFRI antibody (R&D Systems) or IgG1 control immunoglobulins, and these antibodies were detected using 7-amino-4-methylcoumarin-3-acetic acid (AMCA)-conjugated rabbit anti-mouse immunoglobulins. Following this, staining of the TGN with a sheep anti-TGN46 monoclonal antibody (Chemicon) or sheep control immunoglobulins (Sigma) was detected using Alexafluor 594-conjugated donkey anti-sheep immunoglobulins.
For FACS analyses, unfixed infected or control cells were stained with an anti-TNFRI antibody (R&D Systems) or IgG1 isotype-matched control antibody, and this antibody was detected with PE-conjugated rabbit anti-mouse immunoglobulins. If cells were infected with AD169 or RV798, an aliquot of each cell population was fixed as described above and stained with a FITC-conjugated mouse anti-IE72/IE86 antibody (Biosys) to determine the level of HCMV infection. Alternatively, if cells were infected with GFP-tagged HCMV, IE expression was detected by analysis of GFP levels. Cells were analyzed using a Becton Dickinson FACsort.
For FACS analysis of fixed cells, fixed and permeabilized infected or control cells were stained with a goat anti-TNFRI (R&D Systems) or goat control immunoglobulins (Santa Cruz) which were detected using PE-conjugated donkey anti-goat immunoglobulins.
Cell surface MHC class I expression was determined using a PE-conjugated mouse anti-HLA class I A, B, and C (IgG1) or PE-conjugated isotype-matched control antibody (R&D Systems).
For FACS analysis of TNFRI on THP1 cells, differentiated THP1 cells were infected with GFP-tagged HCMV, TNFRI was analyzed with a mouse anti-TNFRI antibody (R&D) or IgG1 isotype-matched control antibody, and these antibodies were detected with PE-conjugated sheep anti-mouse immunoglobulins.
TNF-α assays.
TNF-α release from infected cells was analyzed in an L929 bioassay (30). Briefly, supernatants from infected or uninfected cells or control medium with and without TNF-α was added to L929 mouse cells in 96-well microtiter plates and incubated for 12 h at 37°C. Cells were stained for 10 min with 0.5% crystal violet in methanol-water (1:4) and then treated with 33% acetic acid to solubilize the crystal violet indicator. Absorbance was read at 580 nm using an enzyme-linked immunosorbent assay plate reader. To ensure that the cytotoxicity observed was due to TNF-α, cell supernatants were also treated with 15 μg of anti-TNFRI antibody or control antibody per ml for 30 min prior to assay.
JNK assays.
Glutathione S-transferase (GST) and GST-Jun fusion proteins on glutathione-Sepharose beads were prepared as previously described (8) and used as targets for Jun kinase (JNK), also as previously described (22). Briefly, U373 cells were infected overnight with 5 PFU of HCMV (AD169) per cell. Infected or uninfected control cells were treated with 10 ng of TNF-α per ml for 10 min and then quickly lysed in EBCN (200 mM NaCl, 10 mM NaF, 250 mM Na3VO4, 50 mM Tris-HCl [pH 8.0], and 0.5% NP-40) (8). Cell extracts were incubated with 200 ng of GST-Jun or GST on glutathione-Sepharose beads for 4 h at 4°C. After the glutathione beads were washed three times with EBCN, the beads were washed once in kinase buffer (22) and then incubated for 30 min in kinase buffer containing 5 μCi of [γ-32P]ATP at 30°C. After three washes in kinase buffer, the glutathione beads were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by autoradiography.
Radiolabeled detection of TNF-α binding.
A total of 107 uninfected or infected cells were harvested 15 h postinfection using trypsin-free dissociation medium and resuspended in binding medium (RPMI cell culture medium with 20 mM HEPES [pH 7.5], 0.1% fetal calf serum, and 125I-labeled TNF-α). TNF-α binding was analyzed as previously described (2).
RESULTS
HCMV infection decreases cell surface expression of TNFRI in monocytic cells.
It has puzzled us that HCMV infection of monocytic cells is known to induce TNF-α (12, 38) but that TNF-α is also known to inhibit HCMV production in some cell types (4, 10) and repress the HCMV major IE promoter in permissive differentiated cells (16, 40). Consequently, we initially asked whether HCMV infection had any effect on the expression of TNFRI in infected monocytic cell lines.
