Abstract
Libraries of RNA helicase-coupled randomized ribozymes are a powerful tool for the identification of functional genes. We have demonstrated the usefulness of this functional gene-discovery system by identifying genes involved in tumor invasion, a process that is an essential feature of tumor metastasis: the spread of cancer cells from the original tumor to other sites in the body that imposes serious problems in the prognosis and treatment of cancer. Using a filter-based invasion assay in vitro, we isolated ribozymes that enhanced the invasive properties of NIH 3T3 fibroblasts. Sequence analysis of selected clones and a database search revealed that genes such as the gene for Gem GTPase and uncharacterized genes that resemble genes for myosin phosphatase and protein-tyrosine-phosphatase are involved in cell invasion. Our system for gene identification by using ribozymes and the functional analysis of target genes should help to clarify the complex mechanisms of invasion and metastasis and might provide information that is relevant to cancer therapy.
Hammerhead ribozymes are RNA enzymes that cleave complementary substrate RNAs with high specificity (1, 2). We have been able to improve the accessibility of target sites to ribozymes and, thus, the efficiency of cleavage by ribozymes in vivo (3–5) by generating unique hybrid ribozymes that exploit the unwinding activity of an endogenous RNA helicase, which facilitates access to target sites (6–8). Hybrid ribozymes connected to poly(A) were able to recruit an RNA helicase and had substrate-unwinding activity in addition to strong cleavage activity. We subsequently created ribozymes of this type with randomized substrate-binding arms for use as tools in the identification of functional genes via selection of specific biological phenotypes (6–11). We established and demonstrated the usefulness of such a system for the identification of functional genes in the signaling pathway that leads to Fas-induced apoptosis in Fas-expressing HeLa cells and tumor necrosis factor-α-induced apoptosis in MCF-7 cells (6, 7).
In metastatic cancer, cells migrate from the primary tumor and invade other sites where they give rise to secondary tumors (12), which represent the most serious problem for cancer patients. Investigations of the mechanisms of metastasis have involved a variety of approaches (13–15), but underlying mechanisms remain unclear because metastasis is a complex phenomenon that involves many genes and pathways (16, 17). In this study, we identified functional genes involved in cell invasion, an essential process in the establishment of secondary tumors, using a library of RNA helicase-associated ribozymes with randomized substrate-binding arms. Conversion of NIH 3T3 cells with weak invasive potential (18) to cells with strong invasive potential via inactivation of the products of functional genes by transfected ribozymes was used for selection of ribozymes in an assay of cell invasion in vitro.
The assay allowed the identification and partial characterization of various invasion-related genes, which included several as yet uncharacterized genes, as candidates that might be involved in cell invasion and metastasis.
Materials and Methods
Construction of a Library of Randomized Hybrid Ribozymes.
The library of hybrid ribozymes was generated by using the retrovirus expression vector pMXpuro (19). Fragments of DNA that encoded randomized hammerhead ribozymes and a RNA polymerase III termination signal were generated by PCR with, as template, the sequence 5′-TCC CCg gTT CgA AAC Cgg gCA CTA CAA AAA CCA ACT TTN NNN NNNN CTg Atg Agg CCg AAA ggC CgA AAN NNN NNNg gTA CCC Cgg ATA TCT TTT TTT-3′ plus primers 5′-TCC CCg gTT CgA AAC Cgg gCA-3′ (sense) and 5′-gCT TgC Atg CCT gCA ggT CgA CgC gAT AgA AAA AAA gAT ATC Cgg ggT-3′ (antisense). After digestion with Csp45I and KpnI, the fragments were cloned downstream of the tRNA promotor in pPUR-KE (20). An RNA helicase-binding motif, namely, a poly(A) sequence of 60 nucleotides, was inserted between the ribozyme and the RNA polymerase III termination signal. Then, the fragments encoding tRNAVal-fused randomized hybrid ribozymes were inserted into the EcoRI and BamHI sites in pMXpuro.
Culture and Infection of Cells.
