Abstract
Pseudomonas aeruginosa controls the secretion of extracellular virulence factors, including rhamnolipids and LasB elastase, by the las and rhl quorum-sensing systems. Here, we mutated the dksA gene of P. aeruginosa by insertion of an Ω-Hg cassette. The mutant displayed growth rates similar to that of the wild type in rich medium but was impaired in growth in defined minimal medium. Production of rhamnolipids and LasB elastase by the dksA mutant was only 4 and 10%, respectively, of wild-type levels. These defects could be partially complemented by introduction of the plasmid-encoded dksA genes from P. aeruginosa or Escherichia coli. In the dksA mutant, the expression of rhlI was increased early during exponential growth, but expression of other quorum-sensing regulator genes—lasR, lasI, and rhlR—was not affected. Although the transcription of the lasB and rhlAB genes was comparable between the dksA mutant and the wild-type strain in peptone tryptic soy broth medium, we observed reduced translation of both genes in the dksA mutant. Similarly, we found that full translation of lasB and rhlAB genes in E. coli also requires the dksA gene. DksA is therefore a novel regulator involved in the posttranscriptional control of extracellular virulence factor production in P. aeruginosa.
The opportunistic pathogen Pseudomonas aeruginosa is responsible for severe nosocomial infections in immunocompromised and intubated patients (4). In addition, P. aeruginosa is the most commonly found pathogen in cystic fibrosis (CF) patients and is responsible for progressive lung tissue destruction leading to respiratory failure (4). P. aeruginosa produces a wide spectrum of secreted virulence factors, including LasB elastase, rhamnolipids, pyocyanin, lipase, and hydrogen cyanide (30). The las and rhl quorum-sensing systems regulate the production of these factors in a cell density-dependent manner. This regulation relies on the accumulation in the medium of two autoinducer (AI) molecules, 3-oxo-C12-homoserine lactone (3-oxo-C12-HSL) and C4-HSL, that induce the las and rhl quorum-sensing systems when the bacterial cell density reaches a certain threshold (quorum), leading to transcription of specific genes and the production of the virulence factors cited above. Both systems involve a transcriptional regulator (RhlR and LasR, respectively) and an AI synthase (RhlI and LasI, respectively) (30). A hierarchy was proposed in which the las system activates the rhl system by inducing the transcription of the activator gene rhlR (12, 20).
Additional layers of regulation of the quorum-sensing circuitry have been described. These include the transcriptional regulator vfr (1), a homologue of the Escherichia coli cyclic AMP-binding protein, the GacA two-component regulator (23), the enzyme polyphosphate kinase (22), and the mvaT gene product which modulates the timing of quorum-sensing activation (5). Recently, we have shown that overexpression of the starvation response regulator encoded by relA, leading to increased production of the nutrient stress signal ppGpp, is able to induce the quorum-sensing circuitry of P. aeruginosa even at low cell densities (29). Furthermore, we also demonstrated that overexpressing dksA reduces the expression of the rhlI gene by an as-yet-unknown mechanism (2). DksA was discovered initially as a protein able to suppress the temperature-sensitive growth of an E. coli dnaK mutant (10). Subsequently, dksA homologues were identified in Salmonella enterica serovar Typhimurium (28) and recently in Shigella flexneri (16). DksA plays a role in virulence of both organisms. In serovar Typhimurium, dksA controls the expression of the stationary-phase sigma factor rpoS (33), and a dksA mutant was less virulent than the parental strain when tested in 3-week-old hatched chickens (28). In S. flexneri, dksA is involved in intercellular spread upon infection of epithelial cell layers. Unlike in Salmonella, the effect of dksA does not depend on the rpoS sigma factor in S. flexneri (16). From these observations, it appears that dksA could be a general regulator of virulence. In P. aeruginosa, regulation of rpoS expression is extremely complex and controversial. Earlier work suggested that RhlR regulates the transcription of the stationary-phase sigma factor RpoS (12), whereas it was proposed more recently that rhlI expression is inhibited early during exponential growth by RpoS (34). In E. coli, RpoS is regulated by the nutrient stress signal ppGpp. Furthermore, deletion of dksA in E. coli was shown to block rpoS induction by ppGpp (3), suggesting that DksA indirectly regulates rpoS expression.
To further characterize the role of DksA in the nosocomial pathogen P. aeruginosa, we constructed a dksA knockout mutant and analyzed its quorum-sensing-dependent virulence properties. We found that DksA is required for full translation of the lasB elastase gene and the rhamnosyltransferase encoding rhlAB gene.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this work are described in Table 1. Bacteria were grown at 37°C with aeration in peptone tryptic soy broth (PTSB) (17), unless otherwise indicated.
TABLE 1.
