Abstract
Pseudomonas pseudoalcaligenes KF707 possesses a biphenyl-catabolic (bph) gene cluster consisting of bphR1A1A2-(orf3)-bphA3A4BCX0X1X2X3D. The bphR1 (formerly orf0) gene product, which belongs to the GntR family, is a positive regulator for itself and bphX0X1X2X3D. Further analysis in this study revealed that a second regulator belonging to the LysR family (designated bphR2) is involved in the regulation of the bph genes in KF707. The bphR2 gene was not located near the bph gene cluster, and its product (BphR2) exhibited a high level of similarity to NahR (the naphthalene- and salicylate-catabolic regulator belonging to the LysR family) in plasmid NAH7 of Pseudomonas putida. A strain containing a disrupted bphR2 gene failed to grow on biphenyl as a sole source of carbon, and the BphD (2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoic acid hydrolase) activity was significantly reduced compared to that of wild-type strain KF707. Furthermore, the same strain exhibited extremely low transcription of bphR1, bphA1, bphC, bphX0, and bphD. However, when the bphR2 gene was provided in trans to the bphR2-disrupted strain, the transcription level of these genes was restored. These results indicate that bphR2 regulates the bph genes positively as a second regulator together with BphR1.
A number of degradative pathways of aromatic compounds, such as xylene, toluene, naphthalene, and phenol, have been found in soil bacteria. The degradative genes responsible for these pathways have been analyzed, and their respective transcriptional regulators have been also characterized. In general, the expression of these degradative genes is controlled by one or more regulatory proteins (7, 24, 28). Biphenyl-utilizing bacteria have been isolated from various environmental samples. Because these organisms are able to degrade polychlorinated biphenyls (PCBs), which are known to be some of the most serious environmental pollutants, their biphenyl-catabolic (bph) genes have been extensively studied with respect to PCB degradation (13, 14). Despite the detailed biochemical and genetic analyses of these bph genes of various soil bacteria, their regulation remains to be elucidated.
The following items have been reported to date. The bpdC1C2BADEF operon in gram-positive Rhodococcus sp. strain M5 is regulated by the two-component signal transduction system of bpdS and bpdT. Transcription of these bpd genes is induced by biphenyl. In this system, BpdS and BpdT act as a sensor histidine kinase and a response regulator, respectively (23). The bph gene clusters in Tn4371 of gram-negative Ralstonia eutropha strain A5 and Pseudomonas sp. strain KKS102 (bphSEFGA1A2A3BCDA4R) are negatively regulated by a repressor encoded by the bphS gene (25, 27). In addition to BphS, BphR has been proposed to be a regulator of the LysR family in R. eutropha A5 Tn4371, Pseudomonas sp. strain KKS102, and Sphingomonas aromaticivorans F199, but its function in these strains remains unclear (25, 27, 29). In Pseudomonas azelaica HBP1, on the other hand, the transcriptional regulation of 2-hydroxybiphenyl degradative genes was reported to be mediated by HbpR belonging to the XylR/DmpR subclass within the NtrC family (20, 21).
We previously characterized the function of bphR1 (formerly orf0) in the P. pseudoalcaligenes KF707 bph gene cluster consisting of bphR1A1A2-(orf3)-bphA3A4BCX0X1X2X3D (Fig. 1). Its product (BphR1), which belongs to the GntR family, positively regulates its own expression and bphX0X1X2X3D in the presence of biphenyl (41). In this study, we report the second regulatory bphR2 gene involved in the biphenyl catabolism of P. pseudoalcaligenes KF707.
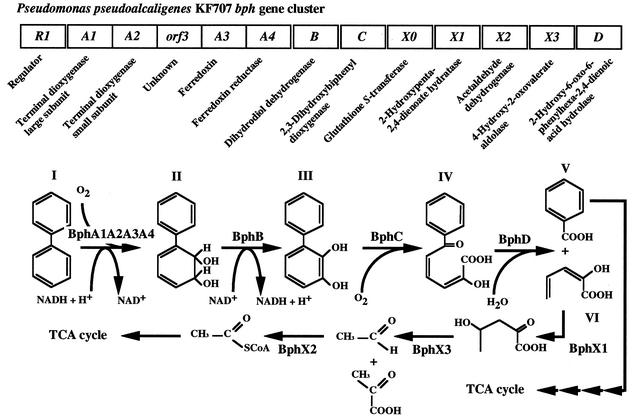
FIG. 1.