NIH 3T3 fibroblast and B16F1 melanoma cells were maintained in DMEM (Sigma) supplemented with 10% FBS (GIBCO/BRL) and a mixture of antibiotics (GIBCO/BRL) at 37°C in an atmosphere of 5% CO2 in air. The retroviral vector pMXpuro used for infections and the packaging cell line Plat-E (based on the 293T cell line) were gifts from T. Kitamura (Institute of Medical Science, Tokyo University, Tokyo). Preparation of retrovirus and infection were performed as described (19). Infected cells were selected by culturing for 1 wk in medium that contained puromycin (7.5 μg/ml).
Invasion Assay.
Invasion assays were performed with a cell invasion assay kit (Chemicon) as described (18). For confirmatory assays, we coated individual 12-μm pore Transwell inserts (Costar) with 50 μg of Matrigel (Becton Dickinson). Ribozyme-infected cells (2 × 105) suspended in DMEM plus 0.5% BSA (Sigma) were placed in the upper compartment of each chamber. Lower compartments were filled with DMEM that contained 50 μg/ml fibronectin from bovine plasma (Sigma) and 10% FBS. After 24 h, cells that had penetrated the matrigel-coated membrane and passed into the lower compartment were recovered and counted.
Preparation of Selected Ribozymes.
Hybrid ribozymes were recovered from infected cells and amplified by RT-PCR. Total RNA was prepared from cells at 70–80% confluency by using the Isogen reagent (Nippon Gene, Toyama, Japan) according to manufacturer protocol. cDNA was synthesized by using 2 μg of total RNA and Maloney murine leukemia virus reverse transcriptase (Promega). PCR was performed with primers specific for poly(A)-linked ribozymes, namely, 5′-TCC CCg gTT CgA AAC Cgg gCA-3′ (sense) and 5′-TTT TTT TTT TTT TTT TTT TTG GTA C-3′ (antisense), and for β-actin, namely, 5′-gca cgg cat cgt cac caa ct-3′ (sense) and 5′-aag gct gga aga gtg cct ca-3′ (antisense). The amplified fragments of DNA that encoded selected ribozymes were cloned into the pGEM-T vector (Promega), sequenced and subcloned into pMXpuro.
Wound Migration Assay.
Aliquots of 1 × 106 cells that harbored ribozymes were suspended in serum-free DMEM and plated in individual wells of six-well plates (Becton Dickinson) that had been coated with 10 μg/ml fibronectin. After 4 h, a line of adherent cells was scraped from the bottom of each well with a p-200 pipette tip to generate a “wound,” and the medium was replaced by DMEM that contained 5% FBS and 5 μg/ml puromycin (Sigma). Cells were allowed to proliferate and migrate into the wound for 20 h. Then they were fixed in 4% formaldehyde in PBS (Takara Shuzo, Shiga, Japan) and stained with Giemsa solution (GIBCO/BRL). The extent of migration of cells into the region from which cells had been scraped was determined from photographs. Each experiment was repeated three times.
Gelatin Zymography.
Cells that harbored selected ribozymes were seeded at 8 × 105 cells per 100-mm dish and incubated in DMEM that contained 10% FBS for 24 h. The cells were then rinsed with PBS, and culture supernatants were prepared after incubation of cells in serum-free DMEM for 48 h. Proteins in the supernatants were concentrated by filtration a Centricon system (Millipore) and were fractionated, without previous boiling, by electrophoresis on a 10% polyacrylamide gel that contained 0.5 mg/ml gelatin (Sigma). The proteins in the gel were renatured and stained as described (21).
Search of DNA Databases.
The target genes of hybrid ribozymes were identified from searches in mouse genome and cDNA databases by using the blast program (22) (www3.ncbi.nlm.nih.gov/blast/). The parameters were set automatically to optimize searches for short sequences with the “EXPECT” threshold set at 100.
Small Interfering RNA (siRNA) Preparation and Transfection.