Bacterial strains, plasmids, and bacteriophage
| Strain, plasmid, or bacteriophage | Genotype or relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| S17-1 | thi pro hsdR recA chr::RP4-2 | 26 |
| MG4λI14 | λ lysogen carrying a chromosomal lasI::lacZ fusion | 24 |
| PK201 | MG1655 (F−) ΔdksA Kanr | EGSC (10)b |
| MC1061 | F−araD139 Δ(ara-leu)7696 galE15 galK16 Δ(lac)X74 rpsL thi | Laboratory collection |
| MC1061D | MC1061 ΔdksA Kanr | This study |
| P. aeruginosa | ||
| PT5 | PAO1 wild-type | Laboratory collection |
| PAO-JP2 | PDO100ΔlasI Tetr Hgr | 19 |
| PAO-RC1 | PT5 ΔdksA Hgr | This study |
| Plasmids | ||
| pBluescript II SK(+) | General-purpose cloning vector, ori (ColE1); Apr | Stratagene |
| pME3088 | Suicide plasmid; Tetr | 32 |
| pVD99.3 | pUCP24 containing dksA on a 960-bp SmaI fragment; Gmr | 2 |
| pRK7813 | RP4-based cosmid vector; Tetr | 9 |
| pRKD1 | dksA in pRK7813; Tetr | This study |
| pRKDE1 | dksA (E. coli) in pRK7813; Tetr | This study |
| pPB101 | dksA in pME3088; Tetr | This study |
| pPB102 | dksA::ΩHg in pME3088; Hgr Tetr | This study |
| pLP170 | Promoterless lacZ fusion vector; Apr | 21 |
| pSW205 | Promoterless lacZ fusion vector; Apr | 7 |
| p101.170 | lasB::lacZ (transcriptional) on pLP170; Apr | This study |
| pECP65 | rhlAB::lacZ (transcriptional) on pLP170; Apr | E. C. Pesci |
| pTS400 | lasB::lacZ (translational) on pSW205; Apr | 18 |
| pECP60 | rhlAB::lacZ (translational) on pSW205; Apr | 20 |
| pPCS1001 | lasR::lacZ (transcriptional) on pLP170; Apr | 20 |
| pPCS223 | lasI::lacZ (transcriptional) on pLP170; Apr | 31 |
| pPCS1002 | rhlR::lacZ (transcriptional) on pLP170; Apr | 20 |
| pLPRI | rhlI::lacZ (transcriptional) on pLP170; Apr | 31 |
| pECP61.5 | rhlAB::lacZ (translational) ptac::rhlR Apr | 19 |
| pECP64 | lasB::lacZ (translational) ptac::lasR Apr | 19 |
| Bacteriophage | ||
| P1 | Transducing phage | S. Raina |
Resistance phenotypes: Tet, tetracycline; Ap, ampicillin; Kan, kanamycin; Gm, gentamicin.
EGSC, E. coli Genetic Stock Center, Yale University, New Haven, Conn.
DNA techniques.
Restriction endonucleases and DNA-modifying enzymes were purchased from Q-BioGene or Gibco-BRL. Plasmids were introduced into E. coli by transformation (13) and into P. aeruginosa by conjugation or electroporation (27). Other DNA manipulations were performed according to standard protocols (13).
Construction of a P. aeruginosa dksA mutant.
To generate a P. aeruginosa dksA mutant, a 960-bp SmaI fragment from plasmid pVD99.3 (2), carrying the dksA gene, was cloned into the suicide vector pME3088 (32), yielding plasmid pPB101. A 130-bp deletion between the EcoRI and SphI sites within the dksA gene was generated. After end polishing with T4 polymerase, the Ω-Hg cassette from plasmid pHP45ΩHg (6) was inserted as a SmaI fragment. The resulting plasmid, pPB102, was mobilized by conjugation from E. coli strain S17-1 into P. aeruginosa wild-type strain PT5. Clones which were mercury resistant (Hgr; encoded by the dksA::Hgr allele) and tetracycline susceptible (encoded by the plasmid vector) were considered putative dksA mutants. The replacement of the wild-type dksA gene with the dksA::Hgr allele was verified by Southern hybridization (data not shown). One clone presenting the expected hybridization pattern was selected for further studies and named PAO-RC1.
Construction of an E. coli dksA mutant.
The E. coli dksA deletion allele was transferred from E. coli strain PK201 (MG1655 dksA::Kan) (10) to E. coli strain MC1061 by P1 transduction. Growth of phage P1 on dksA deletion strain PK201 was obtained after 3 h of culture in Luria-Bertani (LB) medium with aeration. The lysate was filtered (0.22-μm pore size) and conserved at 4°C. Recipient strain MC1061 (108 CFU/ml) was incubated in TNC buffer (0.01 M Tris-HCl, pH 7.4; 0.15 M NaCl, 0.01 M CaCl2) with P1 lysate (5 × 108 PFU/ml) for 15 min at 42°C. P1 transductants appeared on plates containing 25 μg of kanamycin/ml and 0.001 M sodium citrate after incubation at 37°C for 48 h. Replacement of the wild-type dksA gene by the dksA::Kan allele was verified by Southern hybridization with three different restriction endonucleases (data not shown). The obtained hybridization patterns were identical between the three transductants and the original dksA mutant PK201. One transductant, MC1061D, was used for further experiments.
Plasmid constructions.
For complementation experiments, the P. aeruginosa dksA gene was cloned as a 960-bp SmaI fragment, obtained from plasmid pVD99.3, into the SmaI site of pBluescript II SK(+). The resulting construct was then digested with HindIII and BamHI, and the generated fragment, containing the dksA gene, was subsequently ligated into HindIII-BamHI-digested cosmid vector pRK7813 (9), yielding plasmid pRKD1. In this construct, the P. aeruginosa dksA gene conserved its own promoter and Shine-Dalgarno sequence.
Similarly, the E. coli dksA gene was cloned into plasmid pRK7813 by first ligating the 520-bp NheI-PstI fragment from plasmid pMPM31 into EcoRV-PstI-digested pBluescript II SK(+) after the NheI 5′ protruding end was filled in with the Klenow fragment. From this construct, a 530-bp HindIII-BamHI fragment, which contains the E. coli dksA ORF with its own Shine-Dalgarno sequence, was ligated into the HindIII-BamHI-digested plasmid pRK7813, downstream of the plac promoter, yielding plasmid pRKDE1.
To construct a transcriptional lasB::lacZ fusion containing the same regulatory DNA sequence as the translational lacZ fusion pTS400, a 400-bp fragment was amplified by PCR with pTS400 plasmid DNA as a template. An EcoRI and a BamHI site were inserted at the 5′ and 3′ ends, respectively, by using the primers LasBEco (5′-CGGAATTCCAGAAAGCGTGCAACTGAT-3′) and LacZPoly (5′-GACGGGATCCCCGGG-3′). The PCR fragment was digested with these enzymes and ligated into EcoRI-BamHI-cleaved pLP170 to yield plasmid p101.170. Insertion sites were verified by DNA sequencing.