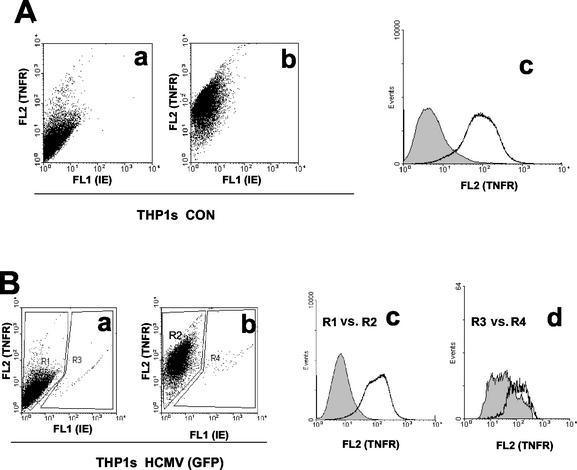
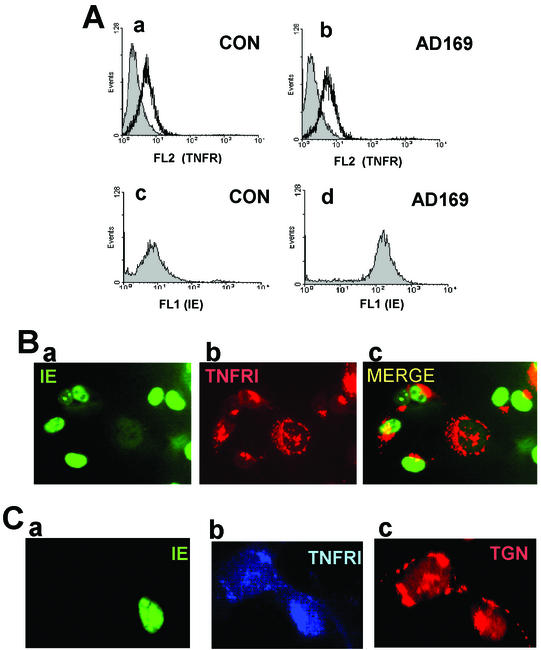
First, we confirmed that differentiated THP1 cells expressed high levels of TNFRI. Figure 1A clearly shows that, as expected, mock-infected cells show extensive specific cell surface staining of TNFRI. To allow us to stain live infected cells for cell surface TNFRI, we infected differentiated THP1 cells with a GFP-tagged HCMV. Infection of differentiated THP1 cells with this GFP-tagged virus was inefficient, resulting in only about 2 to 5% infection. Nevertheless, infected cells clearly showed differences in levels of TNFRI expression. For instance, despite the higher background nonspecific staining of the infected cells within this population (a well-established phenomenon on HCMV infection of myeloid cells), cell surface TNFRI was clearly reduced on infected cells (Fig. 1B, panel d). In contrast, the noninfected cells within this population showed no reduction in cell surface TNFRI (Fig. 1B, panel c). This also argues that reduction in cell surface TNFRI is specific to the infected cell and does not occur on uninfected bystander cells in the population.
FIG. 1.
HCMV decreases cell surface TNFRI expression in THP1 cells. (A) Uninfected THP1 cells (CON) were stained with a PE-conjugated control antibody (a) or anti-TNFRI antibody (b) as detailed in Materials and Methods. Cells were analyzed by FACS for TNFRI (FL2) and GFP-IE (FL1) staining. The data from the dot plots shown in panels a and b are also presented as a histogram plot (c), with control antibody staining shown by the shaded area and anti-TNFRI antibody staining shown by the white area. (B) THP1 cells infected overnight with GFP-tagged HCMV (5 PFU/cell) were stained with a PE-conjugated control antibody (a) or anti-TNFRI antibody (b) as detailed in Materials and Methods. Cells were analyzed by FACS for TNFRI (FL2) and GFP-IE (FL1) staining. The data from the dot plots shown in panels a and b are also presented as a histogram plot where the specific staining for TNFRI in the uninfected cells in the population is shown as R1 versus R2 (c), and the specific staining for TNFRI in the infected cells in the population is shown as R3 versus R4 (d). In both these histogram plots, control antibody staining is shown by the shaded area, and anti-TNFRI antibody staining is shown by the white area.
We also confirmed this observation using indirect immunofluorescence after infection of differentiated THP1 cells with GFP-tagged HCMV (data not shown).
HCMV infection of U373 cells also results in decreases in cell surface TNFRI.
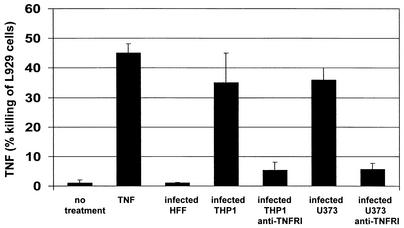
The extremely low levels of infection of differentiated THP1 cells with GFP-tagged HCMV precluded a thorough analysis of this virus-mediated reduction of TNFRI in this cell type and led us to test other fully permissive cell types for HCMV-mediated reduction of TNFRI. As the basis of our analysis was to address how infected cells that release TNF-α were apparently immune to TNF-α-mediated antiviral effects, it was important for us to use a cell type that, upon infection, exhibited biological properties with respect to the release of TNF-α that were similar to the properties of THP1 cells. Consequently, we tested whether HCMV (HFF) infection of two other fully permissive cell types, fibroblasts and U373 cells, also resulted in release of TNF-α. Figure 2 shows that, consistent with previous analyses (33), HCMV infection of fibroblast cells does not induce TNF-α. In contrast, infection of U373 cells clearly results in release of levels of TNF-α similar to that seen upon infection of THP1 cells. In this assay, killing of L929 cells was clearly mediated by TNF-α, as preincubation of target L929 cells with anti-TNFRI antibody drastically reduced L929 cell death induced by virus-infected cell supernatants.
FIG. 2.
U373 cells and monocytic cells release TNF-α upon infection with HCMV. Using an L929 bioassay, supernatants from uninfected and infected U373, HFF, and THP1 cells were assayed for release of TNF-α. Supernatants were also treated with anti-TNFRI antibody to ensure that the killing observed was TNF-α specific. L929 cells were also treated with purified TNF-α (20 ng/ml) alone. In all cases, values were corrected for any killing observed with control medium.
