Abstract
Vaccines that elicit systemic and mucosal immune responses should be the choice to control human immunodeficiency virus (HIV) infections. We have previously shown that prime-boost immunizations with influenza virus Env and vaccinia virus (VV) WR Env recombinants induced an enhanced systemic CD8+ T-cell response against HIV-1 Env antigen. In this report, we analyzed in BALB/c mice after priming with influenza virus Env the ability of two VV recombinants expressing HIV-1 Env B (VV WR Env and the highly attenuated modified VV Ankara [MVA] Env) to boost cellular immune responses in the spleen and in the lymph nodes draining the genital and rectal tracts. Groups of mice were primed by the intranasal route with 104 PFU of influenza virus Env and boosted 14 days later by the intraperitoneal or intranasal route with 107 PFU of MVA Env or VV WR Env, while the control group received two immunizations with influenza virus Env. We found that the combined immunization (Flu/VV) increased more than 60 times the number of gamma interferon-specific CD8+ T cells compared to the Flu/Flu scheme. Significantly, boosting with MVA Env by the intraperitoneal route induced a response 1.25 or 2.5 times (spleen or genital lymph nodes) higher with respect to that found after the boost with VV WR Env. Mice with an enhanced CD8+ T-cell response also had an increased Th1/Th2 ratio, evaluated by the cytokine pattern secreted following in vitro restimulation with gp160 protein and by the specific immunoglobulin G2a (IgG2a)/IgG1 ratio in serum. By the intranasal route recombinant WR Env booster gave a more efficient immune response (10 and 1.3 times in spleen and genital lymph nodes, respectively) than recombinant MVA Env. However, the scheme influenza virus Env/MVA Env increased four times the response in the spleen, giving a low but significant response in the genital lymph nodes compared with a single intranasal immunization with MVA Env. These results demonstrate that the combination Flu/MVA in prime-booster immunization regimens is an effective vaccination approach to generate cellular immune responses to HIV antigens at sites critical for protective responses.
Heterologous prime-boost immunization regimens employing poxvirus vectors for the booster immunization have been shown to be very efficient vaccination approaches in different animal models, specially in their ability to induce specific cellular immune responses and also to trigger protection to pathogens (13, 17, 26, 37, 38). In these studies, the immunogens mostly used for priming were DNA vectors. However, other immunogens such as proteins, peptides, viruslike particles, and attenuated viral vectors have also been employed. In this sense, studies pioneered with influenza virus and vaccinia virus (VV) vectors have shown that sequential immunization with influenza virus and VV recombinants expressing rodent malaria antigens results in enhanced CD8+ T-cell-specific immune responses that protected mice against sporozoite-induced malaria. Interestingly, the order in which these two vectors were administered was critical to efficiently expand specific CD8+ T cells in order to achieve protection from a lethal challenge (23, 44). Prime-boost immunization approaches with heterologous vectors are now widely used against different pathogens and several phase I/II clinical trials are under way (6, 19, 29).
Routes that trigger systemic immune responses (17, 37, 38) have been carried out by most of the prime-boost vaccination studies. Moreover, measurements of immunological parameters were focused on components of systemic immunity. Vaccines capable of protecting against human immunodeficiency virus (HIV) most likely need to induce long-term mucosal immune responses, as the mucosal route is the most natural route for transmission of the virus. An immunization strategy that can generate anti-HIV cytotoxic T cells at mucosal tissues or in lymph nodes draining the genital and rectal tracts may limit the spread of HIV after initial infection. Even after intravenous inoculation of simian immunodeficiency virus (SIV) in monkeys, the mucosal lymphoid tissue is a major initial area of viral proliferation. (42).
The mucosa of the vagina and rectum is drained by iliac nodes, as demonstrated in studies performed in nonhuman primates in which homing of different population of immune cells (CD4, CD8, and B cells) from the iliac lymph nodes to the rectal, cervical, and vaginal mucosa was documented (28). Moreover, studies performed in the SIV model demonstrated that after intravaginal inoculation of SIV in rhesus monkeys (40), the virus was detected in macrophages and dendritic cells of the lamina propria at short times postinoculation, and with time it was detected in the draining iliac lymph nodes. In the chimpanzee model, a rapid transmission of HIV-1 across the vaginal mucosa was also demonstrated (11). Thus, it appears that the first immune protective barriers against HIV are those of the mucosa and the regional lymph nodes, implying that induction of immunity in such places is crucial to control infection.
The stimulation of the mucosal immune response can be achieved by the administration of immunogens at mucosal inductive sites, where specialized organized lymphoepithelial follicular structures exist. The use of the intranasal route of immunization to stimulate inductive sites in the respiratory tract has been of considerable interest in the last few years. Various studies have demonstrated that both the oral and intranasal routes of administration of antigens are capable of inducing immune responses at distant effector sites (33). In this sense, live viral vaccines administered by the intranasal route efficiently stimulate humoral and cell-mediated immune responses in both mucosal and systemic compartments (27). For example, attenuated influenza virus vectors have the capacity to induce mucosal immune responses, as the natural route of infection of the virus is the nasal mucosa (8, 9, 32).
Modified vaccinia virus Ankara (MVA) is one of the most promising live viral vectors to be applied as a recombinant vaccine due to its safety and ability to trigger protection against a wide spectrum of pathogens (1, 2, 17, 24, 25, 30, 39, 44). Several studies have shown the immunogenicity of MVA recombinants when inoculated by systemic routes. However, the immunogenicity elicited after administration of MVA by mucosal routes has not been well characterized. (3).
The aim of this study was to establish immunization regimens that enhance mucosal immune responses against HIV antigens. Due to the ability of influenza virus to target the mucosal tissue, we have used prime-booster immunizations with a recombinant influenza virus vector expressing the well-characterized CD8+ T-cell epitope from the V3 loop of HIV-1 (clade B) and two VV recombinants expressing the entire HIV-1 Env protein (VV WR Env and MVA Env). We have compared in mice how these vectors activate specific cellular immune responses when they were administered by systemic and mucosal routes. Our findings showed that the combination of influenza virus Env and MVA Env (Flu/MVA) effectively activates cellular immune responses in the genitorectal draining lymph nodes and in the spleen.
MATERIALS AND METHODS
Viruses and cells.
The recombinant VV Env (WR strain) and MVA Env employed in this study have a foreign gene inserted in the thymidine kinase locus and have been described previously (12, 35). Both viruses express the complete HIV-1 IIIB gp160. WR derivatives were grown in human HeLa cells, and MVA derivatives were grown in primary chicken embryo fibroblasts. Sucrose cushion-purified viral stocks of recombinant MVA and recombinant WR were titrated in baby hamster kidney BHK-21 cells or African green monkey kidney BSC-40 cell monolayers by immunostaining of fixed infected cultures with polyclonal serum reactive against VV proteins and by plaque assays, respectively. Kinetics of expression of the recombinant gp160 was performed in BHK-21 and 3T3 (a mouse fibroblast-derived cell line) cell monolayers. The chimeric Fluenv IIIB influenza A virus has been described previously, and it was grown and titrated by plaque assay in MDBK cells (15). This virus encodes a 12-amino-acid peptide, IQRGPGRAFVTI, corresponding to the V3 loop of gp120 of HIV-1 IIIB inserted into the antigenic site B of its hemagglutinin protein.
Immunizations of mice and serum sample collection.