Catabolic pathway for degradation of biphenyl and organization of the bph gene cluster in P. pseudoalcaligenes KF707. Compounds: I, biphenyl; II, 2,3-dihydroxy-4-phenylhexa-4,6-diene (dihydrodiol compound); III, 2,3-dihydroxybiphenyl; IV, HOPD (the biphenyl meta-cleavage compound); V, benzoic acid; VI, 2-hydroxypenta-2,4-dienoic acid. Enzymes: BphA1A2A3A4, biphenyl dioxygenases; BphB, dihydrodiol dehydrogenase; BphC, 2,3-dihydroxybiphenyl dioxygenase; BphX0, glutathione S-transferase; BphX1, 2-hydroxypenta-2,4-dienoate hydratase; BphX2, acetaldehyde dehydrogenase (acylating); BphX3, 4-hydroxy-2-oxovalerate aldolase; BphD, 2-hydroxy-6-oxo-6-phenylhexa-2,4-dieonic acid hydrolase. The BphR1 (formerly Orf0) protein, which belongs to the GntR family, is a transcriptional regulator involved in the expression of bphR1 and bphX0X1X2X3D (41). The function of orf3 remains unclear. TCA, tricarboxylic acid.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains and plasmids used in this study are listed in Table 1. The biphenyl-utilizing strain P. pseudoalcaligenes KF707 was grown at 30°C in basal salt medium (BSM) supplemented with 0.2% (wt/vol) biphenyl as the sole source of carbon and energy as described previously (11). Strain KF707dR29 (bphR2 disruptant, bphR2::Kmr), constructed in this study, was grown in BSM supplemented with biphenyl, 0.1% (wt/vol) sodium succinate, and kanamycin (50 μg/ml). For agar plates (1.5% [wt/vol]), biphenyl was supplied as vapor in the inverted lid of a petri dish. An Escherichia coli strain was grown at 37°C in Luria-Bertani medium. The following concentrations of antibiotics were used: for E. coli, ampicillin, 50 μg/ml; chloramphenicol, 34 μg/ml; gentamicin, 20 μg/ml; and kanamycin, 50 μg/ml; for P. pseudoalcaligenes, ampicillin, 25 μg/ml; gentamicin, 20 μg/ml; and kanamycin, 50 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain/plasmid | Genotype/descriptiona | Source or reference |
|---|---|---|
| Strains | ||
| P. pseudoalcaligenes | ||
| KF707 | Bph+, wild type | 11 |
| KF707dR29 | Bph−bphR2::Kmr | This study |
| KF707dRC01 | KF707dR29 carrying pTWF17; Bph+ Ampr Kmr | This study |
| KF7095 | Bph−bphR1::Tcr | 41 |
| P. putida KF715 | Bph+, wild type | 16 |
| Burkholderia sp. strain LB400 | Bph+, wild type | 2 |
| E. coli | ||
| JM109 | Host strain for DNA manipulation | Takara Shuzo |
| S17-1 | pro thi recA hsdR; chromosomally integrated RP4-2-Tc::Mu-Km::Tn7 | 38 |
| Plasmids | ||
| NAH7 | Template DNA for amplification of nahR by PCR; nah operon, sal operon | 44 |
| pSPT18 | Cloning vector; Ampr | Roche Diagnostics |
| pUC118 | Cloning vector; Ampr | Takara Shuzo |
| pUC19 | Cloning vector; Ampr | Takara Shuzo |
| pTV118N | Expression vector; Ampr | Takara Shuzo |
| pHP45Ω-Km | Source of Kmr gene fragment | 9 |
| pMMB66EH | IncQ, tac promoter; Ampr | 10 |
| pSUP102::Tn5-B30 | pACYC184 derivative with RP4-specific Mob site and transposon Tn5-B30; Cmr Gmr Tcr | 39 |
| pSUP102::Tn5-B30ΔTcr | pSUP102::Tn5-B30 without Tcr (4.3-kb XhoI fragment); Cmr Gmr | This study |
| pTWF11 | 0.9-kb EcoRI PCR fragment in pUC118; nahR | This study |
| pTWF12 | 0.64-kb EcoRI-HindIII fragment from pTWF11 in pSPT18; nahR | This study |
| pTWF14 | 1.8-kb EcoRI fragment from KF707 in pUC19; bphR2 | This study |
| pTWF15 | 1.8-kb SmaI fragment (Kmr) from pHP45Ω-Km in blunted SacII site of pTWF14; bphR2::Kmr | This study |
| pTWF16 | 3.6-kb EcoRI fragment from pTWF15 in pSUP102::Tn5-B30ΔTcr; bphR2::Kmr Cmr Gmr | This study |
| pTWF17 | 1.8-kb EcoRI fragment from pTWF14 in pMMB66EH; bphR2 | This study |
| pTWF21 | 0.95-kb NcoI-KpnI fragment from KF707 in pTV118N; bphR2 | This study |
Bph+/−, phenotype able/unable to grow on biphenyl as the sole carbon source; Ampr, ampicillin resistance; Cmr, chloramphenicol resistance; Gmr, gentamicin resistance; Kmr, kanamycin resistance; Tcr, tetracycline resistance.
DNA manipulation and sequencing.
DNA manipulations were performed essentially as described by Sambrook et al. (30). Plasmids were prepared by the rapid alkaline procedure. The DNA fragments to be sequenced were cloned into pUC19 (Takara Shuzo). Nucleotide sequencing was carried out by the chain termination method with a DNA sequencer (Li-Cor model 4000) with Base ImagIR software, version 2.30 (Li-Cor), according to the manufacturer's instructions. The nucleotide sequences obtained were analyzed with GENETYX-MAC software, version 10.1 (Software Development).
Amplification of nahR and synthesis of RNA probe.
The nahR gene was amplified from the naphthalene/salicylate-degradative plasmid NAH7 of P. putida with the following primers. For the forward sequence, primer 5′-GATCGAATTCATGGAACTGCGTGACCTG-3′ was used. (The EcoRI site is underlined and the start codon ATG is in boldface.) For the reverse sequence, primer 5′-GATCGAATTCTCAATGCGTAAACAGGTC-3′ was used (The EcoRI site is underlined, and the stop codon [complementary] is in boldface.) Amplification of nahR was carried out for 25 cycles under the following conditions: denaturation at 94°C for 1 min, primer annealing at 52°C for 1.5 min, and primer extension at 72°C for 1.5 min. The PCR product was digested by EcoRI and inserted at the EcoRI site of pUC118 (Takara Shuzo) to generate pTWF11 (Table 1).
The nahR RNA probe labeled with digoxigenin (DIG)-11-UTP was synthesized by an in vitro transcription method with the DIG RNA labeling kit according to the manufacturer's instructions (Roche Diagnostics). A 0.64-kb EcoRI-HindIII fragment from pTWF11 was ligated to EcoRI- and HindIII-digested pSPT18 (Roche Diagnostics) to generate pTWF12. A linearized plasmid, pTWF12, with HindIII as a template was used in the in vitro transcription reaction. The synthesized RNA was analyzed by formaldehyde-denatured gel electrophoresis.
Southern blot analysis.