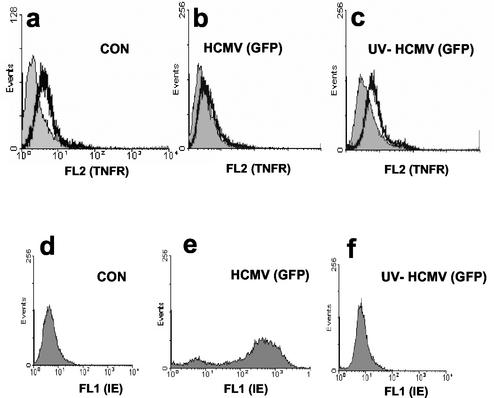
Consequently, we next tested whether infection of U373 cells also resulted in a decrease in cell surface expression of TNFRI (Fig. 3). Although U373 cells expressed lower levels of cell surface TNFRI than THP1 cells did, Fig. 3b clearly shows that, as with infected THP1 cells, infection of U373 cells with GFP-tagged HCMV resulted in a substantial decrease in cell surface staining with an anti-TNFRI antibody. Interestingly, infection with UV-inactivated virus had little or no effect on cell surface expression of TNFRI (Fig. 3 c), arguing that this effect was due to viral gene expression and not just virus binding.
FIG. 3.
HCMV down-regulates cell surface TNFRI expression on U373 cells. Uninfected control (CON) U373 cells (a and d), U373 cells infected overnight with GFP-tagged HCMV (5 PFU/cell) (b and e), or U373 cells infected with UV-inactivated GFP-tagged HCMV (c and f) were stained with a PE-conjugated anti-TNFRI antibody (white area) or a PE-conjugated, isotype-matched control antibody (shaded area) and analyzed by FACS for TNFRI staining (FL2) or GFP-IE staining (FL1).
Viral gene products associated with perturbation of MHC class I expression are not involved in relocalization of TNFRI.
HCMV infection of fibroblasts has already been shown to result in the inhibition in cell surface expression of MHC class I (1, 28, 29). These elegant experiments have shown that viral gene products encoded in the region of the viral genome from US2 to US11 are able to down-regulate cell surface expression of cellular MHC class I by a variety of mechanisms (26). These viral genes are believed to play an important role in immune avoidance of infected cells by cytotoxic T cells (27-29, 31, 39). Figure 4A shows that infection of fibroblast cells with a recombinant HCMV with US2 to US11 deleted (RV798), encompassing all viral gene products known to be involved in MHC class I down-regulation (26), was still able to down-regulate expression of cell surface expression of TNFRI (Fig. 4A, panel b), even though it was unable to inhibit cell surface class I expression, as expected (26) (Fig. 4B, panel c). Consequently, inhibition of cell surface expression of TNFRI by HCMV appears to be mediated by a novel viral function not involved with the well-documented down-regulation of cell surface MHC class I.
FIG. 4.
HCMV deletions devoid of all MHC class I down-regulating genes still down-regulateTNFRI. (A) Uninfected control (CON) U373 cells (a and d), U373 cells infected overnight with AD169 (5 PFU/cell) (b and e), or U373 cells infected with RV798 (c and f) were stained with a PE-conjugated anti-TNFRI antibody (white area) or a PE-conjugated isotype-matched control antibody (shaded area) and analyzed by FACS for TNFRI staining (FL2) (a, b, and c). A small aliquot of each population was also fixed and stained for viral IE expression (d, e, and f). (B) A small aliquot of all the unfixed cell populations shown in panel A were also stained with a PE-conjugated antibody specific for MHC class I (white area) or with a PE-conjugated isotype-matched control (shaded area) and analyzed by FACS for cell surface MHC class I expression.
HCMV reduces TNF-α binding to infected cells.
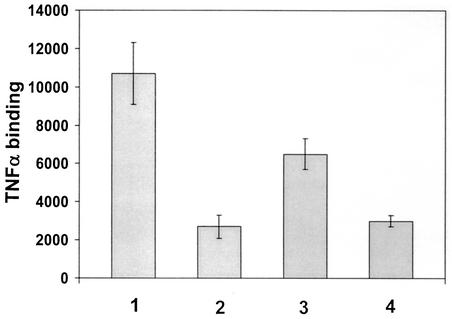
To confirm down-regulation of cell surface TNFRI, we directly tested for binding of TNF-α in uninfected and infected cells using radiolabeled TNF-α. However, the observation that U373 cells actually released TNF-α precluded the use of these cells for this assay, as it could be argued that any decrease in radiolabeled TNF-α binding to infected cells could be due to competition between the radiolabeled TNF-α and the TNF-α released from the infected cells. Consequently, we performed these experiments in fibroblast cells which do not release TNF-α upon infection (33) (Fig. 2) but do express TNFRI (32). In these experiments, radioiodinated TNF-α was added to uninfected or HCMV-infected fibroblast cells in the presence or absence of cold TNF-α competitor. Figure 5 shows that fibroblast cells bind TNF-α, as expected (bar 1). In contrast, cells infected overnight with HCMV show clearly reduced levels of TNF-α binding (bar 3). The binding of the radiolabeled TNF-α is specific, as assays performed in the presence of cold TNF-α show a specific inhibition of binding (bars 2 and 4). This is entirely consistent with a reduction of functional cell surface TNFRI. The fact that the reduction of TNF-α binding by HCMV infection is only partial is almost certainly due to the fact that TNFRII, which also binds TNF-α and is present on fibroblasts (32), is not altered by HCMV infection (unpublished observation).
FIG. 5.
HCMV infection reduces levels of TNF-α binding. Uninfected fibroblasts (bars 1 and 2) or fibroblasts infected with AD169 (5 PFU/cell) (bars 3 and 4) were incubated with radiolabeled TNF-α without cold competitor TNF-α (bars 1 and 3) or with 300-fold excess (bars 2 and 4) of TNF-α cold competitor.
HCMV infection results in relocalization of TNFRI.