Elastase and rhamnolipid production assays.
Elastase production was measured by elastin Congo red assays as previously described (31). Rhamnolipid production was measured on SW blue plates by inoculating strains in M9-based (13) agar plates supplemented with 0.2% glycerol (vol/vol), 2 mM MgSO4, trace elements, 5 mM KNO3 instead of NH4Cl as an N source, 0.0005% (vol/vol) methylene blue, and 0.02% (vol/vol) cetyltrimethylammonium bromide (25). Plates were incubated first at 37°C for 24 h and then for at least 48 h at room temperature until a blue halo appeared around the colony. For quantitative assays, rhamnolipids were extracted from culture supernatants and then grown in M9 minimal medium supplemented with 2% glycerol (vol/vol), 2 mM MgSO4, trace elements, 0.05% glutamate (instead of NH4Cl), and 0.05% Casamino Acids. After ether extraction, rhamnolipids were quantified by the orcinol procedure (19).
β-Galactosidase activity assays.
β-Galactosidase activity was measured as previously described (15), with the following modifications. P. aeruginosa cultures harboring lacZ fusion plasmids were grown for 18 h at 37°C with vigorous shaking in PTSB medium (17) containing the appropriate antibiotics and then inoculated into the same medium without antibiotic to a starting optical density at 660 nm (OD660) of 0.05. Samples were harvested at regular intervals during growth for determination of the turbidity at 660 nm and β-galactosidase measurements. All experiments were done in triplicate and performed at least twice.
Overnight cultures of E. coli strains MC1061 and MC1061D containing plasmid pECP61.5 or pECP64 were diluted to an OD600 of 0.08 and grown at 37°C with shaking to an OD600 of 0.3. Then, 1-ml aliquots of cultures were then grown for 90 min with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) in the presence or absence of AIs. The β-galactosidase activity was measured as described previously (15).
Determination of AI concentrations.
Culture supernatants were extracted with ethyl acetate, and AI concentrations were determined in bioassays as previously described, by using E. coli MG4λI14(pPCS1) for 3-oxo-C12-HSL (24) and P. aeruginosa PAO-JP2(pECP61.5) for C4-HSL (29).
Production of DksA antibodies.
The E. coli dksA gene was expressed in E. coli strain MC1061 under the control of an arabinose-inducible promoter (14). Exponentially growing cells were induced for 5 to 6 h at 37°C by the addition of 67 μM (final concentration) l-arabinose. The cell paste (12.5 g) was resuspended in 375 ml of buffer A (50 mM Tris-HCl [pH 7.5], 1 mM EDTA, 10 mM β-mercaptoethanol) containing 1 mM phenylmethylsulfonide fluoride and then disrupted by sonication. Cell debris was removed by centrifugation, and the supernatant was subjected to fractionated polyethyleneimine precipitation (0.003 and 0.008%). The 0.008% pellet was extracted twice with 15 ml of 1 M NaCl in buffer A, and the pooled supernatants were precipitated with 60% ammonium sulfate. The ammonium sulfate pellet was dissolved in 1 ml of buffer B (100 mM Tris-HCl [pH 7.5], 0.5 mM EDTA, 10 mM β-mercaptoethanol) and loaded onto a Superdex 200 (Hiload 16/60; Amersham) gelfiltration column equilibrated in buffer B. The DksA-containing fractions were pooled, dialyzed against buffer C (20 mM NaxHyPO4 [pH 7.0], 10% glycerol), and loaded onto a heparin-agarose column (Bio-Rad). DksA was eluted with a linear gradient from 50 to 400 mM NaCl. The DksA-containing fractions were dialyzed against 10 mM NaxHyPO4 (pH 7.0)-10% glycerol, loaded onto a hydroxyapatite column, and eluted with 100 mM NaxHyPO4 (pH 7.0)-10% glycerol. Purified DksA protein (63 mg) was frozen in liquid N2 and stored at −80°C. The purity, estimated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, was >95%. For antiserum production, 100 μg of purified DksA protein was mixed with aluminum hydroxide and injected into New Zealand White rabbits. After three injections, the antibody titer was sufficient, and the final bleed was made after a fourth injection.
Protein extraction, electrophoresis, and Western blot analyses.
To prepare cell lysates for such assays, overnight cultures in LB medium were diluted 1:100 into fresh LB medium, and the cultures were incubated at the appropriate temperature with shaking until the OD600 (E. coli) or OD660 (P. aeruginosa) of the cultures reached ca. 0.6 to 0.7. The cells were then harvested and washed in 30 mM MOPS (morpholinepropanesulfonic acid) and 200 mM NaCl (pH 8.0) buffer. Cells were resuspended in 30 mM MOPS, 200 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 2 mM benzamidine, and 10 mM 2-β-mercaptoethanol buffer, with a concentration of 0.1 g of cells/ml. Cells were broken by sonication on ice, and cytoplasmic and membrane extracts were separated by ultracentrifugation at 35,000 rpm during 1 h at 4°C.
Protein samples were subjected to electrophoresis in 12.5% polyacrylamide gels containing 0.4% SDS. Equal amounts of protein samples (10 μg) were loaded per lane, except for purified E. coli DksA protein (50 ng/lane). Gels were stained with Coomassie brilliant blue. Alternatively, the proteins were transferred to nitrocellulose membrane and detected by using polyclonal rabbit anti-DksA serum diluted 1:1,000. Protein-antibody complexes were visualized by using a chemiluminescent detection method (Lumi-Light Western blotting substrate; Roche).
