Abstract
Pulsed field gradient (pfg)-NMR spectroscopy was utilized to determine lipid lateral diffusion coefficients in oriented bilayers composed of 25 mol % sterol and equimolar amounts of dioleoylphosphatidylcholine and sphingomyelin. The occurrence of two lipid diffusion coefficients in a bilayer was used as evidence of lateral phase separation into liquid ordered and liquid disordered domains. It was found that cholesterol, ergosterol, sitosterol, and lathosterol induced domains, whereas lanosterol, stigmasterol, and stigmastanol resided in homogeneous membranes in the temperature interval of 24–70°C. Among the domain-forming sterols, differences in the upper miscibility temperature indicated that the stability of the liquid ordered phase could be modified by small changes in the sterol structure. The domain-forming capacity for the different sterols is discussed in terms of the ordering effect of the sterols on the lipids, and it is proposed that the driving force for the lateral phase separation is the reduced solubility of the unsaturated lipid in the highly ordered phase.
INTRODUCTION
Cholesterol (CHOL) is the most abundant sterol in mammalian plasma membranes (1). One of the more demanding questions to be answered in membrane biology is the feature exercised by CHOL in the lipid bilayer. CHOL solubilized in sphingolipids triggers lateral phase separation into domains in the lipid bilayer, often referred to as “rafts” that are believed to be involved in diverse membrane processes such as signal transduction, protein stabilization, protein and lipid sorting, and membrane fusion (2,3). Moreover, CHOL modulates the packing of the phospholipid molecules in the membrane, thereby increasing both bilayer rigidity and mechanical durability as well as reducing passive permeability (4). Obviously, to get a better understanding of the effect of CHOL and other sterols on membrane properties and functions requires investigations of the phase behavior of sterol/lipid systems.
It is well known that increasing the CHOL concentration in appropriate lipid bilayers will cause a phase separation to occur into liquid ordered (lo) and liquid disordered (ld) phases (5,6). Hitherto, the number of lipid systems investigated, emphazising phase behavior, has been quite limited and mainly comprises a couple of phase diagrams for some phosphatidylcholines (PCs) or sphingomyelins (SMs) with CHOL in excess water (6–9) and CHOL solubility in various lipids (10). On the other hand, an almost overwhelming number of studies of various effects of CHOL on both model and cell membranes have been published, in particular after the proposal of the importance of domains for membrane functions in the biological cells at the end of the 1980s. However, no detailed mechanism underlying the formation of these “rafts”, neither in model membranes nor in living cells, has so far been reached. We have, therefore, undertaken a program, in which systematic studies (11–18) of the dynamics and phase behavior of a variety of lipid/sterol systems are performed. In particular, we utilize a pulsed field gradient (pfg)-NMR method that we have developed to directly determine the lateral diffusion coefficients of lipids in bilayers (17,19). From such measurements, both the dynamics and the phase behavior, such as lateral phase separation, can be obtained.
Recently we proposed that the packing order plays a crucial role in the formation of domains in dioleoylphosphatidylcholine (DOPC)/sphingomyelin (SM)/CHOL systems, in which the distribution of the hydrocarbon chains differed for the natural SMs (14). From these previous studies, we concluded that the lateral phase separation in the systems studied could be rationalized in terms of lipid order and immiscibility of unsaturated lipids, such as DOPC, in the lo phase. The interpretation of our experimental findings was based on the assumption that the lipid lateral diffusion in bilayers was strongly dependent on the lipid packing order and that no specific interactions between the molecules had to be included (15). Under these assumptions it was concluded that saturated lipids, such as egg yolk SM (eSM), formed more ordered phases than unsaturated lipids and that the addition of CHOL greatly enhanced the ordering, especially for the lipids with saturated chains. We then proposed that the driving force for the lateral phase separation into ld and lo phases is the increasing difficulty for the unsaturated lipid to reside in a highly ordered phase. Our findings suggested that the unsaturated lipids had a preference to be located in the ld phase, whereas eSM preferred the lo phase. CHOL, on the other hand, seemed to partition into both phases to roughly the same extent, indicating that CHOL had no particular preference for any of these phases in a ternary system with saturated and unsaturated lipids, probably because of the rigid sterol structure, making it rather insensitive to the molecular order of the environment. Therefore, the role of CHOL in the phase separation process is to increase the ordering of the (homogeneous) lipid membrane to such an extent that the system finally favors a phase separation, where most of the unsaturated lipid is squeezed out from the lo phase into the ld phase (15). Note that the situation is slightly different for a binary system with only a saturated lipid, where there is a fast chemical exchange of molecules between the lo and ld phases, indicating that the domains are small (12). To investigate the mechanism(s) behind the formation of domains in bilayers, utilizing the pfg-NMR method, this simple order and packing model of the lipid bilayer has also been applied in this investigation, where we have studied the effect of the structure of the sterols (Fig. 1) on the lipid lateral diffusion in DOPC/eSM bilayers. As will be seen, just moving a double bond one step in the sterol skeleton, seemingly a small alteration, can have a rather strong effect on the phase behavior.