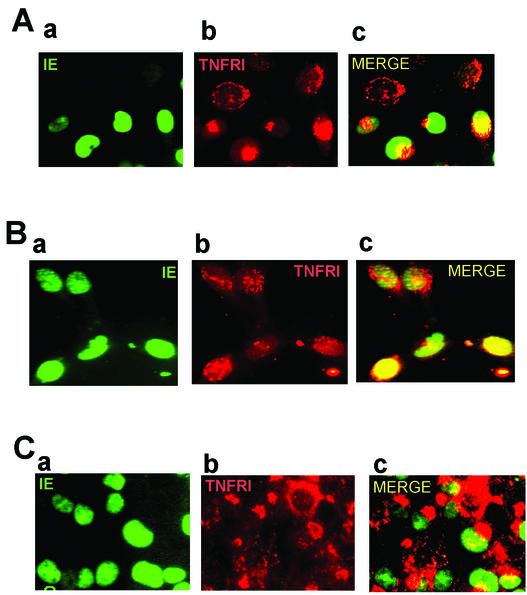
We next investigated the mechanism by which HCMV infection resulted in down-regulation of cell surface TNFRI. Intriguingly, analysis of levels of TNFRI by immunoprecipitation of [35S]methionine-labeled infected and uninfected cells showed no overall decrease in the level of expression of TNFRI (data not shown). Similarly, FACS analysis of TNFRI in fixed and permeabilized cells (Fig. 6A) showed no decrease in the level of total cellular expression of TNFRI, which would be consistent with HCMV causing a relocalization of cell surface TNFRI and not a decrease in overall TNFRI expression. To confirm this, we analyzed TNFRI in uninfected and infected cells by indirect immunofluorescence.
FIG. 6.
Down-regulation of TNFRI by HCMV is due to receptor relocalization. (A) Uninfected control (CON) U373 cells (a and c) or U373 cells infected overnight with AD169 (5 PFU/cell) (b and d) were fixed and stained with a goat anti-TNFRI antibody (white area) or control goat immunoglobulins (shaded area), and antibodies were detected with PE-conjugated donkey anti-goat immunoglobulins and analyzed by FACS (a and b). A small aliquot of fixed cells were also stained for IE expression (c and d). (B) Cells were also analyzed by indirect immunofluorescence. U373 cells on eight-well compartment slides were infected overnight with AD169 (1 PFU/cell). Slides were fixed and then stained with a mouse monoclonal antibody to TNFRI or an IgG1 isotype-matched control antibody and detected using PE-conjugated sheep anti-mouse immunoglobulins. Cells were then stained with a FITC-conjugated mouse anti-IE72/IE86 monoclonal antibody. A representative field of view from the infected cell well that contains both infected and uninfected cell types is shown. IE staining is shown in panel a, TNFRI staining is shown in panel b, and the merged images are shown in panel c. Control immunoglobulins showed no specific cross-staining (data not shown). (C) U373 cells on eight-well compartment slides were infected overnight with GFP-tagged HCMV (1 PFU/cell). Slides were fixed and then stained with a mouse monoclonal antibody to TNFRI or an IgG1 isotype-matched control antibody,and antibodies were detected using AMCA-conjugated rabbit anti-mouse immunoglobulins. Cells were then stained with an anti-TGN46 antibody which was detected using Alexafluor 56-conjugated donkey anti-sheep immunoglobulins. A representative field of view from the infected cell well that contains both an infected and uninfected cell type is shown. GFP-IE staining (a), TNFRI staining (b), and TGN46 staining (c) are shown. Control immunoglobulins showed no specific cross-staining (data not shown).
As expected, uninfected cells (Fig. 6B) show extensive cell and cell surface staining of TNFRI consistent with the known localization of TNFRI to the TGN as well as the cell surface (25, 46). In contrast, infected cells positive for HCMV IE72 and IE86 antigens (Fig. 6B) showed little or no cell surface staining and intense localization of TNFRI at the perinucleus, reminiscent of the known changes in morphology of the TGN in HCMV-infected cells (21, 42). Consequently, we analyzed this further using a TGN-specific antibody. Figure 6C shows that, as expected, in uninfected cells, staining for TNFRI and the TGN colocalize, which is entirely consistent with the known colocalization of TNFRI and TGN (25, 46). In infected cells, the perinuclear staining of TNFRI also colocalized to the TGN, again entirely consistent with the known relocalization of TGN in HCMV-infected cells (21, 42) and the established colocalization of TNFRI with TGN (25, 46). Consequently, it appears that HCMV infection may result in incorrect trafficking of TNFRI through the TGN to the cell surface. While it could be suggested that the known changes in the morphology of human fibroblast cells upon infection with HCMV, namely, rounding up, could explain the apparent changes in the morphology of the TGN upon infection, this is not the case for infection of U373 cells, which show no major changes in morphology upon HCMV infection (unpublished observations).
No such relocalization of TNFRI was observed using UV-inactivated virus (Fig. 3 and data not shown), arguing that soluble factors present in the viral inoculum or virus binding is not responsible for this phenomenon.
HCMV early gene products appear to be responsible for TNFRI relocalization.
