Abstract
Salmonella enterica has two antigenically distinct flagellin genes, fliC and fljB, that are alternatively expressed. The fljA gene is cotranscribed with fljB and encodes a protein that has been characterized as a transcriptional repressor of the unlinked fliC gene when FljB is expressed. In this study we report genetic evidence that FljA prevents the production of FliC protein through an interaction with the 5′-untranslated region of the fliC mRNA transcript. Studies with operon and gene fusions, Western analyses, and T2 RNase protection assays were performed for strains with the fljBA promoter locked in either the on or the off orientation. β-Galactosidase assays of fliC transcriptional and translational fusions to the lac operon demonstrated that while FljA inhibits fliC transcription fivefold in the fljBAON orientation, it has a 200-fold effect on both fliC transcription and translation, indicating that the FljA inhibitor might act at both the transcriptional and translational level. T2 RNase protection assays also demonstrated a fivefold decrease in fliC transcript levels for cells locked in the fljBAON orientation compared to those in the fljBAOFF orientation, and an eightfold decrease in FliC protein levels was observed by Western analysis. This reduction in FliC protein levels is greater than the decrease observed for the transcript. These results are consistent with a new model whereby FljA inhibits FliC expression by an attenuation or translational control mechanism.
Bacterial flagella facilitate the mobility of the organism within its environment, allowing it to move towards attractants and away from repellants (reviewed in reference 4). Salmonella enterica serovar Typhimurium has approximately 6 to 10 flagella that are peritrichously arranged around the cell. The individual flagella are composed of three distinct substructures: the basal body, a transmembrane motor; a hook that links the motor and the filament; and the filament that acts as a propeller (reviewed in references 1 and 36). The filament is approximately 10 μm in length and composed of approximately 20,000 subunits of flagellin protein, either FliC or FljB. During assembly, structural subunit proteins are secreted by a flagellum-specific type III secretion system (36a), assembled at the base of the flagellum, through the elongating structure, and added onto the distal tip (9, 24).
Flagellum biogenesis is a highly ordered process whereby gene expression closely parallels expression and assembly of the subunit proteins (5, 27). The expression of more than 50 genes is required for the assembly, function, and maintenance of these structures. The promoters of the flagellar regulon can be organized into three classes that determine their temporal expression. The class 1 promoter directs transcription of the flhDC master operon and includes six known transcriptional start sites that respond to different environmental signals (52). FlhD and FlhC form a heterotetrameric complex that is a transcriptional activator for σ70-dependent transcription of the class 2 promoters (3, 34, 35). Class 2 promoters mediate the transcription of genes required for the structure and assembly of the hook-basal body (HBB) in addition to flagellum-specific sigma factor σ28, FliA (41), and its cognate anti-sigma factor FlgM (42). Class 3 promoters require σ28-RNA polymerase for their transcription (21). FlgM has been found to associate with σ28 and prevent class 3 transcription until completion of the intermediate HBB structure (13, 29). Upon HBB completion, FlgM is secreted outside of the cell, resulting in σ28-dependent transcription from the class 3 promoters (22, 30) which direct transcription of the hook-associated genes, flagellin genes, and genes whose products are required for chemotaxis and flagellar rotation.
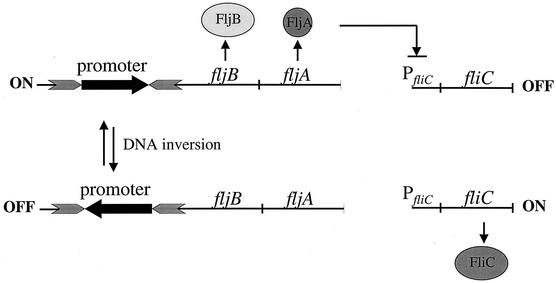
S. enterica alternately expresses two different flagellar filament proteins, FljB and FliC, in a process known as flagellar phase variation (2) (Fig. 1). The molecular mechanism mediating flagellar phase variation occurs by a site-specific DNA inversion event in the chromosome (reviewed in reference 20). The promoter for the FljB flagellin protein is flanked by the recombination sites hixL and hixR (Fig. 1). The Hin recombinase, in conjunction with the recombination enhancer proteins Fis (factor for inversion stimulation) and HU, mediates a reversible recombination reaction between the hix sites, resulting in the inversion of the DNA segment containing the fljBA promoter. In one orientation the fljBA promoter directs transcription of the fljBA operon and FljB flagellin is produced. The fljA gene is cotranscribed with fljB and encodes a transcriptional inhibitor of the unlinked fliC gene (11, 31, 33, 43, 46, 49). In the alternate orientation, the fljBA operon is not expressed and transcription of the fliC gene ensues.
FIG. 1.
A schematic representation of flagellar phase variation in S. enterica. The promoter for the fljBA operon is located within an invertible DNA segment whereby inversion of the promoter is mediated by the Hin recombinase. In one orientation, the fljBA operon is expressed and FljB flagellin is produced along with FljA, repressor of the unlinked fliC gene that encodes FliC flagellin. In the opposite orientation, the fljB gene is not expressed, nor is the repressor FljA, thus allowing transcription of the fliC gene.
The FliC and FljB flagellin proteins themselves are identical for the first 71 amino acids and last 46 amino acids, but surface-exposed amino acids in the middle are divergent, resulting in distinct antigenicities (40). S. enterica alternates between expressions of these proteins at a rate of 10−3 to 10−5 per cell generation (14, 48). In fact, most Salmonella phase vary, at similar rates, between expression of two different flagellin genes (10). As with FliC and FljB, there is a great deal of variation in the central portion of these flagellins, while the amino- and carboxy-terminal domains are highly conserved (51). Flagellin protein itself is a potent antigen that stimulates the innate immune response in many plants and animals. Recently, Hayashi et al. (19) showed that stimulation of the TLR5 toll-like receptor by bacterial flagellin protein, including the Salmonella FliC protein, resulted in the mobilization of the nuclear factor NF-κB and stimulation of tumor necrosis factor alpha production. In addition, most Salmonella-specific CD4+ T lymphocytes generated in response to a Salmonella infection have been shown to be directed at flagellin epitopes (6). Although flagellar phase variation has been postulated to play a role in the pathogenesis of the organism by providing a mechanism for the bacteria to temporarily avoid cellular immunity (10, 25), its role in S. enterica pathogenesis is not understood.