BALB/c mice (H-2d) (6 to 8 weeks old) were immunized intraperitoneally or intranasally with different doses (indicated as PFU) of the different viruses in 200 μl (by the intraperitoneal route) or 25 μl (by the intranasal route) of sterile phosphate-buffered saline (PBS). Fourteen days after immunization, blood was obtained from the retroorbital plexus by a heparinized capillary tube, collected in an Eppendorf tube, and centrifuged, and serum was obtained and stored at −20°C.
Antibody measurements by ELISA.
Enzyme-linked immunosorbent assay (ELISA) was used to determine the presence of antibodies against the V3 loop of gp160 and against β-galactosidase in serum samples. β-galactosidase employed to coat 96-well flat-bottomed plates was used at a concentration of 5 μg/ml. The V3 peptide (IQRGPGRAFVTI) employed for coating the plates was first conjugated to bovine serum albumin following standard methods. Antigens were suspended in carbonate buffer pH 9.6, plated at 50 μl/well, and incubated overnight at 4°C. Afterwards, the contents of the wells were discarded and washed three times with PBS plus 0.05% Tween 20 (PBS-T), and blocking buffer (PBS with 10% fetal calf serum) was added at 200 μl/well and incubated for 1 h at 37°C. The plates were washed once with PBS-T, and samples diluted in blocking buffer were added in a volume of 50 μl/well and incubated for 1 h at 37°C. Then, plates were washed three times before the detection antibody was added. Peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG1) or IgG2a (Southern Biotechnology Associates, Birmingham, Ala.) antibodies were diluted 1:1,500 and 1:2,000, respectively, in blocking buffer and incubated 1 h at 37°C. After washing the plates three times with PBS-T, the wells were reacted with the peroxidase substrate O-phenylenediamine dihydrochloride (OPD) (Sigma Chemical, St. Louis, Mo.). After 10 to 15 min of incubation at room temperature, adding 2N H2SO4 stopped the reaction, and absorbance values were measured at 450 nm on a Labsystems Multiskan Plus plate reader.
Evaluation of CD8+ T cells by the ELISPOT assay.
The ELISPOT assay to detect antigen-specific CD8+ T cells was performed as previously described (12). Briefly, 96-well nitrocellulose plates were coated with 8 μg/ml of anti-mouse gamma interferon (IFN-γ) monoclonal antibody R4-6A2 (PharMingen, San Diego, Calif.) in 100 μl of PBS. After overnight incubation at room temperature, wells were washed three times with RPMI 1640, and 100 μl of complete medium supplemented with 10% fetal calf serum were added to each well. Afterwards, the plate was incubated at 37°C for 1 h. Splenocytes cells (depleted of red blood cells) and lymphocytes from iliac lymph nodes from different groups of mice were added in triplicate of twofold dilutions. P815 cells (a mastocytoma cell line which expresses only major histocompatibility complex class I molecules) were used as antigen-presenting cells.
To evaluate the number of CD8+ IFN-γ-secreting cells specific for the V3 loop epitope of the HIV-1 Env protein, P815 cells were pulsed with 10−6 M synthetic peptide RGPGRAFVTI (10 Env) and treated with mitomycin C (30 μg/ml) for 20 min. After several washes with culture medium, 105 P815 cells were added to each well. As a control, P815 cells not pulsed with the peptide but treated under similar conditions were used. Plates were incubated for 24 h in a 37°C incubator with a 5% CO2 atmosphere, washed extensively with PBS-T, and incubated for 2 h with a solution of 2 μg of biotinylated anti-mouse IFN-γ monoclonal antibody XMG1.2 (PharMingen) per ml in PBS-T. Thereafter, plates were washed with PBS-T, and 100 μl of peroxidase-labeled avidin (Sigma Chemical) at a 1:800 dilution in PBS-T was added to each well and incubated at room temperature. One hour later, wells were washed with PBS-T and PBS. The spots were developed by adding 1 μg of the substrate 3,3′-diaminobenzidine tetrahydrochloride (Sigma Chemical) per ml in 50 mM Tris-HCl, pH 7.5, containing 0.015% hydrogen peroxide. Then spots were counted with the aid of a stereomicroscope.
T-cell proliferation assays.
Lymphocytes were removed from spleens and iliac lymph nodes by passing tissues through a sterile mesh to obtain cell suspensions. Cells were suspended in complete medium (RPMI 1640 supplemented with 10% fetal calf serum, 2 mM l-glutamine, and 10 mM 2-β-mercaptoethanol). Red blood cells in preparations of spleen cells were lysed with 0.1 M ammonium chloride buffer. Lymphocytes were cultured in triplicate (106 cells/well) in 96-well microtiter flat-bottomed plates and stimulated with purified gp160 protein (Intracel Corporation, Cambridge, Mass.) (1 μg/ml) or concanavalin A (1 μg/ml) (Sigma Chemical). Plates were incubated for 3 days at 37°C in 5% CO2. After this incubation period, cytokine levels (IFN-γ and interleukin-4 [IL-4]) in culture supernatants from triplicate cultures were evaluated following the manufacturer's instructions (Pharmingen).
Western blot.
BHK-21 and 3T3 monolayer cells were infected at 5 PFU/cell with the indicated VV or MVA viruses, collected, and lysed at 6, 18, and 24 h postinfection in cold buffer (50 mM Tris-HCl [pH 8.0], 0.5 M NaCl, 10% NP-40, 1% sodium dodecyl sulfate). Equal amounts of protein lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 10% gels, transferred to nitrocellulose membranes, and reacted with primary rabbit polyclonal anti-gp120 protein and with secondary antibodies (anti-rabbit immunoglobulin-peroxidase conjugates). Protein expression was detected with ECL Western blotting reagents (Amersham). Relative quantities of gp160 and gp120 proteins in Western blot were determined by densitometric analysis with the NIH Image 1.62 software.
Immunofluorescence.
3T3 and BHK-21 cells cultured on coverslips were infected at 5 PFU/cell. Twenty-four hours postinfection, cells were washed with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde, and permeabilized by treatment with 0.1% Triton X-100 in PBS (room temperature, 10 min). After the PBS wash, coverslips were blocked with a PBS solution containing 20% bovine serum albumin. Cells were incubated (1 h, 37°C) with antibodies directed to the gp160 HIV-1 IIIB protein. Coverslips were washed extensively with PBS and incubated (1 h at 37°C) with secondary anti-rabbit immunoglobulin conjugated with fluorescein isothiocyanate. The DNA staining reagent To-Pro (Molecular Probes) was included in this incubation. To analyze the intracellular localization of gp160 in infected cells, we used antibodies against the wheat germ antigen, a specific marker for Golgi structures. After several washes with PBS, coverslips were mounted on microscope slides with Mowiol (Calbiochem). Images were obtained with a Bio-Rad Radiance 2100 confocal laser microscope.
Flow cytometry.
3T3 cells in monolayers were infected with the indicated virus at 1 PFU/cell. At 18 h postinfection, cells were trypsinized and extensively washed with PBS. After blocking with PBS-10% fetal calf serum, cells were stained with a rabbit polyclonal anti-gp120 antibody, washed, and incubated with the secondary antibody, anti-rabbit immunoglobulin conjugated with fluorescein isothiocyanate. Following staining, cells were washed, fixed, and analyzed with a FACScan (Becton Dickinson, Mountain View, Calif.).
RESULTS
Strong cellular immune responses to HIV-1 Env in spleen and genital lymph nodes after intranasal priming with influenza virus Env and intraperitoneal boosting with VV WR Env or MVA Env.
In a previous study (15), we showed that combined immunization with influenza virus and VV (WR strain) vectors induced an enhanced CD8+ T-cell response against HIV-1 Env in the spleen. We have now examined whether booster with an attenuated VV vector, MVA Env, potentiates the cellular immune response in a manner comparable to that with the VV WR Env recombinant in mice primed with influenza virus Env.
