Abstract
Shewanella oneidensis is a metal reducer that can use several terminal electron acceptors for anaerobic respiration, including fumarate, nitrate, dimethyl sulfoxide (DMSO), trimethylamine N-oxide (TMAO), nitrite, and insoluble iron and manganese oxides. Two S. oneidensis mutants, SR-558 and SR-559, with Tn5 insertions in crp, were isolated and analyzed. Both mutants were deficient in Fe(III) and Mn(IV) reduction. They were also deficient in anaerobic growth with, and reduction of, nitrate, fumarate, and DMSO. Although nitrite reductase activity was not affected by the crp mutation, the mutants failed to grow with nitrite as a terminal electron acceptor. This growth deficiency may be due to the observed loss of cytochromes c in the mutants. In contrast, TMAO reduction and growth were not affected by loss of cyclic AMP (cAMP) receptor protein (CRP). Fumarate and Fe(III) reductase activities were induced in rich medium by the addition of cAMP to aerobically growing wild-type S. oneidensis. These results indicate that CRP and cAMP play a role in the regulation of anaerobic respiration, in addition to their known roles in catabolite repression and carbon source utilization in other bacteria.
Shewanella oneidensis (formerly Shewanella putrefaciens [24]) is a gram-negative metal reducer and facultative anaerobe that belongs to the γ-group of the proteobacteria. During anaerobic growth, S. oneidensis can use a large number of terminal electron acceptors, including nitrate, nitrite, fumarate, trimethylamine N-oxide (TMAO), dimethyl sulfoxide (DMSO), and insoluble Fe(III) and Mn(IV) oxides or oxyhydroxides (16). S. oneidensis produces a large number of cytochromes c (17) and at least 38 cytochrome c-type genes have been identified in its genome sequence (23). Some of these cytochromes are located in the outer membrane of the cell (15). At least one of these outer membrane cytochromes, MtrC, is involved in metal reduction (2). The unusual location of cytochromes c in S. oneidensis may account for its ability to use insoluble electron acceptors for respiration.
The mechanisms that regulate anaerobic respiration in S. oneidensis are not known. In Escherichia coli and other bacteria, FNR and its homologues mediate global changes in gene expression in response to anaerobiosis (for reviews, see references 8 and 9). Structural studies have shown that the regulatory activity of FNR is controlled by the formation of 4Fe-4S clusters under anaerobic conditions (12). S. oneidensis has an FNR homologue, EtrA, which contains the conserved cysteines that are involved in redox sensing in FNR (19). Although phenotypic analysis of an etrA mutant did not result in detection of major defects in anaerobic respiration (14), recent microarray analysis suggests that EtrA may be involved in the regulation of proteins involved in electron transport, iron uptake, and intermediary carbon metabolism (3).
Isolation and analysis of crp mutants.
SR-558 and SR-559, two mutants that are deficient in anaerobic respiration, were isolated, and the sites of Tn5 insertions were identified by using previously described procedures (1). Both mutants had insertions in the crp gene that encodes the cyclic AMP (cAMP) receptor protein (CRP) (ORF SO0624 [http://www.tigr.org]). S. oneidensis CRP exhibits 88% amino acid identity to CRP from Vibrio cholerae and E. coli. A putative cAMP-binding domain is located at the N terminus of the protein (amino acids 19 to 113) and exhibits 87% identity and 95% similarity to the cAMP-binding domain of the E. coli CRP (amino acids 18 to 112). The C-terminal portion of the S. oneidensis CRP contains a putative DNA-binding domain that is 100% identical to the helix-turn-helix motif of E. coli CRP. These similarities suggest that CRP from S. oneidensis may function as the E. coli protein by binding to similar consensus sequences and that it may require cAMP for activity.
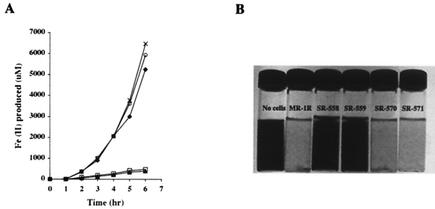
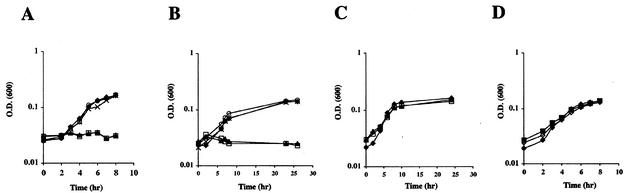
The two crp mutants, SR-558 and SR-559, were tested for anaerobic growth or reduction of various terminal electron acceptors by using a basal medium (22) supplemented with 50 mM lactate, 0.02% Casamino Acids, and one of the following electron acceptors: 10 mM ferric citrate, 10 mM TMAO, 10 mM DMSO, 5 mM nitrate, 0.5 mM nitrite, 2 mM MnO2, or 10 mM fumarate. Both mutants were deficient in Fe(III) and Mn(IV) reduction (Fig. 1). They also did not grow with fumarate, nitrate (Fig. 2A and B), nitrite, or DMSO (data not shown). In contrast, inactivation of crp did not affect growth with TMAO (Fig. 2C).