Southern blot analysis was performed with the DIG DNA labeling and detection kit according to the manufacturer's instruction (Roche Diagnostics). Hybridization was performed with the DIG-11-UTP-labeled nahR RNA probe and a DIG-11-dUTP-labeled BamHI-EcoRI fragment (3.9 kb) from pSUP102::Tn5-B30 (12, 38, 39).
RNA preparation and quantitative RT-PCR.
RNA was prepared for the cells grown to an A600 of 0.7 as described by Ausubel et al. (1). A reverse-transcribed reaction mixture in 25 μl contained 2 μg of total RNA, 1 μg of each forward and reverse primer, 1 mM deoxyribonucleotide triphosphate, 4 mM sodium pyrophosphate, 40 U of RNase inhibitor (Toyobo), 15 U of avian myeloblastosis virus (AMV) reverse transcriptase, and 1× AMV reverse transcriptase buffer (Promega). The primer sequences for bphR2, bphR1, bphA1, bphC, bphX0, and bphD used in the quantitative reverse transcription-PCR (RT-PCR) will be provided upon request. RT was carried out for 1 h in a thermal cycler (PC-700; Astec) at the following temperatures: 50°C for bphR1; 53°C for bphR2, bphC, bphX0, and bphD; and 60°C for bphA1. A real-time PCR was performed with Light Cycler-Fast Start DNA Master SYBR Green I in the Light Cycler Quick System 350S with Light Cycler software, version 3.5, according to the manufacturer's instructions (Roche Diagnostics). Using a LightCycler-Control kit DNA with human genomic DNA and a β-globin primer (Roche Diagnostics), a SYBR Green PCR was performed to draw a standard curve. After the standard curve was drawn, a SYBR Green PCR was performed with cDNAs obtained by the RT-PCR against the total RNAs of KF707 and its derivatives. The concentrations of Mg2+ used in the PCR were as follows: 3 mM for bphR1 and 4 mM for bphR2, bphA1, bphC, bphX0, and bphD. All reactions were conducted at least three times independently to ensure the reproducibility of the results.
Preparation of E. coli cell extracts.
E. coli strain JM109(pTWF21) was grown in Luria-Bertani medium containing ampicillin (50 μg/ml) to obtain an A600 of 0.6. The proteins were inducibly expressed by 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 4 h. The cells were then suspended in 50 mM 3-(N-morpholino) propanesulfonic acid buffer containing 5% (vol/vol) glycerol and disrupted by a French pressure cell (Ohtake). Cell debris was removed by centrifugation. The supernatant as cell extract was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the gel was stained with Coomassie brilliant blue.
Construction of a suicide plasmid for bphR2 disruption and a broad-host-range plasmid containing bphR2.
Because pUC19-bphR2 (pTWF14) has a unique SacII site in the middle of the inserted bphR2-EcoRI fragment, the plasmid was digested by SacII and then blunt ended with T4 DNA polymerase (Toyobo). The fragment obtained was ligated to a kanamycin resistance (Kmr) gene (ca. 1.8 kb) removed from pHP45Ω-Km by SmaI digestion (9). The resultant plasmid (pTWF15) was digested with EcoRI, and a 3.6-kb EcoRI fragment (bphR2::Kmr) was purified and inserted into the EcoRI site of pSUP102::Tn5-B30ΔTcr to generate pTWF16. This plasmid, which contains bphR2 disrupted by the Kmr gene, was transformed into E. coli S17-1 (38).
An EcoRI fragment (ca. 1.8 kb) from pTWF14, which contains the bphR2 gene, was ligated to an EcoRI-digested broad-host-range plasmid, pMMB66EH, to get pTWF17, in which the bphR2 gene was confirmed to be located downstream of the tac promoter (10).
BphD enzyme assay.
Strain KF707 and the derivatives were pregrown in BSM supplemented with biphenyl or succinate for 24 h and subsequently diluted 1/100 in the same fresh medium and grown to the stationary phase. Cells were disrupted with a French pressure cell and centrifuged at 14,700 × g for 30 min; the supernatant was used as a crude extract. BphD (2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoic acid [HOPD] hydrolase) activities were assayed as described previously (11).
Nucleotide sequence accession number.
The nucleotide sequence reported in this paper has been submitted to the DDBJ/EMBL/GenBank database under accession no. AB088347, D85852, M83673, D85853, and D85851.
RESULTS
Southern blot analysis of the nahR-like genes (bphR2) in biphenyl-degrading strains.
The nahR gene has been found in the intrinsic plasmid NAH7 and the chromosome of naphthalene/salicylate-utilizing Pseudomonas strains, and its product has been assigned to the LysR family (3-5, 15, 32, 33, 36, 37, 42-44). The functions of NahR and its transcriptional regulation mechanism have been analyzed in detail (6, 19, 31, 32, 34, 35).
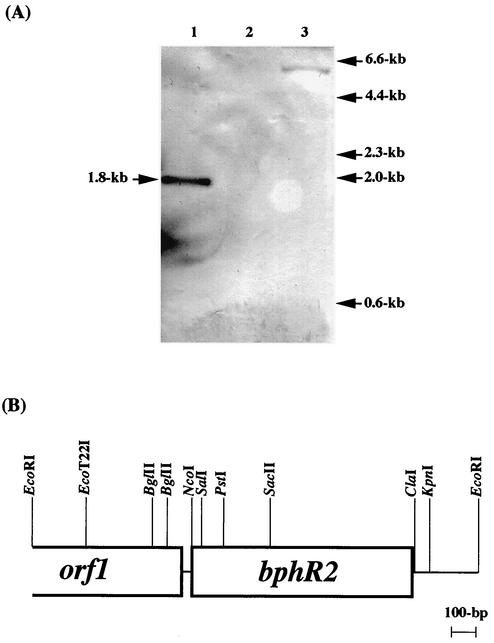
It was previously reported that a homolog nearly identical to nahR (previously termed “bphR” but renamed “bphR2” in this study) exists in the chromosome of P. pseudoalcaligenes KF707 (24). Since then, no further study of bphR2 has been reported. Therefore, we first cloned the bphR2 gene from KF707. We performed Southern blot hybridization with a DIG-labeled nahR RNA probe and confirmed that the bphR2 gene is present on the 1.8-kb EcoRI DNA fragment in KF707 (Fig. 2A, lane 1). On the other hand, no hybridization signal was detected in Burkholderia (formerly Pseudomonas) sp. strain LB400, which possesses a bph gene cluster nearly identical to that of KF707 (Fig. 2A, lane 2) (2, 8, 17, 18, 22). A weak signal (ca. 6-kb) was also detected in P. putida KF715, which possesses a bph gene cluster nearly identical to that of KF707, except that the bphX region was deleted (Fig. 2A, lane 3) (16, 26).