In contrast to our limited understanding of the biological significance of phase variation, the molecular mechanisms mediating this phenomenon have been well elucidated. For example, current dogma suggests that the FljA protein, encoded by the fljA gene downstream of fljB, is a transcriptional repressor of the unlinked fliC gene when FljB is expressed (16, 36, 47). In this study we provide evidence to support a mechanism by which FljA prevents production of FliC protein at the posttranscriptional level. Our results indicate that in addition to inhibiting fliC transcription, FljA regulates fliC translation. Because previous studies have identified mutations in the 5′-untranslated region (UTR) of the fliC transcript that bypass FljA regulation, we propose a model in which FljA regulates both fliC transcription and translation via interactions with the 5′-UTR of the transcript.
MATERIALS AND METHODS
Strains.
The bacterial strains used in this study are presented in Table 1. Unless noted otherwise, all strains were constructed for this work.
TABLE 1.
List of strains
| Strain | Genotype | Reference or sourcea |
|---|---|---|
| TH1059 | IS200-5548::Tn10dCm | |
| TH1077 | fliC5050::MudJ | 12 |
| TH1208 | fliC5050::MudA | 12 |
| TH6594 | fliC5469::MudK | |
| TH6596 | fliC5469::MudCm | |
| TH6595 | fliC5469::MudB | |
| TH331 | proAB47 pyrB64/F′ 128 zzf-1066::MudA | J. Roth |
| TH2748 | thrA49 leuBCD39 ara-7/F+zzf-6820::MudCm | T. Elliot |
| TH1123 | nadA56/F′ 152 zzf-1093::MudB | J. Roth |
| TH4702 | LT2 pKD46 | |
| TH5855 | ΔfljA5576::FRT-Km-FRT | |
| TH5361 | ΔfliA5648::FRT-Km-FRT | |
| TH5947 | fliC5050::MudJ Δhin-5717::FRT-Cm-FRT | |
| TH5951 | fliC5469::MudK Δhin-5717::FRT-Cm-FRT | |
| TH5955 | fliC5050::MudJ Δhin-5718::FRT-Cm-FRT | |
| TH5959 | fliC5469::MudK Δhin-5718::FRT-Cm-FRT | |
| TH5979 | fliC5050::MudA Δhin-5718::FRT-Cm-FRT fljA5576::FRT-Km-FRT | |
| TH5983 | fliC5469::MudB Δhin-5718::FRT-Cm-FRT fljA5576::FRT-Km-FRT | |
| TH6592 | Δhin-5717::FRT-Cm-FRT fliA5648::FRT-Km-FRT | |
| TH6593 | Δhin-5718::FRT-Cm-FRT fliA5648::FRT-Km-FRT | |
| TH5949 | fliC5050::MudJ Δhin-5717::FRT-Cm-FRT ΔflgM5301 | |
| TH5953 | fliC5469::MudK Δhin-5717::FRT-Cm-FRT ΔflgM5301 | |
| TH5957 | fliC5050::MudJ Δhin-5718::FRT-Cm-FRT ΔflgM5301 | |
| TH5961 | fliC5469::MudK Δhin-5718::FRT-Cm-FRT ΔflgM5301 | |
| TH5980 | fliC5050::MudA Δhin-5718::FRT-Cm-FRT ΔflgM5301 fljA5576::FRT-Km-FRT | |
| TH5984 | fliC5469::MudB Δhin-5718::FRT-Cm-FRT ΔflgM5301 fljA5576::FRT-Km-FRT | |
| TH5971 | Δhin-5717::FRT-Cm-FRT | |
| TH5975 | Δhin-5717::FRT-Cm-FRT fljA5576::FRT-Km-FRT | |
| TH5862 | Δhin-5718::FRT-Cm-FRT | |
| TH5990 | Δhin-5718::FRT-Cm-FRT fljA5576::FRT-Km-FRT |
Unless otherwise noted, all strains were constructed for this work.
Culture and growth medium conditions.
Strains were cultured in Luria-Bertani medium with aeration as described by Davis et al. (8). Strains containing the pKD46, pKD3, or pKD4 plasmids (7) were grown at either 30°C (pKD46) or 37°C (pKD3 and pKD4) in Luria-Bertani medium with aeration in the presence of 100 μg of ampicillin (Sigma, St. Louis, Mo.)/ml.
Construction of S. enterica strains.
Markers were mobilized between Salmonella strains by generalized transduction using the mutant P22 HT/int bacteriophage (37). Resistance markers were selected for by using the following antibiotic concentrations: ampicillin, 30 or 100 μg/ml; chloramphenicol, 12.5 μg/ml; kanamycin, 50 μg/ml; and tetracycline, 12.5 μg/ml.
Quantitative immunoblot assays for FliC.
Cells were grown overnight with aeration and then subcultured and grown to an optical density at 600 nm (OD600) of ∼0.6. One milliliter of culture was centrifuged, and the pellet was resuspended in 50 μl of sample buffer (32). Samples were run on 10% Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels (44), and proteins were transferred to polyvinylidene difluoride membranes (Schleicher & Schuell, Inc., Keene, N.H.) in 3-(cyclohexylamine)-1 propanesulfonic buffer (38) and probed with rabbit anti-FliC antibody (Becton Dickinson, Sparks, Md.) purified according to the methods of Hughes et al. (22). Primary antibody was detected, and protein levels were determined as previously described (28). Protein levels for each sample were recorded as phosphorimager units per OD600.
Isolation of a translational fusion to the fliC gene.
