Abstract
We apply the technique of second-harmonic generation (SHG) microscopy to obtain large area submicron resolution image of Type I collagen from rat tail tendon as it is heated from 40°C to 70°C for 0–180 min. The change in the collagen structure as reflected in its SHG image is observed at length scales from submicron to hundreds of microns. We observed that heating the tendon below the temperature of 54°C does not produce any change in the averaged SHG intensity. At the heating temperature of 54°C and above, we find that increasing the heating temperature and time leads to decreasing SHG intensity. As the tendon is heated above 54°C, the regions where the SHG signal vanish and form a tiger-tail like pattern. In addition, a decrease in the SHG signal occurs uniformly throughout the tendon. By comparing the relative SHG intensities in small and large areas, we found that the denaturation process responsible for forming the tiger-tail like pattern occurs at a higher rate than the global denaturation process occurring throughout the tendon. We also measured the fibril spacing and found that it remains constant at 1.61 ± 0.04 micron for all heating temperature and times. The constant fibril density shows that the global denaturation process occurs at a length scale smaller than the size of the fibril. Our results show that second-harmonic generation microscopy is effective in monitoring the thermal damage to collagen and has potential applications in biomedicine.
INTRODUCTION
Collagen, the most abundant protein in mammals, is the main component of connective tissues. It is responsible for the tensile strength in ligaments and tendons, the elasticity for skin, and the transparency and structural support for the cornea. The most prevalent collagen is Type I collagen, which is found in bones, tendons, and scar tissues. The triple helix of Type I collagen molecules are composed of polypeptide chains, each contains the repeated G-X-Y sequence, where G represents glycine, and X and Y usually correspond to usually praline or hydroxyproline (1). They combine to form microfibrils few nanometers in diameter, which then combine to form collagen fibrils that are few hundreds nanometers in diameter. The fibrils further bundles to form collagen fibers that are a few to hundreds of microns in diameter.
Thermally induced conformational changes in collagen have been actively studied, not only because collagen is the most abundant protein of the extracellular matrices, but also due to its relation to the application of several medical procedures. Examples include the use of heating to change cornea curvature, and the use of laser heating to stabilize shoulder joint and to rejuvenate skin (2–4). The response of collagen to heating has been studied using different methods including differential scanning calorimetry (DSC), x-ray diffraction, NMR, and spectroscopy (1,5,6). The denaturation of collagen is complicated among other variables by its polymeric nature and cross-linking. Despite numerous efforts, the precise mechanism of collagen denaturation remains unknown (1).
Among the various methods that can be used to study collagen denaturation, second-harmonic generation (SHG) microscopy is unique in that it is a nonlinear optical technique that has potential to be applied to collagen studies under in vivo conditions (7–13). In short, second-harmonic generation (SHG) is a coherent process in which two photons at the fundamental frequency are converted into one photon at twice the fundamental frequency. Due to the nonlinearity of the process, SHG occurs only in structures that lack inversion symmetry, and it has been demonstrated that Type I collagen is efficient in generating second-harmonic signals (7–13).
In this study, we used high resolution SHG microscopy to investigate the thermal denaturation of collagen from rat tail tendon. In particular, we obtain high resolution SHG images of heated collagen fibrils. We attempt to characterize the change in the SHG image as the collagen fibril undergoes thermally induced structural changes.
EXPERIMENTAL PROCEDURE
Sample preparation
Fresh rat tails obtained from the National Taiwan University Hospital were frozen at −80°C until before the experiment. Previous studies have found that freezing and thawing rat tail tendons do not affect their SHG signals (8,10). On the day of the experiment, the rat tails were first allowed to thaw at room temperature before the individual tendon fascicles were removed and placed in phosphate buffer saline (PBS) solutions (product specifications 0.01 M phosphate buffer, 0.0027 M potassium chloride, and 0.137 M sodium chloride, pH 7.4, at 25°C). The fascicles were then placed inside a tube filled with PBS solution and heated for a specific time in a thermal bath that was maintained at a given temperature. The heating temperature and time were chosen based on a previous study where the SHG signal was found to change significantly between 50°C and 60°C for a heating time of 10 min (7). After heating, the fascicles were placed on top of a PBS wetted tissue paper, which was then placed on top of the glass slide to keep the fascicles moist. Finally a glass coverslip was added and sealed to prevent the tissue moisture from evaporation.