FIG. 2.
(A) Southern blot analysis of bphR2 in the chromosomal DNA of KF707 and other biphenyl-degrading strains. The probe used was an antisense RNA of nahR labeled with DIG-11-UTP by the in vitro transcription method. Lanes: 1, P. pseudoalcaligenes KF707; 2. Burkholderia (formerly Pseudomonas) sp. strain LB400; 3, P. putida KF715. The arrows on the right indicate molecular sizes. (B) Physical maps of the bphR2 gene and adjacent region in KF707. E, EcoRI; K, KpnI; N, NcoI; S, SacII. The truncated orf1 product has similarity to A. tumefaciens IS-3-like transposase (GenBank accession no. U96413).
Cloning and expression of bphR2.
The DNA fragments of ca. 1.8 kb detected in the Southern blot analysis described above were purified and ligated to pUC19, which was then transformed into E. coli. Using the same probe, we screened two E. coli clones carrying the bphR2 gene (data not shown). The 1.8-kb EcoRI fragment thus obtained was sequenced. One open reading frame (ORF) of 903 bp was found (accession no. AB088347). The G+C content of this ORF was 55.5%, which is lower than the 61.1% found with other bph genes of KF707. A purine-rich region of AGCGAGG, which could be a putative ribosome-binding site, was identified at approximately 8 nucleotides upstream of the start codon. This ORF product corresponds to a polypeptide of 300 amino acids with a predicted molecular mass of 33,881 Da and has a high similarity (81.3%) to NahR (accession no. J04233). To confirm whether this ORF is translated with the predicted size, the cell extract from the recombinant E. coli strain carrying pTWF21 (which contains bphR2) was subjected to SDS-PAGE. Expression of the ORF (BphR2) yielded a peptide with a molecular mass of 33 kDa (Fig. 3A, lane 2). This value was in agreement with the predicted molecular mass. The amino acids responsible for the functional domains in NahR were completely conserved in BphR2, except that Ile-116 in NahR is substituted for with Met in BphR2 (6, 34-36). In addition, BphR2 possesses 45 basic amino acids and 33 acidic ones, indicating that BphR2 is a basic protein. A helix-turn-helix motif was predicted in the amino-terminal DNA-binding domain of BphR2. These characteristics strongly indicate that BphR2 is a regulatory protein belonging to the LysR family.
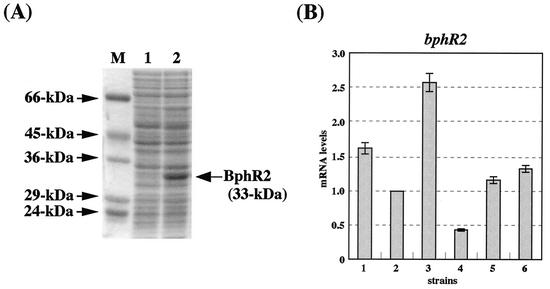
FIG. 3.
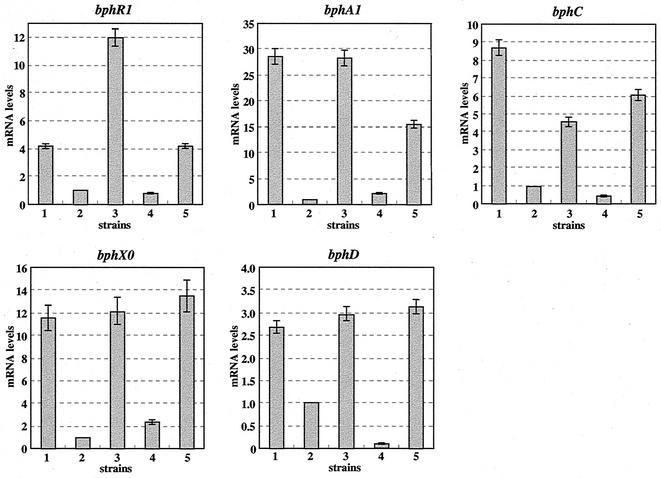
Expression of BphR2 in E. coli and quantitative RT-PCR analysis of bphR2 transcripts. (A) Supernatants of crude extracts were subjected to SDS-PAGE. Lanes: 1, IPTG-uninduced JM109(pTWF21) cells; 2, IPTG-induced JM109(pTWF21) cells; M, molecular mass standards. (B) Quantitative RT-PCR analysis of bphR2 mRNA. The mRNA levels of bphR2 in KF707 and its derivatives are normalized to that of succinate-grown KF707 cells (level of 1.0). Strains: 1, biphenyl-grown KF707 cells; 2, succinate-grown KF707 cells; 3, biphenyl-succinate-grown KF707 cells; 4, biphenyl-succinate-grown KF707dR29 cells (bphR2 disruptant); 5, biphenyl-succinate-grown KF707dRC01 cells (KF707dR29 carrying pMMB66EH-bphR2 in trans [pTWF17]); 6, biphenyl-succinate-grown KF7095 cells (bphR1 disruptant). The error bar represents the standard deviation calculated from at least triplicate assays.