The siRNA corresponding to LOC223433 (GenBank accession no. XM127968) was designed as recommended (23) and chemically synthesized by Japan Bio Service (Saitama, Japan). The targeting sequence for LOC223433 was from position 57 to 79 relative to the start codon. Annealing for duplex siRNA formation was also performed as described (23). Oligofectamine (Invitrogen)-mediated transfection of siRNA was carried out in six-well plates according to manufacturer instructions. Transfection mixtures containing 200 pmol or 20 pmol of siRNA and 4 μl of oligofectamine in 200 μl of Opti-MEM (Invitrogen) were added directly to preincubated cells in 800 μl of serum and antibiotic-free DMEM. Then cells were incubated for 4 h and cultured further in DMEM supplemented with 10% FBS. At 48 h after transfection, cells were harvested and subjected to the invasion assay and RT-PCR analysis with primers specific for LOC223433, namely, 5′-atc tgt gtt gtt cca gat ct-3′ (sense) and 5′-gac tgc aaa gtc tct gga gt-3′ (antisense).
Results and Discussion
Invasion Assay in Vitro with a Hybrid Ribozyme Library.
Invasion of cells through the extracellular matrix (ECM) is a critical step in tumor metastasis. Invasion by cancer cells involves several steps. Cells must adhere to and spread along the walls of blood vessels, and the proteolytic enzymes such as matrix metalloproteinases must generate tiny holes in the basement membrane that surrounds the blood vessels to allow invasion by malignant cells (12, 16, 17). An in vitro invasion assay has been developed that allows the evaluation and quantitation of the invasive potential of mammalian cells (18). The assay is performed in Boyden chambers with a porous filter coated with ECM gel, which serves as a barrier to block noninvasive cells from migrating through the filter. Invasive cells are able to migrate from the top of the filter, through the ECM layer, to the bottom of the filter (Fig. 1A). In our assays, we used nontumorigenic and noninvasive mouse NIH 3T3 fibroblasts (24) that are barely able to penetrate the ECM barrier and normally remain in the upper compartment of Boyden chambers.
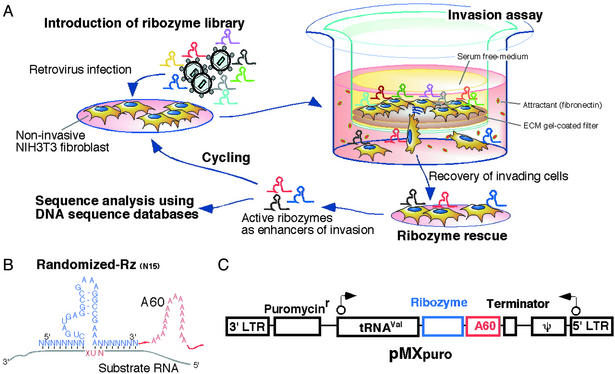
Figure 1.
Schematic representation of the gene-discovery system that included a library of poly(A)-linked ribozymes and involved an assay for determination of the invasive potential of cells. (A) Schematic illustration of the gene-discovery system. (B) The library of poly(A)-linked hammerhead ribozymes. A catalytic RNA molecule, namely, a hammerhead ribozyme (Rz), binds to specific target RNA molecules via Watson–Crick base pairs and cleaves them. The recognition arms of the ribozymes in the library are randomized and substrate RNAs contain a NUX triplet (where N and X represent A, U, G, or C and A, U, or C, respectively) for cleavage by the ribozymes (33). (C) Construction and structure of the vector that carried members of the ribozyme library. Poly(A)-linked ribozymes were cloned into the retroviral vector pMXpuro, and ribozymes were expressed as tRNAVal-fused RNAs. In this study, NIH 3T3 cells were used to isolate active ribozymes.