SHG microscope setup
The arrangement of the SHG microscope is similar to the one previously described (14). The output of a diode pumped and frequency doubled solid state laser (Millennia X, Spectra Physics, Mountain View, CA) pumped femtosecond ti:sapphire laser (Tsunami, Spectra Physics) was tuned to 760 nm, with the power of the scanning laser controlled by the combination of a half wave plate followed by a linear polarizer. To account for any changes in the laser condition, a rat tail tendon sample at ambient temperature was scanned each day of the experiment, and its mean SHG intensity used to normalize the data obtained on that day. For high resolution imaging, a high NA objective (S Fluor 40×, NA 1.3, Nikon, Tokyo, Japan) was used. The back scattered SHG signal from the sample is collected by the focusing objective and isolated using the combination of a dichroic mirror (435dcxr, Chroma Technology, Rockingham, VT) and a 380-nm band pass filter (Chroma, HQ380/20) before being detected by the photomultiplier tube (R7400P, Hamamatsu, Hamamatsu City, Japan). In our single photon counting detection scheme, the output from the PMT is processed by a discriminator before being sent to the computer for image processing. For large area scanning, a computer controlled motorized stage (H101, Prior Scientific, UK) is used to translate the specimen after each optical scan. The sequentially acquired images can then be assembled to form a large area SHG image of the thermally treated rat tail samples.
RESULTS
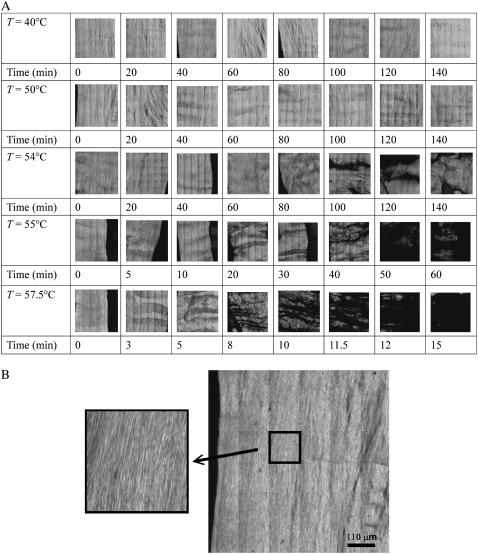
Fig. 1 A shows the 660 μm × 660 μm SHG images of rat tail tendon fibril heated from 40°C to 57.5°C for different heating times. Each image is a montage of 36 nonoverlapping 110 μm × 110 μm image. The fibrils in the images are oriented along the vertical direction, and they are visible in the magnified view of an untreated specimen shown in Fig. 1 B. For heating below T = 54°C, increasing the amount of heating time up to 140 min did not produce observable structural alterations as shown by the SHG images. At temperatures including and higher than 54°C, SHG images show the fibrils start to disintegrate into a “tiger-tail” like band pattern perpendicular to the collagen fibers. The areas with little or no SHG signal tends to extend horizontally in a direction perpendicular to that of the fiber. The pattern suggests that there are positions along the fibers that are more prone toward thermal denaturation. The effects of different heating temperatures and times are shown with greater detail and contrast in Fig. 2, where we display a larger view of the 660 μm × 660 μm SHG images of the tendon at the temperatures of 40°C, 55°C, and 60°C which corresponds to below, just above, and above the apparent critical temperature of 54°C. For each of the temperatures, images for two different heating times are shown.
FIGURE 1.
(A) Second-harmonic generation images of rat tail tendon treated at different heating temperatures and times. Structural change at the fibril level of the rat tail tendon is visible in the SHG signal as the fibril is heated at or above 54°C. Very little structural change is observed when the heating temperature is below 54°C. The images are 660 μm × 660 μm in size, and each one is a montage of 36 individually scanned images. (B) A large image of the untreated 50°C specimen from A, with a magnified view showing the resolved tendon fibrils.
FIGURE 2.
SHG images, 660 μm × 660 μm, of rat tail tendon treated at temperatures below, just above, and far above the critical temperature of 54°C. At 40°C, the average intensity remains constant even for long heating time. At 55°C, just above the critical temperature, the SHG intensity from the tendon decreases with increasing heating time. At 60°C, 16°C above the critical temperature of 54°C, the SHG drops to near zero within few minutes of thermal treatment.
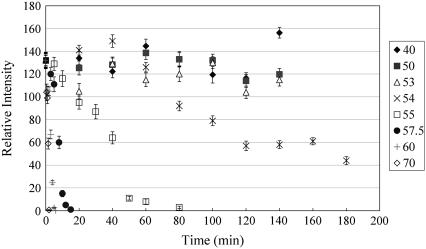
To quantify our observation, we computed and plotted the relative intensities with respect to the unheated 57.5°C data, measured over the 660 μm × 660 μm images. In cases such as the 40 min heating of the 54°C data, where the edge of the fiber is shown, the intensity is averaged only over the fiber areas of the images. Fig. 3 shows the plots at the heating temperature of 40–70°C, for the heating time intervals of 0–180 min. For temperatures at 54°C and higher, there is a steady decrease in the SHG signal as the heating time lengthens.