The transcription of bphR2 was investigated by normal RT-PCR against the total RNA of KF707. An RT-PCR product corresponding to the size of bphR2 (ca. 0.9 kb) was detected when KF707 was grown on either biphenyl or succinate (data not shown). The quantitative RT-PCR analysis revealed that the bphR2 mRNA levels in KF707 cells grown on biphenyl plus succinate are 2.5-fold higher than those in the cells grown on succinate alone (Fig. 3B), indicating that the transcription of the bphR2 gene is inducibly enhanced in the presence of biphenyl.
Disruption and complementation of bphR2.
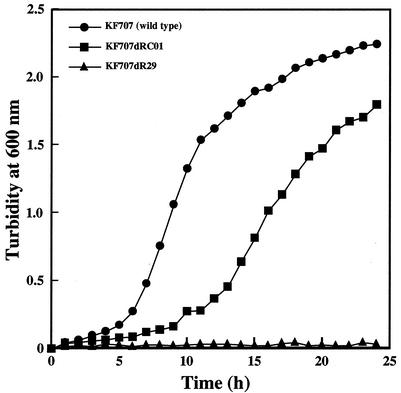
In order to investigate the function of bphR2 in KF707, we disrupted the gene by insertion of the Kmr gene. For this purpose, a suicide plasmid, pTWF16, in which the Kmr gene was inserted within bphR2, was constructed as described in Materials and Methods. E. coli S17-1 cells carrying pTWF16 were filter mated with KF707. This suicide plasmid (pTWF16) cannot replicate in Pseudomonas strains. Therefore, the single-crossover recombinants were first screened on BSM agar plates supplemented with succinate, kanamycin, and gentamicin. The single-crossover recombinants were repeatedly subcultured to obtain the double-crossover recombinants (bphR2 disruptants). These were examined for growth on BSM plates without or supplemented with kanamycin and gentamicin. The loss of the vector-borne Gmr gene was confirmed by Southern blot analysis (data not shown). The bphR2 disruptant, designated KF707dR29, failed to grow on biphenyl (Fig. 4). We then introduced pTWF17 carrying the bphR2 gene into KF707dR29. The resultant strain, KF707dRC01, restored the ability to grow on biphenyl, albeit at a reduced growth rate compared to that of strain KF707 (Fig. 4). This strain exhibited almost the same transcriptional level of bphR2 as the biphenyl-grown strain KF707 did (Fig. 3B). These observations revealed that bphR2 acts in trans and that its product is absolutely involved in biphenyl catabolism.
FIG. 4.
Acquisition of growth capability of KF707 bphR2 disruptant with pTWF17. Cells of strains KF707 (wild type), KF707dR29 (bphR2 disruptant), and KF707dRC01 (KF707dR29 carrying pMMB66EH-bphR2 [pTWF17]) were cultured on BSM supplemented with biphenyl as the sole source of carbon and with antibiotics when necessary. We confirmed by colony PCR analysis that KF707dRC01 contains the bphR2::Kmr fragment (ca. 2.7 kb) derived from its chromosome and a complete bphR2 gene (0.9 kb) fragment from pTWF17 (data not shown).
Involvement of BphR2 in the expression of BphD.
We first investigated how BphR2 is involved in the expression of the bphD gene. Previously, we showed that BphR1 (formerly Orf0) is absolutely required for the expression of bphD (encoding HOPD hydrolase) (41). The BphD activity in KF707dR29 was also very low, although the activity was a little higher than that of KF7095 (Table 2). The expression of BphD is induced in the presence of biphenyl (41), but the BphD activity in KF707dR29 cells grown on biphenyl plus succinate was much lower than those of KF707 cells grown on biphenyl plus succinate or succinate alone (Table 2). On the other hand, the BphD activity of KF707dRC01 expressing bphR2 in trans was restored to the level of KF707 biphenyl-grown cells (Table 2). These results revealed that both bphR1 and bphR2 are absolutely required for the expression of bphD.
TABLE 2.
BphD activities of KF707 bphR2 disruptant with or without pTWF17
| Strain | Carbon sourcea | Activity (U/mg of protein)b |
|---|---|---|
| KF707 | Bph | 2.82 ± 0.02 |
| KF707 | Suc | 1.06 ± 0.08 |
| KF707 | Bph and Suc | 7.06 ± 0.01 |
| KF707dR29 | Bph and Suc | 0.27 ± 0.04 |
| KF707dRC01(pTWF17) | Bph and Suc | 3.47 ± 0.05 |
| KF7095 | Bph and Suc | 0.02 ± 0.00 |
Bph, biphenyl; Suc, succinate.
The BphD enzyme activity data represent the means of triplicate assays.
Expression of other bph genes in the bphR2-disrupted strain.
In order to investigate how the disruption of bphR2 affects the expression of bph genes, quantitative RT-PCR analyses were performed. For this purpose, the mRNAs of the bphR1, bphA1, bphC, bphX0, and bphD genes were measured because the transcriptional initiation sites were found upstream of these genes, except for bphC (40, 41). The amounts of mRNA of bphR1, bphA1, bphC, bphX0, and bphD in the bphR2 disruptant (KF707dR29) cells grown on biphenyl plus succinate were much lower than that of KF707 (Fig. 5). In KF707dRC01 cells (KF707dR29 carrying bphR2 in trans), however, the mRNA levels of these bph genes (especially bphX0 and bphD) were restored to almost the same level as those in KF707 cells (grown on biphenyl alone or biphenyl plus succinate). These results revealed that bphR2 is also involved in the transcription of bphR1, bphA1, bphC, and bphX0, as well as that of bphD.
FIG. 5.
Quantitative RT-PCR analyses of bph genes in KF707. For definitions, refer to the legend to Fig. 3B.