The fact that TNFRI relocalization occurred as early as 15 h postinfection argued that viral IE or early gene expression was responsible for relocalization of TNFRI. To address this, we infected cells in the presence of cycloheximide and actinomycin D as well as phosphonoformate under conditions which prevented specific stages of viral gene expression (Fig. 7). Figure 7A clearly shows that treating cells with phosphonoformate to inhibit viral DNA replication and hence viral late gene expression had no effect on TNFRI relocalization. Consequently, it appeared that the viral functions responsible for the relocalization of TNFRI were associated with viral IE or early gene expression. To investigate this further, we performed cycloheximide block and release experiments in the presence of actinomycin D to allow IE but not early gene expression (Fig. 7B). In these experiments, no relocalization of TNFRI was observed, not even in cells expressing IE72 or IE86, arguing that viral early gene expression was responsible for receptor relocalization. As expected, treatment with cycloheximide, actinomycin D, or phosphonoformate had no effect on IE gene expression (Fig. 7A and B, panels a), and cells treated with cycloheximide or actinomycin D did not express UL44 and UL56 (viral early gene products) as determined by indirect immunofluorescence, confirming a lack of viral early gene expression in these infected cells (data not shown).
FIG. 7.
HCMV early gene products are required for TNFRI relocalization. (A) U373 cells were seeded onto eight-well compartment slides and infected with AD169 in the continual presence of phosphonoformate for 72 h (5 PFU/cell). Slides were fixed and then stained with a mouse anti-TNFRI monoclonal antibody or with an IgG1 isotype-matched control antibody and detected using PE-conjugated sheep anti-mouse immunoglobulins. Cells were then stained with a FITC-conjugated mouse anti-IE72/IE86 monoclonal antibody. A representative field of view from the infected cell well that contains both infected and uninfected cell types is shown. IE staining is shown in panel a, TNFRI staining is shown in panel b, and the merged images are shown in panel c. Control immunoglobulins showed no specific cross-staining (data not shown). (B) U373 cells were seeded onto eight-well compartment slides and infected with AD169 (5 PFU/cell) in the presence of cycloheximide and actinomycin D to permit only IE expression, as described in Materials and Methods. Slides were fixed and then stained with a mouse anti-TNFRI monoclonal antibody or with an IgG1 isotype-matched control antibody and detected using PE-conjugated sheep anti-mouse immunoglobulins. Cells were then stained with a FITC-conjugated mouse anti-IE72/IE86 monoclonal antibody. A representative field of view from the infected cell well that contains both infected and uninfected cell types is shown. IE staining is shown in panel a, TNFRI staining is shown in panel b, and the merged images are shown in panel c. Control immunoglobulins showed no specific cross-staining (data not shown). (C) U373 cells were seeded onto eight-well compartment slides and infected overnight with RV798 (5 PFU/cell). Slides were fixed and then stained with a mouse anti-TNFRI monoclonal antibody or an IgG1 isotype-matched control antibody and detected using PE-conjugated sheep anti-mouse immunoglobulins. Cells were then stained with a FITC-conjugated mouse anti-IE72/IE86 monoclonal antibody. A representative field of view from the infected cell well that contains both infected and uninfected cell types is shown. IE staining is shown in panel a, TNFRI staining is shown in panel b, and the merged images are shown in panel c. Control immunoglobulins showed no specific cross-staining (data not shown).
Consistent with our FACS analyses, RV798 had no effect on the relocalization of TNFRI (Fig. 7C), arguing that early gene products other than those associated with MHC class I down-regulation were responsible for this TNFRI relocalization.
HCMV infection inhibits TNF-α induction of JNK.
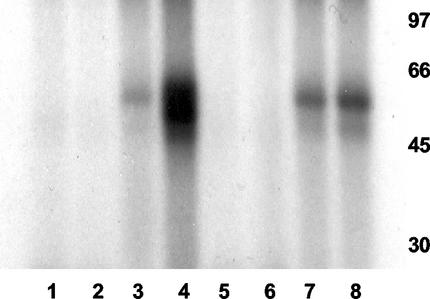
One known effect of TNF-α is the induction of the JNK pathway, resulting in phosphorylated c-Jun (11). It has already been shown that HCMV infection itself has no effect on JNK activity (24, 41). Consequently, if HCMV infection were to result in a reduction in TNFRI, then we would predict that TNF-α induction of JNK activity would be blocked by HCMV infection. Therefore, we analyzed the levels of JNK in TNF-α-treated U373 cells after mock infection or infection with HCMV using a GST-JNK assay (Fig. 8). Figure 8 clearly shows that, as expected, TNF-α treatment of U373 cells results in increased levels of JNK as detected by phosphorylation of GST-Jun substrate (lane 4) and that infection with HCMV had little effect on JNK activity (lane 7). In contrast, prior infection with HCMV substantially reduced TNF-α-induced JNK (compare lanes 4 and 8), which is entirely consistent with an HCMV-mediated reduction in functional cell surface TNFRI.
FIG. 8.
HCMV infection prevents TNF-α-mediated induction of JNK. Control U373 cells (lanes 1 to 4) or U373 cells infected overnight with AD169 (5 PFU/cell) (lanes 5 to 8) were left untreated (lanes 1, 3, 5, and 7) or treated with TNF-α (lanes 2, 4, 6, and 8). Cell extracts were then assayed for JNK activity using GST (lanes 1, 2, 5, and 6) or GST-Jun (lanes 3, 4, 7, and 8) as targets for in vitro phosphorylation with [32P]ATP. Phosphorylation of GST or GST-Jun was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography. The positions of molecular mass markers (in kilodaltons) are shown to the right of the gel.
DISCUSSION
The disruption of key cellular functions by viral gene products appears essential for viruses to optimize the cell for productive infection, and this disruption often involves direct physical and functional interactions between viral proteins and key cellular regulatory proteins (15). Such interactions specifically target cellular processes, such as cellular transcription, cell cycle, immunomodulation, and regulation of chemokine and cytokine gene expression, and this removes their normal cellular control (15, 34).
