Abstract
There is a remarkable array of new chemical entities in the current antiepileptic drug (AED) development pipeline. In some cases, the compounds were synthesized in an attempt improve upon the activity of marketed AEDs. In other cases, the discovery of antiepileptic potential was largely serendipitous. Entry into the pipeline begins with the demonstration of activity in one or more animal screening models. Results from testing in a panel of such models provide a basis to differentiate agents and may offer clues as to the mechanism. Target activity may then be defined through cell-based studies, often years after the initial identification of activity. Some pipeline compounds are believed to act through conventional targets, whereas others are structurally novel and may act by novel mechanisms. Follow-on agents include the levetiracetam analogs brivaracetam and seletracetam that act as SV2A-ligands; the valproate-like agents valrocemide, valnoctamide, propylisopropyl acetamide, and isovaleramide; the felbamate analog flurofelbamate, a dicarbamate, and the unrelated carbamate RWJ-333369; the oxcarbazepine analog licarbazepine, which probably acts as a use-dependent sodium channel blockers, and its prodrug acetate BIA 2-093; and various selective partial benzodiazepine receptor agonists, including ELB139, which is a positive allosteric modulator of α3-containing GABAA receptors. A variety of AEDs that may act through novel targets are also in clinical development: lacosamide, a functionalized amino acid; talampanel, a 2,3-benzodiazepine selective noncompetitive AMPA receptor antagonist; NS1209, a competitive AMPA receptor antagonist; ganaxolone, a neuroactive steroid that acts as a positive modulator of GABAA receptors; retigabine, a KCNQ potassium channel opener with activity as a GABAA receptor positive modulator; the benzanilide KCNQ potassium channel opener ICA-27243 that is more selective than retigabine; and rufinamide, a triazole of unknown mechanism.
Keywords: antiepileptic drug, drug discovery, epilepsy models, maximal electroshock test, pentylenetetrazol test, kindling model
1. Introduction
Since 1993, regulatory authorities have approved ten new chemical entities for the treatment of epilepsy. The most recent drug to receive marketing approval for an epilepsy indication is pregabalin. A new drug application for rufinamide is pending before the U.S. Food and Drug Administration. Antiepileptic drug (AED) development is less vigorous today than it had been a decade ago and most major pharmaceutical companies no longer have AED discovery programs. Nevertheless, spin-offs from research in other programs along with work in academia and smaller companies continue to provide a steady stream of new molecules with activity in preclinical epilepsy models to maintain a robust pipeline and selected agents continue to enter clinical trials.
The engine that drives antiepileptic drug (AED) discovery is screening in animal models. For practical reasons, most testing, at least at the compound identification stage, is carried out in normal rodents induced to have a seizure by an electrical or chemical stimulus. Chronic models, in which the animals have a genetic or acquired propensity for seizures, are arguably closer to human epilepsy and commonly are included in the battery of tests for early stage characterization AEDs (White et al., 2002). Although there are a large number of models that could potentially be used to screen for anticonvulsant activity, the maximal electroshock model (MES) and the subcutaneous pentylenetetrazol model (PTZ) remain the “gold standards” in early stages of testing (Schmidt and Rogawski, 2002). Compounds active in either the MES or PTZ tests have generally been efficacious in clinical trials, although inactivity in these tests does not necessarily indicate lack efficacy. An alternative electroshock test—the 6-Hz model, in which the endpoint is limbic seizure activity rather than tonic hindlimb extension as in the MES test—may detect compounds like levetiracetam that fail to be identified by the MES or PTZ models (Barton et al., 2001). Kindling models, where animals have an acquired susceptibility for limbic seizures, are now routinely a part of the battery for preclinical characterization. Other models commonly used in early stage development include audiogenic seizure susceptible mice, various chemoconvulsant models other than the PTZ model, and rat and mouse strains that exhibit absence-like seizures.
The use of animal screening models that are unbiased with respect to mechanism provides an opportunity to identify compounds that act in new ways and on novel molecular targets (Rogawski, 2006). As clinical experience is acquired postmarketing, a consensus develops as to the uses of the new drug for different seizure types and epilepsy syndromes. It is not uncommon for the new drug to exhibit a “personality” that differs from that of other marketed AEDs. Often clinical applications are found in nonepilepsy conditions, such as migraine or bipolar disorder. Thus, experience has shown that screening in a standard panel of animal models does not necessarily lead to “me-too” drugs. Moreover, because the animal models used in the early stage identification of AEDs are mechanism neutral, they do not necessarily detect compounds that act through traditional AED targets, such as voltage-activated sodium channels or components of the GABA system (Rogawski and Löscher, 2004a). In fact, compounds can emerge from animal testing programs that protect against seizures through actions on entirely novel brain targets. Recently, this principal has been reinforced by the identification of the target for gabapentin and pregabalin as members of the α2δ family of calcium channel subunits (Rogawski and Taylor, 2006) and, as discussed below, for levetiracetam as the synaptic vesicle protein SV2A.
All of the molecules in today’s AED pipeline were identified and characterized in animal models. However, there are two pathways through which they entered the screening process. In some cases the compounds are new chemical entities that were submitted for testing in order to characterize their biological activity and were serendipitously found to protect against seizures. In other cases, the molecules were more-or-less rationally designed based on currently marketed agents, with the objective of enhancing efficacy or, more commonly, eliminating problematic side effects. In the present review, I consider what is known about the mechanisms of the various classes of agents currently in the development pipeline.