Translational fusions of the fliC gene to the lacZ gene were made using the MudK-lac(MudII1734) fusion vector (17a). Strain TH1059 contains a Tn10dCm insertion in the IS200 element adjacent to the fliC gene. Random MudK insertion mutants were introduced into TH1059 by the transitory cis-complementation method (23). The MudK insertion mutants were pooled, P22 transducing lysates were prepared on these pools, and MudK insertions linked to the Tn10dCm insertion were identified. Isolates with linked insertions were tested for motility and phase variation. Potential MudK insertions within the fliC gene were confirmed by PCR amplification using a primer homologous to the adjacent fliD gene reading towards fliC (fliD 5′ out, 5′-ACAGAAGCTTCATAGGCGGTTAGCTTTGC; Life Sciences, Boston, Mass.) and a primer homologous to end of the MudK (mur4, 5′-ATGTAATGAATAAAAAGC; Life Sciences). Products were sequenced using an ABI 377 apparatus (Life Sciences). One MudK insertion (fliC5469::MudK) was found to be inserted 567 nucleotides into the fliC gene, resulting in a translational fusion of the first 189 amino acids of FliC to LacZ, and was used for further studies.
Construction of the fljA null mutation.
A deletion of the fljA gene was constructed using the λ-red system as described by Murphy et al. (39) and modified by Datsenko and Wanner (7). The kanamycin Flp recombinase target site (FRT) cassette was amplified from pKD4 (7) using the following oligonucleotides: 5′ fljA-FRT (5′-CGGGGCTTTTTCATTTAGCATAGATGAATATATATTTTGTAGGCTGGAGCTGCTTCG) and 3′ fljA-FRT (5′-CTTTTCTCACGGAATTTTTTATTACCGTAGGCGCATATGAATATCCTCCTTAG; MWG Biotech, Inc., High Point, N.C.) that contain homology to the upstream and downstream DNA immediately adjacent to the fljA gene. TH4702 (LT2/pKD46) (7) was prepared for electroporation such that the cells were concentrated 250-fold and transformed with 50 to 100 ng of PCR product. Recombination into the chromosome of the FRT cassette and loss of the pKD46 plasmid were simultaneously selected for by growing the cells in the presence of 50 μg of kanamycin/ml at 37°C. Constructs were confirmed using the K1-test primer (7) and the hinSspI primer (homologous to the hin gene upstream of fljA; 5′-CGGCAGCAATTAGCTATTATTTTTAATATTG; MWG Biotech, Inc.).
Construction of phase-variation mutants.
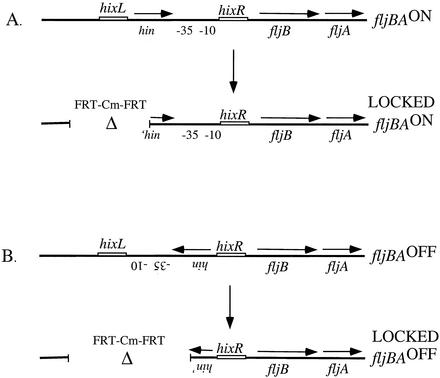
The chloramphenicol FRT cassette was amplified from pKD3 using the following oligonucleotides: hin-A-FRT, 5′-CCGCTCTGCGATTTTTATAGCGCATCAGCCACACGATTTTGTAGGCTGGAGCTGCTTCG, and hin-C-FRT, 5′-TCCTGTTCGTGTCTATTGATCGCCCGAGGGTGCCCTCCCAGCATATGAATATCCTCCTTAG (Life Technologies, Boston, Mass.). The hin-A-FRT oligonucleotide contains DNA homologous to the region upstream of the hixL site, and the hin-C-FRT primer contains DNA homologous to the middle of the hin gene reading toward the 5′ end of the gene. The PCR product was transformed into TH4702 with the fljBA promoter in the on orientation. Recombination into the chromosome resulted in a deletion of the hixL site and 448 nucleotides of the hin gene. The hixR site and the fljBA promoter were still present. The FRT chloramphenicol cassette was also amplified using the hin-A-FRT primer in conjunction with the hin-B-FRT primer (5′-CTGGGAGGGCACCCTCGGGCGATCAATAGACACGAACAGGACATATGAATATCCTCCTTAG). The hin-B-FRT primer has homology to the middle of the hin gene reading towards the 3′ end of the gene. Transformation and recombination of this PCR product into TH4702 with the fljBA promoter in the off orientation for fljBA expression resulted in the deletion of the hixL site, the 3′ end of the hin gene, and the fljBA promoter. Both of these strains are unable to undergo phase variation, with the former constructs being locked in the on orientation for fljBA expression and the latter constructs locked in the off orientation.
β-Galactosidase assays.
β-Galactosidase assays were performed as described by Maloy (37). Cells were grown to an OD600 of ∼0.8. Each sample was assayed in triplicate, and the values were recorded as β-galactosidase units (nanomoles per minute per OD650 unit per milliliter).
T2 RNase protection assays.
Cells were grown to an OD600 of ∼0.6, and RNA was isolated as described previously (17). RNase T2 protection assays of transcripts from the bacterial chromosome were performed as described elsewhere (50). A radiolabeled RNA probe complementary to the first 200 nucleotides of the fliC transcript was synthesized with T7 polymerase and the Riboprobe in vitro transcription system (Promega, Madison, Wis.). Template DNA for the in vitro transcription reaction was amplified using the following primers: fliC200R-T7P, 5′-TAATACGACTCACTATAGGGCCTGCCGCATCGTCTTTCG, and fliC60F, 5′-CGGTGAGAAACCGTGGGC (Integrated DNA Technologies, Inc., Coralville, Iowa). A 15-μg aliquot of total RNA from each strain was added to the hybridization mixture. Transcript levels were quantified with a Storm 840 Imager (Molecular Dynamics), and band intensity was determined using ImageQuant software (Molecular Dynamics).
RESULTS
FliC expression during phase variation is posttranscriptionally regulated.
FliC production is known to be repressed when the fljBA promoter is in the on orientation (46), but to evaluate the contribution of transcriptional and posttranscriptional mechanisms in the regulation of fliC gene expression during phase variation we examined transcription and translation of fliC in strains that were locked in either the fljBAON orientation or the fljBAOFF orientation (Fig. 2). Transcription of the fliC promoter was measured by determining β-galactosidase activities of a lac operon fusion to the fliC gene. Translation was determined using a fliC-lacZ gene fusion, where both transcription and translation of the lacZ gene are dependent upon fliC gene transcription and translation.
FIG. 2.