FIGURE 3.
SHG intensity of rat tail tendon treated for different heating temperatures and times. For temperature above 54°C, the SHG intensity falls off rapidly with increasing heating time. Below 54°C, the intensities remain fairly constant with heating time. The relative intensities are normalized with respect to the SHG intensity measured at 57.5°C (0 min treatment).
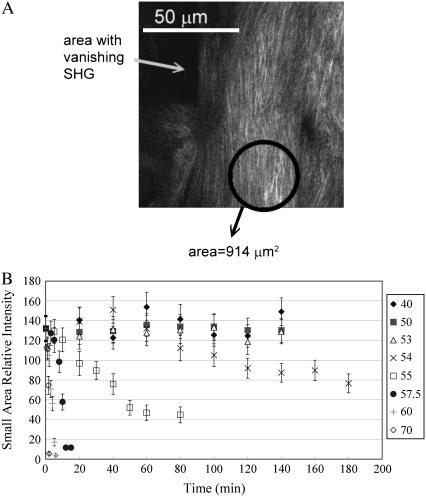
As shown in Fig. 1, the decrease of SHG intensity with increasing heating time for heating temperature at and above 54°C are caused by the increase in regions lacking SHG signal. The location and pattern of these dark areas suggest that a mechanism of the thermal denaturation of tendon fibril occurred along the tiger-tail patterns running perpendicular to the direction of the tendon. To further investigate the thermal denaturation process, we calculated the SHG intensities in smaller regions where the signal has not completely disappeared, and a sample calculation of our approach is shown in Fig. 4 A, where the average SHG intensity was calculated for the circled region. Note that several areas of the nearby regions have little or no SHG signal. The average intensities of these smaller, selected areas for various heating conditions are shown in Fig. 4 B. Under each heating condition, the relative intensities were calculated by averaging over five smaller selected regions each being 914 μm2 in area. The results of our calculations (Fig. 4 B) show that regardless of the selected regions, the average SHG intensity begins to drop off at long heating times for heating temperature at and higher than 54°C.
FIGURE 4.
(A) SHG image from a thermally treated rat tail tendon specimen where parts of image have little or no second-harmonic signal. The circled area shows an example of selected small area used to calculate the SHG intensity. (B) The SHG intensity average over five selected small areas for different heating temperatures and times. The SHG intensity falls off more slowly with increasing heating time than the case shown in Fig. 3.
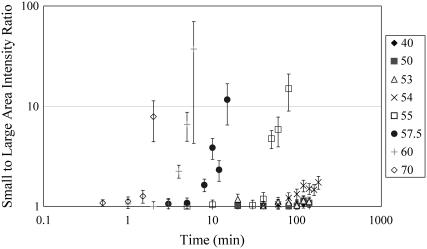
This observation is qualitatively similar to that observed when the average SHG intensity is determined from the large area calculations which include the tiger-tail patterned regions where the collagen has denatured. Qualitatively, the rate of intensity drop off is higher for Fig. 3 which corresponds to the global change of the SHG signal. These results suggest that although Fig. 1 shows the denaturation process appears to first occur only along certain selected regions, a global melting of the collagen fibril also occurs concurrently. To perform further analysis, we plot in Fig. 5 the ratio of the small to large area average intensity (shown in Figs. 3 and 4 B) for the different heating conditions. For heating temperatures above 54°C, the calculated ratio rises with increasing heating time. With increasing temperatures, the ratio increases more quickly with the heating times. The rise in the ratio with heating time suggests that the denaturation along the tiger-tail pattern occurs at a higher rate than the global melting of the collagen fibril.
FIGURE 5.
Ratio of small to large area SHG intensity for different heating conditions plotted on logarithmic scale. For heating temperature above 54°C, the ratio rise with increasing heating time. The rise in the ratio with heating time suggests that the denaturation along the tiger-tail pattern occurs at a higher rate than the global melting of the collagen fibril.