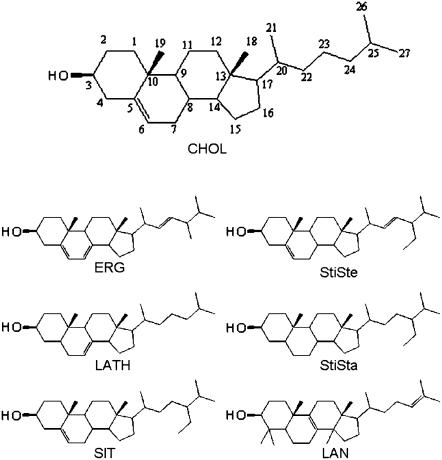
FIGURE 1.
Structures of the sterols utilized in this study.
MATERIALS AND METHODS
Materials
DOPC was purchased from Avanti Polar Lipids (Alabaster, AL); eSM ∼99%, CHOL grade 99+%, lanosterol 50–60% (LAN) with the major impurity being dihydrolanosterol, stigmasterol 95% (StiSte), stigmastanol 95% (StiSta), ergosterol 95% (ERG), and lathosterol 98% (LATH) were all from Sigma Aldrich (St. Louis, MO); β-sitosterol SIT 78% was from Steraloids (Newport, Rhode Island). Deuterium oxide, 99.9% in 2H, was obtained from Larodan Fine Chemicals AB (Malmö, Sweden).
Preparation of oriented samples
The macroscopically aligned samples were prepared with 25 mol % sterol and equimolar amounts of the phospholipids DOPC and eSM. The appropriate amount of each compound was weighed and dissolved in a 1:4 volume ratio of methanol/propanol solvent to a concentration of 15 mg/mL of total lipid; 25 μL of the solution was then put on each of ∼30 glass plates (5 × 14 mm, Marienfeldt, Germany). The solvent was evaporated at atmospheric pressure, and the sample was then kept under vacuum at room temperature for at least 6 h.
The glass plates were stacked in a special sample holder and put in a 2H2O atmosphere at 45°C for 5 days. After the formation of hydrated, oriented bilayers, excess deuterated water was added to a filter paper at each end of the sample to ensure maximum hydration during the experiments. The sample tube was sealed and left to equilibrate for an additional 2 days before the measurements were performed.
Diffusion measurements
Pfg-NMR measurements were performed mainly on a 100-MHz Chemagnetics Infinity (Varian, Fort Collins, CO) NMR spectrometer. Some measurements were also made on a 400-MHz Chemagnetics Infinity (Varian) NMR spectrometer. The macroscopically aligned samples were oriented at the magic angle with respect to the main magnetic field in a goniometer probe. Details of the pfg-NMR method can be found elsewhere (17).
The stimulated echo pulse sequence (20) was used for all measurements; in this procedure, the attenuation of the amplitude of the echo, A, and its dependence on translational diffusion are described by a sum over all the different diffusion components present in the sample
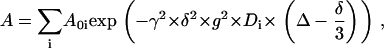 |
(1) |
where Aoi is the initial amplitude of component i without applied magnetic field gradients, γ is the gyromagnetic ratio, δ and g represent the duration and amplitude of the pulsed field gradients, Δ is the time interval between the two gradient pulses, and Di is the self-diffusion coefficient of component i (17,21).
The experimental settings were as follows: the time between the first two 90° pulses, τ, and the time interval between the second and the third 90° pulses, τ1, were kept constant in each experiment but varied for different experiments between 10–14 ms and 90–288 ms, respectively. The majority of the experiments were performed with τ = 12 ms, and τ1 = 188 ms. Δ was typically 200 ms, and δ varied in each experiment between 1 and 11 ms. The number of accumulations ranged from 16 to 512 to achieve an acceptable signal/noise ratio. Measurements were performed between 24 and 60°C in steps of 3° with a waiting time of 20 min before each measurement. The obtained diffusion coefficients did not depend on the thermal history of the sample.