FIG. 1.
(A) Fe(III) reduction by S. oneidensis MR-1R (⧫), crp mutants SR-558 (□) and SR-559 (▴), and complemented mutants SR-570 (×) and SR-571 (○). (B) Mn(IV) reduction by MR-1R, crp mutants, and complemented mutants. Reduction of Mn(IV) is indicated by the disappearance of the dark color that is characteristic of the oxide.
FIG. 2.
(A to C) Anaerobic growth of MR-1R, crp mutants, and mutants complemented with S. oneidensis crp in basal medium supplemented with 10 mM fumarate (A), 5 mM nitrate (B), and 10 mM TMAO (C). The symbols are as described in the legend to Fig. 1A. (D) Anaerobic growth of MR-1R and crp mutants complemented with E. coli crp with 10 mM fumarate as the electron acceptor. Symbols: ⧫, MR-1R; ▪, SR-637; ◊, SR-638.
The loss of anaerobic respiration by the crp mutants was restored by S. oneidensis and E. coli crp. The S. oneidensis crp gene was amplified by using the primers CRPF (5′-GTTGCTGCTCGGCAGCGAGGG-3′) and CRPR (5′-CTCGGTAGTGCGGTGGAGTG-3′). The E. coli crp gene was amplified by using the primers ECRPE (GATAGAATTCGATTCGCCCAGAAAAGTTAACCCT) and ECRPRP (GAATCTGCAGCATAGCACCAGCGTTTGTCGAAGTGC) whichcontain EcoRI and PstI restriction sites, respectively. The resulting fragments were cloned into pJB3Cm6 (4) and then transferred to SR-558 and SR-559 by conjugation. Both mutants containing S. oneidensis crp (strains SR-570 and SR-571) regained the ability to reduce Fe(III) and Mn(IV), as shown in Fig. 1. They were also able to grow with nitrate, fumarate (Fig. 2), and nitrite (data not shown). Similar results were obtained with mutants complemented with E. coli crp. A representative growth curve of the E. coli crp-complemented strains, SR-637 and SR-638, is shown in Fig. 2D.
Heme c levels in crp mutants.
In addition to loss of anaerobic respiration, both crp mutants exhibited lower levels of heme c than the wild type. Heme c content was measured by the pyridine hemochrome assay as described by Paul et al. (18). Wild-type S. oneidensis, the mutant SR-558, and the complemented mutant SR-570 were grown anaerobically in LM (0.02% peptone, 0.01% yeast extract, 10 mM HEPES [pH 7.5]) supplemented with 50 mM lactate and 10 mM TMAO for 18 h. The cells were harvested and then lysed with bacterial protein extraction reagent (B-PER; Pierce Chemical). The amount of heme c was determined by measuring the absorbance at 550 nm (ɛ550 = 29.1 mM−1 cm−1 [7]). Anaerobically grown SR-558 contained a 0.84 μM concentration of heme c per g of protein compared to 2.1 and 2.8 μM/g for the wild type and the complemented mutant SR-570, respectively.
Analysis of anaerobic reductase activities.
To determine if the deficiency in anaerobic growth in the crp mutants was due to the loss of cytochromes c or terminal reductases, cell extracts were tested for DMSO, nitrate, nitrite, and fumarate reductase activities with methyl viologen used as the electron donor. Strains of S. oneidensis were grown in a Coy anaerobic chamber in Luria-Bertani (LB) medium containing 50 mM lactate and the appropriate electron acceptor. Cell extracts were prepared by using B-PER lysis reagent or as described previously (1). Bands of enzyme activity were visualized on native polyacrylamide electrophoresis gels by using reduced methyl viologen (8 mM) (13) followed by the addition of 10 mM fumarate, 10 mM DMSO, 10 mM nitrate, or 5 mM nitrite. The crp mutants lacked DMSO reductase activity (Fig. 3 A) and nitrate reductase activity (data not shown). Surprisingly, bands that corresponded to nitrite reductase activity were detected (Fig. 3B). Our results suggested that the loss of DMSO and nitrate reduction by whole cells is due to the loss of terminal reductases and possibly other electron transport chain components. The loss of anaerobic growth with nitrite appears to be due to loss of electron transport chain components such as cytochromes c and not to loss of the terminal reductase.