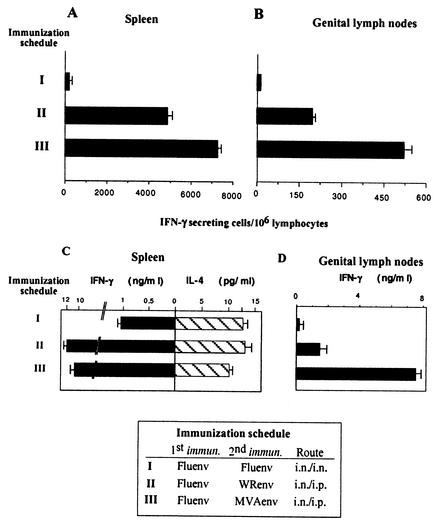
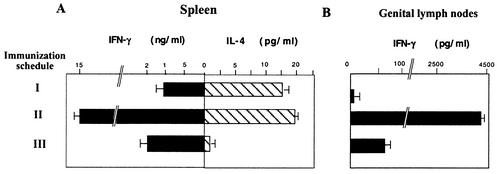
Groups of BALB/c mice were first intranasally primed with 104 PFU of a recombinant influenza virus A vector (Fluenv) that expresses a 10-amino-acid peptide, IQRGRAFVTI, corresponding to the V3 loop of gp120 of HIV-1 IIIB (15). Fourteen days after priming, mice received a booster dose given intranasally with influenza virus Env or intraperitoneally with either VV WR Env (107 PFU/mouse) or MVA Env (107 PFU/mouse). Both VV vectors expressed the complete gp160 sequence of HIV-1 strain IIIB (35). Fourteen days after the booster, the number of IFN-γ-secreting T cells in the spleen was measured. Figure 1A shows that VV-based vectors induced a significant enhancement of splenic CD8+ T-cell response against the Env antigen. Numbers of specific IFN-γ CD8+ T cells after Flu/VV immunizations were more than 50 times higher than those induced by Flu/Flu immunization. Interestingly, booster with MVA Env induced a response 1.5 times higher (P < 0.01) than that induced by VV WR Env boost. The CD8+ T-cell response was also evaluated in genitorectal draining lymph nodes (iliac lymph nodes). As shown in Fig. 1B, a pattern similar to that detected in the spleen was observed in the lymph node tissues, where boosting with MVA Env also induced a response 2.5 times higher than that found in mice boosted with VV WR Env (P < 0.01).
FIG. 1.
Evaluation of specific cellular immune responses against HIV-1 Env antigen. Quantification of Env peptide specific IFN-γ-secreting CD8+ T cells. Four BALB/c mice per group were first intranasally (i.n.) immunized with 104 PFU of influenza virus Env, and 14 days later the animals were intranasally boosted with 104 PFU of influenza virus Env or intraperitoneally (i.p.) boosted with 107 PFU of VV WR Env or MVA Env. Cell suspensions of the spleens (A) or genitorectal lymph nodes (B) obtained 14 days after the immunization were evaluated for Env-specific IFN-γ-secreting cells by ELISPOT assay. The number of antigen-specific IFN-γ-secreting cells with standard deviation from triplicate cultures is shown. The pattern of cytokine secretion after gp160 restimulation of cell suspensions of spleens (C) or genitorectal lymph nodes (D) was determined. After 72 h of culture, cell culture supernatants were harvested and evaluated for IFN-γ and IL-4 by ELISA. Bars represent the median ± standard deviation of triplicate samples.
To further analyze the Th type of immune response induced in the different groups of immunized mice, we evaluated the cytokines secreted in cell culture supernatants derived from lymphocytes of spleen and genital draining lymph nodes after in vitro restimulation with the specific antigen (purified gp160 protein). This is shown in Fig. 1C and D. Whereas influenza virus Env/influenza virus Env immunization induced a Th1/Th2 ratio of 0.1 (measured as the IFN-γ/IL-4 ratio), priming with influenza virus Env and boosting with VV vectors increased the ratio 10-fold. Similar ratios were also found in cell supernatants from splenocytes stimulated with concanavalin A (data not shown). In supernatants from lymphocytes of genital lymph nodes, the levels of IL-4 were not significant, and hence, Fig. 1D only shows IFN-γ levels.
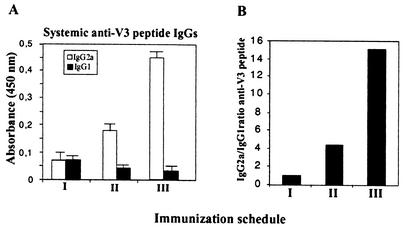
We further characterized specific IgG2a/IgG1 antibodies against the V3 peptide in serum. Sera from mice employed in the experiment described above were used to assay for IgG antibodies against the V3 region of the HIV-1 Env antigen. As shown in Fig. 2, the levels of IgGs (evaluated as net absorbance) in the different groups were not high, but we observed a higher ratio of specific IgG2a/IgG1 subclasses (Th1 and Th2 dependent, respectively) in mice immunized with the Flu/MVA scheme. Thus, the IgG2a/IgG1 ratio correlated with the CD8+ induction and Th1/Th2 cytokine secretion pattern (see Fig. 1) found in the different groups.
FIG. 2.
Induction of anti-V3 Env antibodies and IgG2a/IgG1 ratios after immunization with influenza virus and VV vectors. Sera from mice of the experiment described in Fig. 1 were evaluated for specific anti-gp160 antibodies 14 days after booster. Reactivity of individual serum samples against V3 peptide was assayed by ELISA. (A) Absorbances for IgG1 and IgG2a subclasses in 1:50 serum dilutions from mice of the different groups (absorbance from sera of nonimmunized mice was subtracted). (B) IgG2a/IgG1 ratios in the different groups.
Similar levels and pattern of expression of HIV-1 Env antigen found in cells infected with MVA Env and VV WR Env.
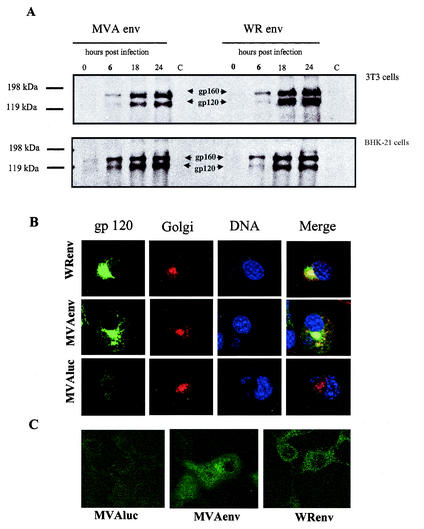
The experiments shown in Fig. 1 revealed that systemic administration of MVA Env to mice previously primed with influenza virus Env results in stronger cellular immune responses than those boosted by VV WR Env administration. To determine if these differences could be attributed to different levels of expression of Env, we carried out a kinetic analysis in cells infected with MVA Env and VV WR Env. Env expression was measured by Western blot in murine 3T3 cells, which are nonpermissive for MVA (Fig. 3A, upper panel), and in MVA-permissive BHK-21 cells (Fig. 3A, lower panel). Cell monolayers were infected with 5 PFU/cell, and at different times postinfection, proteins in cell extracts were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, blotted, and reacted with a polyclonal anti-gp120 antibody. No considerable differences in the kinetic of synthesis of Env protein were observed between 3T3 cells infected with VV WR Env or MVA Env (Fig. 3A, upper panel). At 18 h and 24 h postinfection, higher levels of Env antigen were expressed from VV WR Env (two times higher levels of gp120 and 1.4 times higher levels of gp160). However, in BHK-21 cells (lower panel) at short times (6 h) postinfection, gp120 and gp160 expression levels from cells infected with MVA Env were approximately 2.8 and 1.6 times higher than those observed in cells infected with VV WR Env, but these differences disappear by 18 h and 24 h postinfection.