RESULTS
Cellular localization of the DksA protein.
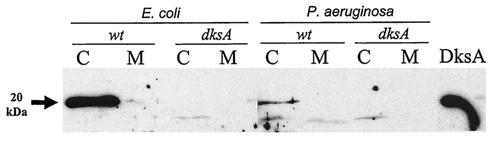
Despite the increasing amount of data regarding DksA, the cellular localization of this protein has never been analyzed. We therefore attempted to detect the DksA protein in cytoplasmic and membrane fractions from exponentially growing E. coli and P. aeruginosa cells. A polyclonal antiserum raised against the E. coli DksA protein was used to detect the protein in Western blots. The antiserum strongly reacted with the purified DksA protein from E. coli (deduced molecular weight of 17,528) (Fig. 1) and with a protein of similar molecular weight present in cytosolic but not in membrane fractions of E. coli strain MC1061 (Fig. 1). As expected, this protein band was absent in extracts from the dksA mutant MC1061D. A 20-kDa protein reacting with DksA antiserum was also found in cytosolic extracts of the P. aeruginosa wild-type strain PT5, albeit with lower signal intensity (Fig. 1). Since this signal was absent in extracts from the dksA mutant PAO-RC1, we considered that the protein corresponded to DksA of P. aeruginosa. Hence, DksA is a cytosolic protein both in exponentially growing E. coli and in P. aeruginosa cells and therefore is expected to interact with a target also localized in the cytosol.
FIG. 1.
Western blot analyses of supernatants and cell sonic extracts from E. coli and P. aeruginosa wild type (wt) (MC1061 and PT5, respectively) and dksA mutants (dksA) (MC1061D and PAO-RC1, respectively). A total of 10 μg of protein from cell membrane and cytosolic fractions was collected, separated by SDS-polyacrylamide gel electrophoresis, and transferred to nitrocellulose membranes. The blot was probed with anti-DksA antiserum, and reactive bands were visualized by chemiluminescence, as described in Materials and Methods. Lanes: C, cytosolic fraction; M, membrane fraction. The arrow indicates the 20-kDa protein corresponding to DksA. The lane marked DksA contains 50 ng of purified E. coli DksA protein.
Construction and growth of a P. aeruginosa dksA mutant.
Recently, we described the effect of dksA overexpression on the quorum-sensing circuit of P. aeruginosa. dksA expressed on a multicopy plasmid inhibited the transcription of the rhlI gene, leading to a reduced production of C4-HSL (2). In order to further analyze the role played by dksA in the regulation of quorum-sensing-dependent virulence factor production, we constructed a dksA deletion mutant in the P. aeruginosa wild-type strain PT5, as described in Materials and Methods. The dksA mutant of PT5 was designated PAO-RC1. We first compared the growth of this mutant in rich and defined media since growth defects had been reported for the E. coli dksA mutant (3). Whereas both strains displayed similar growth rates in PTSB medium (Fig. 2A), we observed reduced growth of the P. aeruginosa dksA mutant in M9 minimal medium (13) (Fig. 2B). This growth defect was almost completely relieved by the addition of 0.05% Casamino Acids (Fig. 2C), suggesting that, as in E. coli, dksA is involved in amino acid biosynthesis in P. aeruginosa.
FIG. 2.
Growth curves of PT5 (▪) and dksA mutant PAO-RC1 (•) in PTSB medium (A) and in M9-glucose medium, 2 mM MgSO4, and trace elements without (B) or with (C) 0.05% Casamino Acids. Shown are the means ± the standard deviations of three independent experiments.
Production of quorum-sensing-regulated virulence factors is affected in the dksA mutant.
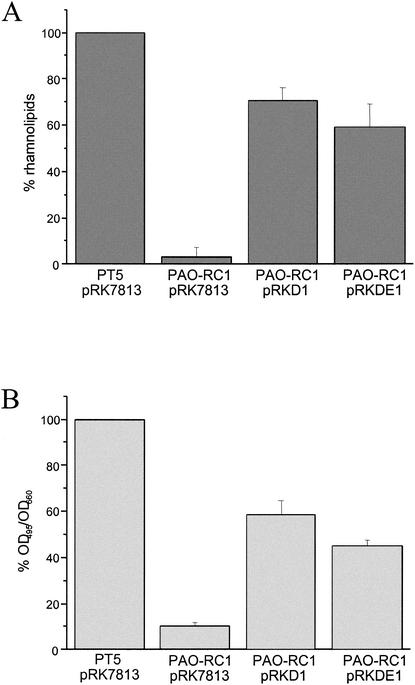
Since we previously found that dksA overexpression interferes with quorum sensing (2), we tested in both the wild-type and the dksA mutant the production of two quorum-sensing-regulated virulence factors, namely, rhamnolipids and LasB elastase. Surprisingly, the dksA mutant PAO-RC1 produced no rhamnolipids, as measured by our plate assay (data not shown). Rhamnolipids were further extracted from 36-h culture supernatants and then quantified by the orcinol assay. The amount of rhamnolipid produced by the mutant was only 4% of that of the wild-type strain (Fig. 3A). We further determined the production of LasB elastase after growth in PTSB medium after 16 h growth at 37°C by using the elastin-Congo red assay. We found that the LasB elastase activity in supernatants of the dksA mutant was only 10% of that found in wild-type supernatants (Fig. 3B).
FIG. 3.
(A) Rhamnolipid production as determined by orcinol assay. Strains to be tested were grown for 36 h in M9-glycerol medium as described in Materials and Methods. Means of triplicate determinations are expressed as the percentage of wild-type production. (B) Elastase production was determined on filtered culture supernatants of strains grown in PTSB medium for 16 h. Elastin-Congo red determinations (OD495) were performed on three different occasions. The means of these results, divided by the cell density measured at OD660, are represented as the percentage of wild-type activity. Plasmid pRKDE1 and pRKD1 carry the dksA genes of E. coli and P. aeruginosa on the low-copy-number plasmid pRK7813, respectively.