Construction of strains locked in either the fljBAON or fljBAOFF orientation. An FRT-chloramphenicol-FRT cassette (see Materials and Methods) was amplified using oligonucleotides containing DNA homologous to the region upstream of the hixL site and to the middle of the hin gene reading either towards the 5′ end of the gene (A) or towards the 3′ end of the gene (B). Recombination of the FRT-Cm-FRT cassette into the chromosome of an isolate with the fljBA promoter in the on orientation resulted in a deletion of the hixL site and a portion of the hin gene. The hixR site and the fljBA promoter are still present. In contrast, recombination of the second FRT-Cm-FRT cassette into an isolate with the fljBA promoter in the off orientation resulted in a deletion of the hixL site, the fljBA promoter, and the 3′ end of the hin gene. Both of these strains are unable to undergo phase variation with the constructs locked in either the on orientation (A) or the off orientation (B) for fljBA expression.
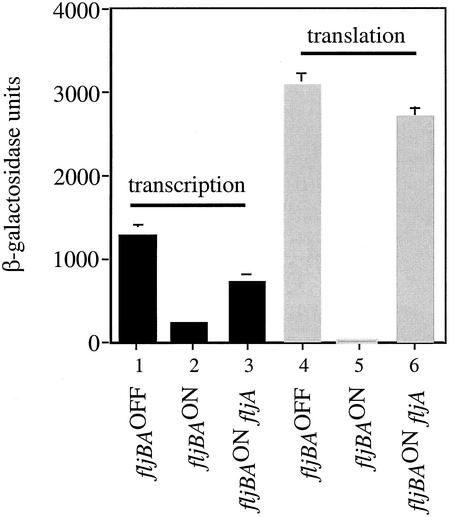
β-Galactosidase levels were down fivefold for the fliC-lac transcriptional fusion when the cells were locked in the fljBAON orientation compared to cells locked in the fljBAOFF orientation (Fig. 3, columns 1 and 2), indicating that fliC transcription is somewhat inhibited when FljB is expressed. In contrast, a 200-fold decrease in β-galactosidase activity was observed with the FliC-LacZ translational fusion for cells locked in the fljBAON orientation compared to the fljBAOFF orientation (Fig. 3, columns 4 and 5). The fact that there is only a fivefold effect of FljA on fliC transcription, but a 200-fold effect of FljA on both fliC transcription and translation, suggested that posttranscriptional regulation is a key factor mediating FliC expression during phase variation and that the FljA inhibitor might act at both the level of fliC transcription and translation.
FIG. 3.
β-Galactosidase activities for fliC-lac transcriptional and translational fusions in the fljBAOFF and fljBAON orientations and for the fljBAON orientation in the absence of fljA. The levels of transcription and translation are recorded as β-galactosidase units. The average of three independent experiments and the standard deviations are shown. β-Galactosidase units for the fliC-lac transcriptional fusion were as follows: fljBAOFF, 1,300 ± 75; fljBAON, 250 ± 32; fljBAON fljA, 730 ± 114. β-Galactosidase units for the fliC-lac translational fusion were as follows: fljBAOFF, 3,100 ± 170; fljBAON, 15 ± 2; fljBAON fljA, 2,700 ± 175.
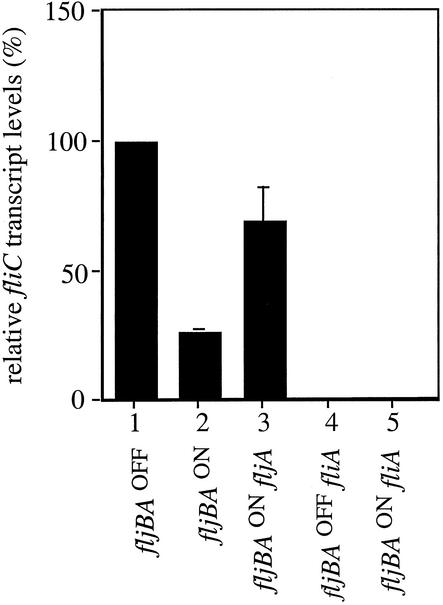
To verify that the high basal level of fliC-lac transcription in the fljBAON strains was not an artifact of the fliC-lac reporter systems, we performed T2 RNase protection assays to measure actual fliC mRNA transcript levels in the presence and absence of FljA. A fivefold decrease in fliC transcript levels was observed in cells locked in the fljBAON orientation compared to fliC transcript levels in the fljBAOFF orientation (Fig. 4, columns 1 and 2), thus corroborating our studies with the transcriptional fusions. As a negative control, T2 RNase protection assays were performed in fljBAON and fljBAOFF phase-locked strains containing a deletion of the flagellum-specific sigma factor FliA that is required for fliC transcription. The fliC transcript detected in these backgrounds was negligible (Fig. 4, columns 4 and 5).
FIG. 4.
Quantification of fliC transcript levels in the fljBAOFF and fljBAON orientations in the presence and absence of FljA using T2 RNase protection assays. Radiolabeled RNA probes covering the first 200 nucleotides of the fliC transcript were hybridized to 15 μg of total RNA for each strain tested. Band intensities were quantified using a Storm 840 PhosphorImager, and relative transcript levels were recorded as a percentage of the amount observed in the fljBAOFF orientation. The averages of three independent experiments and the standard deviations are shown.
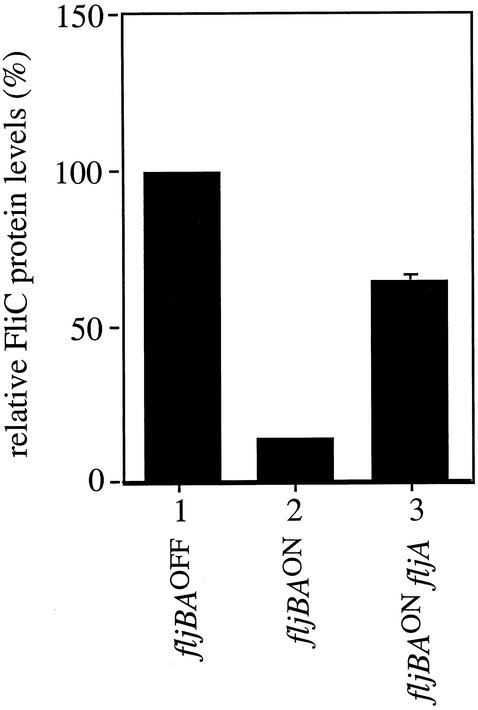
FliC protein levels were directly determined by Western analysis with anti-FliC antibody in the fljBAON and fljBAOFF orientations (Fig. 5, columns 1 and 2). An eightfold decrease in FliC protein levels in the fljBAOFF orientation was observed compared to that in the fljBAON orientation. This reduction is greater than that observed for the transcripts, suggesting that fliC translation in addition to its transcription is regulated during phase variation and, therefore, FljA might not only inhibit fliC transcription but also inhibit its translation. However, the decrease of protein levels indicated by Western analysis was not as large as the decrease observed with the translational fusions (Fig. 3, columns 4 and 5). This could be due to posttranscriptional effects on FliC levels.