FIG. 3.
Enzyme activity assays of cell extracts with methyl viologen as the electron donor and DMSO (A), nitrite (B), or fumarate (C). Forty micrograms of total protein was used for the DMSO and nitrite reductase assays, and 20 μg of protein was used for the fumarate reductase assay. Lanes 1, wild-type; lanes 2, SR-558; lanes 3, SR-559; lanes 4, SR-570; lanes 5, SR-571, lane 6, SR-515 (fumarate reductase mutant).
Although the crp mutants did not grow with fumarate, low levels of fumarate reductase activity were detected in cell extracts of the mutants (Fig. 3C). This activity may be due to constitutive expression or the presence of another fumarate reductase that is not subject to CRP regulation. To distinguish between these two possibilities, we generated a mutation in fccA, which encodes a fumarate reductase (23). An fccA internal fragment, amplified by using the primers FCC4F (5′-GTACCTGTTGATGCAGAC-3′) and FCC4R (5′-GTCATGTCGGCACCCATAGAG-3′) was cloned into the suicide vector pVIK165 (11). The resulting plasmid was transferred into MR-1R (a rifampin-resistant strain of S. oneidensis) (1) by conjugation. Insertion of the suicide vector into fccA was confirmed by PCR. The resulting fccA mutant, SR-515, was unable to grow anaerobically with fumarate but grew with other terminal electron acceptors used by the wild type (data not shown). Additionally, fumarate reductase activity was not detected in SR-515 cell extracts (Fig. 3C, lane 6). This suggests that S. oneidensis contains a single fumarate reductase that is under CRP control but that is constitutively expressed at low levels.
Induction of fumarate and Fe(III) reductase activities by cAMP in aerobic cultures.
In E. coli, cAMP is required for CRP activity, and its addition overcomes the catabolite repression of glucose (6). Because anaerobic respiratory enzymes such as the fumarate reductase are expressed primarily under anaerobic conditions, we tested the effect of the addition of cAMP on their expression under aerobic conditions. Wild-type S. oneidensis was grown aerobically in 200 ml of LB medium by shaking at 150 rpm in 2.8-liter Fernbach flasks. After 2 to 3 h (optical density at 600 nm [OD600], 0.2 to 0.3), 5 mM cAMP was added and incubation was continued. Cells were harvested at different times and assayed for fumarate, Fe(III), DMSO, and nitrate reductase activities as described above. The addition of cAMP to aerobic cultures resulted in increased fumarate reductase activity to levels similar to those found in anaerobically grown cells (Fig. 4A). The faint band of enzyme activity seen in aerobic cell extracts may represent constitutive levels of fumarate reductase observed in the crp mutants.
FIG. 4.

Effect of exogenous cAMP on fumarate and DMSO reductase activities. (A) Fumarate reductase activity of S. oneidensis determined by using cell extracts from cells grown anaerobically with fumarate (lane 1); aerobically for 3 h (lane 2); or aerobically for 3 h followed by exposure to cAMP for 15 min (lane 3), 30 min (lane 4), 45 min (lane 5), or 60 min (lane 6). Twenty micrograms of protein was used for each lane. Cultures were grown in LB medium. (B) Fumarate reductase activity of S. oneidensis determined by using cell extracts from cells grown anaerobically with fumarate (lane 1), aerobically for 3 h (lane 2), or aerobically for 3 h followed by exposure to cAMP for 60 min (lane 3). Cultures were grown in basal medium supplemented with lactate. (C) DMSO reductase activity of S. oneidensis cell extracts from cells grown anaerobically with DMSO (lane 1), aerobically for 3 h (lane 2), or aerobically for 3 h and then exposed to cAMP for 60 min (lane 3). Lane 4 is the same as lane 3 except that DMSO was included in the growth medium. Cultures were grown in LB medium.
Fe(III) reduction was also induced in aerobic cultures by the addition of cAMP. Aerobically grown cells produced 58 μM Fe(II)/min/unit of OD600, compared to 320 μM Fe(II)/min/OD600produced by anaerobic cells. cAMP treatment of aerobically growing cells led to a threefold increase in Fe(III) reduction [169 μM Fe(II)/min/OD600].