The production of both rhamnolipids (Fig. 3A) and elastase (Fig. 3B) could be partially restored in the dksA mutant by complementation in trans with the low-copy-number plasmid pRKD1 containing the P. aeruginosa dksA gene and with the E. coli dksA gene provided on plasmid pRKDE1. In agreement with the high degree of sequence similarity, this result further demonstrates a functional conservation between the two DksA homologues. The lack of complete complementation of PAO-RC1 might be due to nonoptimal expression of dksA from the plasmid constructs. Together with our previous observations from overexpression of dksA (2), these results suggest that the precise amount of DksA during growth is crucial for its regulatory effects.
Expression of quorum-sensing regulator genes in the dksA mutant.
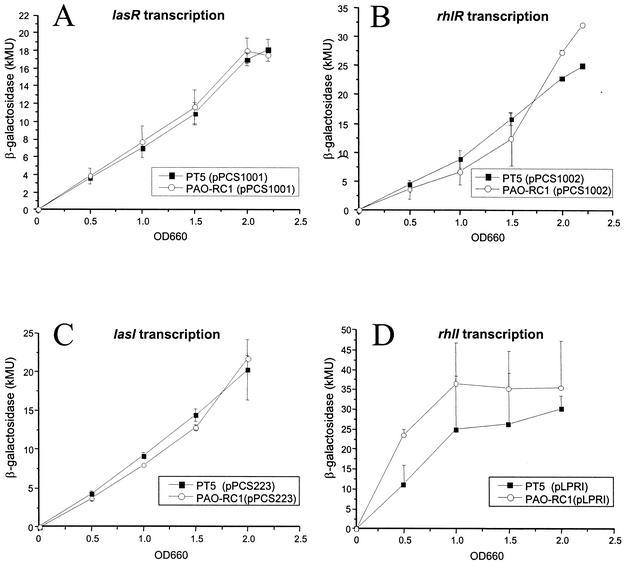
Both lasB and rhlAB are controlled by the las and rhl quorum-sensing systems. To determine whether the dramatically decreased expression of rhamnolipids and elastase resulted from altered expression of the quorum-sensing regulatory genes, we introduced plasmid encoded transcriptional lacZ fusions to the lasR (pPCS1001), rhlR (pPCS1002), lasI (pPCS223), and rhlI (pLPRI) genes into wild-type PT5 and mutant PAO-RC1. No differences in the transcription of lasR, rhlR, and lasI were found when β-galactosidase levels were measured during growth in PTSB medium (Fig. 4A to C). rhlI transcription was increased in mutant PAO-RC1 compared to PT5 during early growth, but this difference decreased after an OD660 of 1.0 (Fig. 4D). The increase in rhlI expression in the dksA mutant correlates with our previous observation of decreased rhlI expression when dksA was overexpressed from a plasmid (2). These experiments suggest that dksA does not influence the transcription of lasR, lasI, and rhlR under these conditions but seems to inhibit the transcription of rhlI during the early logarithmic phase.
FIG. 4.
β-Galactosidase activities, expressed from lasR-lacZ (pPCS1001) (A), rhlR-lacZ (pPCS1002) (B), lasI-lacZ (pPCS223) (C), and rhlI-lacZ (pLPRI) (D) fusions, were determined during growth in PTSB medium. Experiments were repeated on three different occasions in triplicate. Error bars represent the standard deviations of three determinations.
DksA affects translation but not transcription of lasB and rhlAB genes.
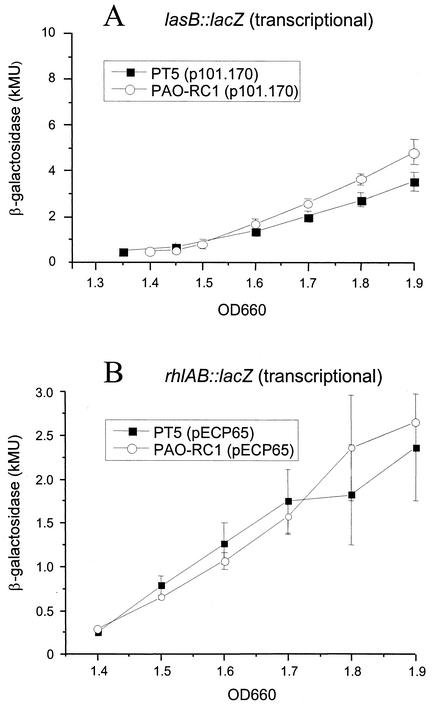
Since the transcription of the quorum-sensing regulatory genes was not reduced in mutant PAO-RC1, we measured the transcription of the elastase (lasB) and rhamnosyltransferase (rhlAB) genes in the wild-type PT5 and the dksA mutant PAO-RC1. For the lasB gene, the transcriptional lacZ fusion p101.170 was constructed and introduced into the two strains. Surprisingly, in PTSB medium we observed the same levels of expression in the wild type and in the dksA mutant (Fig. 5A), suggesting that transcription of lasB was not affected by the dksA mutation. To test the transcription of rhlAB, we introduced plasmid pECP65 (kindly provided by E. Pesci) carrying a rhlAB-lacZ fusion, into the wild type and the dksA mutant. Again, there was no significant difference in the expression profile of the rhlAB-lacZ fusion between the two strains (Fig. 5B).
FIG. 5.