FIG. 5.
Western blot analysis of FliC protein levels in strains locked in the fljBAON and fljBAOFF orientations and the fljBAON in the absence of FljA protein. Relative FliC protein levels are shown as the percentage of those observed for cells locked in the fljBAOFF orientation. The values represent the averages of three independent experiments, and standard deviations are shown.
FljA is a translational regulator of fliC expression.
The above results with the operon and gene fusions suggested that FljA might inhibit fliC transcription by 5-fold and fliC translation by an additional 40-fold. To test if inhibition of fliC translation in the fljBAON phase is mediated by FljA, the activities from fliC-lac transcriptional and translational fusions were assayed in the presence and absence of FljA protein in strains that have the fljBA promoter locked in the on orientation. A 3-fold increase in fliC-lac transcription was observed in the absence of FljA protein (Fig. 3, columns 2 and 3). In contrast, a 180-fold increase was observed for the FliC-LacZ translational fusion in the absence of FljA (Fig. 3, columns 5 and 6). These results implicate FljA as a negative regulator of both fliC transcription and translation, with a greater effect on fliC translation.
We performed T2 RNase protection assays to measure transcript levels in the presence and absence of FljA in the fljBAON orientation. As with the fliC-lac transcriptional fusion, a threefold increase in fliC transcript levels was observed in the absence versus the presence of FljA (Fig. 4, columns 2 and 3). Because the fljA gene is cotranscribed with fljB and thus not expressed in the fljBAOFF orientation, we did not expect to observe a FljA affect on fliC transcription in strains with the fljBA promoter in the off orientation. As predicted, fliC transcript levels were not significantly different in the presence or absence of FljA in these strains (data not shown).
To further characterize the role of FljA in regulating FliC expression, FliC protein levels were also determined by Western analysis in the presence and absence of FljA in the fljBAON orientation. In the absence of FljA, we observed a fivefold increase in FliC protein levels (Fig. 5, columns 2 and 3). This increase is greater than the threefold increase in transcription observed with both the fusion studies and the RNase protection assays (Fig. 3 and 4), consistent with the model that FljA inhibits both fliC transcription and translation when FljB flagellin is expressed, i.e., when the fljBA promoter is in the ON orientation. However, the increase in FliC protein levels is not as large as the 180-fold increase observed with the translational fusions (Fig. 3, columns 5 and 6).
Regulation of FliC expression by FljA functions independently of the anti-sigma factor FlgM.
The FlgM protein is known to inhibit σ28-dependent transcription of the fliC gene. We examined the effect of uncoupling fliC transcription from the regulatory control of FlgM (flgM deletion mutants) on the regulation of fliC-lac transcription and translation by FljA protein. In the absence of the anti-sigma factor FlgM, levels of flagellin transcription have been shown to increase above those observed in a wild-type background (15) (Table 2). We wanted to determine if the absence of FlgM would allow for fliC expression in the fljBAON orientation in the presence of FljA.
TABLE 2.
Effects of fljA5576 or flgM5301 disruptions on β-galactosidase activities of transcriptional and translational fusions of the fliC gene to the lac operon
| Presence of: | Orientation of fljBA promoter | β-Galactosidase activity (β-galactosidase units)
|
||
|---|---|---|---|---|
| fljA | flgM | Transcription | Translation | |
| + | + | OFF | 830 (± 140) | 2,000 (± 150) |
| + | − | OFF | 1,100 (± 170) | 2,300 (± 480) |
| + | + | ON | 270 (± 60) | 24 (± 1) |
| + | − | ON | 260 (± 30) | 17 (± 2) |
| − | + | ON | 710 (± 140) | 4,010 (± 1,100) |
| − | − | ON | 1,800 (± 210) | 3,900 (± 1,300) |
β-Galactosidase assays with the fliC-lac transcriptional and translational fusions were used to measure FliC expression. Introduction of a flgM null allele had no significant effect on fliC transcription or translation in the presence of FljA (Table 2). These data suggest that the modulation of FliC expression by FljA is maintained in the absence of the regulatory control of FlgM. We did not observe an increase in β-galactosidase activity for the fliC::MudK fusion in the absence of FlgM in the fljA fljBAON background (Table 2). This is because, in the absence of FlgM, the translational fusion is unstable and throws off Lac− revertants at a high frequency, which resulted in a reduction in β-galactosidase levels.
fliC transcription and translation were still tightly regulated by FljA in the fljBAON orientation in flgM null strains, suggesting that FljA-dependent inhibition of FliC expression is not easily titratable. That is, in the presence of increased σ28 and, thus, increased initiation of fliC transcription, FljA is able to maintain both transcriptional and translational fliC inhibition. However, the fljB promoter belongs to the late promoter class and is regulated by the interaction between σ28 and FlgM and, therefore, the levels of FljA protein are likely elevated in flgM null strains, and this alone may account for the maintenance of FliC inhibition. However, if σ28-independent factors were required for FliC inhibition during FljB expression, we would have expected to observe an increase in fliC-lac transcription or translation in the absence of FlgM. Alternatively, excess fliC transcripts or FliC protein in the flgM mutant backgrounds might be hypersensitive to degradation.