In the quantitative RT-PCR analysis (Fig. 5), the mRNA levels of bphR1 (4-fold), bphA1 (30-fold), bphC (9-fold), bphX0 (12-fold), and bphD (2.5-fold) were all enhanced in the presence of biphenyl, as indicated in parentheses. This result suggests that BphR2 acts as an activator for the transcription of the bph genes in the presence of biphenyl or its metabolites.
DISCUSSION
In this study, we first confirmed the presence of the bphR2 gene in the chromosome of P. pseudoalcaligenes KF707 (Fig. 2A), although its location remains unknown. The BphR2 protein has a high level of similarity to NahR, belonging to the LysR-type transcriptional regulatory family. The quantitative RT-PCR analyses permitted us to measure the bphR2 mRNA, revealing that transcription is enhanced in the presence of biphenyl (Fig. 3B).
We have previously reported that the bphR1-disrupted strain KF7095 accumulated large amounts of biphenyl ring meta-cleavage yellow compounds (HOPD). This is due to the lack of BphD activity; hence, BphR1 is absolutely required to express the bphD. In addition, the same protein regulates the expression of bphR1 and bphX0X1X2X3 (41). On the other hand, the bphR2-disrupted strain KF707dR29 did not produce HOPD from biphenyl and hardly transcribed bphR1, bphA1, bphC, bphX0, and bphD (Table 2 and Fig. 5). The poor mRNA levels of bphA1 and bphC in KF707dR29 indicate the poor transcription of bphA1A2-(orf3)-bphA3A4BC, because the bphA1A2-(orf3)-bphA3A4BC genes are polycistronically transcribed (41). However, introduction of bphR2 in trans allowed KF707dR29 to restore the ability to grow on biphenyl, where the bphR1, bphA1, bphC, bphX0, and bphD genes were fully expressed (Table 2 and Fig. 4 and 5). These results indicate that BphR2 is also absolutely required for the expression of bph genes together with BphR1.
Because BphR1 is necessary for the expression of bphD as described previously (41), low expression of bphD might be due to the extremely low expression of bphR1 caused by the disruption of bphR2 in KF707dR29 (Table 2 and Fig. 5). The reasons for low levels of bphR1 expression in KF707dR29 can be considered as follows. (i) BphR1 positively regulates its own expression in the presence of HOPD (41). (ii) KF707dR29 hardly transcribes bphA1A2-(orf3)-bphA3A4BC genes, and, therefore, the level of production of HOPD from biphenyl is very low (if any is produced). (iii) Finally, poor production of HOPD results in the poor expression of bphR1. The low level of expression of bphR1 further leads to poor expression of bphD. In contrast to the fact that bphR1 is hardly transcribed in KF707dR29, bphR2 was transcribed in bphR1-disrupted strain KF7095 (Fig. 3B and 5). Based on the facts that the transcription of bphA1A2-(orf3)-bphA3A4BC is bphR1 independent and that bphR1 and bphA1 are not cotranscribed (41), it is likely that the transcription of bphA1A2-(orf3)-bphA3A4BC is positively regulated by bphR2, but not by bphR1.
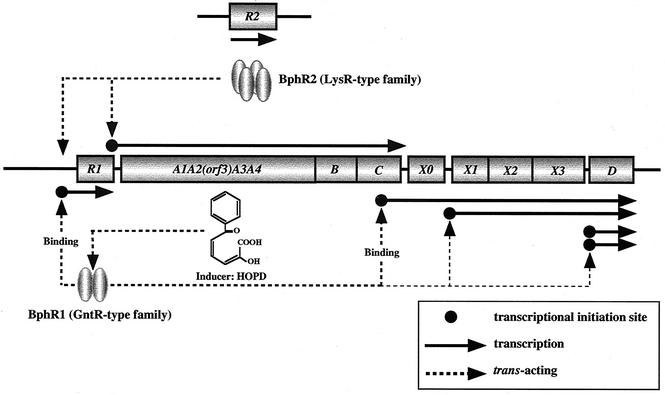
The results obtained in the previous and present studies allow us to propose the mechanism of transcription of the KF707-bph gene clusters as shown in Fig. 6. At least six transcriptional initiation sites exist upstream of bphR1, bphA1, bphX0, bphX1, and bphD, including two sites upstream of bphD (thereby six transcripts) (40, 41). The bphA1A2-(orf3)-A3A4BC genes are polycistronically transcribed. The bphC and bphX0 genes, as well as the bphR1 and bphA1 genes, are independently transcribed (41). The BphR1 protein, belonging to the GntR-type family, is absolutely required for the expression of bphR1 itself and bphX0X1X2X3D. This protein functions as a dimer (T. Watanabe et al., unpublished data) and directly binds to the bphR1 operator region. This binding is greatly enhanced by HOPD as an inducer (41). BphR1 also binds to the upstream region of bphX0 (Watanabe et al., unpublished). Although the location of bphR2 in the chromosome of KF707 remains unknown, its product (BphR2), which belongs to the LysR-type family, acts as a positive regulator to activate the transcription of bphA1A2-(orf3)-bphA3A4BC. Thus, the P. pseudoalcaligenes KF707-bph genes are likely to be regulated by two regulatory systems: (i) bphR2-dependent transcription for bphA1A2-(orf3)-bphA3A4BC; and (ii) bphR1-dependent transcription for bphR1 itself, bphX0X1X2X3, and bphD. In these systems, BphR2 first activates the transcription of bphA1A2-(orf3)-bphA3A4BC to convert biphenyl to HOPD, which binds to BphR1 to activate this protein. The activated BphR1 then promotes the transcription of bphX0X1X2X3 and bphD.
FIG. 6.
Proposed transcriptional regulation of the bph genes in P. pseudoalcaligenes KF707. See the text for details. The relationship indicated by the arrow linking BphR2 with the bphR1 promoter has not been confirmed, but we cannot rule out the possibility that the BphR2 protein regulates the transcription of bphR1.