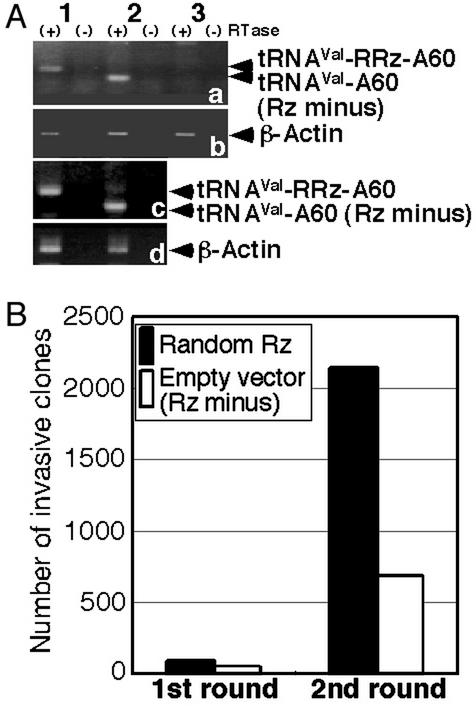
For the identification of genes involved in cell invasion, we prepared a library of hybrid poly(A)-linked ribozymes with randomized target-recognition arms composed of a total of 15 random nucleotides in a retroviral expression system (19) (Fig. 1 B and C). After selection with puromycin, we obtained >5 × 106 independent colonies. We confirmed the expression of transfected ribozymes in cells by RT-PCR with primers specific for the ribozymes (Fig. 2A). Then we subjected cells that harbored transfected ribozymes to the invasion assay (Fig. 1A). Cells were incubated in the upper compartment of Boyden chambers for 24 h. Then we collected the cells that had migrated successfully to the lower compartment. In the first round of the assay, with a total of 1.2 × 106 ribozyme-harboring cells, we isolated 95 clones of cells that had migrated to the lower compartment (Fig. 2B). However, 54 clones of cells that harbored the empty vector also invaded the lower compartment. The isolated cells were allowed to proliferate and were then subjected to a second invasion assay for the concentration and isolation of invasion-promoting ribozymes. The second round of screening yielded 2,135 clones of ribozyme-containing cells and 683 clones of empty vector-containing cells that had migrated to the lower compartment (Fig. 2B). The increased rate of migration of empty vector-containing cells might have been due to the nonspecific selection of a subpopulation of more invasive cells by the invasion assay (18).
Figure 2.
Assay of invasion by NIH 3T3 cells that harbored ribozymes. (A) Confirmation of the expression of poly(A)-linked ribozymes in randomized ribozyme (RRz)-infected cells (1), cells infected with the empty vector (2), and WT NIH 3T3 cells (3). As internal controls, results for β-actin are also shown. The symbols (+) and (−) refer to assays with and without reverse transcriptase, respectively. (B) Numbers of clones isolated after the invasion assay.
The presence of hybrid ribozymes clearly enhanced the invasiveness of NIH 3T3 cells, which was by many fold higher than that of cells that harbored the empty vector. The results indicated that the library included ribozymes that acted on the transcripts of genes involved in cell invasion. We recovered ribozymes from cells that had been identified as positive (invasive) in the second round of screening by amplification of ribozymes from the total RNA of these cells by RT-PCR (Fig. 2A). We cloned the amplified cDNAs that included the positive ribozyme sequences into the retroviral vector pMXpuro and the plasmid vector pGEM-T for further analysis of the effects of the hybrid ribozymes and for determination of their sequences, respectively.
Effects of Selected Ribozymes.
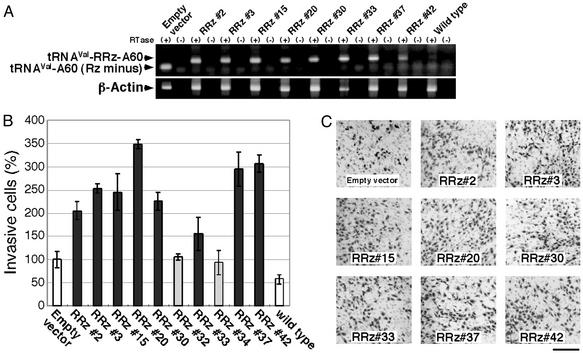
Sequence analysis of ≈10% of the total population (250 clones) revealed that the selected ribozymes had converged to ten types of hybrid ribozyme with different substrate-binding arms in the second round of the invasion assay. We reintroduced representatives of each of the 10 types of positive ribozyme into NIH 3T3 cells (Fig. 3A) and subjected the cells to the invasion assay to confirm the effects of the ribozymes on cell invasion. Eight of the 10 selected ribozymes enhanced the invasive activity of NIH 3T3 cells, as expected (Fig. 3 B and C). The remaining two ribozymes, which had little effect on invasion, were considered to represent false-positive results in the invasion assay.
Figure 3.