DISCUSSION
Since the early 1920s (2), it has been known that S. enterica undergoes a phenomenon known as phase variation, a molecular mechanism that allows for the alternate expression of the two different unlinked flagellar filament proteins, FliC and FljB (16). Transcription of the fljB gene is initiated from an invertible promoter, and a reversible site-specific DNA recombination event turns fljB transcription on and off (Fig. 1) (45). It has been postulated that the FljA protein, which is cotranscribed with the fljB gene (11, 43, 49), represses fliC transcription when the fljB promoter is in the on orientation (31, 43).
We found that fliC transcription is indeed regulated by FljA during phase variation. However, FljA-dependent inhibition of FliC expression is at the level of transcription and translation (Fig. 3, 4, and 5). Specifically, β-galactosidase assays with fliC-lac operon and gene fusions were performed for strains with the fljBA promoter locked in either the on or the off orientation. These experiments demonstrated that while FljA inhibits fliC transcription fivefold in the fljBAON orientation compared to the fljBAOFF orientation, it has a 200-fold effect on both fliC transcription and translation, indicating that the FljA inhibitor might act at both the transcriptional and translational levels. T2 RNase protection assays also demonstrated a fivefold decrease in fliC transcript levels, while FliC protein levels as determined by Western analysis showed an eightfold decrease in FliC protein levels in the fljBAOFF orientation compared to fljBAON, further suggesting that FljA not only regulates fliC transcription in the fljBAON orientation but also inhibits its translation.
An eightfold decrease in FliC protein levels was observed by Western analysis (Fig. 5), while a 200-fold decrease in FliC-LacZ levels was observed in strains locked in the fljBAON orientation compared to that in the fljBAOFF orientation (Fig. 3). The differences in FliC expression between the fusion studies and Western analysis likely represent differential stability of fliC-lacZ transcript or FliC-LacZ protein fusions compared to that of wild-type fliC transcript or FliC protein. It is important that wild-type flagellin is secreted and polymerized into flagellar filaments where it is highly stable (data not shown) while, in contrast, the fusion protein cannot be secreted and assembled (data not shown) but is retained within the cell, where it would be subject to proteolysis. Thus, the FliC-LacZ fusion protein may be turned over more rapidly than wild-type FliC protein, resulting in lower levels of the reporter protein in the presence of translational regulation by the FljA inhibitor.
In the absence of FljA protein in the fljBAON orientation, both fliC transcription and translation were reduced compared to the fljBAOFF orientation (Fig. 3, 4, and 5). Because the fljBA promoter is maintained in strains locked in the fljBAON orientation, competition for σ28-RNA polymerase is likely occurring between the fliC and fljBA promoters. In fact, both FliC and FljB flagellin can be detected by Western blotting in this genetic background (data not shown). It is possible that factors other than FljA are regulating FliC expression during phase variation and are responsible for the remaining inhibition of FliC expression in the absence of FljA.
Previous experiments demonstrated that strains locked in the fljBAON orientation but containing a defective fljB gene were nonmotile due to FljA-dependent inhibition of fliC gene expression (12). We were initially surprised that only a 5-fold reduction in fliC transcription by FljA activity could prevent motility, but that the further 40-fold reduction in fliC translation in the presence of FljA could account for the complete loss of motility. But yet, by Western blotting we were able to detect FliC protein within strains locked in the fljBAON orientation, indicating that FliC expression is not completely inhibited during phase variation. In fact, it has been shown that in the absence of fljB expression, these strains do not produce enough flagellin to allow motility (12). Thus, other mechanisms may be regulating the secretion and assembly of FliC protein during phase variation, or the amount of protein that is expressed is insufficient for full filament assembly.
Model of posttranscriptional regulation of FliC expression by FljA.
Because the regulation of FliC expression by FljA during phase variation was thought to occur at the transcriptional level, genetic experiments were performed to identify the operator site for the FljA protein (12, 26). This work involved the isolation of motile revertants from strains which were transcribing fljBA but contained a nonfunctional fljB allele. Because these strains were also blocked for phase variation, mutations alleviating the negative regulation of FliC expression would result in a motile phenotype. The authors of this study identified nine cis-acting mutations that mapped downstream of the fliC promoter within the 5′-UTR of the fliC mRNA transcript. These mutations did not conform to the classical operator regulatory sequences observed in bacteria, which are often located close to or within transcriptional promoter regions, but instead clustered to a 15-bp sequence within the 5′-UTR of the fliC transcript immediately adjacent to and overlapping the ribosome binding sequence. In fact, one operator-constitutive fliC mutant (SJW57) was found to have a 28-bp tandem duplication that included 13 bases upstream of the fliC translational initiation site through base 15 of the coding sequence. This fliC-Oc mutant was dependent upon the presence of FljA for motility, consistent with an interaction of FljA with the mRNA to allow fliC gene expression. The 5′-UTR of bacterial transcripts has often been implicated in the translational regulation of protein expression. The work presented here suggests that the FljA protein functions as both a transcriptional and translational regulator of FliC expression (Fig. 3, 4, and 5) and, therefore, these “operator mutants” may be defective in FljA binding to mRNA as a regulator of transcription (attenuation) and translation.
Therefore, we put forward the following model for the transcriptional and posttranscriptional regulation of FliC expression during phase variation. We propose that FljA regulates fliC gene expression by binding to the 5′-UTR of the transcript, within or near the Shine-Dalgarno sequence, to inhibit transcription by an attenuation mechanism and to inhibit ribosome binding and thus translation. The presence of ribosomes at or near the Shine-Dalgarno site has previously been demonstrated to be particularly important for mRNA stability by protecting the 5′-terminal extremity from initiation of mRNA degradation (18). In addition, decreased stability of the fliC transcript would further reduce fliC translation. Finally, if FljA does indeed bind to fliC transcripts and prevent ribosome binding, it may also affect FliC expression by masking positive regulatory sequences contained within the 5′-UTR of the fliC transcript.
Importance of posttranscriptional regulation of FliC expression during phase variation.
Historically, translational regulation was not thought to be an important factor mediating protein expression because mRNA half-lives in bacteria are typically on the order of a few minutes, but recent studies have demonstrated that mRNA turnover alone is often insufficient to provide necessary regulation of protein levels (18), as may be the case for regulation of FliC expression during phase variation. For example, translational regulation would allow for the inhibition of FliC expression from existing transcripts after inversion of the fljBA promoter to the on orientation and a more rapid transition to filaments composed of FljB flagellin. Although the central domain of a particular flagellin sequence is the peripheral molecular region exposed on a flagellar filament and helps define the diameter of the filament and the character of its surface, including topography, physiochemistry, and antigenicity, the importance of filament diameter and surface properties and the reasons for variability are not well understood (51). Further investigations into the biological significance of S. enterica phase variation may elucidate the importance of posttranscriptional regulatory mechanisms to mediate flagellar phase variation.