2. Enhancement of Presently Marketed Drugs
2.1. Levetiracetam Analogs
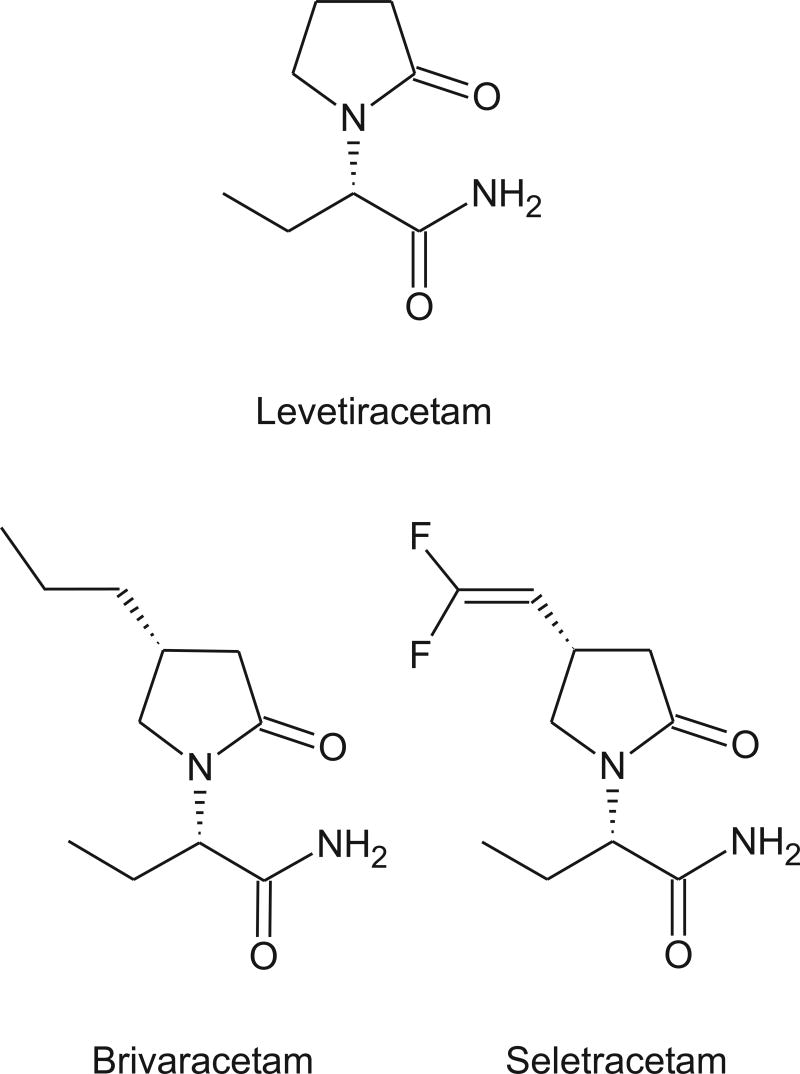
SV2A, a synaptic vesicle protein, has recently been identified as the likely target for levetiracetam (Lynch et al., 2004) and two more potent follow-on structural analogs seletracetam (ucb 44212) and brivaracetam (ucb 34714). The mechanism whereby binding to SV2A results in anticonvulsant activity is unknown. SV2A is an abundant protein component of synaptic vesicles that is structurally similar to 12-transmembrane domain transporters, although a transporter activity for SV2A has not yet been identified. SV2A is not essential for synaptic transmission, but mice in which the protein has been deleted by gene targeting exhibit seizures (Janz et al., 1999). It seems reasonable that the SV2A ligands could protect against seizures through effects on synaptic release mechanisms. In fact, for AEDs that target voltage-activated sodium and calcium channels (including α2δ) inhibition of glutamate release is likely to be a critical downstream action for seizure protection (Rogawski and Löscher, 2004a; Rogawski and Taylor, 2006).
Levetiracetam is not active in the traditional MES or PTZ models when conducted according to standard protocols (Klitgaard et al., 1998; Klitgaard, 2001). Its anticonvulsant activity was discovered in audiogenic seizures in susceptible mice, and it was subsequently found to have activity in a range of chemoconvulsant models, including seizures induced by submaximal PTZ doses (Gower et al., 1992), the 6-Hz model, a rat genetic model of absence epilepsy (Gower et al., 1995) and also various kindling models (Löscher and Hönack, 1993). Levetiracetam also seems to have “antiepileptogenic” activity, in that it retards the development of pentylenetetrazol-kindled seizures (Gower et al., 1992) as well as conventional amygdala-kindled seizures (Löscher et al., 1998).
Seleteracetam and brivaracetam are derivatives of levetiracetam that are substituted at the 4-position on the 2-pyrrolidinone ring (Kenda et al., 2004). Both have 10-fold greater affinity for SV2A than does levetiracetam, but brivaracetam may also have modest sodium-channel blocking activity. Like levetiracetam, seleteracetam is inactive in the MES and PTZ tests. However, it is 10-fold more potent that levetiracetam in the corneal kindling model and 25-fold more potent in the genetic absence epilepsy rat of Strasbourg (GAERS). In contrast, brivaracetam is slightly less potent than seletracetam in corneal kindling, but is weakly active in the MES and PTZ tests. Overall, seletracetam seems to have a similar pharmacological profile to levetiracetam, but is more potent. In animal models, it exhibits an even higher protective index than levetiracetam. (Protective index, the ratio of TD50 for rotorod toxicity to ED50 for seizure protection, is a measure of the separation between anticonvulsant potency and potency to induce motor impairment; Löscher and Nolting, 1991). In contrast, brivaracetam has a broader spectrum of activity and may be more effective than levetiracetam in the rat amygdala kinding model for suppressing both behavioral kindled seizures and the afterdischarge. However, it has a lower protective index than levetiracetam. In a placebo-controlled phase IIa clinical trial in patients with photosensitive epilepsy brivaracetam was effective in reducing the photoparoxysmal electroencephalogram response (Kasteleijn-Nolst Trenitè et al., 2004). Seleteracetam and brivaracetam provide examples of how it is possible for analogs of a prototype anticonvulsant structure to either optimize the inherent activity of the molecule and maintain its high target site selectivity (seleteracetam) or, alternatively, broaden its target specificity so that it exerts therapeutically-desirable actions on additional targets. I have argued previously that it can be advantageous for an AED to act through more than one anticonvulsant mechanism (Rogawski, 1998). However, the price paid in the case of brivaracetam is tolerability, where the protective index slips from the very high value associated with pure SV2A ligands toward the much lower value seen with sodium channel-blockers.