One factor induced to high levels by HCMV infection is TNF-α (12, 37). TNF-α is a pleiotropic cytokine involved in inflammation and immunoregulation and has cytotoxic and antiviral activities (14). It can stimulate or prevent cell proliferation, depending on the cell type, and induce lysis of virus-infected cells (20). Consequently, many viruses have developed strategies to combat the effects of TNF-α (3). It seemed paradoxical to us, therefore, that HCMV would induce a powerful chemokine which in differentiated, fully permissive cells has been shown to repress HCMV IE gene expression (40) and inhibit virus production (4, 10). However, our experiments clearly show that, like other viruses, HCMV also prevents TNF-α signaling and that this is mediated by a virus-induced reduction of TNFRI from the cell surface. It is now well established that TNFRI resides in the TGN and on the cell surface (25, 46). Consequently, the observation that TNFRI staining appears intensely localized only to the TGN and is not present on the surface of the infected cell suggests that the viral gene products responsible for this perturbation of TNFRI during infection may target trafficking of TNFRI. This is currently under investigation.
HCMV has recently been shown to encode a gene product (UL37x1) that acts as an inhibitor of apoptosis and can prevent TNF-α-mediated cell death by targeting the mitochondria, similar to the antiapoptotic mechanism of Bcl-2 (17). However, our data would also suggest that reduction of TNFRI early in infection may play an important role in inhibition of apoptosis and that HCMV uses more than one strategy to prevent the cell from responding to such proapoptotic signals which may change during the course of infection. It is interesting that, recently, the HCMV UL144 reading frame of clinical isolates of HCMV has been shown to encode a structural homolog of a member of the TNFR superfamily (6). However, no known ligand of the TNF family binds UL144, suggesting that it may be constitutively active. It is therefore possible that HCMV infection results in the expression of a viral TNFRI homolog that mediates some, but not all, aspects of TNF-α-mediated signaling and concomitantly induces down-regulation of cellular TNFRI to ensure that these viral signals are dominant. Consistent with this, HCMV Toledo strain and clinical isolates of HCMV also down-regulate TNFRI (data not shown).
We also confirmed this reduction of TNFRI cell surface expression directly using radiolabeled TNF-α binding assays and FACS analysis of TNFRI cell surface staining. Clearly, this relocalization of TNFRI was dependent on viral gene expression, as UV-inactivated virus showed no such receptor relocalization. Similarly, time course analysis and drug inhibition of specific phases of infection suggested that viral early gene products were responsible for this relocalization. Interestingly, a viral deletion encompassing all viral gene products known to be involved in down-regulation of cell surface MHC class I still resulted in TNFRI relocalization. Consequently, it appears that a novel early viral function that is not associated with this well-documented down-regulation of MHC class I is responsible for TNFRI relocalization. Work is in progress to identify the viral gene products responsible for this relocalization.
It is becoming increasingly clear that efficient productive infection with HCMV is critically dependent on the ability of the virus to express genes that optimize the cellular environment for virus production. These viral genes target specific cellular functions and remove their normal control. We believe that an important part of optimizing the cell for virus infection involves the ability of the virus to isolate the infected cell from host-specific signals. This forces the cell to ignore cellular signals and respond solely to viral signals which specifically optimize the cellular environment for viral gene expression and productive infection. An understanding of what mechanisms are used by the virus to hijack the cell and modify its response to cell signals will be important in fully understanding the biology and pathogenesis of HCMV.
Acknowledgments
We are indebted to Tom Jones for the gift of RV798 and Antonio Alcami for help with the TNF-α and radiolabeled TNFR assays and critically reading the manuscript.
This work was supported by the British Medical Research Council.
REFERENCES
- 1.Ahn, K., A. Angulo, P. Ghazal, P. A. Peterson, Y. Yang, and K. Fruh. 1996. Human cytomegalovirus inhibits antigen presentation by a sequential multistep process. Proc. Natl. Acad. Sci. USA 93:10990-10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcami, A., A. Khanna, N. L. Paul, and G. L. Smith. 1999. Vaccinia virus strains Lister, USSR and Evans express soluble and cell-surface tumour necrosis factor receptors. J. Gen. Virol. 80:949-959. [DOI] [PubMed] [Google Scholar]
- 3.Alcami, A., and U. H. Koszinowski. 2000. Viral mechanisms of immune evasion. Trends Microbiol. 8:410-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allan-Yorke, J., M. Record, C. de Preval, C. Davrinche, and J. L. Davignon. 1998. Distinct pathways for tumor necrosis factor alpha and ceramides in human cytomegalovirus infection. J. Virol. 72:2316-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes, P. D., and J. E. Grundy. 1992. Down-regulation of the class I HLA heterodimer and beta 2-microglobulin on the surface of cells infected with cytomegalovirus. J. Gen. Virol. 73:2395-2403. [DOI] [PubMed] [Google Scholar]