Acknowledgments
We thank Phillip Aldridge for generously providing the fliA deletion allele. We are grateful to members of the Hughes laboratory for critical reading of the manuscript.
This work was supported by PHS grant GM62206 from the National Institutes of Health awarded to K.T.H. H.R.B. is a recipient of a PHS National Research Service Award (T32 GM07270) from the National Institute of General Medical Sciences.
REFERENCES
- 1.Aizawa, S.-I. 1996. Flagellar assembly in Salmonella typhimurium. Mol. Microbiol. 20:1-4. [DOI] [PubMed] [Google Scholar]
- 2.Andrewes, F. W. 1922. Studies on group-agglutination. I. The Salmonella group and its antigenic structure. J. Pathol. Bacteriol. 25:1509-1514. [Google Scholar]
- 3.Bartlett, D. H., B. B. Frantz, and P. Matsumura. 1988. Flagellar transcriptional activators FlbB and FlaI: gene sequences and 5′ consensus sequences of operons under FlbB and FlaI control. J. Bacteriol. 170:1575-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blair, D. F. 1995. How bacteria sense and swim. Annu. Rev. Microbiol. 49:489-522. [DOI] [PubMed] [Google Scholar]
- 5.Chilcott, G. S., and K. T. Hughes. 2000. Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar Typhimurium and Escherichia coli. Microbiol. Mol. Biol. Rev. 64:694-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cookson, B. T., and M. J. Bevan. 1997. Identification of a natural T cell epitope presented by Salmonella-infected macrophages and recognized by T cells from orally immunized mice. J. Immunol. 158:4310-4319. [PubMed] [Google Scholar]
- 7.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis, R. W., D. Botstein, and J. R. Roth. 1980. Advanced bacterial genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 9.Emerson, S. U., K. Tokuyasu, and M. I. Simon. 1970. Bacterial flagella: polarity of elongation. Science 169:190-192. [DOI] [PubMed] [Google Scholar]
- 10.Fierer, J., and D. G. Guiney. 2001. Diverse virulence traits underlying different clinical outcomes of Salmonella infection. J. Clin. Investig. 107:775-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujita, H., S. Yamaguchi, and T. Iino. 1973. Studies on H-O variants in Salmonella in relation to phase variation. J. Gen. Microbiol. 76:127-134. [DOI] [PubMed] [Google Scholar]
- 12.Fujita, H., S. Yamaguchi, T. Taira, T. Hirano, and T. Iino. 1987. Isolation and genetic analysis of operator-constitutive mutants of the H1 operon in Salmonella typhimurium. J. Gen. Microbiol. 133:3071-3080. [DOI] [PubMed] [Google Scholar]
- 13.Gillen, K. L., and K. T. Hughes. 1991. Molecular characterization of flgM, a gene encoding a negative regulator of flagellin synthesis in Salmonella typhimurium. J. Bacteriol. 173:6453-6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillen, K. L., and K. T. Hughes. 1991. Negative regulatory loci coupling flagellin synthesis to flagellar assembly in Salmonella typhimurium. J. Bacteriol. 173:2301-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillen, K. L., and K. T. Hughes. 1993. Transcription from two promoters and autoregulation contribute to the control of expression of the Salmonella typhimurium flagellar regulatory gene flgM. J. Bacteriol. 175:7006-7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glasgow, A. C., K. T. Hughes, and M. I. Simon. 1989. Bacterial DNA inversion systems, p. 636-659. In M. M. Howe (ed.), Mobile DNA. American Society for Microbiology, Washington, D. C.
- 17.Goluszko, P., S. L. Moseley, L. D. Truong, A. Kaul, J. R. Williford, R. Selvarangan, S. Nowicki, and B. Nowicki. 1987. Development of experimental model of chronic pyelonephritis with Escherichia coli O75:K5:H-bearing Dr fimbriae: mutation in the dra region prevented tubulointerstitial nephritis. J. Clin. Investig. 99:1662-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Groisman, E. A. 1991. In vivo genetic engineering with bacteriophage Mu. Methods Enzymol. 204:180-212. [DOI] [PubMed] [Google Scholar]
- 18.Grunberg-Manago, M. 1999. Messenger RNA stability and its role in control of gene expression in bacteria and phages. Annu. Rev. Genet. 33:193-227. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 20.Haykinson, M. J., L. M. Johnson, J. Soong, and R. C. Johnson. 1996. The Hin dimer interface is critical for Fis-mediated activation of the catalytic steps of site-specific DNA inversion. Curr. Biol. 6:163-177. [DOI] [PubMed] [Google Scholar]
- 21.Helmann, J. D., and M. J. Chamberlin. 1987. DNA sequence analysis suggests that expression of flagellar and chemotaxis genes in Escherichia coli and Salmonella typhimurium is controlled by an alternative sigma factor. Proc. Natl. Acad. Sci. USA 84:6422-6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes, K. T., K. L. Gillen, M. J. Semon, and J. E. Karlinsey. 1993. Sensing structural intermediates in bacterial flagellar assembly by export of a negative regulator. Science 262:1277-1280. [DOI] [PubMed] [Google Scholar]
- 23.Hughes, K. T., and J. R. Roth. 1988. Transitory cis complementation: a method for providing transposition functions to defective transposons. Genetics 119:9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iino, T. 1969. Polarity of flagellar growth in Salmonella. J. Gen. Microbiol. 56:227-239. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda, J. S., C. K. Schmitt, S. C. Darnell, P. R. Watson, J. Bispham, T. S. Wallis, D. L. Weinstein, E. S. Metcalf, P. Adams, C. D. O'Connor, and A. D. O'Brien. 2001. Flagellar phase variation of Salmonella enterica serovar Typhimurium contributes to virulence in the murine typhoid infection model but does not influence Salmonella-induced enteropathogenesis. Infect. Immun. 69:3021-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoue, Y. H., K. Kutsukake, T. Iino, and S. Yamaguchi. 1989. Sequence analysis of operator mutants of the phase-1 flagellin-encoding gene, fliC, in Salmonella typhimurium. Gene 85:221-226. [DOI] [PubMed] [Google Scholar]