Confirmatory analysis of the promotion by selected ribozymes of invasion by NIH 3T3 cells. (A) Expression of the selected ribozymes in NIH 3T3 cells was confirmed by RT-PCR (see Fig. 2 legend for details). (B) NIH 3T3 cells that harbored the empty vector and cells that expressed selected ribozymes were allowed to migrate toward fibronectin for 24 h. Results are averages (±SD) of results from two independent experiments. (C) Cells that penetrated the matrigel-coated membrane were visualized by Giemsa staining. (Scale bar, 250 μm.)
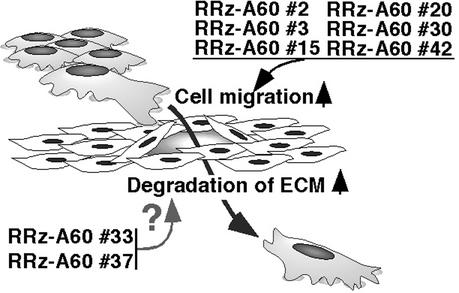
Cell invasion requires cell migration and dissolution of the ECM (12, 25). Therefore, in our initial characterization of positive ribozymes, we examined their effects on cell migration. We measured the ability of the cells to migrate by a “wound assay” (26), details of which are described in Materials and Methods (Fig. 4 A and B). Six of the eight ribozymes that we had isolated as enhancers of cell invasion accelerated the migration of NIH 3T3 cells. By contrast, ribozymes nos. 33 and 37, which enhanced cell invasion, had no effects on cell migration and appeared to enhance invasiveness via some mechanism(s) other than that involved in cell migration.
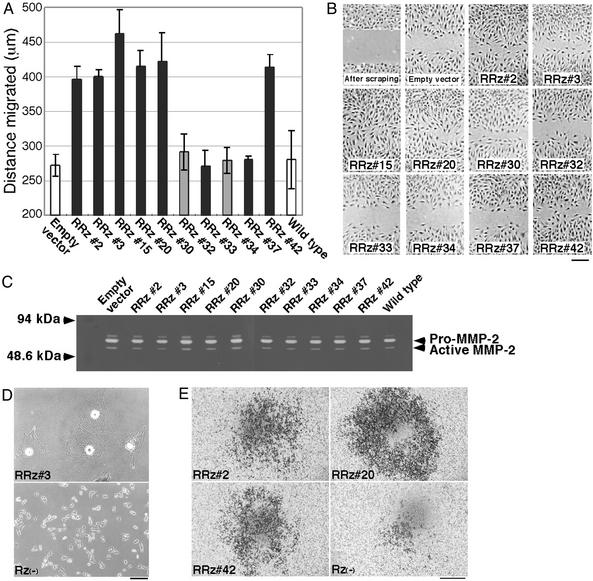
Figure 4.
Effects of selected poly(A)-linked ribozymes. (A) The selected ribozymes nos. 2, 3, 15, 20, 30, and 42, but not nos. 33 and 37, enhanced the migration of NIH 3T3 cells. Each bar represents the mean ± SD of results of three independent experiments. (B) Scraped areas and migrating cells were visualized by Giemsa staining. (Scale bar, 250 μm.) (C) The ribozymes had no significant effect on MMP-2 activity, as monitored by gelatin zymography. (D) Morphological difference between B16F1 melanoma cells treated with the ribozyme no. 3 or without ribozymes, defined Rz(−). (Scale bar, 200 μm.) (E) Effect of the selected ribozymes on invasion of B16F1 cells. Invading cells treated with the ribozyme nos. 2, 20, and 42 or without ribozymes were also visualized by Giemsa staining. (Scale bar, 700 μm.) The expression of the ribozymes in B16F1 cells was confirmed by RT-PCR (data not shown).
We next examined the ability of cells that harbored the selected ribozymes to dissolve the ECM (Fig. 4C). When cells dissolve the ECM, they release proteases, such as metalloproteases, and the activity of one well characterized protease, known as MMP-2 or gelatinase A, is correlated with the malignancy of cancer cells (25). We had anticipated the activation of MMP-2/gelatinase A by the active ribozymes, in particular by nos. 33 and 37, but we detected no significant changes of the gelatinase activities of ribozyme-harboring NIH 3T3 cells, as determined by gelatin zymography (Fig. 4C). However, numerous proteases, such as MT-MMPs, stromelysins, and matrix serine proteases are known to contribute to invasion and metastasis (27) and further investigations of proteases that cleave, for example, laminin, collagens and heparan sulfate proteoglycans might reveal how the ribozymes nos. 33 and 37 enhance the invasiveness of NIH 3T3 cells.