In contrast to fumarate and Fe(III) reduction, neither DMSO reductase activity (Fig. 4C) nor nitrate reductase activity (data not shown) was detected in cAMP-supplemented aerobic cultures. It is clear that although CRP and cAMP play a role in regulating the expression of several reductases in S. oneidensis, other regulatory proteins or factors may also be involved.
The increased reductase activities described above were observed when cAMP-treated cultures were grown in a rich medium (LB medium). We performed similar experiments using cells grown aerobically in basal medium supplemented with lactate and Casamino Acids as described above. The addition of cAMP did not result in an increase in fumarate reductase activity under these conditions (Fig. 4B). These results suggest that cAMP addition does not act simply by relieving possible catabolite repression caused by nutrients in LB medium. If these nutrients act as catabolite repressors, growth in basal medium should relieve this inhibition and result in increased enzyme activity even in the absence of exogenous cAMP. The fumarate reductase of S. oneidensis is a flavocytochrome c (23), and it is possible that the gene is expressed but is not translated. Alternatively, the protein may be present in an inactive form due to lack of other components such as flavins or heme c. Further analyses of gene expression and differences in components of cells grown in LB or minimal medium should help elucidate other factors, in addition to CRP and cAMP, that control fumarate reductase expression.
CRP belongs to the CRP/FNR family of global regulators. In both E. coli and Salmonella enterica serovar Typhimurium, CRP regulates gene expression in response to glucose levels (6, 21). When glucose levels are low, cAMP levels become elevated (5, 8). cAMP binds and activates CRP, resulting in increased expression of genes involved in carbon catabolism (for a review, see reference 20). The isolation of S. oneidensis mutants deficient in CRP allowed us to begin a preliminary investigation of its role in this organism. Although we have shown that mutations in crp result in loss of anaerobic reductase activities, we cannot determine if CRP acts by binding to consensus sequences upstream of the reductase genes, and directly activating gene expression, or if it affects the expression of other regulatory proteins. The availability of the genome sequence of S. oneidensis (10) made it possible to identify possible CRP-binding sites upstream of putative terminal reductase genes. We found a potential CRP binding site upstream of the predicted nitrate reductase operon (TGAGA------TCACG), which matches very closely the E. coli CRP consensus sequence (TGTGA------TCACA). A similar sequence was identified upstream of a putative DMSO reductase operon (TGTAA------TTACA). Potential half sites for CRP binding were also found upstream of the fumarate reductase gene. Although it is tempting to speculate that CRP regulates expression of anaerobic reductases directly by binding to these sequences, evidence for such interactions is not yet available. Additionally, the assigned functions for most of the putative reductase genes have not been confirmed by experimental evidence. Nonetheless, the regulation of anaerobic respiration in S. oneidensis appears to be quite different from the regulation in other studied bacteria. Further work will be needed to identify other regulators of genes involved in anaerobic growth and to identify the exact role of CRP and cAMP in anaerobic respiration.
Acknowledgments
This work was supported by Department of Energy grant DE-FG02-00ER15068 and National Science Foundation grant MCB 9896375.
We thank M. McBride for helpful discussions and critical reading of the manuscript.
REFERENCES
- 1.Beliaev, A., and D. Saffarini. 1998. Shewanella putrefaciens mtrB encodes an outer membrane protein required for Fe(III) and Mn(IV) reduction. J. Bacteriol. 180:6292-6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beliaev, A., D. Saffarini, J. McLaughlin, and D. Hunnicut. 2001. MtrC, an outer membrane decaheme c cytochrome required for metal reduction in Shewanella putrefaciens MR-1. Mol. Microbiol. 39:722-730. [DOI] [PubMed] [Google Scholar]
- 3.Beliaev, A., D. Thompson, M. Fields, L. Wu, D. Lies, K. Nealson, and J. Zhou. 2002. Microarray transcription profiling of a Shewanella oneidensis etrA mutant. J. Bacteriol. 184:4612-4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blatny, J. M., T. Brautaset, H. C. Winther-Larsen, K. Haugan, and S. Valla. 1977. Construction and use of a versatile set of broad-host-range cloning and expression vectors based on the RK2 replicon. Appl. Environ. Microbiol. 63:370-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Botsford, J., and J. Harman. 1992. Cyclic AMP in prokaryotes. Microbiol. Rev. 56:100-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busby, S., and A. Kolb. 1996. The CAP modulon, p. 255-279. In E. C. Lin and A. Lynch (ed.), Regulation of gene expression in Escherichia coli. Chapman & Hall, New York, N.Y.
- 7.Fuhrhop, J.-H., and K. M. Smith. 1975. Laboratory methods, p. 757-869. In K. M. Smith (ed.), Porphyrins and metalloporphyrins. Elsevier, Amsterdam, The Netherlands.