A recent paper from our laboratory revealed that the large bph-sal conjugative element coding for the biphenyl and salicylate catabolisms can be transferred among soil bacteria (26). If this is the case in KF707, the bph genes could be foreign genes derived from other strains. It should be interesting to investigate how such foreign bph genes are regulated in various hosts.
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. D. Seidman, J. A. Smith, and K. Struhl. 1994. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 2.Bopp, L. H. 1986. Degradation of highly chlorinated PCBs by Pseudomonas strain LB400. J. Ind. Microbiol. 1:23-29. [Google Scholar]
- 3.Bosch, R., E. R. Moore, E. Garcia-Valdés, and D. H. Pieper. 1999. NahW, a novel, inducible salicylate hydroxylase involved in mineralization of naphthalene by Pseudomonas stutzeri AN10. J. Bacteriol. 181:2315-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosch, R., E. Garcia-Valdes, and E. R. Moore. 1999. Genetic characterization and evolutionary implications of a chromosomally encoded naphthalene-degradation upper pathway from Pseudomonas stutzeri AN10. Gene 236:149-157. [DOI] [PubMed] [Google Scholar]
- 5.Bosch, R., E. Garcia-Valdes, and E. R. Moore. 2000. Complete nucleotide sequence and evolutionary significance of a chromosomally encoded naphthalene-degradation lower pathway from Pseudomonas stutzeri AN10. Gene 245:65-74. [DOI] [PubMed] [Google Scholar]
- 6.Cebolla, A., C. Sousa, and V. de Lorenzo. 1997. Effector specificity mutants of the transcriptional activator NahR of naphthalene degrading Pseudomonas define protein sites involved in binding of aromatic inducers. J. Biol. Chem. 272:3986-3992. [DOI] [PubMed] [Google Scholar]
- 7.Diaz, E., and M. A. Prieto. 2000. Bacterial promoters triggering biodegradation of aromatic pollutants. Curr. Opin. Biotechnol. 11:467-475. [DOI] [PubMed] [Google Scholar]
- 8.Erickson, B. D., and F. J. Mondello. 1992. Nucleotide sequencing and transcriptional mapping of the genes encoding biphenyl dioxygenase, a multicomponent polychlorinated-biphenyl-degrading enzyme in Pseudomonas strain LB400. J. Bacteriol. 174:2903-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fellay, R., J. Frey, and H. Krisch. 1987. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene 52:147-154. [DOI] [PubMed] [Google Scholar]
- 10.Furste, J. P., W. Pansegrau, R. Frank, H. Blocker, P. Scholz, M. Bagdasarian, and E. Lanka. 1986. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene 48:119-131. [DOI] [PubMed] [Google Scholar]
- 11.Furukawa, K., and T. Miyazaki. 1986. Cloning of a gene cluster encoding biphenyl and chlorobiphenyl degradation in Pseudomonas pseudoalcaligenes. J. Bacteriol. 166:392-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furukawa, K., S. Hayashida, and K. Taira. 1991. Gene-specific transposon mutagenesis of the biphenyl/polychlorinated biphenyl-degradation-controlling bph operon in soil bacteria. Gene 98:21-28. [DOI] [PubMed] [Google Scholar]
- 13.Furukawa, K. 1994. Molecular genetics and evolutionary relationship of PCB-degrading bacteria. Biodegradation 5:289-300. [DOI] [PubMed] [Google Scholar]
- 14.Furukawa, K. 2000. Biochemical and genetic bases of microbial degradation of polychlorinated biphenyls (PCBs). J. Gen. Appl. Microbiol. 46:283-296. [DOI] [PubMed] [Google Scholar]
- 15.Harayama, S., M. Rekik, A. Wasserfallen, and A. Bairoch. 1987. Evolutionary relationships between catabolic pathways for aromatics: conservation of gene order and nucleotide sequences of catechol oxidation genes of pWW0 and NAH7 plasmids. Mol. Gen. Genet. 210:241-247. [DOI] [PubMed] [Google Scholar]
- 16.Hayase, N., K. Taira, and K. Furukawa. 1990. Pseudomonas putida KF715 bphABCD operon encoding biphenyl and polychlorinated biphenyl degradation: cloning, analysis, and expression in soil bacteria. J. Bacteriol. 172:1160-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofer, B., L. D. Eltis, D. N. Dowling, and K. N. Timmis. 1993. Genetic analysis of a Pseudomonas locus encoding a pathway for biphenyl/polychlorinated biphenyl degradation. Gene 130:47-55. [DOI] [PubMed] [Google Scholar]
- 18.Hofer, B., S. Backhaus, and K. N. Timmis. 1994. The biphenyl/polychlorinated biphenyl-degradation locus (bph) of Pseudomonas sp. LB400 encodes four additional metabolic enzymes. Gene 144:9-16. [DOI] [PubMed] [Google Scholar]
- 19.Huang, J. Z., and M. A. Schell. 1991. In vivo interactions of the NahR transcriptional activator with its target sequences. Inducer-mediated changes resulting in transcription activation. J. Biol. Chem. 266:10830-10838. [PubMed] [Google Scholar]
- 20.Jaspers, M. C., M. Sturme, and J. R. van Der Meer. 2001. Unusual location of two nearby pairs of upstream activating sequences for HbpR, the main regulatory protein for the 2-hydroxybiphenyl degradation pathway of Pseudomonas azelaica HBP1. Microbiology 147:2183-2194. [DOI] [PubMed] [Google Scholar]
- 21.Jaspers, M. C. M., W. A. Suske, A. Schmid, D. A. M. Goslings, H.-P. E. Kohler, and J. R. van Der Meer. 2000. HbpR, a new member of the XylR/DmpR subclass within the NtrC family of bacterial transcriptional activators, regulates expression of 2-hydroxybiphenyl metabolism in Pseudomonas azelaica HBP1. J. Bacteriol. 182:405-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura, N., A. Nishi, M. Goto, and K. Furukawa. 1997. Functional analyses of a variety of chimeric dioxygenases constructed from two biphenyl dioxygenases that are similar structurally but different functionally. J. Bacteriol. 179:3936-3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labbé, D., J. Garnon, and P. C. K. Lau. 1997. Characterization of the genes encoding a receptor-like histidine kinase and a cognate response regulator from a biphenyl/polychlorobiphenyl-degrading bacterium, Rhodococcus sp. strain M5. J. Bacteriol. 179:2772-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lau, P. C. K., Y. Wang, D. Labbé, H. Bergeron, and J. Garnon. 1996. Two-component signal transduction systems regulating toluene and biphenyl-polychlorinated-biphenyl degradations in a soil pseudomonad and an actinomycete, p. 176-187. In T. Nakazawa, K. Furukawa, D. Haas, and S. Silver (ed.), Molecular biology of pseudomonads. ASM Press, Washington, D.C.