β-Galactosidase activities expressed from transcriptional lasB-lacZ (A) and rhlAB-lacZ (B) fusions. Expression was monitored during growth in PTSB medium by using the plasmids p101.170 and pECP65, respectively. Experiments were repeated on three different occasions and performed in triplicate. Error bars represent the standard deviations of three determinations.
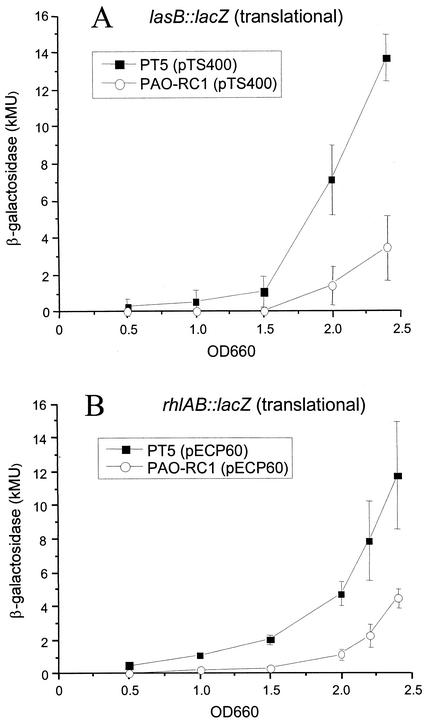
Since it has been suggested that the mechanism of action of DksA could involve translational control in S. enterica serovar Typhimurium (28), we decided to measure translational fusions to the lasB and rhlAB genes carried on plasmids pTS400 and pECP60, respectively. This time we observed an almost fourfold reduction of lasB expression in the dksA mutant compared to the wild type toward the end of the exponential growth phase in PTSB medium (Fig. 6A). Likewise, expression of the rhlAB operon encoding rhamnosyltransferase was decreased threefold in the dksA mutant PAO-RC1 compared to the levels detected in the parent strain PT5 (Fig. 6B). These results suggest that the drastically reduced LasB elastase and rhamnolipid production in mutant PAO-RC1 (Fig. 2) might be caused by a reduction in both lasB and rhlAB translation and under these conditions support a role for DksA in the posttranscriptional control of lasB and rhlAB gene expression.
FIG. 6.
β-Galactosidase activities expressed from translational lasB-lacZ (A) and rhlAB-lacZ (B) fusions. Expression was monitored during growth in PTSB medium by using plasmids pTS400 and pECP60, respectively. Experiments were repeated on three different occasions and performed in triplicate. Error bars represent the standard deviations of three determinations.
DksA is required for translation of lasB and rhlAB in E. coli.
In order to confirm the effect of dksA on lasB and rhlAB expression in a heterologous background, we decided to measure the expression of the lasB-lacZ and rhlAB-lacZ translational reporter fusions in an E. coli dksA mutant. We therefore monitored β-galactosidase activities in E. coli strain MC1061 and in its dksA mutant derivative MC1061D (see Materials and Methods) containing plasmid pECP64 (lasB-lacZ ptac-lasR) or plasmid pECP61.5 (rhlAB-lacZ ptac-rhlR) in the presence or absence of synthetic 3-oxo-C12-HSL or C4-HSL, respectively. As previously described (18), we found that E. coli containing either pECP64 or pECP61.5 in the absence of exogenous AIs yielded a low basal expression of lasB-lacZ and rhlAB-lacZ (Fig. 7). The addition of 3-oxo-C12-HSL at a final concentration of 50 nM caused LasR to activate lasB-lacZ ca. 10-fold in the wild-type MC1061(pECP64), whereas no activation was observed in mutant MC1061D(pECP64) (Fig. 7A). Similarly, expression of rhlAB-lacZ increased 10-fold when wild-type MC1061(pECP61.5) was grown with 1 μM C4-HSL but remained at basal level in mutant MC1061D(pECP61.5) (Fig. 7B). These results clearly demonstrate that the respective AI/transcriptional activator couples are not sufficient for full lasB and rhlAB expression but also require the presence of the dksA gene both in E. coli and in P. aeruginosa.
FIG. 7.
E. coli strain MC1061 and its derived dksA mutant MC1061D were grown to an OD600 of 0.3. β-Galactosidase activities expressed from lasB-lacZ ptac-lasR (pECP64) (A) and from rhlAB-lacZ ptac-rhlR (pECP61.5) (B) fusions were determined after a 90-min induction with 1 mM IPTG in the presence (+) or absence (−) of 50 nM 3-oxo-C12-HSL or 1 μM C4-HSL. Experiments were repeated on three different occasions in triplicate. Error bars represent the standard deviations of three determinations.
DISCUSSION
In the present study, we show that DksA exerts a posttranscriptional control on quorum-sensing-dependent virulence genes in P. aeruginosa. Interestingly, expression of lasR, rhlR, and lasI was not altered in the dksA mutant, whereas rhlI expression was slightly increased during early logarithmic phase. Since the lacZ fusions to these genes were all transcriptional, one cannot completely exclude an effect of dksA on their translation. This is, however, very unlikely, since a reduced translation of these quorum-sensing regulators, resulting in decreased amounts of LasR and RhlR protein, would have affected the transcription of lasB and rhlAB. However, in our experimental conditions, we observed no difference in lasB and rhlAB transcription and no significant difference in the production of AIs between the wild type and the dksA mutant (data not shown). In view of these results, it is more likely that DksA regulates expression of lasB and rhlAB independently of the regulator complexes LasR/3-oxo-C12-HSL and RhlR/C4-HSL.
Earlier results concerning the expression of quorum-sensing genes in E. coli suggested that LasR and the corresponding AI 3-oxo-C12-HSL are required and sufficient for lasB expression in E. coli (18). Our results with the dksA E. coli mutant now clearly demonstrate that dksA is also required for full expression of lasB and rhlAB genes in E. coli.