FIG. 3.
Comparison of levels of expression of gp160 between cells infected with VV WR Env and MVA Env. (A) Western blot. 3T3 and BHK-21 cells were infected (5 PFU/cell) with VV WR Env or MVA Env, and at various times postinfection cells were collected and Env expression was analyzed by Western blot with a specific anti-gp160 IIIB antibody. (B) Immunofluorescence analysis under permeable conditions. 3T3 cells were infected (5 PFU/cell) with recombinant VV WR Env, MVA Env, or MVAluc, and at 24 h postinfection cells were fixed, permeabilized, and incubated with polyclonal gp120 IIIB antibody to show Env, with antibody against wheat germ to show the Golgi, or with To-Pro to show the DNA. To the right is the color merging to show colocalization of gp160 and Golgi compartments. (C) Immunofluorescence analysis under nonpermeable conditions. 3T3 cells were infected (1 PFU/cell) with recombinant VV WR Env, MVA Env, or MVAluc, and at 18 h postinfection cells were fixed, nonpermeabilized, and incubated with rabbit polyclonal gp120 IIIB antibody.
To define if differences could be observed in the intracellular localization of Env between the two VV vectors, 3T3 cells at twenty-four hours postinfection were analyzed by immunofluorescence with Env specific antibodies. Env antigen was predominantly found in the Golgi of cells infected with MVA Env or VV WR Env, as it can be expected for a protein that is glycosylated (Fig. 3B). Cells infected with the unrelated recombinant MVAluc served as control. Staining of the DNA permits the visualization of the cell nuclei and of typical cytoplasmic VV factories. In Fig. 3C, it can be seen that in 3T3 cells infected with MVA Env or VV WR Env and fixed under nonpermeable conditions, the gp160 antigen is exposed on the cell surface. FACS analysis of 3T3 cells infected with the recombinant viruses and doubly stained on the surface with anti-gp120 antibody and with a conjugated secondary antibody-fluorescein isothiocyanate (see Materials and Methods), showed that after 18 h and 24 h postinfection 18% (VV WR Env) and 11.2% (MVA Env) of the cells were positively stained (data not shown).
The findings in Fig. 3 suggest that the differences in immune response to Env between MVA Env and VV WR Env are not due to different levels of Env expression, but to an intrinsic property of the MVA vector, such as the lack of immunomodulatory molecules or its ability to interact with the host.
Mucosal (intranasal) delivery of VV vectors is an effective route to boost specific cellular immune responses after priming with influenza virus Env.
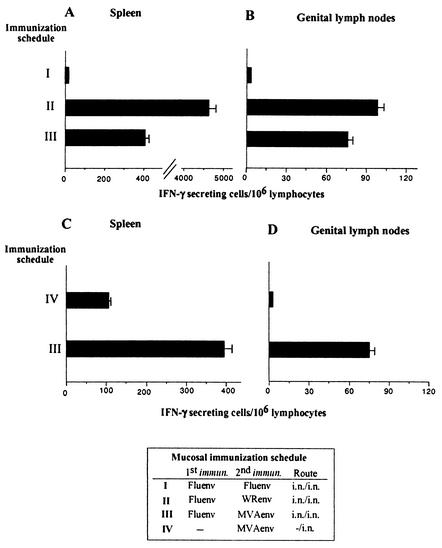
The results depicted above corroborated our previous experiments in which the WR-based vector was employed at booster (15) and demonstrated that MVA Env can be as efficient as or even a better immunogen than VV WR Env to boost the anti-Env CD8+ T-cell response primed with the influenza virus Env vector. Our next experiments were conducted to prove if a mucosal immunization scheme, priming and booster by the intranasal route, can induce a CD8+ T-cell response against Env in the spleen and genitorectal lymph nodes. Delivery intranasally of VV WR Env induced a booster effect in the spleen (Fig. 4A) and genitorectal lymph nodes (Fig. 4B) of a magnitude comparable to that obtained with the systemic (intraperitoneal) inoculation (Fig. 1). By contrast, boosting intranasally with MVA Env induced lower responses than those triggered by VV WR Env. Nevertheless, an intranasal booster with MVA Env induced a significant response at distal places from the inoculation site, such as the spleen and iliac-inguinal lymph nodes. Moreover, influenza virus Env/MVA Env induced a fourfold higher CD8+ T-cell response in the spleen and a low but significant response in genitorectal lymph nodes than that obtained after a single intranasal immunization with MVA Env, proving the boosting capacity of MVA Env when delivered by intranasal route (Fig. 4C and 4D).
FIG. 4.
Evaluation of the CD8+ T-cell responses against Env after a mucosal immunization scheme. Four BALB/c mice per group were first intranasally immunized with 104 PFU of influenza virus Env, and 14 days later they were intranasally boosted with 107 PFU of VV WR Env or MVA Env. Fourteen days after boosting, Env peptide-specific IFN-γ-secreting cells in spleen cells (A) and genitorectal lymph nodes (B) were quantified. Shown are the mean numbers of antigen-specific IFN-γ-secreting cells with standard deviation from triplicate cultures. (C) Potentiation of the CD8+ T-cell anti-Env response induced after intranasal MVA Env immunization by priming mice with influenza virus Env. Splenocytes of both groups were obtained 14 days after immunization and evaluated by ELISPOT. Shown are the mean numbers of antigen-specific IFN-γ-secreting cells with standard deviation from triplicate cultures.
The Th type of immune response induced in the immunized groups was also evaluated. Figure 5 shows the cytokine pattern secreted in cell culture supernatants from lymphocytes of spleen (Fig. 5A) and genital draining lymph nodes (Fig. 5B) after in vitro restimulation with the specific antigen (gp160 protein). Mucosal (intranasal) delivery of the recombinant VV vectors induced a Th1 immune response, as measured by the IFN-γ/IL-4 ratio. The levels of IFN-γ found in supernatants from gp160-stimulated splenocytes correlated with the levels of IFN-γ-secreting CD8+ T cells analyzed by the ELISPOT assay (compare Fig. 4 and 5). However, such a direct correlation was not observed for genitorectal lymph nodes lymphocytes, as the ratio of group II to group III for levels of IFN-γ in supernatants from gp160-stimulated lymphocytes was about 60 (Fig. 5B), while the ratio for IFN-γ-secreting cells evaluated in the ELISPOT was about 1.4 (Fig. 4B).
FIG. 5.
Pattern of specific cytokine secretion after mucosal immunization. Cell suspensions of spleens (A) or genitorectal lymph nodes (B) of mice in Fig. 4, obtained 14 days after the booster, were in vitro restimulated with gp160 or RPMI (negative control), and 72 h later cell culture supernatants were harvested and evaluated for IFN-γ and IL-4 by ELISA. Bars represent the mean with standard deviation of triplicates from cell cultures gp160-stimulated; nonspecific cytokine levels found in negative controls were subtracted.
Booster effect of poxvirus vectors in heterologous prime-boost regimens is dependent of the VV strain and dose of the vector.