- 25.Mouz, S., C. Merlin, D. Springael, and A. Toussaint. 1999. A GntR-like negative regulator of the biphenyl degradation genes of the transposon Tn4371. Mol. Gen. Genet. 262:790-799. [DOI] [PubMed] [Google Scholar]
- 26.Nishi, A., K. Tominaga, and K. Furukawa. 2000. A 90-kilobase conjugative chromosomal element coding for biphenyl and salicylate catabolism in Pseudomonas putida KF715. J. Bacteriol. 182:1949-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohtsubo, Y., M. Delawary, K. Kimbara, M. Takagi, A. Ohta, and Y. Nagata. 2001. BphS, a key transcriptional regulator of bph genes involved in polychlorinated biphenyl/biphenyl degradation in Pseudomonas sp. KKS102. J. Biol. Chem. 276:36146-36154. [DOI] [PubMed] [Google Scholar]
- 28.Parsek, M. R., S. M. McFall, and A. M. Chakrabarty. 1996. Evolution of regulatory systems of biodegradative pathways, p. 135-152. In T. Nakazawa, K. Furukawa, D. Haas, and S. Silver (ed.), Molecular biology of pseudomonads. ASM Press, Washington, D.C.
- 29.Romine, M. F., L. C. Stillwell, K.-K. Wong, S. J. Thurston, E. C. Sisk, C. Sensen, T. Gaasterland, J. K. Fredrickson, and J. D. Saffer. 1999. Complete sequence of a 184-kilobase catabolic plasmid from Sphingomonas aromaticivorans F199. J. Bacteriol. 181:1585-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 31.Schell, M. A. 1985. Transcriptional control of the nah and sal hydrocarbon-degradation operons by the nahR gene product. Gene 36:301-309. [DOI] [PubMed] [Google Scholar]
- 32.Schell, M. A. 1986. Homology between nucleotide sequences of promoter regions of nah and sal operons of NAH7 plasmid of Pseudomonas putida. Proc. Natl. Acad. Sci. USA 83:369-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schell, M. A., and P. E. Wender. 1986. Identification of the nahR gene product and nucleotide sequences required for its activation of the sal operon. J. Bacteriol. 166:9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schell, M. A., and M. Sukordhaman. 1989. Evidence that the transcription activator encoded by the Pseudomonas putida nahR gene is evolutionarily related to the transcription activators encoded by the Rhizobium nodD genes. J. Bacteriol. 171:1952-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schell, M. A., P. H. Brown, and S. Raju. 1990. Use of saturation mutagenesis to localize probable functional domains in the NahR protein, a LysR-type transcription activator. J. Biol. Chem. 265:3844-3850. [PubMed] [Google Scholar]
- 36.Schell, M. A. 1993. Molecular biology of the LysR family of transcriptional regulators. Annu. Rev. Microbiol. 47:597-626. [DOI] [PubMed] [Google Scholar]
- 37.Simon, M. J., T. D. Osslund, R. Saunders, B. D. Ensley, S. Suggs, A. Harcourt, W. C. Suen, D. L. Cruden, D. T. Gibson, and G. J. Zylstra. 1993. Sequences of genes encoding naphthalene dioxygenase in Pseudomonas putida strains G7 and NCIB 9816-4. Gene 127:31-37. [DOI] [PubMed] [Google Scholar]
- 38.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vitro genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 39.Simon, R., J. Quandt, and W. Klipp. 1989. New derivatives of transposon Tn5 suitable for mobilization of replicons, generation of operon fusions and induction of genes in gram-negative bacteria. Gene 80:161-169. [DOI] [PubMed] [Google Scholar]
- 40.Taira, K., J. Hirose, S. Hayashida, and K. Furukawa. 1992. Analysis of bph operon from the polychlorinated biphenyl-degrading strain of Pseudomonas pseudoalcaligenes KF707. J. Biol. Chem. 267:4844-4853. [PubMed] [Google Scholar]
- 41.Watanabe, T., R. Inoue, N. Kimura, and K. Furukawa. 2000. Versatile transcription of biphenyl catabolic bph operon in Pseudomonas pseudoalcaligenes KF707. J. Biol. Chem. 275:31016-31023. [DOI] [PubMed] [Google Scholar]
- 42.Yen, K. M., and I. C. Gunsalus. 1982. Plasmid gene organization: naphthalene/salicylate oxidation. Proc. Natl. Acad. Sci. USA 79:874-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yen, K.-M., and I. C. Gunsalus. 1985. Regulation of naphthalene catabolic genes of plasmid NAH7. J. Bacteriol. 162:1008-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.You, I.-S., D. Ghosal, and I. C. Gunsalus. 1988. Nucleotide sequence of plasmid NAH7 gene nahR and DNA binding of the nahR product. J. Bacteriol. 170:5409-5415. [DOI] [PMC free article] [PubMed] [Google Scholar]