- 6.Benedict, C. A., K. D. Butrovich, N. S. Lurain, J. Corbeil, I. Rooney, P. Schneider, J. Tschopp, and C. F. Ware. 1999. Cutting edge: a novel viral TNF receptor superfamily member in virulent strains of human cytomegalovirus. J. Immunol. 162:6967-6970. [PubMed] [Google Scholar]
- 7.Boldogh, I., S. AbuBakar, and T. Albrecht. 1990. Activation of proto-oncogenes: an immediate early event in human cytomegalovirus infection. Science 247:561-564. [DOI] [PubMed] [Google Scholar]
- 8.Caswell, R., L. Bryant, and J. Sinclair. 1996. Human cytomegalovirus immediate-early 2 (IE2) protein can transactivate the human hsp70 promoter by alleviation of Dr1-mediated repression. J. Virol. 70:4028-4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cebulla, C. M., D. M. Miller, Y. Zhang, B. M. Rahill, P. Zimmerman, J. M. Robinson, and D. D. Sedmak. 2002. Human cytomegalovirus disrupts constitutive MHC class II expression. J. Immunol. 169:167-176. [DOI] [PubMed] [Google Scholar]
- 10.Cheeran, M. C., S. Hu, G. Gekker, and J. R. Lokensgard. 2000. Decreased cytomegalovirus expression following proinflammatory cytokine treatment of primary human astrocytes. J. Immunol. 164:926-933. [DOI] [PubMed] [Google Scholar]
- 11.Chen, G., and D. V. Goeddel. 2002. TNF-R1 signaling: a beautiful pathway. Science 296:1634-1635. [DOI] [PubMed] [Google Scholar]
- 12.Dudding, L., S. Haskill, B. D. Clark, P. E. Auron, S. Sporn, and E. S. Huang. 1989. Cytomegalovirus infection stimulates expression of monocyte-associated mediator genes. J. Immunol. 143:3343-3352. [PubMed] [Google Scholar]
- 13.Fairley, J. A., J. Baillie, M. Bain, and J. H. Sinclair. 2002. Human cytomegalovirus infection inhibits epidermal growth factor (EGF) signalling by targeting EGF receptors. J. Gen. Virol. 83:2803-2810. [DOI] [PubMed] [Google Scholar]
- 14.Fiers, W. 1991. Tumor necrosis factor. Characterization at the molecular, cellular and in vivo level. FEBS Lett. 285:199-212. [DOI] [PubMed] [Google Scholar]
- 15.Fortunato, E. A., A. K. McElroy, I. Sanchez, and D. H. Spector. 2000. Exploitation of cellular signaling and regulatory pathways by human cytomegalovirus. Trends Microbiol. 8:111-119. [DOI] [PubMed] [Google Scholar]
- 16.Geist, L. J., M. M. Monick, M. F. Stinski, and G. W. Hunninghake. 1994. The immediate early genes of human cytomegalovirus upregulate tumor necrosis factor-alpha gene expression. J. Clin. Investig. 93:474-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldmacher, V. S., L. M. Bartle, A. Skaletskaya, C. A. Dionne, N. L. Kedersha, C. A. Vater, J. Han, R. J. Lutz, S. Watanabe, E. D. McFarland, E. D. Kieff, E. S. Mocarski, and T. Chittenden. 1999. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc. Natl. Acad. Sci. USA 96:12536-12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffiths, P. D., and J. E. Grundy. 1988. The status of CMV as a human pathogen. Epidemiol. Infect. 100:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard, O. M., K. A. Clouse, C. Smith, R. G. Goodwin, and W. L. Farrar. 1993. Soluble tumor necrosis factor receptor: inhibition of human immunodeficiency virus activation. Proc. Natl. Acad. Sci. USA 90:2335-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito, M., and J. A. O'Malley. 1987. Antiviral effects of recombinant human tumor necrosis factor. Lymphokine Res. 6:309-318. [PubMed] [Google Scholar]
- 21.Jarvis, M. A., K. N. Fish, C. Soderberg-Naucler, D. N. Streblow, H. L. Meyers, G. Thomas, and J. A. Nelson. 2002. Retrieval of human cytomegalovirus glycoprotein B from cell surface is not required for virus envelopment in astrocytoma cells. J. Virol. 76:5147-5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johns, L. D., T. Sarr, and G. E. Ranges. 1994. Inhibition of ceramide pathway does not affect ability of TNF-alpha to activate nuclear factor-kappa B. J. Immunol. 152:5877-5882. [PubMed] [Google Scholar]
- 23.Johnson, D. C., and N. R. Hegde. 2002. Inhibition of the MHC class II antigen presentation pathway by human cytomegalovirus. Curr. Top. Microbiol. Immunol. 269:101-115. [DOI] [PubMed] [Google Scholar]
- 24.Johnson, R. A., S. M. Huong, and E. S. Huang. 2000. Activation of the mitogen-activated protein kinase p38 by human cytomegalovirus infection through two distinct pathways: a novel mechanism for activation of p38. J. Virol. 74:1158-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones, S. J., E. C. Ledgerwood, J. B. Prins, J. Galbraith, D. R. Johnson, J. S. Pober, and J. R. Bradley. 1999. TNF recruits TRADD to the plasma membrane but not the trans-Golgi network, the principal subcellular location of TNF-R1. J. Immunol. 162:1042-1048. [PubMed] [Google Scholar]