- 27.Kalir, S., J. McClure, K. Pabbaraju, C. Southward, M. Ronen, S. Leibler, M. G. Surette, and U. Alon. 2001. Ordering genes in a flagella pathway by analysis of expression kinetics from living bacteria. Science 292:2080-2083. [DOI] [PubMed] [Google Scholar]
- 28.Karlinsey, J. E., J. Lonner, K. L. Brown, and K. T. Hughes. 2000. Translation/secretion coupling by type III secretion systems. Cell 102:487-497. [DOI] [PubMed] [Google Scholar]
- 29.Karlinsey, J. E., S. Tanaka, V. Bettenworth, S. Yamaguchi, W. Boos, S.-I. Aizawa, and K. T. Hughes. 2000. Completion of the hook-basal body complex of the Salmonella typhimurium flagellum is coupled to FlgM secretion and fliC transcription. Mol. Microbiol. 37:1220-1231. [DOI] [PubMed] [Google Scholar]
- 30.Kutsukake, K. 1994. Excretion of the anti-sigma factor through a flagellar substructure couples flagellar gene expression with flagellar assembly in Salmonella typhimurium. Mol. Gen. Genet. 243:605-612. [DOI] [PubMed] [Google Scholar]
- 31.Kutsukake, K., and T. Iino. 1980. Inversions of specific DNA segments in flagellar phase variation of Salmonella and inversion systems of bacteriophages P1 and Mu. Proc. Natl. Acad. Sci. USA 77:7238-7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laemmli, U. K., and M. Favre. 1973. Maturation of the head of bacteriophage T4. I. DNA packaging events. J. Mol. Biol. 80:575-599. [DOI] [PubMed] [Google Scholar]
- 33.Lederberg, J., and T. Iino. 1956. Phase variation in Salmonella. Genetics 41:743-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu, X., and P. Matsumura. 1995. The C-terminal region of the alpha subunit of Escherichia coli RNA polymerase is required for transcriptional activation of the flagellar level II operons by the FlhD/FlhC complex. J. Bacteriol. 177:5186-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu, X., and P. Matsumura. 1994. The FlhD/FlhC complex, a transcriptional activator of the Escherichia coli flagellar class II operons. J. Bacteriol. 176:7345-7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macnab, R. M. 1996. Flagella and motility, p. 123-145. In F. C. Neidhart et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 36a.Macnab, R. M. 1999. The bacterial flagellum: reversible rotary propellor and type III export apparatus. J. Bacteriol. 181:7149-7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maloy, S. R. 1990. Experimental techniques in bacterial genetics. Jones and Bartlett, Boston, Mass.
- 38.Matsudaira, P. 1987. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J. Biol. Chem. 262:10035-10038. [PubMed] [Google Scholar]
- 39.Murphy, K. C., K. G. Campellone, and A. R. Poteete. 2000. PCR-mediated gene replacement in Escherichia coli. Gene 246:321-330. [DOI] [PubMed] [Google Scholar]
- 40.Namba, K. 2001. Roles of partly unfolded conformations in macromolecular self-assembly. Genes Cells 6:1-12. [DOI] [PubMed] [Google Scholar]
- 41.Ohnishi, K., K. Kutsukake, H. Suzuki, and T. Iino. 1990. Gene fliA encodes an alternative sigma factor specific for flagellar operons in Salmonella typhimurium. Mol. Gen. Genet. 221:139-147. [DOI] [PubMed] [Google Scholar]
- 42.Ohnishi, K., K. Kutsukake, H. Suzuki, and T. Iino. 1992. A novel transcriptional regulatory mechanism in the flagellar regulon of Salmonella typhimurium: an anti sigma factor inhibits the activity of the flagellum-specific sigma factor, σF. Mol. Microbiol. 6:3149-3157. [DOI] [PubMed] [Google Scholar]
- 43.Pearce, U. B., and B. A. D. Stocker. 1967. Phase variation of flagellar antigens in Salmonella: abortive transduction studies. J. Gen. Microbiol. 49:335-347. [DOI] [PubMed] [Google Scholar]
- 44.Schägger, H., and G. Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 45.Silverman, M., and M. Simon. 1980. Phase variation: genetic analysis of switching mutants. Cell 19:845-854. [DOI] [PubMed] [Google Scholar]
- 46.Silverman, M., J. Zieg, and M. Simon. 1979. Flagellar-phase variation: isolation of the rh1 gene. J. Bacteriol. 137:517-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simon, M., and M. Silverman. 1983. Recombinational regulation of gene expression in bacteria, p. 211-227. In J. Beckwith, J. Davies, and J. A. Gallant (ed.), Gene function in procaryotes. Cold Spring Harbor Laboratory, Cold Spring Harbor.
- 48.Stocker, B. A. D. 1949. Measurement of the rate of mutation of flagellar antigenic phase in Salmonella typhimurium. J. Hyg. 47:398-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki, H., and T. Iino. 1973. In vitro synthesis of phase-specific flagellin of Salmonella. J. Mol. Biol. 81:57-70. [DOI] [PubMed] [Google Scholar]
- 50.Tsui, H. C. T., A. J. Pease, T. M. Koehler, and M. E. Winkler. 1994. Detection and quantitation of RNA transcribed from bacterial chromosomes. Methods Mol. Genet. 3:179-204. [Google Scholar]
- 51.Wilson, D. R., and T. J. Beveridge. 1993. Bacterial flagellar filaments and their component flagellins. Can. J. Microbiol. 39:451-472. [DOI] [PubMed] [Google Scholar]
- 52.Yanagihara, S., S. Iyoda, K. Ohnishi, T. Iino, and K. Kutsukake. 1999. Structure and transcriptional control of the flagellar master operon of Salmonella typhimurium. Genes Genet. Syst. 74:105-111. [DOI] [PubMed] [Google Scholar]