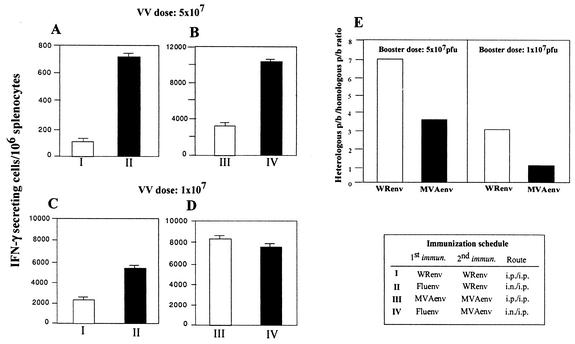
Next we compared, in mice primed with influenza virus Env, the dosage effect of MVA Env and VV WR Env to boost a specific immune response against Env antigen. Different groups of mice were immunized with a heterologous or homologous immunization scheme, employing VV WR Env or MVA Env vectors at two different doses, inoculated by the intraperitoneal route. Figure 6 shows the CD8+ T-cell response against Env antigen induced after homologous and heterologous immunizations with 5 × 107 PFU (Fig. 6A, upper panel) or 107 PFU (Fig. 6A, lower panel) of VV vectors. Using 5 × 107 PFU/mouse, both VV WR Env and MVA Env vectors produced a higher response in the heterologous immunization scheme compared to that obtained in the homologous prime-boost protocol. By contrast, administration of 107 PFU/mouse of VV vector in heterologous immunizations resulted in higher response only in the case of VV WR Env-immunized mice. MVA Env induced higher levels of CD8+ T cells than VV WR Env, as also shown in Fig. 1. It should be noted that the ELISPOT assay in panels B, C, and D was carried out independently of the ELISPOT in panel A, and hence, the important feature of Fig. 6 is not the total number of spots that vary between assays, but the difference in ratio of IFN-γ-secreting cells between the groups of immunized animals evaluated in the same assay.
FIG. 6.
Booster effect of poxvirus vectors in heterologous prime-boost schemes is dependent on the VV strain and dose of virus vector inoculated. Groups of four BALB/c mice were immunized as indicated. Fourteen days after the booster immunization, the number of IFN-γ-secreting CD8+ T cells in the spleen from the different groups of mice was evaluated by ELISPOT. The VV vector (MVA Env and VV WR Env) dose applied was 5 × 107 PFU/mouse (A and B) or 107 PFU/mouse (C and D). Shown are the mean numbers of antigen-specific IFN-γ-secreting cells with standard deviation from triplicate cultures. Panel E shows the increase obtained in the specific CD8+ T-cell response after the heterologous prime-boost scheme, represented as the ratio of heterologous prime-boost response versus the homologous prime-boost response.
If we define the increase in the cell-mediated immune response after recombinant VV boost in heterologous prime-boost schemes as the ratio of heterologous (influenza virus Env/VV Env) to homologous (VV Env/VV Env) response, we found that when a higher booster dose was administered (5 × 107 PFU) both WR and MVA heterologous immunizations induced an increased (seven- and threefold, respectively) immune response (Fig. 6E). When 107 PFU were used, the WR strain produced a threefold increase in the CD8+ T-cell response in heterologous immunizations. However, at this booster dose, the MVA Env vector produced a response that was of similar magnitude after homologous or heterologous prime-boost schemes, ratio = 1. This could be explained if, after priming with a low dose of MVA, the magnitude of the immune response induced against the vector is low, resulting in efficient boosting after a second administration of MVA. An indirect measurement of the extent of immunity against the vector triggered after one recombinant VV immunization will be the increase in antibodies against a recombinant antigen expressed from the VV vector (like β-galactosidase) after a second homologous immunization dose. Thus, the induction of an efficient immune response against the recombinant VV after the first dose would inhibit replication, β-galactosidase expression, and enhancement of β-galactosidase specific antibodies after a second administration of recombinant VV.
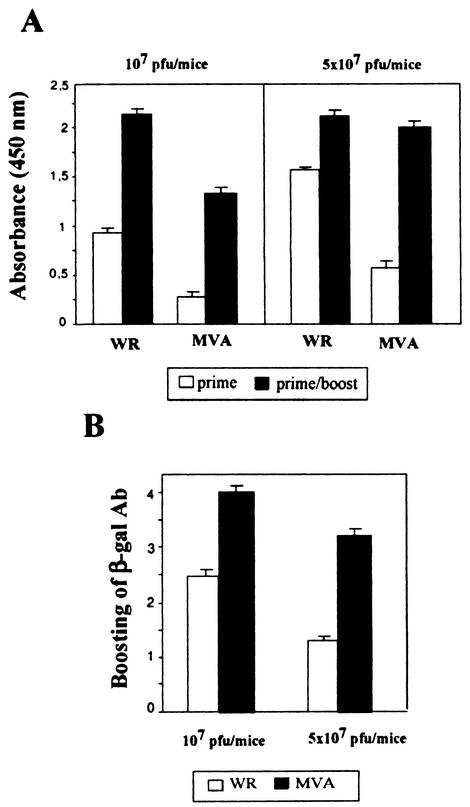
We measured the antibody levels against β-galactosidase (expressed by both VV vectors) in sera from mice immunized intraperitoneally with one or two doses of recombinant VV and evaluated the relative increase in the antibody levels after the second immunization dose. As can be seen in Fig. 7, when 107 PFU/mouse of MVA was used, a fourfold increase in β-galactosidase antibodies was observed after the second administration, contrasting with the minor 2.4-fold increase detected after WR boost. As expected, at the higher dose (5 × 107 PFU/mouse), almost no booster effect against β-galactosidase was detected after WR, whereas 3.3-fold increase in MVA immunized mice was found. Thus, the measurement of β-galactosidase antibodies suggested that after immunization with 107 PFU of MVA Env, a low immune response against the vector was produced and, hence, a second immunization with the same vector gave a booster effect directed against the recombinant antigen.
FIG. 7.
Increase in the level of antibodies against β-galactosidase after a second dose with recombinant VV. Fourteen days after intraperitoneal immunization with one or two doses of the indicated recombinant VV vectors, VV WR Env (white bars) or MVA Env (black bars), pooled sera from four mice were analyzed to evaluate the specific antibodies against β-galactosidase (a recombinant gene expressed from both recombinant VV) by standard ELISA. (A) Levels of anti-β-galactosidase (β-gal) IgG (optical density of 1:200 dilution of sera). (B) Bars represent, in optical densities, the relative increase (optical density of 1:200 serum dilution after second immunization/optical density of 1:200 serum dilution after one immunization) in the level of antibody (Ab) against β-galactosidase after the second immunization with recombinant VV. Data are representative of three different ELISA determinations.
DISCUSSION
In this study, we showed that prime-boost immunization schemes employing a recombinant influenza virus vector (influenza virus Env) at priming and recombinant VVs (VV WR Env and MVA Env) at booster is a competent immunization protocol to induce cellular immune responses against an HIV antigen. We demonstrated that both the intraperitoneal and intranasal routes of administration of the recombinant VV vectors are effective immunization approaches. Previous works have largely proved in the murine malaria model that the combination of these live viral vectors and in the same order of immunization (i.e., giving recombinant VV at booster) was an excellent vaccination approach, resulting in high levels of protection against challenge with parasites (44). Other studies have also shown that boosting with VV vectors after priming with a heterologous live virus recombinant induced a higher immune response than sequential immunizations with homologous vectors (17, 37, 38).