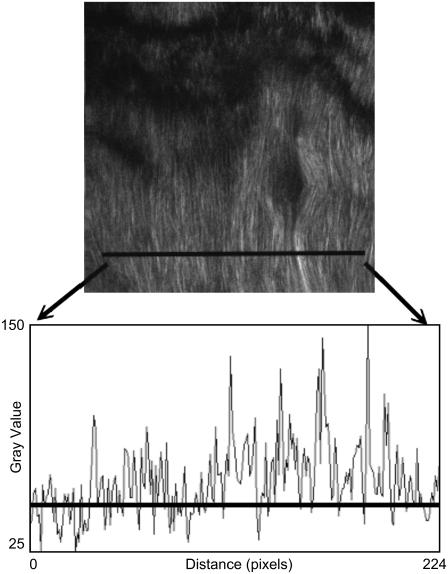
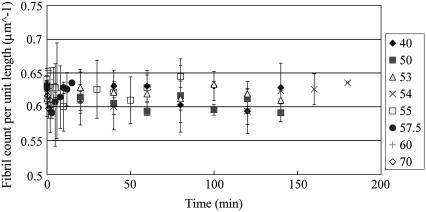
An additional analysis we performed was to determine the fibril spacing as a function of the heating time and temperature. In our approach illustrated in Fig. 6, we plotted the SHG intensity profile along selected lines perpendicular to the fibril direction and by choosing the average intensity along that line as the threshold intensity, the fibril count per unit length was determined by counting the number of peaks over the threshold intensity. We computed and averaged the collagen fibril spacing over five selected regions where SHG are nonvanishing for different heating temperature and duration and the results is shown in Fig. 7. The averaged fibril spacing is 1.62 ± 0.04 μm for all heating conditions even when the SHG intensities within the selected regions decreased. The decrease in the SHG signal as we increase the amount of heating can therefore partly be attributed to changes of collagen molecular or bundling structures that occur within the individual fibrils rather than the change in inter-fibril spacing.
FIGURE 6.
Fibril counting procedure involves plotting the profile of the intensities along a selected line perpendicular to the fibril orientation. The line in the SHG image shows an example of such a selection. The profile plot corresponding to that selection is shown below the SHG image. The horizontal line in the profile plot is the average intensity along the selected line. It is used as a reference for counting the number of fibrils.
FIGURE 7.
Number of fibril per unit length for different heating temperature and time. Five regions were selected for the counting. The count per unit length remains close to 0.62 counts per micron regardless of the heating temperatures and times.
DISCUSSION
We have obtained large area high resolution SHG images of rat tail tendon that have been heated at 40–70°C from 0 to 180 min. We found that below the critical temperature of 54°C, the SHG intensity remains constant up to heating times of 140 min. At temperatures of 54°C and above, the SHG signal dropped as the heating time increased. SHG images acquired under higher heating temperature show that the SHG signal first vanishes in a banding pattern along the direction perpendicular to the fibril direction, instead of occurring uniformly over the tendon fascicle (Fig. 1). Previous studies have found that when viewed under crossed polarizers, rat tail tendon fascicles show banding patterns perpendicular to the fiber direction with the bands spaced by ∼100 microns (15). Since the banding pattern is likely formed by the macromolecular ordering of collagen assembly, it is possible that the location most susceptible to bond breaking also occurs in a banding pattern perpendicular to the fiber, much like the tiger-tail pattern SHG images we observed in tendon fibrils undergoing denaturation.
The similarity in the way the average SHG intensities falls between Figs. 3 and 4 B, however, suggest that bond breaking or structural change of collagen fibril occur not only along the dark tiger-tail bands, but also in areas where SHG signals are nonvanishing. Our results suggest that Type I collagen in rat tail tendon undergo thermal denaturation by two mechanisms. First, thermal effects can induce breakages at weak points along the tendon fiber and additional heating would result in collagen denaturation spreading from the broken positions. In addition, collagen can undergo a global denaturation independent of the presence of the break points. Furthermore, since increasing the heating time above the critical temperature leads to an increase in the ratio of the small to large area average intensity (Fig. 5), the denaturation process responsible for the tiger-tail pattern proceed at a higher rate than the global denaturation process.
Since we measured the fibril density to be a constant (Fig. 7), whereas the SHG signal decreases with heating, the structural change of the rat tail tendon leading to the loss of the SHG signal due to heating most likely occur within the collagen fibrils. This suggests that the bonds within the individual fibrils are broken more easily than the molecular forces holding the fibrils together. It is likely that both the global denaturation process and the denaturation process responsible for the tiger-tail pattern involve the breaking of the crosslinking forces holding the collagen molecules into microfibrils, and that the process occurs more easily at the ends of the fibril where not all collagen molecules are linked due to their staggered arrangement within a fibril bundle. As the tendons are heated, thermal denaturation then takes places from the broken ends of the fibril and moves progressively along the fibers. This is consistent with the increasing size of the bands of vanishing SHG as the heating time increases above the critical temperature (Fig. 1).