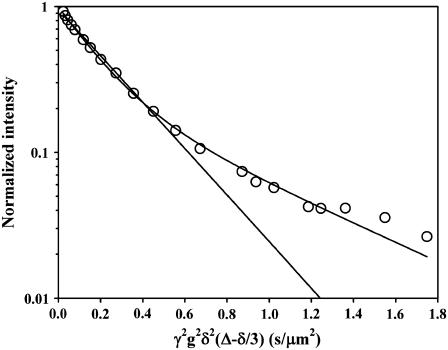
The data was Fourier transformed, and the total integral and/or peak intensities were used in a nonlinear fit to Eq. 1. For each sample and temperature, one or two diffusion coefficients corresponding to the lipid translational diffusion (1–20 μm2/s) were obtained. At low temperatures the fast diffusion of water (100–300 μm2/s) was also sometimes observed. Fig. 2 gives an example of the results obtained for LATH at 30°C. The relative errors as reported from the nonlinear fits were generally below 5% for the monoexponential data and between 5% and 20% for the biexponential decays. The choice of Δ did not affect D, and it was thus independent of the diffusion time. Because the bilayer normal and the magnetic field gradient form an angle of 54.7°, the lateral diffusion coefficient, DL, is obtained by multiplying the measured diffusion coefficient D by 1.5 (22).
FIGURE 2.
Obtained decay of the lipid signal intensity for DOPC/eSM bilayers containing LATH at 30°C. The lines in the plot show the best fits to one and two exponential decays, respectively.
RESULTS AND DISCUSSION
Fig. 1 shows the sterol molecules utilized in this investigation. It can be inferred from this figure that the structural differences, compared to CHOL, are rather small. LATH has a Δ7 double bond instead of a Δ5, SIT and StiSte are ethylated at carbon 24, and StiSte also posesses a Δ22 double bond. StiSta is the saturated form of StiSte. ERG has two extra double bonds (Δ7 and Δ22) and is methylated at carbon 24. Finally, LAN has a Δ8(9) double bond instead of a Δ5 and is also 4,4,14-trimethylated.
It turns out that although the changes in structure among the different sterols can be considered as quite small, the phase behavior differs in that some of them are able to induce domains in DOPC/SM bilayers, whereas some of them are not. The goal with our investigation is thus to try to make clear what kind of physicochemical properties of these sterols are determining such a diverse phase behavior.
Sterols in homogeneous lipid bilayers
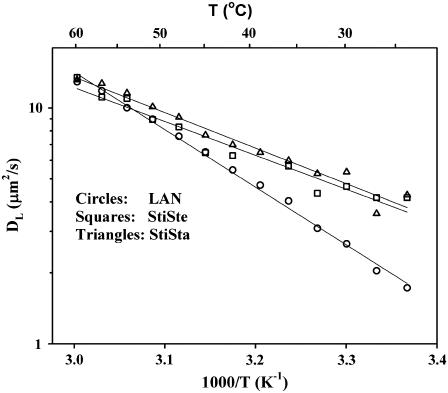
The NMR diffusion data for StiSte and StiSta were well described by one lipid diffusion coefficient, and DL increased monotonically with increasing temperature (Fig. 3). The apparent activation energies obtained for the lipid membranes containing StiSte (33 kJ/mol) and StiSta (28 kJ/mol) were typical for an ld phase in accordance with previous investigations (12). The DL values were close to those for a DOPC/eSM membrane with no sterol (11).
FIGURE 3.
Temperature dependence of DL for the homogeneous DOPC/eSM/sterol bilayers containing 25 mol % StiSte (squares), StiSta (triangles), and LAN (circles). The lines are linear least-square fits to the points.
The diffusion data for the LAN system were also well described by one lipid diffusion coefficient (Fig. 3), with an activation energy of 47 kJ/mol. This was higher than for StiSte and StiSta, but it is still within the values obtained in previous studies of other ld phases (11,12).
The observation of a single diffusion coefficient does not in itself rule out the possibility of microscopic phase separation with domain sizes much smaller than 1 μm because then only a weighted mean value of the DLs would be observed due to a fast exchange between the lo and ld phases (23). In earlier studies of binary systems of saturated lipids and CHOL, the plot of the temperature dependence showed curved features that were attributed to the presence of small domains in the bilayers (12). No such effect was observed in Fig. 3, and it could be concluded that the sterols resided in an ld phase and that no domains were present in the lipid bilayers containing StiSte, StiSta, or LAN.