Since complementation with plasmid-encoded copies of the dksA gene resulted only in partial restoration (60 to 70% of wild-type activity) of rhamnolipids and elastase production in the mutant, it was conceivable that this resulted from effects unrelated to dksA inactivation, namely, (i) polar effects on genes located downstream of dksA and (ii) mutation by the Ω-Hg cassette of an overlapping ORF (ORF2 in an earlier study [2]) transcribed in opposite direction to dksA. However, these effects can be excluded since introduction into the dksA mutant of plasmid pVD99.1 (2), harboring a truncated dksA gene but encoding 1.5 kbp of DNA downstream of dksA, did not restore rhamnolipid production, and plasmid pVD99.0 carrying dksA and 1.5 kb of downstream DNA complemented elastase activity to the same level as did plasmid pVD99.3 carrying only dksA (data not shown). Furthermore, introduction of plasmid pVD99.5 (2), harboring ORF2 and only a truncated dksA gene, did not restore rhamnolipid production (data not shown). We therefore believe that the inactivation of dksA is solely responsible for reduced production of rhamnolipid and LasB elastase and that the correct amount of DksA protein produced and the timing of its expression are critical for optimal complementation.
How could DksA affect the expression of target genes? In E. coli, dksA has been recently suggested to be required for rpoS induction by the nutrient stress signal ppGpp (8). Deleting dksA blocked rpoS induction by ppGpp, whereas overproduction of dksA induced rpoS independently of ppGpp (8). Since RpoS was shown to inhibit rhlI expression during early exponential growth in P. aeruginosa (34), it is conceivable that increased rhlI expression in the dksA mutant during exponential growth is an indirect effect due to reduced expression of rpoS. In the same way, these data suggest that the decreased rhlI transcription, observed when dksA was overexpressed in P. aeruginosa (2), could be secondary to an increase in rpoS expression. However, it seems unlikely that the effect of dksA on rhlI transcription is solely responsible for the drastic reduction of both rhamnolipid and elastase production by the dksA mutant.
Involvement of DksA on translation of rpoS was reported recently in S. enterica serovar Typhimurium (33). The region required for the DksA-mediated translational regulation was found to be located between the 8th and 73rd codons of the rpoS reading frame (33). In contrast, in E. coli the region required for the same translational regulation was far upstream of the AUG initiation codon, similar to HF-1, but different from those required for the regulation by ppGpp (8). It remains unclear whether DksA binds directly to RNA or regulates the expression of another protein exerting a translational control. Interestingly, although dksA affected rpoS expression in S. enterica serovar Typhimurium (33), an RpoS-independent effect of DksA was described in S. flexneri (16). S. flexneri dksA mutants exhibited sensitivity to acid and oxidative stress, and some dksA mutant cells showed abnormal localization of the virulence protein IcsA, which is required for the intercellular spread of Shigella bacteria (16). In light of the remarkable sequence conservation between the dksA genes of different species, one could expect that the DksA proteins play similar roles and recognize similar target(s). DksA contains a C4-zinc finger motif, which has been reported to be involved in binding to RNA, DNA, or as a protein-protein interaction site (11). Experiments to elucidate the molecular mechanism by which DksA affects lasB and rhlAB translation in P. aeruginosa are under way.
DksA was shown to be required for full virulence of S. enterica serovar Typhimurium in a hatched chicken model (28) and for the intercellular spread of epithelial cell layers by S. flexneri (16). Our report on the requirement of DksA for the expression of virulence factors in P. aeruginosa is further evidence for a role of DksA as a global regulator of virulence. It is likely that in P. aeruginosa DksA also regulates the expression of genes other than lasB and rhlAB, since the expression of at least six different proteins was altered in a two-dimensional gel analysis of total proteins from a dksA mutant (P. Branny et al., unpublished results). In this respect DksA deserves further attention as a possible novel target for anti-infective agents that aim at the modulation of virulence properties of pathogenic microorganisms.
Acknowledgments
We are grateful to Everett C. Pesci for providing the transcriptional rhlAB-lacZ fusion plasmid pECP65 and to S. Raina for the gift of P1 phage. We thank C. Georgopoulos, in whose laboratory part of the work was performed.
This work was supported by the Swiss National Science Foundation (grants FN 32-52189.97, 32-051940.97, and 32-67262.01 to C. van Delden and grant FN 31-65403.01 to C. Georgopoulos).