2.2. Valproate-Like Agents
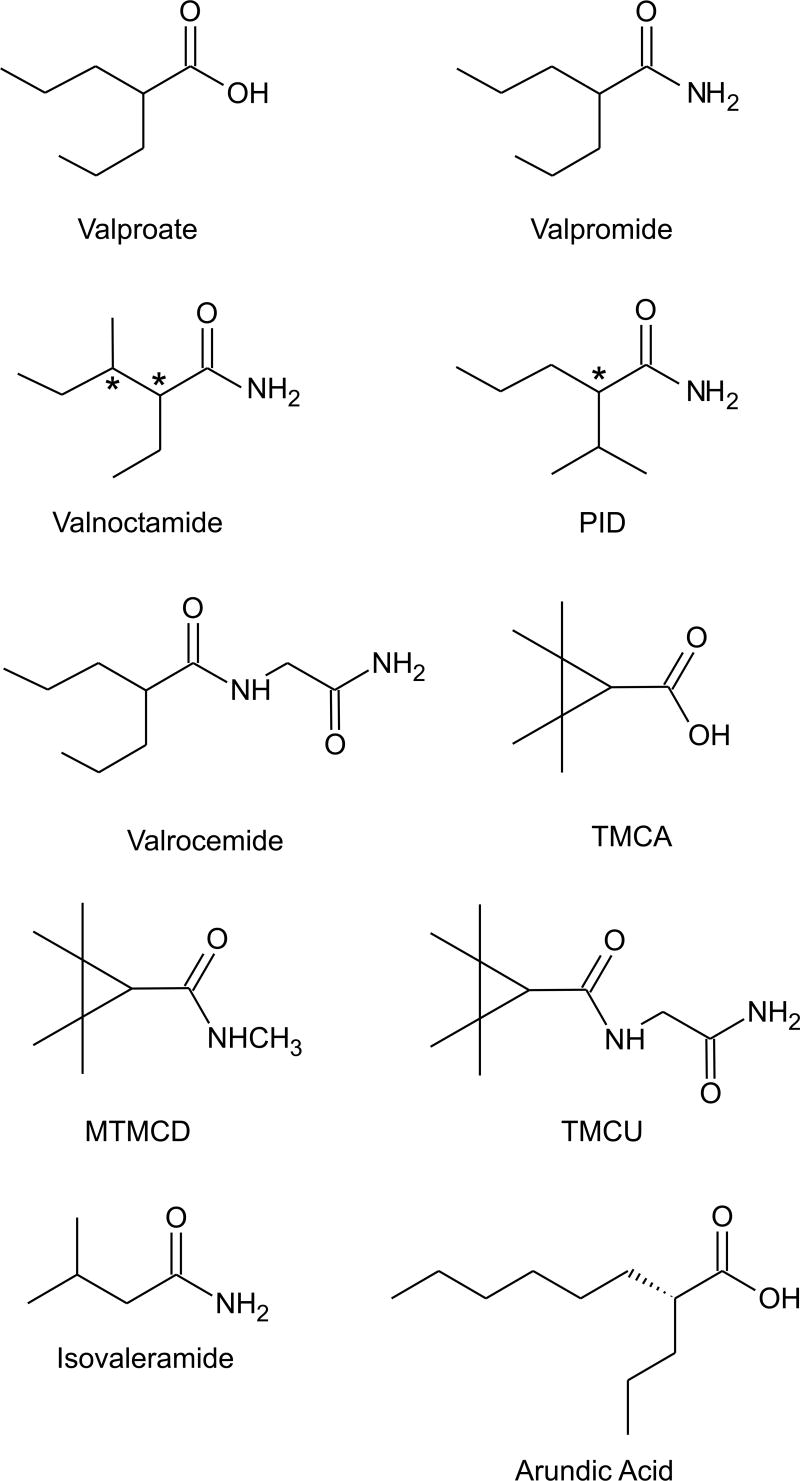
Valproate (di-n-propylacetic acid, isooctanoic acid) has a unique place in epilepsy therapy because of its efficacy in generalized seizures, including absence, primary generalized tonic-clonic, and myoclonic seizures, as well as in partial seizures (Perucca, 2002). It is also an important therapeutic option for migraine prophylaxis and in the treatment of acute mania. However, valproate has substantial disadvantages in that it is a teratogen, causes weight gain and reproductive dysfunction, and occasionally is associated with heptatic and pancreatic toxicity. Therefore, there has been considerable interest in the development of an agent that has the same broad spectrum of clinical efficacy as valproate without these undesirable characteristics. Because the precise mechanism of action of valproate is unknown, it is difficult to predict—based on preclinical studies alone—whether an analog will have the same broad spectrum of clinical efficacy as the parent. Valproate exhibits protective activity in virtually all animal AED screening models, but its potency in any specific model varies depending upon the species and route of administration (Löscher, 2002). These factors complicate attempts to prove that a compound is “valproate-like.” Valproate is especially active in genetic models of absence seizures, such as the “genetic absence epilepsy rat from Strasbourg” (GAERS) (Marescaux et al., 1992). It is also protective against seizures induced by the inverse benzodiazepine agonist DMCM (Petersen, 1993). At higher doses, valproate is protective in the MES and PTZ models. Valproate has diverse pharmacological actions, but none of these actions has been unequivocally linked to its broad protective activity against diverse seizure types in animals and in man. Among these various actions, Löscher (2002) has concluded that increases in GABA turnover which are produced in specific brain regions is of particular importance in the ability of valproate to control of seizure generation and propagation in diverse epilepsy models.
A variety of compounds that are more or less structurally similar to valproate have been shown to have a valproate-like profile in animal seizure models. In some cases, the compounds have greater potency than valproate. For example, valpromide (valproic acid amide), which is marketed as an AED and antipsychotic agent in several European countries (Bialer, 1991), is approximately two-fold more potent than valproate in the MES and PTZ models (Löscher and Nau, 1985). The enhanced potency is believed to be due to greater blood–brain barrier permeability, with corresponding greater brain concentrations (Blotnick et al., 1996). The higher brain penetration also contributes to greater potency for inducing motor impairment, so that, if anything, the protective index of valpromide is reduced compared with valproate. Valpromide is not converted to valproate in mice, and there is no evidence that it is teratogenic in this species (Nau and Löscher, 1986). In humans, however, valpromide is rapidly biotransformed to valproate (Bialer, 1991).
Because of its conversion to valproate, valpromide does not offer a substantial therapeutic advantage. However, Bialer and colleagues have identified analogs of valpromide that resist metabolic hydrolysis to their corresponding acids (Isoherranen et al., 2003b). Two such analogs, valnoctamide (2-ethyl-3-methylpentanamide) and diisopropyl acetamide (PID), exhibit greater anticonvulsant potency than valproate and their corresponding acids (Winkler et al., 2005). Studies in human subjects have confirmed that valnoctamide undergoes only minimal biotransformation to valnoctic acid (Barel et al., 1997). Valnoctamide is a chiral compound with two stereogenic carbons, so that the molecule exists in two enantiomeric pairs. Only small differences were found between the anticonvulsant potencies and pharmacokinetic properties of the (2S,3S) and (2R,3S) forms (Isoherranen et al., 2003a). Valnoctamide (a mixture of both enantiomeric pairs) has been marketed as an anxiolytic and sedative (as Axiquel and Nirvanil) in the U.S., Italy, the Netherlands and Switzerland. Whether it is useful in epilepsy remains to be determined. Despite having been marketed as a sedative, the drug was well tolerated and produced only mild sedation in volunteers (Pisani et al., 1992; F. Pisani, personal communication). Animal studies reveal a striking difference from valproate in terms of teratogenicity. Thus, in pregnant NMRI mice where valproate produces more than 50% embryotoxicity, valnoctic acid and valnoctamide induce embryotoxicity at frequencies no greater than control (Radatz et al., 1998). One disadvantage of valnoctamide is that it is a potent inhibitor of microsomal epoxide hydrolase so that concomitant administration with carbamazepine causes five-fold increases in the levels of carbamazepine epoxide and clinical signs of toxicity (Pisani et al., 1992; 1993).