In conclusion, by use of SHG microscopy, we have shown that both the global and local mechanisms contribute to the thermal denaturation of Type I collagen in rat tail tendon. With additional developments, we foresee potential applications of SHG microscopy in studying the thermal effects of collagen for biomedical applications.
Acknowledgments
This work was supported by the National Science Council of Taiwan (grants NSC 94-3112-B-002-015-Y).
Yen Sun and Wei-Liang Chen contributed equally to this work.
References
- 1.Wright, N. T., and J. D. Humphrey. 2002. Denaturation of collagen via heating: an irreversible rate process. Annu. Rev. Biomed. Eng. 4:109–128. [DOI] [PubMed] [Google Scholar]
- 2.Lyons, T. R., P. L. Griffith, F. H. Savoie, and L. D. Field. 2001. Laser-assisted capsulorrhaphy for multidirectional instability of the shoulder. Arthroscopy. 17:25–30. [DOI] [PubMed] [Google Scholar]
- 3.McDonald, M. B., P. S. Hersh, E. E. Manche, R. K. Maloney, J. Davidorf, and M. Sabry. The Conductive Keratoplasty United States Investigators Group. 2002. Conductive keratoplasty for the correction of low to moderate hyperopia: U.S. clinical trial 1-year results on 355 eyes. Ophthalmology. 109:1978–1989. [DOI] [PubMed] [Google Scholar]
- 4.Fitzpatrick, R. E., M. P. Goldman, N. M. Satur, and W. D. Tope. 1996. Pulsed carbon dioxide laser resurfacing of photoaged facial skin. Arch. Dermatol. 132:395–402. [PubMed] [Google Scholar]
- 5.Leikina, E., M. V. Mertts, N. Kuznetsova, and S. Leikin. 2001. Type I collagen is thermally unstable at room temperature. Proc. Natl. Acad. Sci. USA. 99:1314–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miles, C. A., T. V. Burjanadze, and A. J. Bailey. 1995. The kinetics of the thermal denaturation of collagen in unrestrained rat tail tendon determined by differential scanning calorimetry. J. Mol. Biol. 245:437–446. [DOI] [PubMed] [Google Scholar]
- 7.Lin, S.-J., C.-Y. Hsiao, Y. Sun, W. Lo, W.-C. Lin, G.-J. Jan, S.-H. Jee, and C.-Y. Dong. 2005. Monitoring the thermally induced structural transitions of collagen by use of second-harmonic generation microscopy. Opt. Lett. 30:622–624. [DOI] [PubMed] [Google Scholar]
- 8.Williams, R. M., W. R. Zipfel, and W. W. Webb. 2005. Interpreting second-harmonic generation images of collagen I fibrils. Biophys. J. 88:1377–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campagnola, P. J., A. C. Millard, M. Terasaki, P. E. Hoppe, C. J. Malone, and W. A. Mohler. 2002. Three-dimensional high-resolution second harmonic generation imaging of endogenous structural proteins in biological tissues. Biophys. J. 81:493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoller, P., P. M. Celliers, K. M. Reiser, and A. M. Rubenchik. 2003. Quantitative second-harmonic generation microscopy in collagen. Appl. Opt. 42:5209–5219. [DOI] [PubMed] [Google Scholar]
- 11.Roth, S., and I. Freund. 1981. Optical second-harmonic scattering in rat-tail tendon. Biopolymers. 20:1271–1290. [DOI] [PubMed] [Google Scholar]
- 12.Brown, E., T. McKee, E. DiTomaso, A. Pluen, B. Seed, Y. Boucher, and R. K. Jain. 2003. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat. Med. 9:796–800. [DOI] [PubMed] [Google Scholar]
- 13.Zipfel, W. R., R. M. Williams, R. Christie, A. Y. Nikitin, B. T. Hyman, and W. W. Webb. 2003. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc. Natl. Acad. Sci. USA. 100:7075–7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun, Y., J.-W. Su, W. Lo, S.-J. Lin, S.-H. Jee, and C.-Y. Dong. 2003. Multiphoton polarization imaging of the stratum corneum and the dermis in ex-vivo human skin. Opt. Express. 11:3377–3384. [DOI] [PubMed] [Google Scholar]
- 15.Yeh, A. T., B. Choi, J. S. Nelson, and B. J. Tromberg. 2003. Reversible dissociation of collagen in tissues. J. Invest. Dermatol. 121:1332–1335. [DOI] [PubMed] [Google Scholar]