- 8.Green, J., C. Scott, and J. Guest. 2001. Functional versatility in the CRP-FNR superfamily of transcriptional factors: FNR and FLP. Adv. Microb. Physiol. 44:1-34. [DOI] [PubMed] [Google Scholar]
- 9.Guest, J., J. Green, A. Irvine, and S. Spiro. 1996. The FNR modulon and FNR-regulated gene expression, p. 317-342. In E. C. Lin and A. Lynch (ed.), Regulation of gene expression in Escherichia coli. Chapman & Hall, New York, N.Y.
- 10.Heidelberg, J., I. Paulsen, K. Nealson, E. Gaidos, W. Nelson, T. Read, et al. 2002. Genome sequence of the dissimilatory metal ion-reducing bacterium Shewanella oneidensis. Nat. Biotechnol. 20:1118-1123. [DOI] [PubMed] [Google Scholar]
- 11.Kalogeraki, V., and S. Winans. 1997. Suicide plasmids containing promoterless reporter genes can simultaneously disrupt and create fusions to target genes of diverse bacteria. Gene 188:69-75. [DOI] [PubMed] [Google Scholar]
- 12.Kiley, P. J., and H. Beinert. 1998. Oxygen sensing by the global regulator, FNR: the role of the iron-sulfur cluster. FEMS Microbiol. Rev. 22:341-352. [DOI] [PubMed] [Google Scholar]
- 13.Lund, K., and J. DeMoss. 1976. Association-dissociation behavior and subunit structure of heat-released nitrate reductase from Escherichia coli. J. Biol. Chem. 251:2207-2216. [PubMed] [Google Scholar]
- 14.Maier, T. M., and C. R. Myers. 2001. Isolation and characterization of a Shewanella putrefaciens MR-1 electron transport regulator etrA mutant: reassessment of the role of EtrA. J. Bacteriol. 183:4918-4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myers, C., and J. Myers. 1992. Localization of cytochromes to the outer membrane of anaerobically grown Shewanella putrefaciens MR-1. J. Bacteriol. 174:3429-3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nealson, K., and D. Saffarini. 1994. Iron and manganese in anaerobic respiration: environmental significance, physiology, and regulation. Annu. Rev. Microbiol. 48:311-343. [DOI] [PubMed] [Google Scholar]
- 17.Obuekwe, C., and D. Westlake. 1982. Effects of medium composition on cell pigmentation, cytochrome content and ferric iron reduction in a Pseudomonas sp. isolated from crude oil. Can. J. Microbiol. 28:989-992. [DOI] [PubMed] [Google Scholar]
- 18.Paul, K. G., H. Theorell, and Å. Åkeson. 1953. The molar light absorption of pyridine ferroprotoporphyrin (pyridine haemochromogen). Acta Chem. Scand. 7:1284-1287. [Google Scholar]
- 19.Saffarini, D., and K. Nealson. 1993. Sequence and genetic characterization of etrA, an fnr analog that regulates anaerobic respiration in Shewanella putrefaciens MR-1. J. Bacteriol. 175:7938-7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saier, M., T. Ramseier, and J. Reizer. 1996. Regulation of carbon utilization, p. 1325-1343. In F. C. Neidhardt et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C.
- 21.Spector, M. 1998. The starvation stress response (SSR) of Salmonella. Adv. Microb. Physiol. 40:233-279. [DOI] [PubMed] [Google Scholar]
- 22.Tanner, R. 1997. Cultivation of bacteria and fungi, p. 52-60. In C. Hurst, G. Knudsen, M. McInerney, L. Stetzenbach, and M. Walter (ed.), Manual of environmental microbiology. ASM Press, Washington, D.C.
- 23.Tsapin, A. I., I. Vandenberghe, K. H. Nealson, J. H. Scott, T. E. Meyer, M. A. Cusanovich, E. Harada, T. Kaizu, H. Akutsu, D. Leys, and J. J. Van Beeumen. 2001. Identification of a small tetraheme cytochrome c and aflavocytochrome c as two of the principal soluble cytochromes c in Shewanella oneidensis strain MR1. Appl. Environ. Microbiol. 67:3236-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venkateswaran, K., D. P. Moser, M. E. Dollhopf, D. P. Lies, D. A. Saffarini, B. J. MacGregor, D. B. Ringelberg, D. C. White, M. Nishijima, H. Sano, J. Burghardt, E. Stackebrandt, and K. H. Nealson. 1999. Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int. J. Syst. Bacteriol. 49:705-724. [DOI] [PubMed] [Google Scholar]