In addition, the active ribozymes were introduced into mouse B16F1 melanoma cells, which are known as low-invasive cells (18), to confirm the effect of the ribozymes on another cell line. Interestingly, cells treated with the active ribozyme no. 3 were clearly different from untreated and other ribozymes-treated cells in terms of morphology (Fig. 4D). Cells treated with the ribozyme no. 3 appeared to be spread as “sheets” and to barely proliferate. Currently, the difference effects of the ribozyme no. 3 on NIH 3T3 and B16F1 cells are unclear. The target gene of the ribozyme no. 3 might contribute to cell adhesion or differentiation of B16F1 cells. Next, cells expressing the ribozymes were subjected to the invasion assay. The active ribozyme nos. 2, 20, and 42 clearly promoted the invasion of B16F1 cells (Fig. 4E). Taking NIH 3T3 and B16F1 cases together, target genes of these ribozymes might function universally as invasion suppressors. On the other hand, any significant effect of the other ribozymes (nos. 15, 30, 33, and 42) on the invasion of B16F1 cells was not observed (data not shown). This result indicated a possibility that the target genes of the ribozymes act as invasion suppressors specifically in NIH 3T3 cells and/or several other cell lines, and that the genes could be used as markers for invasive properties of cancer cell lines.
Candidates for Invasion-Related Genes.
For identification of the target genes involved in cell invasion, we determined the sequences of the eight active ribozymes that we had isolated, and then we subjected the sequences to a DNA database search with the BLAST search program (22). The target genes that we identified are shown in Table 1. The search identified a gene for Gem GTPase, which was the target of active ribozyme no. 15. This gene was first cloned in 1994 and its product was recently shown to be a negative regulator of the Rho–Rho kinase pathway in cytoskeletal regulation (28, 29). The gene product has also been shown to suppress the invasiveness of transformed fibroblasts. It is, thus, both not surprising and reassuring that a ribozyme that targeted Gem GTPase mRNA was selected in our assay. The target of active ribozyme no. 42 was the product of an uncharacterized gene, clone LOC240758 (GenBank accession no. XM 136177), with a sequence that is partially similar to that of myosin phosphatase. It has been suggested that this enzyme might be a cytoskeletal regulator required for cell migration (30). The other three genes (targets of active ribozymes nos. 2, 33, and 37) did not appear to resemble any of the sequences in the DNA databases.
Table 1.
Selected ribozymes and their target genes
| Target gene | Target RNA sequence | GenBank no. | Gene/clone name | Function of gene product |
|---|---|---|---|---|
| RRz-A60 2 | UCCUGGAGUNUAAACAGAG | XM127968 | LOC223433 | No data |
| RRz-A60 3 | AGCAAUGGUNAACAAAGAU | XM122517 | AW261723 | No data, contains sugar-transport domain |
| RRz-A60 15 | ACCGCCACUNUACUGCUCC | U10551 | Gem GTPase | Small GTP-binding protein, mediator of cytoskeletal organization |
| RRz-A60 20 | CGAACUGGUNCGCACCGACA | D14636 | PEBP2 | Phosphatidylethanolamine-binding protein, transcription factor |
| RRz-A60 30 | CUAUACGCUNUGAAGGAUC | XM161208 | LOC215382 | No data, partially similar to protein-tyrosine-phosphatase |
| RRz-A60 33 | CUAUACGCUNGACAUCUG | XM156204 | LOC224159 | No data |
| RRz-A60 37 | AGCAAUGGUNAACACAGAU | XM152318 | LOC237596 | No data |
| RRz-A60 42 | AACCACACGUNAUAUAAUACC | XM136177 | LOC240758 | No data, similar to regulatory subunit of myosin phosphatase |
Recently it was revealed that 21-nt siRNAs could be used to suppress expression of endogenous genes in several mammalian cell lines (23, 31, 32). Thus we prepared synthesized-siRNA corresponding to the target gene of active ribozyme no. 2 (LOC223433) to validate functions of the identified gene by an approach different from the ribozyme technology. After confirmation of RNAi effect of the siRNA for no. 2 target gene by RT-PCR (Fig. 5A), cells treated with the siRNA were subjected to the invasion assay. The suppression of expression of the gene by the siRNA led to the promotion of the invasiveness of NIH 3T3 cells (Fig. 5B). As a result, the effect of the siRNA was similar to that of the active ribozymes no. 2. Other genes may be subjected individually to further assays in vitro and in vivo to confirm and characterize their roles in cell invasion and metastasis. The effects of the active ribozymes on invasion of NIH 3T3 cells are summarized schematically in Fig. 6.