PID, which is expected to enter clinical development, is also a chiral compound with a single stereocenter. As in the case of valnoctamide, only small differences were found in the relative anticonvulsant potencies and pharmacokinetic properties of the enantiomers. Thus, while the (R)-enantiomer was found to be modestly more potent than the (S)-enantiomer in the rat MES test (ED50 values, 25 and 16 mg/kg, p.o., respectively; Spiegelstein et al., 1999), this enantioselectivity was not observed in all models (Isoherranen et al., 2003c). In mice, the (R)-enantiomer had a modestly longer half-life, but pharmacokinetic differences between the isomers were less evident in the rat. Whether the enantiomeric selectivity is due to pharmacokinetic differences remains to be determined.
Another potential valproate-like AED is valrocemide (valproyl glycinamide). Valrocemide is modestly more active in the mouse MES test than valproate (ED50, 151 versus 287 mg/kg, i.p., for valproate) and is similarly more active in the mouse PTZ test (ED50, 132 versus 209 mg/kg, i.p. for valproate) (Isoherranen et al., 2001). Valrocemide is also more potent in other chemoconvulsant models, including seizures induced by bicuculline and picrotoxin, but not by strychnine, where valproate is weakly active (ED50, 293 mg/kg, i.p.) but valrocemide is inactive. It has been proposed that the greater potency of valrocemide is due to its increased accumulation in brain. Assuming valrocemide has valproate-like activity in the clinic, the question remains whether the drug will have reduced teratogenicity or side effects. Valrocemide does not cause embryotoxicity in rats and rabbits. The main metabolic pathway is enzymatic amide hydrolysis yielding the acid valproylglycine (Hovinga, 2004; Bialer et al., 2004). In rats and dogs, minimal levels of valproate are produced. However, in humans treated with valrocemide (3 g/day) mean valproate levels are substantial (15 μg/ml), but below the therapeutic range (50–100 μg/ml). Therefore, valrocemide could be safer than valproate, but the risk of teratogenicity is not eliminated. The half-life of valrocemide is relatively short so that three-times daily dosing will be required.
Bialer and colleagues have extensively investigated the anticonvulsant activity of cyclic analogs of valproate in an attempt to avoid the hepatotoxicity and teratogenicity of valproate. Starting with 2,2,3,3-tetramethylcyclopropanecarboxylic acid (TMCA), which itself is not a potent anticonvulsant, several amides were discovered with excellent anticonvulsant activity, including MTMCD (N-methyl-tetramethylcyclopropanecarboxamide) and 2,2,3,3-tetramethylcyclopropanecarbonylurea (TMCU) (Isoherranen et al., 2003b; Sobol et al., 2004). These cyclic analogs possess two quaternary carbons in the β-position to the carbonyl and cannot be biotransformed into metabolites with a terminal double bond, which is presumed to be the souce of hepatotoxicity (Sobol et al., 2005). Like valproate, these compounds are effective in both the MES and PTZ tests, but some show a greater relative potency in the PTZ test raising the possibility that they are mechanistically different from valproate. For example, MTMCD has ED50 values of 98 and 39 mg/kg in the mouse MES and PTZ tests compared with values for valproate of 263 and 220 mg/kg. In contrast, TMCU is preferentially active in the MES test (ED50 values, 90 and 125 mg/kg, respectively).
Eadie (2004) has recently proposed that valerian, the dried root of various Valeriana species with purported antiepileptic properties, is active as a result of a constituent isovaleric acid, a simple branched-chain monocarboxylic acid. Isovaleric acid has undesirable odor and taste, but its amide isovaleramide does not. Indeed, isovaleramide (NPS-1776) has been found to have activity in the MES and PTZ models as well as in kindling models and other chemoconvulsant and absence epilepsy models (Bialer et al., 2001). Overall, it appears similar to valproate but is weaker in potency in animal testing (e.g., MES and PTZ ED50 values, 913 and 748 mg/kg, p.o., respectively). Phase I clinical trials have indicated that isovaleramide is safe and well tolerated; whether it is truly valproate-like will require further clinical evaluation.
An additional valproate analog that is currently in early stage clinical trial is arundic acid [(R)-(–)-2-propyloctanoic acid; ONO-2506 (de Paulis, 2003; Asano et al., 2005). This compound is being evaluated for stroke and neurodegenerative diseases, but not epilepsy.
2.3 Carbamates: Flurofelbamate and RWJ-333369
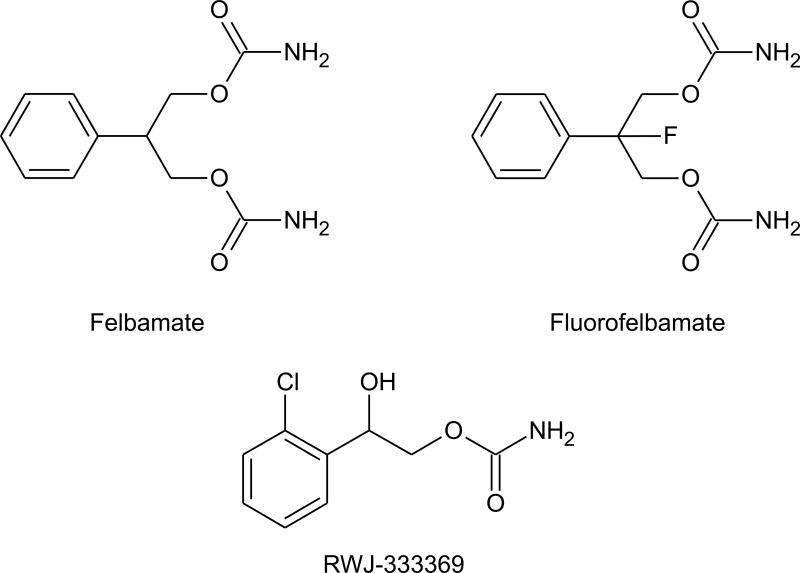
The dicarbamate felbamate, a broad spectrum AED, has a variety of effects on ion channels that could contribute to its therapeutic activity. The drug has been reported to inhibit sustained repetitive spike firing in cultured neurons, suggesting that it modulates voltage-activated sodium channels (White et al., 1992). However, an effect on recombinant sodium channels was observed only at relatively high (1 mM) concentrations (Taglialatela et al., 1996). Felbamate also inhibits high voltage activated calcium currents (Stefani et al., 1996), but the significance of this action for seizure protection is uncertain. At therapeutic serum concentrations (100 to 300 μM), felbamate acts as a positive allosteric modulator of GABAA receptors (Rho et al., 1994; Kume et al., 1996). However, this effect appears to be specific for GABAA receptors containing α1 or α2 subunits along with β2 or β3 subunits (Simeone and McClellan, 2000). In addition, felbamate at a concentration of 100 μM has been found to block NMDA receptor mediated synaptic responses (Pugliese et al., 1996) and to inhibit NMDA receptor currents in isolated neurons (Kd, ~1 mM; Rho et al., 1994; Subramaniam et al., 1995; Kuo et al., 2004). Studies with recombinant NMDA receptor subunit combinations have indicated that felbamate selectively bocks NMDA receptors composed of NR2B subunits at lower concentrations (IC50, 0.5 mM), than other subunit combinations (Kleckner et al., 1999; Harty and Rogawski, 2000). This selectivity could contribute to the relatively low neurobehavioral toxicity of felbamate in relation to other NMDA receptor antagonists (Löscher and Rogawski, 2002).