Domain formation in bilayers
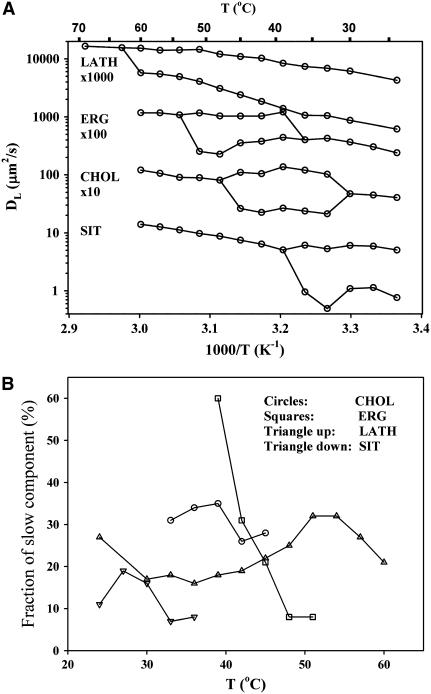
The results from the remaining sterols in this study are summarized in Fig. 4 A. At high temperatures, all systems exhibited a single diffusion coefficient, but at some point all the systems reached an upper miscibility temperature (TM) at which the data were better described by two diffusion coefficients. This temperature was highest for LATH, followed by ERG, CHOL, and SIT. Even though the obtained NMR signal contains overlapping contributions from the two phospholipids, the occurrence of two DLs cannot simply be attributed to the motions of each of the two lipid species. Rather, previous investigations using isotopically labeled molecules have shown that all lipids (phospholipids as well as sterols) have the same DL as long as they reside in the same phase (or domain) in the bilayer (18,24). Instead, two lipid DLs indicate a lateral phase separation into the lo and ld phases in the bilayer. This conclusion is well supported from other studies in several raft-forming systems (11,14), and the observation of a diffusion decay with two components in pfg-NMR therefore provides a convenient method for investigation of the lateral phase separation process.
FIGURE 4.
(A) Temperature dependence of DL for the DOPC/eSM/sterol bilayers forming domains. The curves for CHOL, ERG, and LATH have been shifted upward for clarity. (B) The fraction of the slow diffusion component obtained from the fits to Eq. 1 as a function of temperature.
As the temperature was lowered further, the ERG and CHOL systems returned to a single diffusion component, whereas the two-phase behavior for the LATH and SIT systems remained to the lowest temperature studied. The results for the CHOL system were in good agreement with earlier reports (11).
Within the two-phase region, the relative amplitude of the slow diffusion coefficient varied only slightly within ∼20–30% for CHOL, SIT, and LATH, whereas it decreased monotonically from 60% to 10% for ERG (Fig. 4 B). This relative amplitude is not necessarily a measure of the actual amount of the lo phase because it is weighted with the transverse and longitudinal proton relaxation in the phases, but it can be used as a rough estimate of the relative quantities of ld and lo phases. At lower temperatures we cannot rule out the presence of a solid ordered (so, gel) phase because the very fast relaxation in this phase makes it effectively invisible in the pfg-NMR experiment.
The ratio between the fast and slow diffusion coefficients was found to vary between 2 and 7 for all systems. For CHOL and SIT, it was more or less constant throughout the temperature range at a value of 5 and 6, respectively. For LATH it decreased with increasing temperature from 7 at 20°C to 3 at 60°C. The opposite trend was observed for ERG, in which the ratio increased from 3 at 42°C to 5 at 54°C.
Sterol properties influence domain formation
To get a better understanding of why a certain sterol will induce domains in lipid bilayers, we need to look into what properties of the bilayer are changed by the incorporation of the sterol and the roles played by its chemical structure. Because raft formation seems to be intimately coupled to the packing and ordering of the molecules in the bilayer, it is appropriate to first briefly summarize some relevant data from the literature on the influence that sterols may have on these properties. Here, many methods have been employed, such as order parameter determinations, fluorescence anisotropy and quenching, membrane permeability, and detergent insolubility. Methods that probe bilayer properties such as bending stiffness and area compressibility are also related to the packing properties, and so is the lipid lateral diffusion. See Table 1 for a compilation of some methods used for various systems containing the sterols in our study. In the following we have tried to rank the sterols studied with respect to their ability to promote domain formation by influencing the packing properties in the bilayer. In such a comparison, the nature of the lipid also has to be taken into account because the ordering effect of the sterols depends on the degree of unsaturation of the lipid chains.
TABLE 1.
Summary of experimental methods used to characterize domain formation and some physicochemical properties in bilayers
| Experimental method and measured characteristic | References |
|---|---|
| Deuterium quadrupole splittings: order parameters | 29,31,36,38,39,49 |
| Vesicle deformation and orientation in a magnetic field: bending energy | 29 |
| Micropipet aspiration: area expansion modulus | 29,31,50 |
| Fluorescence microscopy: lateral organization | 44,45 |
| Fluorescence correlation spectroscopy: lateral diffusion | 45 |
| Permeability of water, small molecules, and ions | 33,34,36,51,52 |
| Fluorescence quenching: domain stability | 25,40,43,53 |
| Triton X100 solubility: domain packing | 25,35,40,43 |
| Fluorescence anisotropy: domain packing | 28,40,52,53 |
| Dynamic light scattering: lateral tension, surface viscosity | 32 |
| Differential scanning calorimetry: transition temperatures and enthalpies | 26,35,38,49 |
| Resonance energy transfer: domain formation | 35 |
| Pulsed field gradient NMR: lateral diffusion | 11–15,18,37 |
| Electron paramagnetic resonance: order parameters | 39 |
The lo phase is characterized by a high degree of ordering of the lipids, which results in large order parameters, stiffer and less compressible membranes, low lateral diffusion, low permeability, resistance to Triton X100 solubilization, and high fluorescence anisotropy. The effectiveness of different sterols in changing these characteristics is utilized to rank their domain-forming ability.