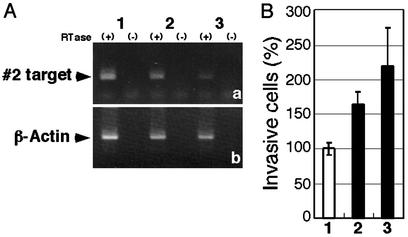
Figure 5.
Validation of target gene by siRNA. Cells were treated without siRNA for mock experiments (1) or with 20 pmol of siRNAs (2) and 200 pmol of siRNAs (3) for target gene no. 2. (A) RNAi effect was confirmed by RT-PCR. (a) Suppression of expression of target gene no. 2 was observed. (b) Results for β-actin are also shown as internal controls. (B) Invasive properties of NIH 3T3 cells were enhanced by the siRNA. Results are means ± SD of the results from two independent experiments.
Figure 6.
Schematic illustration of the effects of the selected ribozymes on invasion of NIH 3T3 cells.
The results of this study demonstrate the potential power of libraries of randomized hybrid ribozymes in the identification of genes involved in cell invasion. Using such a library and a standard assay, we identified eight genes that could be involved in invasion. These candidates seem to be the genes that normally function as metastasis suppressors. In this assay with a ribozyme library, genes that act as metastasis inducers when they are overexpressed would not be identified. Genes involved in invasion and metastasis remain to be uncovered by the assay, and thus further investigations are still required. The selected active ribozymes and potentially more effective siRNAs against each respective target gene identified in this study should help in such further efforts to understand the mechanisms of invasion and metastasis more clearly and might provide information relevant to cancer therapy.
Acknowledgments
We thank Dr. T. Kitamura (University of Tokyo) for providing us a retrovirus-packaging cell line Plat-E and a retroviral plasmid pMXpuro and Drs. R. Wadhwa and L. Nelson (National Institute of Advanced Industrial Science and Technology) for critical reading of the original manuscript.
Abbreviations
- siRNA
small interfering RNA
- ECM
extracellular matrix
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Uhlenbeck O C. Nature. 1987;328:596–600. doi: 10.1038/328596a0. [DOI] [PubMed] [Google Scholar]
- 2.Haseloff J, Gerlach W L. Nature. 1988;334:585–591. doi: 10.1038/334585a0. [DOI] [PubMed] [Google Scholar]
- 3.Krupp G, Gaur R K, editors. Ribozyme: Biochemistry and Biotechnology. Natick, MA: Eaton; 2000. [Google Scholar]
- 4.Kawasaki H, Eckner R, Yao T P, Taira K, Chiu R, Livingston D M, Yokoyama K K. Nature. 1998;393:284–289. doi: 10.1038/30538. [DOI] [PubMed] [Google Scholar]
- 5.Tanabe T, Kuwabara T, Warashina M, Tani K, Taira K, Asano S. Nature. 2000;406:473–474. doi: 10.1038/35020190. [DOI] [PubMed] [Google Scholar]
- 6.Kawasaki H, Onuki R, Suyama E, Taira K. Nat Biotechnol. 2002;20:376–380. doi: 10.1038/nbt0402-376. [DOI] [PubMed] [Google Scholar]
- 7.Kawasaki H, Taira K. EMBO Rep. 2002;3:443–450. doi: 10.1093/embo-reports/kvf098. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8. Taira, K., Warashina, M., Kuwabara, T. & Kawasaki, H. (1999) Japanese Patent Appl. H11-316133.