Felbamate use is limited by hematological and hepatic toxicities which are attributed to the formation of an aldehyde (atropaldehyde or 2-phenylpropenol) intermediate that reacts with proteins (Thompson et al., 2000). Substitution of a fluorine atom for a hydrogen at the 2-position of the propanediol moiety of felbamate to form fluorofelbamate prevents the formation of this reactive aldehyde. In some animal models fluorofelbamate is equivalent in potency to felbamate and in others it is substantially more potent (Bialer et al., 2004). Notably, in the rat MES test, the ED50 of flurofelbamate is 3 mg/kg p.o. whereas felbamate is 8-fold less potent (ED50, 25 mg/kg). There is only limited information to address the question of whether the action of fluorofelbamate on ion channels is similar to that of felbamate. Preliminary evidence indicates that fluorofelbamate does not enhance GABA receptor responses (Bialer et al., 2004). However, like felbamate, fluorofelbamate may inhibit NMDA receptors and might also affect sodium channels.
A series of monocarbamates of the general structure 2-carbamoyl-1-chlorophenylethanol have been described with activity in the mouse MES test (ED50 values, ≥7.4 mg/kg, p.o.; Choi and Kim, 1997, 2000). One of these compounds 2-carbamoyloxy-1-(2-chlorophenyl)ethanol (YKP509, RWJ-333369) was selected for clinical development. RWJ-333369 has activity in the mouse MES (7.9 mg/kg, i.p.) and PTZ (ED50, 20.4 mg/kg, i.p.) tests and is also protective against seizures induced by bicuculline and picrotoxin, and in some kindling models. RWJ-333369 is also active in the GAERS model of absence epilepsy, albeit at doses that are modestly greater than those active in the MES test (doses of 30 and 60 mg/kg attenuated spike-wave discharges but 10 mg/kg was inactive; Nehlig et al., 2005). It therefore differs from other marketed AEDs in having a broader spectrum of activity even than felbamate, which is not active in the bicuculline model. There is preliminary evidence that the drug is effective in protecting against spontaneous recurrent seizures in kainate-treated animals (Grabenstatter and Dudek, 2004) and that it confers antiepileptogenic and neuroprotective activity when administered repeatedly after lithium-pilocarpine status epilepticus in rats (Francois et al., 2005). In the lithium-pilocarpine study, RWJ-333369 was administered at 1 h and 8 h after the onset of status epilepticus and then twice daily for 6 days. At 30 mg/kg, all animals had a spontaneous seizure within 15 days. However, with doses of 60–120 mg/kg many of the animals showed longer delays to the onset of seizures and at the 90 and 120 mg/kg doses 45% of animals failed to show spontaneous seizures during the 150 day observation period. In this study, diazepam did not confer the same antiepileptogenic activity as RWJ-333369, however, it is noteworthy that attenutation of status epilepticus by diazepam in other situations can reduce the incidence and severity of spontaneous seizures (Pitkänen et al., 2005). RWJ-333369 is currently in Phase II clinical development for the treatment of partial epilepsy and migraine headache. Whether the drug will ultimately prove to have clinically useful antiepileptogenic properties is of considerable interest.
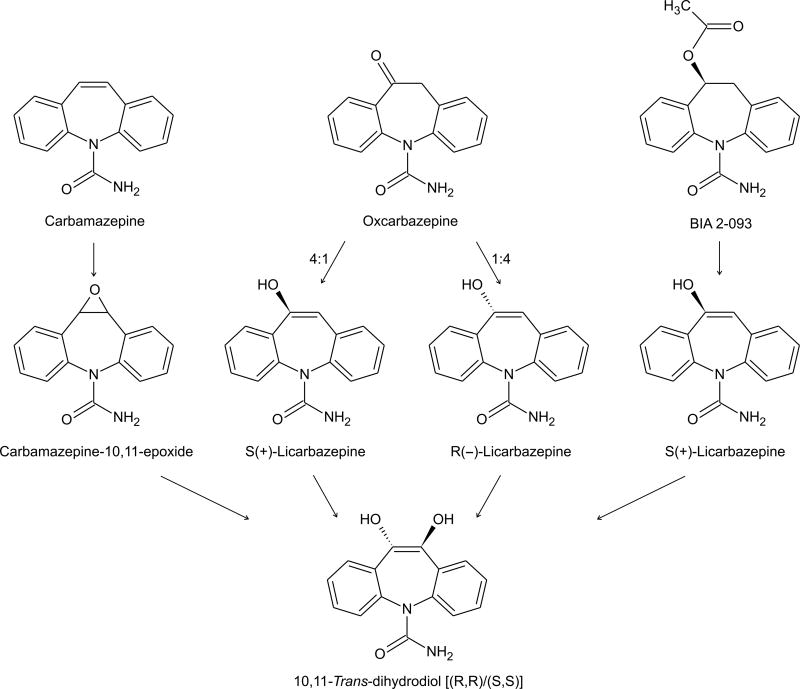
2.4. Carbamazepine Analogs: Licarbazepine and (S)-Licarbazepine Acetate
Carbamazepine is extensively metabolized through several distinct biotransformation pathways. Quantitatively, the most important biotransformation is the expoxide-diol pathway in which carbamazepine is first oxidized by CYP3A4 and CYP2C8 at the 10,11-double bond of the azepine ring to form the chemically stable epoxide (carbamazepine-10,11-epoxide), which has potent anticonvulsant activity in its own right. Several studies have implicated the epoxide in side effects. However, other studies have disputed this conclusion and at least one study suggested an improvement in side-effects when patients are switched to the epoxide (Holmes, 2002). In any case, there has been an interest in analogs of carbamazepine that are not converted to the epoxide, as is the case for oxcarbazepine, the 10-keto analog of carbamazepine. Oxcarbazepine serves as a prodrug for its 10-hydroxy metabolites (R)- and (S)-licarbazepine, which appear in plasma and urine in a 4:1 ratio (Faigle and Menge, 1990; Volosov et al., 1999). Racemic licarbazepine (LIC477) is currently in Phase III clinical trials for the treatment of bipolar mania, but there are no plans to develop it for epilepsy. Since licarbazepine is the main active metabolite of oxcarbazepine, it is unlikely to provide advantages over the parent.