Thus, in a study performed in the SIV macaque model, it was shown that a systemic prime-boost immunization with Semliki Forest virus in combination with MVA, both expressing the env, gag-pol, nef, rev, and tat genes of SIV, was more efficient than multiple immunizations with the same vector for the induction of both humoral and cellular immune responses (30). Other work showed that the combination of recombinant vesicular stomatitis viruses and recombinant VVs expressing gag and env genes induced long-term T-cell responses (16). In the mouse malaria model, Gilbert et al. assayed different combinations of vectors in a prime-boost protocol and demonstrated a complete protective efficacy with an adenovirus prime/MVA boost, both expressing the CS gene from Plasmodium berghei (14).
We have previously shown that it is possible to obtain enhanced CD8+ T-cell responses to HIV-1 Env by sequential immunization with influenza virus and VV (WR) recombinants (15). In this early study, we only used the intraperitoneal route of inoculation and the VV WR Env vector. Here, we extended our previous observations by comparing the boosting capacity of WR and MVA recombinants expressing the entire HIV-1 Env antigen when inoculated by the intraperitoneal or intranasal route. We found that intraperitoneal administration of MVA Env was more effective than VV WR Env in enhancing the specific cellular immune response against Env antigen; MVA Env induced a response 1.25 or 2.5 times (spleen or genitorectal lymph nodes) higher with respect to that found after VV WR Env boost. Other work from our laboratory has previously demonstrated a higher immunogenicity for MVA compared to WR recombinant (34). In this regard, other investigators have found similar results (2, 3), attributed to higher levels of expression from MVA and to its stronger adjuvant capacity due to the loss of several viral anti-immune defense genes. In this study, although relatively higher expression levels of gp160 were detected from MVA Env infected BHK-21 cells at short time postinfection (6 h), at later times comparable expression levels were detected between both VV strains, and thus, the stronger MVA Env immunogenicity may be due to an intrinsic property of the vector.
The pattern of cytokine secretion after lymphocyte (splenocytes or genitorectal lymph node lymphocytes) restimulation with the complete gp160 protein indicated that intraperitoneal boosting with MVA or WR increased the Th1 response. The immunization schedules applied induced antibodies against Env antigen, and the serum specific IgG2a/IgG1 ratios correlated with the cytokine secretion and with the ELISPOT results, indicating that the heterologous prime-boost scheme increased the Th1/Th2 ratio.
Studies performed in macaques and chimpanzees have demonstrated that iliac lymph nodes drain the genitorectal tract (11, 40). More importantly, it was demonstrated that these lymph nodes can function as an inductive site from which T and B cells home preferentially to the vaginal, cervical and rectal mucosa (28). Thus, as the principal route of HIV infection is the genitorectal mucosa, we have considered it important to evaluate the specific cellular immune response induced in the genitorectal lymph nodes. The cellular immune response found in the genitorectal lymph nodes was significant and proportional to that found in the spleen for both systemic (intraperitoneal) and local (intranasal) routes of boosting with recombinant VV, indicating that the prime-boost scheme with these live viral vectors can induce a significant cellular immune response in the regional lymph nodes draining the principal route of infection for HIV.
While numerous studies have been performed with VV recombinants in the vaccine research field, few studies have characterized the immunogenicity of the poxvirus vectors delivered by a mucosal route and the mucosal immune response triggered by the recombinant viral vectors. This is of key relevance because immunity at the mucosal sites may play a major role in protection against HIV infection. The aim of this study was to analyze the impact of immunization at mucosal sites on specific immune responses to HIV Env. This was achieved with a prime-boost scheme by the intranasal route, combining two heterologous live viral vectors for the delivery of the antigen. Prime immunization was performed with an influenza virus-based vector. Several studies (8, 9, 32) have previously demonstrated the mucosal immunogenicity of the influenza virus vectors expressing HIV antigens, but studies in which MVA recombinants have been administered by mucosal routes are limited, in particular the use of MVA in combination with a heterologous live vector delivered at mucosal sites. Indeed, it has been shown that intranasal delivery of an MVA recombinant expressing an influenza virus antigen induced in mice protective immunity against a lethal influenza virus respiratory virus challenge (4) and in rhesus monkeys triggered effective protection in the lower respiratory tract to parainfluenza virus (7). MVA recombinants expressing respiratory syncytial virus proteins also induced in mice an immune response that reduced the replication of a challenge virus (43). Belyakov et al. demonstrated that intrarectal immunization with MVA expressing gp160 of HIV-1 89.6 induced a mucosal and systemic cytotoxic T lymphocyte response (3).
To our knowledge, there is no other report in which it has been demonstrated the immunogenicity of MVA recombinants expressing HIV antigens when delivered intranasally. In our study, when MVA Env was administered in a single intranasal immunization dose, it induced a weak immune response, but if mice were first primed intranasally with influenza virus Env, the intranasal MVA Env boost induced significant CD8+ T-cell responses in both spleen and genitorectal lymph node tissues. It must be pointed out that when the intranasal route was employed to deliver the recombinant VV strains to boost animals that had been primed with influenza virus Env, the booster with recombinant WR Env gave a response that was 10-fold (in spleen) or 1.4-fold (in genitorectal lymph nodes) higher than that obtained after the recombinant MVA Env booster. This can be explained considering that after intranasal delivery, WR replicates extensively in different mouse target tissues, including the spleen, but MVA infection is restricted to epithelial cells of the bronchi and bronchioles (36), leading to a more limited local antigen distribution.
The intranasal route of immunization is a feasible and practical route of vaccination. More importantly, by means of the common mucosal associate lymphocyte tissue, lymphocytes activated in the nasal associated lymphoid tissue can migrate and repopulate other mucosal surfaces. Several reports demonstrated that intranasal immunizations can give rise to immune responses in genital tissues (5, 18, 20, 40). In this study, we have not measured immune responses in the genitorectal mucosa, but instead, we have evaluated the specific cellular immune response induced in the draining lymph nodes. Other investigators in the SIV macaque model have previously demonstrated the importance of the immunity induced at these lymph nodes to potentially confer protection against viral infection (21). Moreover, targeted iliac lymph node immunization experiments in cats prevented vaginal infection with a virulent feline immunodeficiency virus (10). Here we have shown that sequential intranasal immunizations with influenza virus and MVA recombinants expressing Env antigens induce significant cellular immune responses in the genitorectal lymph nodes cells.
We evaluated the effect of the booster dose of recombinant VV in the heterologous Flu/VV prime-boost immunization protocol. When a higher intraperitoneal booster dose (5 × 107 PFU) was administered, we observed that both WR and MVA strains increased the response after the heterologous prime-boost immunization seven- and threefold, respectively, compared with a VV/VV immunization. However, when a reduced recombinant VV booster dose (107 PFU) was inoculated into mice, the heterologous prime-boost (influenza virus Env/VV Env) immunization induced a higher response than the homologous scheme (VV Env/VV Env) only for the VV WR Env vector. Considering that the principal difference between the VV vectors is that the WR strain is fully competent to replicate in mouse tissues, while MVA does not produce progeny virus in this host, we rationalized that the host immune response induced against the WR vector might be stronger, preventing a boosting response after a second immunization with the same vector.