- 26.Jones, T. R., L. K. Hanson, L. Sun, J. S. Slater, R. M. Stenberg, and A. E. Campbell. 1995. Multiple independent loci within the human cytomegalovirus unique short region down-regulate expression of major histocompatibility complex class I heavy chains. J. Virol. 69:4830-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones, T. R., and L. Sun. 1997. Human cytomegalovirus US2 destabilizes major histocompatibility complex class I heavy chains. J. Virol. 71:2970-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones, T. R., E. Wiertz, L. Sun, K. N. Fish, J. A. Nelson, and H. L. Ploegh. 1996. Human cytomegalovirus US3 impairs transport and maturation of major histocompatibility complex class I heavy chains. Proc. Natl. Acad. Sci. USA 93:11327-11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jun, Y., E. Kim, M. Jin, H. C. Sung, H. Han, D. E. Geraghty, and K. Ahn. 2000. Human cytomegalovirus gene products US3 and US6 down-regulate trophoblast class I MHC molecules. J. Immunol. 164:805-811. [DOI] [PubMed] [Google Scholar]
- 30.Keisari, Y. 1992. A colorimetric microtiter assay for the quantitation of cytokine activity on adherent cells in tissue culture. J. Immunol. Methods 146:155-161. [DOI] [PubMed] [Google Scholar]
- 31.Lehner, P. J., J. T. Karttunen, G. W. Wilkinson, and P. Cresswell. 1997. The human cytomegalovirus US6 glycoprotein inhibits transporter associated with antigen processing-dependent peptide translocation. Proc. Natl. Acad. Sci. USA 94:6904-6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mackay, F., J. Rothe, H. Bluethmann, H. Loetscher, and W. Lesslauer. 1994. Differential responses of fibroblasts from wild-type and TNF-R55-deficient mice to mouse and human TNF-alpha activation. J. Immunol. 153:5274-5284. [PubMed] [Google Scholar]
- 33.Michelson, S., J. Alcami, S. J. Kim, D. Danielpour, F. Bachelerie, L. Picard, C. Bessia, C. Paya, and J. L. Virelizier. 1994. Human cytomegalovirus infection induces transcription and secretion of transforming growth factor β1. J. Virol. 68:5730-5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mocarski, E. S., Jr. 2002. Immunomodulation by cytomegaloviruses: manipulative strategies beyond evasion. Trends Microbiol. 10:332-339. [DOI] [PubMed] [Google Scholar]
- 35.Murphy, J. C., W. Fischle, E. Verdin, and J. H. Sinclair. 2002. Control of cytomegalovirus lytic gene expression by histone acetylation. EMBO J. 21:1112-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips, A. J., P. Tomasec, E. C. Wang, G. W. Wilkinson, and L. K. Borysiewicz. 1998. Human cytomegalovirus infection downregulates expression of the cellular aminopeptidases CD10 and CD13. Virology 250:350-358. [DOI] [PubMed] [Google Scholar]
- 37.Pulliam, L., J. A. Clarke, D. McGuire, and M. S. McGrath. 1994. Investigation of HIV-infected macrophage neurotoxin production from patients with AIDS dementia. Adv. Neuroimmunol. 4:195-198. [DOI] [PubMed] [Google Scholar]
- 38.Pulliam, L., D. Moore, and D. C. West. 1995. Human cytomegalovirus induces IL-6 and TNF alpha from macrophages and microglial cells: possible role in neurotoxicity. J. Neurovirol. 1:219-227. [DOI] [PubMed] [Google Scholar]
- 39.Reusch, U., W. Muranyi, P. Lucin, H. G. Burgert, H. Hengel, and U. H. Koszinowski. 1999. A cytomegalovirus glycoprotein re-routes MHC class I complexes to lysosomes for degradation. EMBO J. 18:1081-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ritter, T., C. Brandt, S. Prosch, A. Vergopoulos, K. Vogt, J. Kolls, and H. D. Volk. 2000. Stimulatory and inhibitory action of cytokines on the regulation of hCMV-IE promoter activity in human endothelial cells. Cytokine 12:1163-1170. [DOI] [PubMed] [Google Scholar]
- 41.Rodems, S. M., and D. H. Spector. 1998. Extracellular signal-regulated kinase activity is sustained early during human cytomegalovirus infection. J. Virol. 72:9173-9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanchez, V., K. D. Greis, E. Sztul, and W. J. Britt. 2000. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J. Virol. 74:975-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simmen, K. A., J. Singh, B. G. Luukkonen, M. Lopper, A. Bittner, N. E. Miller, M. R. Jackson, T. Compton, and K. Fruh. 2001. Global modulation of cellular transcription by human cytomegalovirus is initiated by viral glycoprotein B. Proc. Natl. Acad. Sci. USA 98:7140-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sinclair, J., J. Baillie, L. Bryant, and R. Caswell. 2000. Human cytomegalovirus mediates cell cycle progression through G1 into early S phase in terminally differentiated cells. J. Gen. Virol. 81:1553-1565. [DOI] [PubMed] [Google Scholar]
- 45.Stenberg, R. M. 1996. The human cytomegalovirus major immediate-early gene. Intervirology 39:343-349. [DOI] [PubMed] [Google Scholar]
- 46.Storey, H., A. Stewart, P. Vandenabeele, and J. P. Luzio. 2002. The p55 tumour necrosis factor receptor TNFR1 contains a trans-Golgi network localization signal in the C-terminal region of its cytoplasmic tail. Biochem. J. 366:15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Warren, A. P., D. H. Ducroq, P. J. Lehner, and L. K. Borysiewicz. 1994. Human cytomegalovirus-infected cells have unstable assembly of major histocompatibility complex class I complexes and are resistant to lysis by cytotoxic T lymphocytes. J. Virol. 68:2822-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weinshenker, B. G., S. Wilton, and G. P. Rice. 1988. Phorbol ester-induced differentiation permits productive human cytomegalovirus infection in a monocytic cell line. J. Immunol. 140:1625-1631. [PubMed] [Google Scholar]
- 49.Yurochko, A. D., and E. S. Huang. 1999. Human cytomegalovirus binding to human monocytes induces immunoregulatory gene expression. J. Immunol. 162:4806-4816. [PubMed] [Google Scholar]