(S)-licarbazepine acetate (BIA 2-093) is also in Phase III clinical trials. BIA 2-093 only forms (S)-licarbazepine, which is then converted to the trans-diol (Benes et al., 1999; Almeida et al., 2005). In rats, orally administered BIA 2-093 (2 h; ED50, 4.7 mg/kg) is as potent as carbamazepine (ED50, 5.4 mg/kg) in the MES test and more potent than oxcarbazepine (ED50, 10.0 mg/kg). Interestingly, there is some stereoselectivity as (R)-licarbazepine acetate is about one-half as potent (ED50, 10.9 mg/kg). In the mouse MES test BIA 2-093 is less potent than carbamazepine. BIA 2-093, like carbamazepine and oxcarbazepine, is weak or inactive in the PTZ and kindling models. BIA 2-093 causes a voltage-dependent block of voltage-dependent sodium currents with similar potency to carbamazepine (Bonifácio et al., 2001). In addition, BIA 2-093, as is the case for other sodium channel blocking AEDs (Rogawski and Löscher, 2004a), inhibits sodium channel-dependent release of neurotransmitters with similar potency to carbamazepine and oxcarbazepine (Ambrósio et al., 2001; Parada and Soares-da-Silva, 2002). Although BIA 2-093 is chemically distinct from licarbazepine and could potentially have distinct pharmacodynamic properties related to its novel structure, there is little evidence that it is substantially different mechanistically. Since oxcarbazepine forms both licarbazepine enantiomers, the fact that BIA 2-093 only forms (S)-licarbazepine could confer an advantage. However, as yet, there is no evidence that (R)-licarbazepine interferes with efficacy or results in increased side effects. In fact, even though (R)-licarbazepine is less potent than (S)-licarbazepine in the MES test, (R)-licarbazepine has an equivalent or more favorable protective index (rotarod ED50/MES ED50) (Benes et al., 1999).
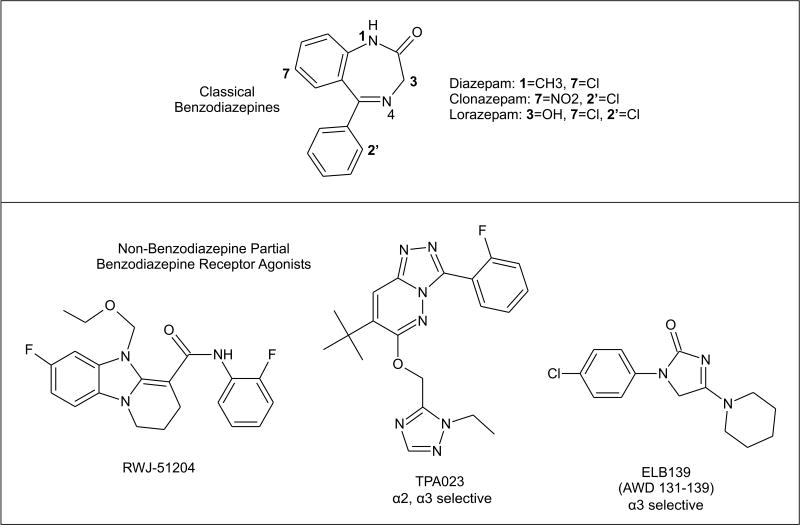
2.5 Benzodiazepine-like Agents
Benzodiazepines which act as positive allosteric modulators of GABAA receptors, including diazepam, lorazepam and clonazepam, have potent anticonvulsant activity in animal seizure models and are used clinically in the treatment of status epilepticus (Rogawski, 1996). These classical benzodiazepines act on GABAA receptors containing γ2 subunits and α1, α2, α3 or α5 subunits. Such benzodiazepine-sensitive GABAA receptors are believed to comprise more than three-quarters of all brain GABAA receptors (Whiting, 2003). The development of tolerance as well as mechanism-related side effects, most notably sedation, limit the use of classical benzodiazepines in chronic epilepsy therapy. Benzodiazepine-like agents that act as partial agonists at the benzodiazepine recognition site, such as bretazenil, imidazenil, divaplon, FG 8205, abecarnil, NS 2710, pagoclone, and RWJ-51204, may show less propensity for tolerance (Costa and Guidotti, 1996). RWJ-51204 is of particular interest as it has highly potent anticonvulsant activity and a favorable protective index (Dubinsky et al., 2002). None of the partial benzodiazepine agonists, however, has been proven to lack tolerance in clinical trials.
Another approach toward obtaining improved clinical performance is through the development of subtype selective benzodiazepine-like agents. It is now well recognized that α1-subunit containing GABAA receptors have a more significant role in the sedative and anticonvulsant effects of benzodiazepine recognition site agonists, while anxiolytic, myorelaxant and motor-impairing activity largely depends on the α2, α3 and α5 subunits (Rudolph et al., 1999; Whiting, 2003). Based on this view, it would be expected that α1-selective benzodiazepine receptor agonists like zolpidem (Kralic et al., 2002) would excel as anticonvulsants. In fact, zolpidem has strong sedative activity as expected by its α1 selectivity, but requires 10–20-fold higher doses for protection against PTZ seizures and the protection conferred is not as robust as for classical benzodiazepines (Depoortere et al., 1986; Crestani et al., 2000). In contrast, classical benzodiazepines protect against PTZ seizures at doses lower than those causing sedation because lower receptor occupancy is required for seizure protection than sedation (see Rogawski, 1996). The basis for the relatively low anticonvulsant potency of zolpidem is obscure, but could indicate that non-α1-containing GABAA receptors also play a role in seizure protection in contrast to sedation which is largely dependent upon α1-containing receptors. In any case, α1-selective agents are not clinically useful anticonvulsants since they are strongly sedative.