Previous studies from our laboratory comparing MVA and WR immunogenicity in mice (34, 35) have shown that WR induced high and similar anti-VV IgG antibody levels after a 107 or 5 × 107 PFU dose, whereas significant antibodies were only detected after inoculation with 5 × 107 PFU of MVA. Importantly, the levels of IgGs evaluated by ELISA correlated with the virus neutralization titers. These previous findings explain the booster effect of MVA in the homologous scheme when a low virus dose (107 PFU) was applied. Indeed, we have also demonstrated that after a booster with MVA, higher humoral and cellular immune responses to foreign antigens were found in mice immunized with 107 PFU of recombinant MVA than in mice primed with the WR strain and boosted with recombinant MVA (35). Previous data obtained in the SIV-macaque model showed an increase in the SIV-Gag/Pol cytotoxic T-lymphocyte responses after repeated recombinant MVA injections, as well as in the specific cytotoxic T-lymphocyte response (39). In this sense, a recent vaccine trial in the macaque model showed that priming and boosting with MVA confer comparable levels of protection than a DNA-MVA vaccine against intrarectal challenge with a SHIV, although different patterns of immune responses were induced by the different immunization schemes (1).
In summary, the results presented in this study showed that prime-boost immunizations with a recombinant influenza virus vector for priming and recombinant VV at boosting (MVA or WR) expressing HIV-1 Env antigen are efficient vaccination protocols inducing potent cellular immune responses (CD8+ T cells and Th1) in the spleen and in the genitorectal draining lymph nodes. Our findings provided evidence that recombinant VVs (WR and MVA) can be immunogenic for HIV antigens if they are delivered by the intranasal route. These observations merit further studies employing the intranasal immunization protocol (Flu/MVA) for its potential mucosal protective immune response in a macaque SHIV model.
Acknowledgments
This investigation was supported, in part, by the Spanish Ministry of Science and Technology BIO2001-2269, the Spanish Foundation for AIDS Research (FIPSE, 36344/02), and the EU (QLRT-PL 1999-01321 and QLK2-CT-2002-01867) to M.E and by an NIH grant to A.G.-S. M.M.G. is a researcher from the Consejo Nacional de Investigaciones Cientificos (CONICET-Argentina). E.P.-J. is a recipient of a fellowship from the Ministerio de Educación y Cultura.
We thank V. Jimenez for expert technical assistance.
REFERENCES
- 1.Amara, R. R., F. Villinger, S. I. Staprans, J. D. Altman, D. C. Montefiori, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Different patterns of immune responses but similar control of a simian-human immunodeficiency virus 89.6P mucosal challenge by modified VV Ankara (MVA) and DNA/MVA vaccines. J. Virol. 76:7625-7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belyakov, I. M., L. S. Wyatt, J. D. Ahlers, P. L. Earl, C. D. Pendleton, B. L. Kelsall, W. Strober, B. Moss, and J. A. Berzofsky. 1998. Induction of a mucosal cytotoxic T-limphocyte response by intrarectal immunization with a replication-deficient recombinant VV expressing human immunodeficiency virus 89.6 envelope protein. J. Virol. 72:8264-8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belyakov, I. M., B. Moss, W. Strober, and J. A. Berzofsky. 1999. Mucosal vaccination overcomes the barrier to recombinant vaccinia immunization caused by preexisting poxvirus immunity. Proc. Natl. Acad. Sci. USA 96:4512-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender, B. S., C. A. Rowe, S. F. Taylor, L. S. Wyatt, B. Moss, and P. A. Small, Jr. 1996. Oral immunization with a replication-deficient recombinant VV protects mice against influenza virus. J. Virol. 70:6418-6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergquist, C., E. L. Johansson, T. Lagergard, J. Holmgren, and A. Rudin. 1997. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect. Immun. 65:2676-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnet, M. C., J. Tartaglia, F. Verdier, P. L. A. Kourilsky, M. Klein, and P. Moingeon. 2000. Recombinant viruses as a tool for therapeutic vaccination against human cancers. Immunol. Lett. 74:11-25. [DOI] [PubMed] [Google Scholar]
- 7.Durbin, A. P., L. S. Wyatt, J. Siew, B. Moss, and B. R. Murphy. 1998. The immunogenicity and efficacy of intranasally or parenterally administered replication-deficient vaccinia-parainfluenza virus type 3 recombinants in rhesus monkeys. Vaccine 16:1324-1330. [DOI] [PubMed] [Google Scholar]
- 8.Ferko, B., D. Katinger, A. Grassauer, A. Egorov, J. Romanova, B. Niebler, H. Katinger, and T. Muster. 1998. Chimeric influenza virus replicating predominantly in the murine upper respiratory tract induces local immune responses against human immunodeficiency virus type 1 in the genital tract. J. Infect. Dis. 178:1359-1368. [DOI] [PubMed] [Google Scholar]
- 9.Ferko, B., J. Stasakova, S. Sereinig, J. Romanova, D. Katinger, B. Niebler, H. Katinger, and A. Egorov. 2001. Hyperattenuated recombinant influenza virus A virus nonstructural-protein-encoding vectors induce human immunodeficiency virus type 1 Nef-specific systemic and mucosal immune responses in mice. J. Virol. 75:8899-8908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finerty, S., C. R. Stokes, T. J. Gruffydd-Jones, T. J. Hillman, F. J. Barr, and D. A. Harbour. 2001. Targeted lymph node immunization can protect cats from a mucosal challenge with feline immunodeficiency virus. Vaccine 20:49-58. [DOI] [PubMed] [Google Scholar]
- 11.Fultz, P. N., et al. 1986. Vaginal transmission of human immunodeficiency virus (HIV) to a chimpanzee. J. Infect. Dis. 154:896-900. [DOI] [PubMed] [Google Scholar]
- 12.Gherardi, M. M., J. C. Ramirez, D. Rodriguez, J. R. Rodriguez, G. Sano, F. Zavala, and M. Esteban. 1999. IL-12 delivery from recombinant VV attenuates the vector and enhances the cellular immune response against HIV-1 Env in a dose-dependent manner. J. Immunol. 162:6724-6733. [PubMed] [Google Scholar]
- 13.Gherardi, M. M., J. C. Ramirez, and M. Esteban. 2001. Towards a new generation of vaccines: the cytokine IL-12 as an adjuvant to enhance cellular immune responses to pathogens for prime-booster vaccination regimens. Histol. Histopathol. 16:655-667. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert, S. C., J. Schneider, C. M. Hannan, J. T. Hu, M. Plebanski, R. Sinden, and A. V. Hill. 2002. Enhanced CD8 T-cell immunogenicity and protective efficacy in a mouse malaria model with a recombinant adenoviral vaccine in heterologous prime-boost immunisation regimes. Vaccine 20:1039-1045. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalo, R. M., D. Rodriguez, A. Garcia Sastre, J. R. Rodriguez, P. Palese, and M. Esteban. 1999. Enhanced CD8+ T-cell response to HIV-1 Env by combined immunization with influenza virus and VV recombinants. Vaccine 17:887-892. [DOI] [PubMed] [Google Scholar]