Binary systems
In systems with a saturated lipid, LATH generated about the same ordering as CHOL, but it produces more stable domains (25,26). For ERG, the ordering seemed to depend on the degree of lipid unsaturation. Thus, for saturated lipids, such as DMPC and DPPC, ERG is more potent than CHOL in creating a highly ordered bilayer (27–30), whereas in systems of varying degree of unstauration (palmitoyloleoylphosphatidylcholine, dielaidoylphosphatidylcholine, and egg phosphatidylcholine), the opposite is true (31–33). However, on lipid extracts from Acholeplasma laidlawii grown on both saturated and unsaturated fatty acids, no difference could be seen between ERG and CHOL (34). It should also be mentioned that the ordering obtained from the 2H NMR quadrupole splitting of the chain methyl showed a large temperature dependence for the ERG system and that the induced ordering, at least in this position, could be larger or smaller than for CHOL, depending on the temperature (29). A similar but opposite difference between saturated and unsaturated lipids was found for SIT; i.e., the ordering capability was smaller than for CHOL in saturated systems (28,35), whereas it was larger for unsaturated systems (36).
A majority of studies showed that LAN was clearly inferior to CHOL in the capacity to pack both saturated and unsaturated systems (27–29,31,32,37–39), although in one study LAN was found to increase ordering in bilayers to the same extent as CHOL (40). One simulation study (41) reported no domain formation for LAN, whereas another found only small differences between LAN and CHOL (42). Also StiSte seemed to be less prone to form domains than CHOL in both saturated and unsaturated systems (27,28,33–36).
Ternary systems
In systems of saturated lipid/unsaturated lipid/sterol, LATH was shown to induce the most stable domains (25), but ERG, StiSte, and SIT also gave results that indicated that these sterols promoted domain formation (43). According to the latter study, all of these three sterols induced more stable domains than CHOL in the corresponding system, although the domains induced by SIT seemed to be less packed than for CHOL. LAN gave somewhat ambiguous results, in which fluorescence microscopy methods indicated that no domains formed in DOPC/DPPC/LAN systems (44), whereas domains were detected in DOPC/stearoyl-SM/LAN systems (45). Other studies indicated that LAN induced only small changes in domain formation compared to the sterol-free membranes (40).
Further indications that CHOL could be replaced by other sterols of similar structure came from in vivo studies showing that SIT could support cell growth, provided that small amounts of CHOL were present. The results suggested that SIT can replace CHOL for bulk membrane functions but that small amounts of CHOL were required for other cellular functions (46). Similar results were obtained with desmosterol, which could replace cholesterol with regard to membrane functions (47).
From this summary, it is clear that our results are quite consistent with the general trends published in the literature for these sterols. Let us now discuss our results obtained for each one of the sterols with the basic understanding that the packing capability of the sterol is the main reason for the domain formation.
It seems reasonable that the extra bulkiness obtained by introducing a side group in the hydrocarbon chain of CHOL will impair the packing in the bilayer, thereby destabilizing the lo phase. SIT shows exactly this effect, where TM is substantially lowered compared to CHOL. Adding a double bond to the hydrocarbon chain, as in StiSte, would further impair the ability to produce tightly packed bilayers (cf. the effect of lipid unsaturation in bilayers), and therefore, StiSte does not induce domain formation. A comparison of SIT and StiSta shows that the double bond of the ring system is essential for domain formation for the chain ethylated compounds. This is rather surprising because dihydrocholesterol, i.e., CHOL lacking the double bond, has very similar properties to CHOL (48).
For ERG it seems that the destabilizing effect of having both a double bond and a methyl group in the end chain is more than compensated for by the Δ7 double bond. Also, the high TM for LATH reflects a tendency for this sterol to form highly stable domains. Thus, changing the Δ5 double bond to a Δ7 acts to stabilize domains. It is rather remarkable that the position of double bonds in the sterol skeleton can have such a large effect. Perhaps this position affects the molecular shape, leading to a different packing of the sterol in the lipid bilayer. Further investigations are needed to clarify this. Finally, the reason why LAN does not induce raft formation is probably related to the bulky methyl groups on the sterol skeleton that will reduce its ability to increase the necessary lipid ordering, or, again, the position of the double bond in the ring and in the chain might play a role.