Confirming the role of α1 in sedation, agents that selectively target non-α1 subunits such as L-838,417 [7-(1,1-Dimethylethyl)-6-(2-methyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2,5-difluorophenyl)-1,2,4-triazolo[4,3-b]pyridazine] and TPA023 [7-(1,1-dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2-fluorophenyl)-1,2,4-triazolo[4,3-b]pyridazine] lack sedative effects in animal models (McKernan et al., 2000; Atack et al., 2006). Both L-838,417 and TPA023 bind to α1-containing GABAA receptors but have no intrinsic activity and indeed act as antagonists at these isoforms; they are partial agonists for other GABAA receptor subtypes. (TPA023 also acts as an antagonist at α5-containing receptors.) Interestingly, however, L-838,417 and TPA023 retain anticonvulsant activity against PTZ seizures and in other seizure models indicating that seizure protection can be conferred in the absence of α1 agonist activity. Another α2-selective positive modulator is the fluoroquinolone antibiotic analog 7-chloro-1-ethyl-6-[(1,2,3,4-tetrahydro-1-naphthylenyl)amino]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid, but it does not appear to act at the benzodiazepine recognition site (Johnstone et al., 2004). Such agents could theoretically be useful AEDs and may have reduced liability for sedation compared with classical benzodiazepines, although this remains to be demonstrated in man. L-838,417 was not advanced to development because of undesirable pharmacokinetic properties, whereas TPA023 appears to have clinical potential. Unfortunately, early stage clinical testing has revealed that these compounds do not lack sedative activity in humans, indicating that the animal models that have been used to assess liability for sedation are not adequately predictive.
Another subtype selective benzodiazepine receptor agonist is ELB139 [1-(4-chlorophenyl)-4-piperidin-1-yl-1,5-dihydro-imidazol-2-on], which has potency equivalent to diazepam on α3-subunit containing GABAA receptors, but is 50-fold weaker at α1 and α5 and inactive at α2 and α4 (Langen et al., 2004, 2005). It has reduced efficacy for potentiation of α3, α1 and α5 receptors, and is therefore a partial agonist. ELB139 is currently in Phase II clinical trials as an anxiolytic. However, it also has anticonvulsant activity albeit with lower potency than classical benzodiazepines (Dost et al., 2005). There is evidence that the partial agonist activity of ELB139 confers reduced tolerance liability. While the animal studies with this compound are encouraging, the human experience to date with subtype selective partial agonists tempers optimism. Nevertheless, careful clinical evaluation of such agents in chronic epilepsy therapy is warranted to determine whether the tolerance and sedation characteristic of classical benzodiazepines are avoided. Patients with epilepsy may tolerate AED side-effects better than non-epileptic individuals, so studies in subjects with epilepsy are imperative.
3. New Scaffolds and Mechanisms
3.1 Functionalized Amino Acids
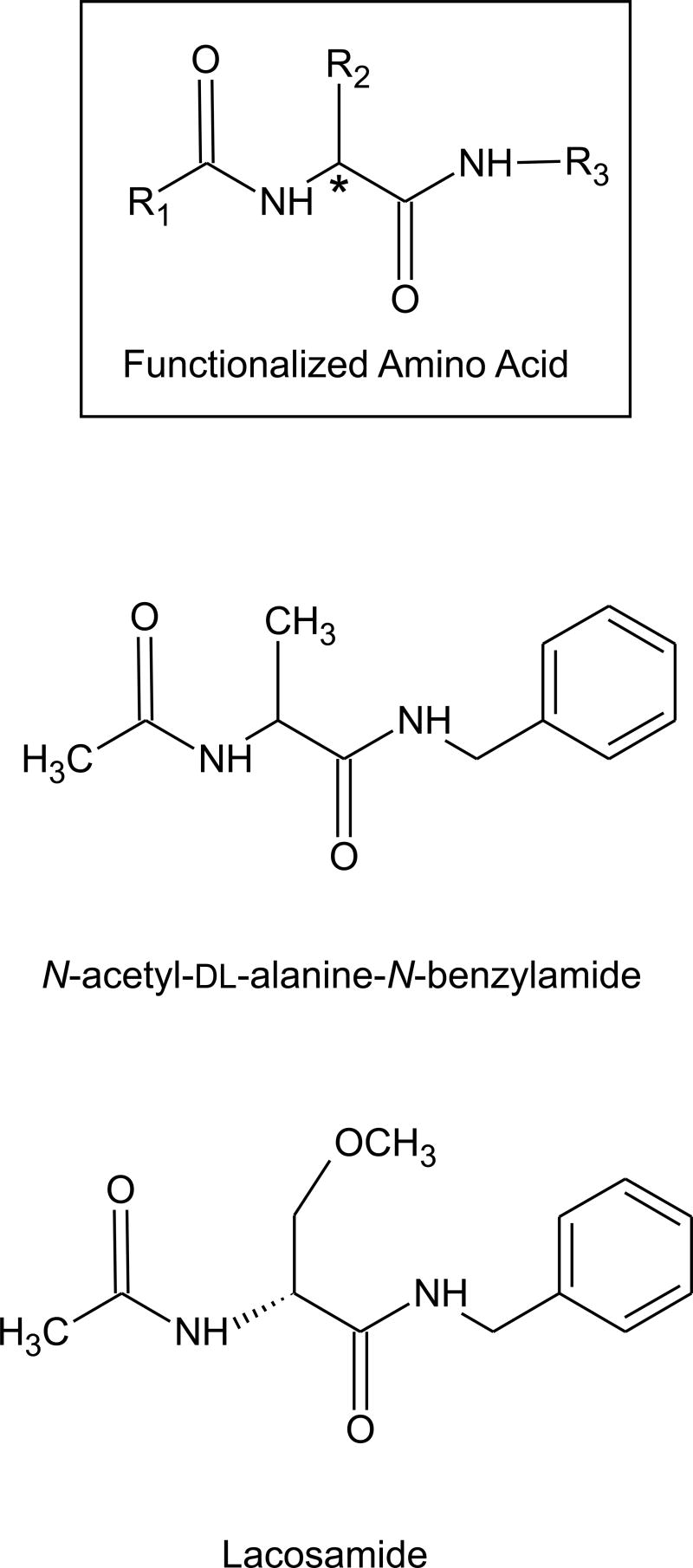
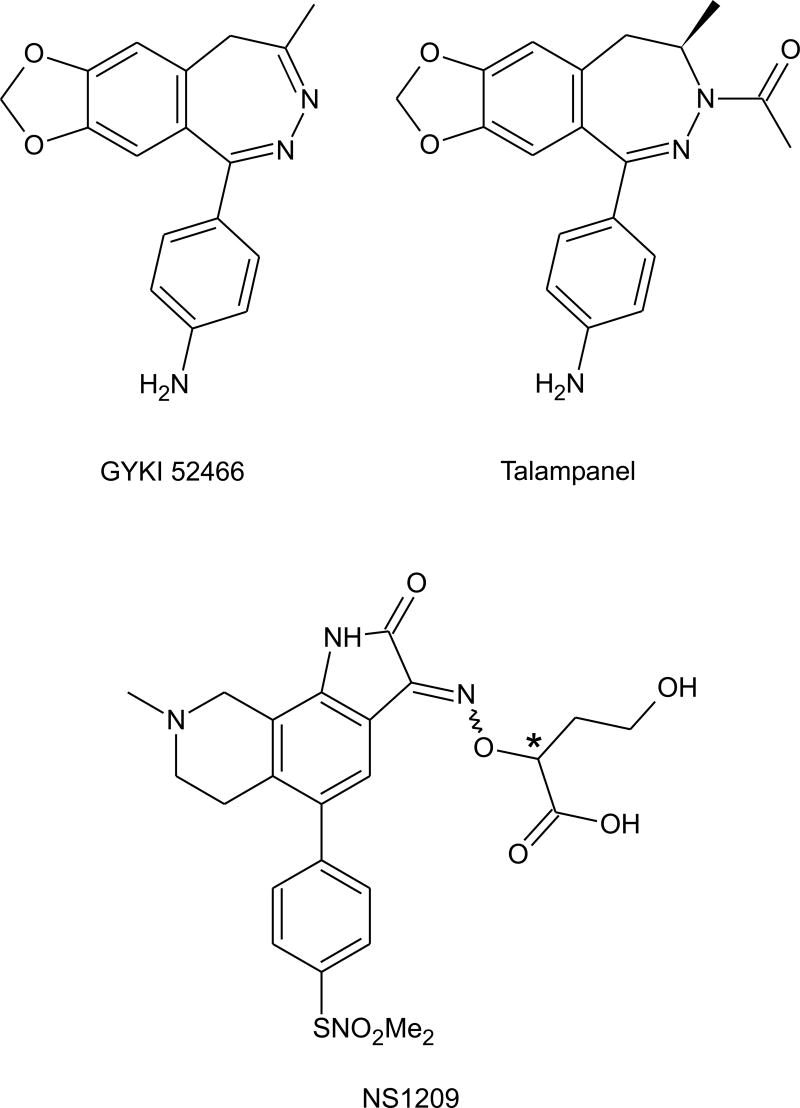