- 16.Haglund, K., I. Leiner, K. Kerksiek, L. Buonocore, E. Pamer, and J. K. Rose. 2002. Robust recall and long-term memory T-cell responses induced by prime-boost regimens with heterologous live viral vectors expressing human immunodeficiency virus type 1 Gag and Env proteins. J. Virol. 76:7506-7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanke, T. 2001. Vehicles for genetic vaccines against human immunodeficiency virus: induction of T-cell-mediated immune responses. Curr. Mol. Med. 1:123-135. [DOI] [PubMed] [Google Scholar]
- 18.Hazama, M., A. Mayumi-Aono, T. Miyazaki, S. Hinuma, and Y. Fujisawa. 1993. Intranasal immunization against herpes simplex virus infection by with a recombinant glycoprotein D fused with immunomodulating proteins, the B subunit of Escherichia coli heat-labile enterotoxin and interleukin-2. Immunology 78:643-649. [PMC free article] [PubMed] [Google Scholar]
- 19.Hill, A. V., W. Reece, P. Gothard, V. Moorthy, K. Flanagan, M. Plebanski, C. Hannan, J. T. Hu, R. Anderson, P. Degano, J. Schneider, E. Prieur, E. Sheu, and S. C. Gilbert. 2000. DNA-based vaccines for malaria: a heterologous prime-boost immunization strategy. Dev. Biol. (Basel) 104:171-179. [PubMed] [Google Scholar]
- 20.Klavinskis, L. S., C. Barnfield, L. Gao, and S. Parker. 1999. Intranasal immunization with plasmid DNA-lipid complexes elicits mucosal immunity in the female genital and rectal tracts. J. Immunol. 162:254-262. [PubMed] [Google Scholar]
- 21.Lehner, T., L. A. Bergmeier, Y. Wang, L. Tao, and E. A. Mitchell. 1999. A rational basis for mucosal vaccination against HIV infection. Immunol. Rev. 170:183-196. [DOI] [PubMed] [Google Scholar]
- 22.Letvin, N. L., J. E. Schmitz, H. L. Jordan, V. M. Hirsch, K. A. Reimann, and M. J. Kuroda. 1999. Cytotoxic T lymphocytes specific for the simian immunodeficiency virus. Immunol. Rev. 170:127-134. [DOI] [PubMed] [Google Scholar]
- 23.Li, S., M. Rodrigues, D. Rodriguez, J. R. Rodriguez, M. Esteban, P. Palese, R. S. Nussenzweig, and F. Zavala. 1993. Priming with recombinant influenza virus followed by administration of recombinant VV induces CD8+ T-cell-mediated protective immunity against malaria. Proc. Natl. Acad. Sci. USA 90:5214-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahnel, H., and A. Mayr. 2002. Experiences with immunization against orthopox viruses of humans and animals with vaccine strain MVA. Berl. Muench. Tierazetl. Wochnschr. 107:253-256. [PubMed] [Google Scholar]
- 25.Mayr, A., H. Stickl, H. K. Muller, K. Danner, and H. Singer. 1978. The smallpox vaccination strain MVA: marker, genetic structure, experience gained with parenteral vaccination and behaviour in organism with a debilitated defense mechanism. Zentbl. Bakteriol. B 167:375-390. [PubMed] [Google Scholar]
- 26.McShane, H. 2002. Prime-boost immunization strategies for infectious diseases. Curr. Opin. Mol. Ther. 4:23-27. [PubMed] [Google Scholar]
- 27.Michalek, S. M., J. H. Eldridge, R. Curtiss III, and K. Rosenthal. 2002. Use of recombinant viral vectors for mucosal immunization, p 383-385. In P. L. Ogra, M. E. Lamm, J. R. McGhee, J. Mestecky, W. Strober, and J. Bienenstock (ed.), Handbook of mucosal immunology. Academic Press Inc., New York, N.Y.
- 28.Mitchell, E. A., L. A. Bergmeier, C. Doyle, R. Brookes, L. A. Hussain, Y. Wang, and T. Lehner. 1998. Homing of mononuclear cells from iliac lymph nodes to the genital and rectal mucosa in non-human primates. Eur. J. Immunol. 28:3066-3074. [DOI] [PubMed] [Google Scholar]
- 29.Newman, M. J. 2002. Heterologous prime-boost vaccination strategies for HIV-1: augmenting cellular immune responses. Curr. Opin. Investig. Drugs 3:374-378. [PubMed] [Google Scholar]
- 30.Nilsson, C., B. Makitalo, P. Berglund, F. Bex, P. Liljestrom, G. Sutter, V. Erfle, P. Haaft, J. Heeney, G. Biberfeld, and R. Thorstensson. 2001. Enhanced simian immunodeficiency virus-specific immune responses in macaques induced by priming with recombinant Semliki Forest virus and boosting with modified VV Ankara. Vaccine 19:3526-3536. [DOI] [PubMed] [Google Scholar]
- 31.Oliveira-Ferreira, J., and C. Daniel-Ribeiro. 2001. Protective CD8+ T-cell responses against the pre-erythrocytic stages of malaria parasites: an overview. Mem. Inst. Oswaldo Cruz 96:221-227. [DOI] [PubMed] [Google Scholar]
- 32.Palese, P., F. Zavala, T. Muster, R. S. Nussenzweig, and A. Garcia Sastre. 1997. Development of novel influenza virus vaccines and vectors. J. Infect. Dis. 176:S45-49. [DOI] [PubMed] [Google Scholar]
- 33.Pearay, L. O., H. Faden, and R. Welliver. 2001. Vaccination strategies for mucosal immune responses. Clin. Microbiol. Rev. 14:430-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramirez, J. C., M. M. Gherardi, and M. Esteban. 2000. The biology of attenuated modified VV Ankara (MVA) recombinat vector in mice: fate and activation of B and T-cell immune responses in comparison with the Western Reserve (WR) strain and advantages as a vaccine. J. Virol. 74:923-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramirez, J. C., M. M. Gherardi, D. Rodriguez, and M. Esteban. 2000. Attenuated modified VV Ankara can be used as an immunizing agent under conditions of preexisting immunity to the vector. J. Virol. 74:7651-7655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramirez, J. C., D. Finke, M. Esteban, J. P. Kraehenbuhl, and H. Acha-Orbea. 2003. Tissue distribution of modified VV Ankara (MVA) after mucosal or systemic administration. Arch. Virol. 148:827-839. [DOI] [PubMed] [Google Scholar]
- 37.Ramshaw, I. A., and A. Ramsay. 2000. The prime-boost strategy: exciting prospects for improved vaccination. Trends Immunol. Today 21:163-165. [DOI] [PubMed] [Google Scholar]
- 38.Schneider, J., S. C. Gilbert, et al. 1999. Induction of CD8+ T cells with heterologous prime-boost immunization strategies. Immunol. Rev. 170:29-38. [DOI] [PubMed] [Google Scholar]
- 39.Seth, A., I. Ourmanov, M. J. Kuroda, J. E. Schmitz, M. W. Carroll, L. S. Wyatt, B. Moss, M. A. Forman, V. M. Hirsch, and N. L. Letvin. 1998. Recombinant modified VV Ankara-simian immunodeficiency virus gag pol elicits cytotoxic T lymphocytes in rhesus monkeys detected by a major histocompatibility complex class I/peptide tetramer. Proc. Natl. Acad. Sci. USA 95:10112-10116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spira, A. I., et al. 1996. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 183:215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staats, H. F., W. G. Nichols, and T. J. Palker. 1996. Mucosal immunity to HIV-1: systemic and vaginal antibody responses after intranasal immunization with the HIV-1 C4/V3 peptide T1SP10MN(A). J. Immunol. 157:462-472. [PubMed] [Google Scholar]
- 42.Veazey, R., M. A. DeMaria, L. V. Chalifoux, et al. 1998. Gastrointestinal tract as a major site of CD4+T-cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 43.Wyatt, L. S., S. S. Whitehead, K. A. Venanzi, B. R. Murphy, and B. Moss. 1999. Priming and boosting immunity to respiratory syncytial virus by recombinant replication-defective VV MVA. Vaccine 18:392-397. [DOI] [PubMed] [Google Scholar]
- 44.Zavala, F., M. Rodrigues, D. Rodriguez, J. R. Rodriguez, R. S. Nussenzweig, and M. Esteban. 2001. A striking property of recombinants poxviruses: efficient inducers of in vivo expansion of primed CD8+ T cells. Virology 280:155-159. [DOI] [PubMed] [Google Scholar]