Let us now try to rationalize the results in terms of the order and packing model mentioned in the introduction. The decrease in the Gibbs free energy on lateral phase separation originates from several sources. The ordering of the sterol in the lo phase leads to a closer packing between the molecules, resulting in an increase in the van der Waals interaction (enthalpy). At the same time the entropy decreases, most for the unsaturated lipid in the ordered phase, because it will lose configurational entropy in the ordering process. However, when the unsaturated lipid leaves the lo phase for the ld phase, a rather large increase in the configurational entropy can be expected. This may be the driving force for domain formation in these kinds of ternary systems, and the phase separation occurs because of a large difference in ordering between the two phases. This reasoning can be used to explain the observed increasing trend in TM for SIT, CHOL, and ERG (Fig. 4 A). The induced order in the mainly saturated lo phase will be largest for ERG and smallest for SIT, whereas in the mainly unsaturated ld phase, the ordering will be largest for SIT and smallest for ERG. Therefore, the difference in ordering between the two phases will increase in the order SIT < CHOL < ERG, and we would expect the same order for TM for these systems, at least as long as the partitioning of the membrane components is similar to that found in the CHOL system.
It should be noted that for the ternary system the tielines in the two-phase region with the lo and ld phases are not very far from being parallel with the base of the triangular phase diagram, CHOL being at the top (8). This means that CHOL has no preference for either of the two phases, as stated in the introduction and also observed previously for our systems (15,18). Thus, the CHOL content is similar in the lo and ld phases in equilibrium. It can be concluded that there is a rather subtle balance between the enthalpic and entropic forces for a domain to form in these systems. This shows why small changes in the sterol structure play such an important role in the ability to induce domains.
Acknowledgments
This work was supported by the Magnus Bergvall Foundation, the Swedish Research Council, and the Knut and Alice Wallenberg Foundation.
References
- 1.Gennis, R. B. 1989. Biomembranes, Molecular Structure and Function. Springer-Verlag, New York.
- 2.Brown, D. A., and E. London. 1998. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14:111–136. [DOI] [PubMed] [Google Scholar]
- 3.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31–39. [DOI] [PubMed] [Google Scholar]
- 4.Simons, K., and E. Ikonen. 2000. How cells handle cholesterol. Science. 290:1721–1726. [DOI] [PubMed] [Google Scholar]
- 5.Ipsen, J. H., G. Kalström, O. G. Mouritsen, H. W. Wennerström, and M. J. Zuckermann. 1987. Phase equilibria in the phosphatidylcholine-cholesterol systems. Biochim. Biophys. Acta. 905:162–172. [DOI] [PubMed] [Google Scholar]
- 6.Vist, M. R., and J. H. Davis. 1990. Phase equilibria of cholesterol/dipalmitoylphosphatidylcholine mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry. 29:451–464. [DOI] [PubMed] [Google Scholar]
- 7.Veatch, S. L., and S. L. Keller. 2003. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys. J. 85:3074–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veatch, S. L., and S. L. Keller. 2005. Seeing spots: Complex phase behavior in simple membranes. Biochim. Biophys. Acta. 1746:172–185. [DOI] [PubMed] [Google Scholar]
- 9.Almeida, R. F. M., A. Fedorov, and M. Prieto. 2003. Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: Boundaries and composition of lipid rafts. Biophys. J. 85:2406–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bach, D., and E. Wachtel. 2003. Phospholipid/cholesterol model membranes: formation of cholesterol crystallites. Biochim. Biophys. Acta. 1610:187–197. [DOI] [PubMed] [Google Scholar]
- 11.Filippov, A., G. Orädd, and G. Lindblom. 2004. Lipid lateral diffusion in ordered and disordered phases in raft mixtures. Biophys. J. 86:891–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filippov, A., G. Orädd, and G. Lindblom. 2003. The effect of cholesterol on the lateral diffusion of phospholipids in oriented bilayers. Biophys. J. 84:3079–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filippov, A., G. Orädd, and G. Lindblom. 2003. Influence of cholesterol and water content on phospholipid lateral diffusion in bilayers. Langmuir. 19:6397–6400. [Google Scholar]
- 14.Filippov, A., G. Orädd, and G. Lindblom. 2006. Sphingomyelin structure influences the lateral diffusion and raft formation in lipid bilayers. Biophys. J. 90:2086–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindblom, G., G. Orädd, and A. Filippov. 2006. Lipid lateral diffusion in bilayers with phosphatidylcholine, sphingomyelin and cholesterol. An NMR study of dynamics and lateral phase separation. Chem. Phys. Lipids. 141:179–184. [DOI] [PubMed] [Google Scholar]
- 16.Orädd, G., and G. Lindblom. 2003. NMR in macroscopically oriented lyotropic systems. In NMR of Orientationally Ordered Liquids. E. Burnell and K. de Lange, editors. Kluwer Academic Publishers, Dordrecht. 399–418.