REFERENCES
- 1.Albus, A. M., E. C. Pesci, L. J. Runyen-Janecky, S. E. West, and B. H. Iglewski. 1997. Vfr controls quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 179:3928-3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Branny, P., J. P. Pearson, E. C. Pesci, T. Köhler, B. H. Iglewski, and C. van Delden. 2001. Inhibition of quorum sensing by a Pseudomonas aeruginosa dksA homologue. J. Bacteriol. 183:1531-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, L., D. Gentry, T. Elliott, and M. Cashel. 2002. DksA affects ppGpp induction of RpoS at a translational level. J. Bacteriol. 184:4455-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns, J. L., B. W. Ramsey, and A. L. Smith. 1993. Clinical manifestations and treatment of pulmonary infections in cystic fibrosis. Adv. Pediatr. Infect. Dis. 8:53-66. [PubMed] [Google Scholar]
- 5.Diggle, S. P., K. Winzer, A. Lazdunski, P. Williams, and M. Camara. 2002. Advancing the quorum in Pseudomonas aeruginosa: MvaT and the regulation of N-acylhomoserine lactone production and virulence gene expression. J. Bacteriol. 184:2576-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fellay, R., J. Frey, and H. Krisch. 1987. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene 52:147-154. [DOI] [PubMed] [Google Scholar]
- 7.Gambello, M. J., S. Kaye, and B. H. Iglewski. 1993. LasR of Pseudomonas aeruginosa is a transcriptional activator of the alkaline protease gene (apr) and an enhancer of exotoxin A expression. Infect. Immun. 61:1180-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsch, M., and T. Elliott. 2002. Role of ppGpp in rpoS stationary-phase regulation in Escherichia coli. J. Bacteriol. 184:5077-5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones, J. D. G., and N. Gutterson. 1987. An efficient mobilizable cosmid vector, pRK7813, and its use in a rapid method for marker exchange in Pseudomonas fluorescens strain HV37a. Gene 61:299-306. [DOI] [PubMed] [Google Scholar]
- 10.Kang, P. J., and E. A. Craig. 1990. Identification and characterization of a new Escherichia coli gene that is a dosage-dependent suppressor of a dnaK deletion mutation. J. Bacteriol. 172:2055-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laity, J. H., B. M. Lee, and P. E. Wright. 2001. Zinc finger proteins: new insights into structural and functional diversity. Curr. Opin. Struct. Biol. 11:39-46. [DOI] [PubMed] [Google Scholar]
- 12.Latifi, A., M. Foglino, K. Tanaka, P. Williams, and A. Lazdunski. 1996. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhIR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol. Microbiol. 21:1137-1146. [DOI] [PubMed] [Google Scholar]
- 13.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 14.Mayer, M. P. 1995. A new set of useful cloning and expression vectors derived from pBluescript. Gene 163:41-46. [DOI] [PubMed] [Google Scholar]
- 15.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 16.Mogull, S. A., L. J. Runyen-Janecky, M. Hong, and S. M. Payne. 2001. dksA is required for intercellular spread of Shigella flexneri via an RpoS-independent mechanism. Infect. Immun. 69:5742-5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohman, D. E., S. J. Cryz, and B. H. Iglewski. 1980. Isolation and characterization of Pseudomonas aeruginosa PAO mutant that produces altered elastase. J. Bacteriol. 142:836-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Passador, L., J. M. Cook, M. J. Gambello, L. Rust, and B. H. Iglewski. 1993. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 260:1127-1130. [DOI] [PubMed] [Google Scholar]
- 19.Pearson, J. P., E. C. Pesci, and B. H. Iglewski. 1997. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 179:5756-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pesci, E. C., J. P. Pearson, P. C. Seed, and B. H. Iglewski. 1997. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 179:3127-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preston, M. J., P. C. Seed, D. S. Toder, B. H. Iglewski, D. E. Ohman, J. K. Gustin, J. B. Goldberg, and G. B. Pier. 1997. Contribution of proteases and LasR to the virulence of Pseudomonas aeruginosa during corneal infections. Infect. Immun. 65:3086-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rashid, M. H., K. Rumbaugh, L. Passador, D. G. Davies, A. N. Hamood, B. H. Iglewski, and A. Kornberg. 2000. Polyphosphate kinase is essential for biofilm development, quorum sensing, and virulence of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 97:9636-9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reimmann, C., M. Beyeler, A. Latifi, H. Winteler, M. Foglino, A. Lazdunski, and D. Haas. 1997. The global activator GacA of Pseudomonas aeruginosa PAO positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol. Microbiol. 24:309-319. [DOI] [PubMed] [Google Scholar]
- 24.Seed, P. C., L. Passador, and B. H. Iglewski. 1995. Activation of the Pseudomonas aeruginosa lasI gene by LasR and the Pseudomonas autoinducer PAI: an autoinduction regulatory hierarchy. J. Bacteriol. 177:654-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegmund, I., and F. Wagner. 1991. New method for detecting rhamnolipids excreted by Pseudomonas species during growth in mineral agar. Biotech. Techniques 5:265-268. [Google Scholar]
- 26.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology 1:784-790. [Google Scholar]
- 27.Smith, A. W., and B. H. Iglewski. 1989. Transformation of Pseudomonas aeruginosa by electroporation. Nucleic Acids Res. 17:10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner, A. K., M. A. Lovell, S. D. Hulme, L. Zhang-Barber, and P. A. Barrow. 1998. Identification of Salmonella typhimurium genes required for colonization of the chicken alimentary tract and for virulence in newly hatched chicks. Infect. Immun. 66:2099-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Delden, C., R. Comte, and A. M. Bally. 2001. Stringent response activates quorum sensing and modulates cell density-dependent gene expression in Pseudomonas aeruginosa. J. Bacteriol. 183:5376-5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Delden, C., and B. H. Iglewski. 1998. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg. Infect. Dis. 4:551-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Delden, C., E. C. Pesci, J. P. Pearson, and B. H. Iglewski. 1998. Starvation selection restores elastase and rhamnolipid production in a Pseudomonas aeruginosa quorum-sensing mutant. Infect. Immun. 66:4499-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voisard, C., C. T. Bull, C. Keel, J. Laville, M. Maurhofer, U. Schnider, G. Défago, and H. Haas. 1994. Biocontrol of root diseases by Pseudomonas fluorescens CHA0: current concepts and experimental approaches, p. 67-89. In F. O'Gara, D. N. Dowling, and B. Boesten (ed.), Molecular ecology of rhizosphere microorganisms. VCH Publishers, Weinheim, Germany.
- 33.Webb, C., M. Moreno, M. Wilmes-Riesenberg, R. Curtiss III, and J. W. Foster. 1999. Effects of DksA and ClpP protease on sigma S production and virulence in Salmonella typhimurium. Mol. Microbiol. 34:112-123. [DOI] [PubMed] [Google Scholar]
- 34.Whiteley, M., M. R. Parsek, and E. P. Greenberg. 2000. Regulation of quorum sensing by RpoS in Pseudomonas aeruginosa. J. Bacteriol. 182:4356-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]