- 9.Welch P J, Marcusson E G, Li Q X, Beger C, Kruger M, Zhou C, Leavitt M, Wong-Staal F, Barber J R. Genomics. 2000;66:274–283. doi: 10.1006/geno.2000.6230. [DOI] [PubMed] [Google Scholar]
- 10.Kruger M, Beger C, Li Q X, Welch P J, Tritz R, Leavitt M, Barber J R, Wong-Staal F. Proc Natl Acad Sci USA. 2000;97:8566–8571. doi: 10.1073/pnas.97.15.8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beger C, Pierce L N, Kruger M, Marcusson E G, Robbins J M, Welcsh P, Welch P J, Welte K, King M C, Barber J R, Wong-Staal F. Proc Natl Acad Sci USA. 2001;98:130–135. doi: 10.1073/pnas.98.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tannock I F, Hill R P, editors. The Basic Science of Oncology. 3rd Ed. New York: McGraw–Hill; 1998. [Google Scholar]
- 13.DeRisi J, Penland L, Brown P O, Bittner M L, Meltzer P S, Ray M, Chen Y, Su Y A, Trent J M. Nat Genet. 1996;14:457–460. doi: 10.1038/ng1296-457. [DOI] [PubMed] [Google Scholar]
- 14.Fidler I J, Radinsky R. J Natl Cancer Inst. 1996;88:1700–1703. doi: 10.1093/jnci/88.23.1700. [DOI] [PubMed] [Google Scholar]
- 15.Clerk E A, Golub T R, Lander E S, Hynes R O. Nature. 2000;406:532–535. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- 16.Liotta L A, Steeg P S, Stetler-Stevenson W G. Cell. 1991;64:327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- 17.Holland J F, Frei E, editors. Cancer Medicine. 5th Ed. ON, Canada: B. C. Decker; Hamilton; 2000. [Google Scholar]
- 18.Albini A, Iwamoto Y, Kleinman H K, Martin G R, Aaronson S A, Kozlowski J M, McEwan R N. Cancer Res. 1987;47:3239–3245. [PubMed] [Google Scholar]
- 19.Morita S, Kojima T, Kitamura T. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- 20.Koseki S, Tanabe T, Tani K, Asano S, Shioda T, Nagai Y, Shimada T, Ohkawa J, Taira K. J Virol. 1999;73:1868–1877. doi: 10.1128/jvi.73.3.1868-1877.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welch D R, Fabra A, Nakajima M. Proc Natl Acad Sci USA. 1990;87:7678–7682. doi: 10.1073/pnas.87.19.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elbashir S M, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 24.Thorgeirsson U P, Turpeenniemi-Hujanen T, Williams J E, Westin E H, Heilman C A, Talmadge J E, Liotta L A. Mol Cell Biol. 1985;5:259–262. doi: 10.1128/mcb.5.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang C, Werb Z. Trends Cell Biol. 2001;11:S37–S43. doi: 10.1016/s0962-8924(01)02122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edin M L, Howe A K, Juliano R L. Exp Cell Res. 2001;270:214–222. doi: 10.1006/excr.2001.5345. [DOI] [PubMed] [Google Scholar]
- 27.Egeblad M, Werb Z. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 28.Maguire J, Santoro T, Jensen P, Siebenlist U, Yewdell J, Kelly K. Science. 1994;265:241–244. doi: 10.1126/science.7912851. [DOI] [PubMed] [Google Scholar]
- 29.Ward Y, Yap S F, Ravichandran V, Matsumura F, Ito M, Spinelli B, Kelly K. J Cell Biol. 2002;157:291–302. doi: 10.1083/jcb.200111026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murata K, Hirano K, Villa-Moruzzi E, Hartshorne D J, Brautigan D L. Mol Biol Cell. 1997;8:663–673. doi: 10.1091/mbc.8.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyagishi M, Taira K. Nat Biotechnol. 2002;20:497–500. doi: 10.1038/nbt0502-497. [DOI] [PubMed] [Google Scholar]
- 32.Kawasaki H, Taira K. Nucleic Acids Res. 2003;31:700–707. doi: 10.1093/nar/gkg158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou D M, Taira K. Chem Rev (Washington, DC) 1998;98:991–1026. doi: 10.1021/cr9604292. [DOI] [PubMed] [Google Scholar]