- 17.Orädd, G., and G. Lindblom. 2004. Lateral diffusion studied by pulsed field gradient NMR on oriented lipid membranes. Magn. Reson. Chem. 42:123–131. [DOI] [PubMed] [Google Scholar]
- 18.Orädd, G., P. W. Westerman, and G. Lindblom. 2005. Lateral diffusion coefficients of separate lipid species in a ternary raft-forming bilayer: a pfg-NMR multinuclear study. Biophys. J. 89:315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindblom, G., and G. Orädd. 1994. NMR studies of translational diffusion in lyotropic liquid crystals and lipid membranes. Prog. Nucl. Magn. Reson. Spectrosc. 26:483–515. [Google Scholar]
- 20.Tanner, J. E. 1970. Use of the stimulated echo in NMR diffusion studies. J. Chem. Phys. 52:2523–2526. [Google Scholar]
- 21.Stejskal, E. O., and J. E. Tanner. 1965. Spin diffusion measurements: spin echoes in the presence of a time-dependent field gradient. J. Chem. Phys. 42:288–292. [Google Scholar]
- 22.Wästerby, P., G. Orädd, and G. Lindblom. 2002. Anisotropic water diffusion in macroscopically oriented lipid bilayers studied by pulsed magnetic field gradient NMR. J. Magn. Reson. 157:156–159. [DOI] [PubMed] [Google Scholar]
- 23.Kärger, J., H. Pfeifer, and W. Heink. 1988. Principles and application of self-diffusion measurements by nuclear magnetic resonance. In Advances in Magnetic and Optical Resonance. W. S. Warren, editor. Academic Press, San Diego, CA. 1–89.
- 24.Orädd, G., G. Lindblom, and P. W. Westerman. 2002. Lateral diffusion of cholesterol in a lipid bilayer measured by pfg-NMR spectroscopy. Biophys. J. 83:2702–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang, J. W., Megha, and E. London. 2004. Relationship between sterol/steroid structure and participation in ordered lipid domains (lipid rafts): implications for lipid raft structure and function. Biochemistry. 43:1010–1018. [DOI] [PubMed] [Google Scholar]
- 26.Nyholm, T. K. M., M. Nylund, and J. P. Slotte. 2003. A calorimetric study of binary mixtures of dihydrosphingomyelin and sterols, sphingomyelins, or phosphatidylcholine. Biophys. J. 84:3138–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urbina, J. A., S. Pekerar, H.-B. Le, J. Patterson, B. Montez, and E. Oldfield. 1995. Molecular order and dynamics of phosphatidylcholine bilayer membranes in the presence of cholesterol, ergosterol and lanosterol: a comparative study using 2H-, 13C- and 31P-NMR spectroscopy. Biochim. Biophys. Acta. 1238:163–176. [DOI] [PubMed] [Google Scholar]
- 28.Bernsdorff, C., and R. Winter. 2003. Differential properties of the sterols cholesterol, ergosterol, b-sitosterol, trans-7-dehydrocholesterol, stigmasterol and lanosterol on DPPC bilayer order. J. Phys. Chem. 107:10658–10664. [Google Scholar]
- 29.Endress, E., S. Bayerl, K. Prechtel, C. Maier, R. Merkel, and T. M. Bayerl. 2002. The effect of cholesterol, lanosterol, and ergosterol on lecithin bilayer mechanical properties at molecular and microscopic dimensions: A solid-state NMR and micropipit study. Langmuir. 18:3293–3299. [Google Scholar]
- 30.Czub, J., and M. Baginski. 2006. Comparative molecular dynamics study of lipid membranes containing cholesterol and ergosterol. Biophys. J. 90:2368–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henriksen, J., A. C. Rowat, E. Brief, Y. W. Hsueh, J. L. Thewaldt, M. J. Zuckermann, and J. H. Ipsen. 2006. Universal behavior of membranes with sterols. Biophys. J. 90:1639–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hildenbrand, M. F., and T. M. Bayerl. 2005. Differences in the modulation of collective membrane motions by ergosterol, lanosterol, and cholesterol: A dynamic light scattering study. Biophys. J. 88:3360–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demel, R. A., K. R. Bruckdorfer, and L. L. M. Van Deenen. 1972. The effect of sterol structure on the permeability of liposomes to glucose, glycerol and Rb+. Biochim. Biophys. Acta. 255:321–330. [DOI] [PubMed] [Google Scholar]
- 34.de Kruyff, B., W. J. de Greef, R. V. W. van Eyk, R. A. Demel, and L. L. M. Van Deenen. 1973. The effect of different fatty acid and sterol composition on the erythritol flux through the cell membrane of Acholeplasma laidlawii. Biochim. Biophys. Acta. 298:479–499. [DOI] [PubMed] [Google Scholar]
- 35.Halling, K. K., and J. P. Slotte. 2004. Membrane properties of plant sterols in phospholipid bilayers as determined by differential scanning calorimetry, resonance energy transfer and detergent-induced solubilization. Biochim. Biophys. Acta. 1664:161–171. [DOI] [PubMed] [Google Scholar]
- 36.Schuler, I., A. Milon, Y. Nakatani, G. Ourisson, A.-M. Albrecht, P. Benveniste, and M.-A. Hartmann. 1991. Differential effects of plant sterols on water permeability and on acyl chain ordering of soybean phosphatidylcholine bilayers. Proc. Natl. Acad. Sci. USA. 88:6926–6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheidt, H. A., D. Huster, and K. Gawrisch. 2005. Diffusion of cholesterol and its precursors in lipid membranes studied by 1H pulsed field gradient magic angle spinning NMR. Biophys. J. 89:2504–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miao, L., M. Nielsen, J. Thewaldt, J. H. Ipsen, M. Bloom, M. J. Zuckermann, and O. G. Mouritsen. 2002. From lanosterol to cholesterol: Structural evolution and differential effects on lipid bilayers. Biophys. J. 82:1429–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huster, D., H. A. Scheidt, K. Arnold, A. Herrmann, and P. Müller. 2005. Desmosterol may replace cholesterol in lipid membranes. Biophys. J. 88:1838–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu, X. L., and E. London. 2000. The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. Biochemistry. 39:843–849. [DOI] [PubMed] [Google Scholar]
- 41.Polson, J. M., I. Vattulainen, H. Zhu, and M. J. Zuckermann. 2001. Simulation study of lateral diffusion in lipid-sterol bilayer mixtures. Eur. Phys. J. E. 5:485–497. [Google Scholar]
- 42.Smondyrev, A. M., and M. L. Berkowitz. 2001. Molecular dynamics simulation of the structure of dimyristoylphosphatidylcholine bilayers with cholesterol, ergosterol, and lanosterol. Biophys. J. 80:1649–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu, X., R. Bittman, G. Duportail, D. Heissler, C. Vilcheze, and E. London. 2001. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (Rafts). J. Biol. Chem. 276:33540–33546. [DOI] [PubMed] [Google Scholar]
- 44.Beattie, M. E., S. L. Veatch, B. L. Slottrup, and S. L. Keller. 2005. Sterol structure determines miscibility versus melting transitions in lipid vesicles. Biophys. J. 89:1760–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bacia, K., P. Schwille, and T. Kurzchalia. 2005. Sterol structure determines the separation of phases and the curvature of the liquid-ordered phase in model membranes. Proc. Natl. Acad. Sci. USA. 102:3272–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu, F. 2005. Dual roles for cholesterol in mammalian cells. Proc. Natl. Acad. Sci. USA. 102:14551–14556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wechsler, A., A. Brafman, M. Shafir, M. Heverin, H. Gottlieb, G. Damari, S. Gozlan-Kelner, I. Spivak, O. Moshkin, E. Fridman, Y. Becker, R. Skaliter, P. Einat, A. Faerman, I. Björkhem, and E. Feinstein. 2003. Generation of viable cholesterol-free mice. Science. 302:2087. [DOI] [PubMed] [Google Scholar]
- 48.McConnell, H. M., and A. Radhakrishnan. 2000. Chemical activity of cholesterol in membranes. Biochemistry. 39:8119–8124. [DOI] [PubMed] [Google Scholar]
- 49.Hsueh, Y.-W., K. Gilbert, C. Trandum, M. Zuckermann, and J. Thewalt. 2005. The effect of ergosterol on dipalmitoylphosphatidylcholine bilayers: A deuterium NMR and calorimetric study. Biophys. J. 88:1799–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tierney, K. J., D. E. Block, and M. L. Longo. 2005. Elasticity and phase behavior of DPPC membrane modulated by cholesterol, ergosterol, and ethanol. Biophys. J. 89:2481–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bittman, R., and L. Blau. 1972. The phospholipid-cholesterol interaction. Kinetics of water permeability in liposomes. Biochemistry. 11:4831–4839. [DOI] [PubMed] [Google Scholar]
- 52.Ranadive, G. N., and A. K. Lala. 1987. Sterol-phospholipid interaction in model membranes: Role of C5-C6 double bond in cholesterol. Biochemistry. 26:2426–2431. [DOI] [PubMed] [Google Scholar]
- 53.Wenz, J. J., and J. Barrantes. 2003. Steroid structural requirements for stabilizing or disrupting lipid domains. Biochemistry. 42:14267–14276. [DOI] [PubMed] [Google Scholar]