In 1985, Cortes et al. reported that the amino acid derivative N-acetyl-DL-alanine-N-benzylamide was protective in the mouse MES test with modest potency (ED50, 77 mg/kg; Conley et al., 1987). Examination of a large number of derivatives that conform to the general N-benzyl-2-acetamidopropionamide structure (R1=CH3; R3=phenyl in the model structure of Fig. 6) revealed that certain substitutions at the 2-carbon markedly increased potency. For example, the 2-CH2OCH3 derivative (N-benzyl-2-acetamido-3-methoxypropionamide) had ED50 of 8.2 mg/kg (mouse) and rotorod toxicity (TD50) of 43 mg/kg, so that its potency and protective index (TD50/ED50, 5.2) was comparable to phenytoin (Choi et al., 1996). These compounds—which are referred to as functionalized amino acids—have an asymmetric carbon at position 2. In general, the (R)-enantiomers exhibit greater potency than the (S)-enantiomers. In the case of the 2-CH2OCH3 derivative, the (R)-enantiomer [lacosamide (formerly harkoseride), SPM 927] has about twice the potency of the racemic mixture (ED50, 4.5 mg/kg in mouse MES test) whereas the (S)-enantiomer is inactive (Hovinga, 2003). Functionalized amino acids generally lack activity in the PTZ test. Indeed, lacosamide is also inactive against clonic seizures induced by bicuculline and picrotoxin at doses below those that cause rotorod impairment. Lacosamide has activity against hippocampal kindled seizures, as do marketed AEDs including phenytoin, carbmazepine and valproate, although there is a suggestion that it may be modestly more efficacious. Overall, lacosamide does not appear to have a profile of activity in animal seizure models that distinguishes it from sodium channel blocking agents. However, cellular electrophysiological studies have failed to reveal an effect of the drug at concentrations as high as 100 μM on voltage-activated sodium channels or on other channel types, including voltage-activated calcium-channels (L-, N-, P- or T-type) or voltage-activated potassium channels (delayed rectifier, KCNQ2/3). The drug also does not block synaptic transmission in the hippocampal slice preparation, although it does produce a stereoselective block of spontaneous and evoked epileptiform discharges in the presence of 4-AP that resembles the action of carbamazepine (Lees et al., 2006). It has been suggested that lacosamide may act as an antagonist of NMDA receptors through an interaction with the strychnine-insensitive glycine site. Radioligand binding experiments have provided inconsistent results and generally do not support this conclusion, as do recordings from recombinant NMDA receptors expressed in Xenopus oocytes (Troughton and Kleckner, 2005). However, these recording indicate that the drug may allosterically block NMDA receptors with a specific action on receptors containing the NR2B subunit. If this observation can be confirmed, lacosamide would have some similarity with felbamate, which also is a low affinity antagonist of NMDA receptors with a preference for the NR2B subunit (Kleckner et al., 1999; Harty and Rogawski, 2000). Lacosamide has good oral bioavailability (~100%), linear pharmacokinetics, a half-life (~12 h) that will allow twice-daily dosing, insignificant protein binding (<1%), and its metabolites are largely eliminated by the kidney so that there are no significant interactions with other AEDs (Hovinga, 2003). Thus, lacosamide has nearly ideal pharmacokinetic properties. Phase II clinical studies have been promising and Phase III trials are in progress.
Fig. 6.
General functionalized amino acid structure, with two examples—N-acetyl-DL-alanine-N-benzylamide, the first compound in the class to be identified with anticonvulsant properties, and lacosamide, which is currently in clinical development. The asymmetric center is indicated by an asterisk.
Harold Kohn’s laboratory has prepared 250 distinct functionalized amino acids. Among these structures, 12 provide protection in the MES test at equal or greater potency to phenytoin (Shen et al., 2004). Compounds within several related structural series including amino acid amides (Béguin et al., 2004) and functionalized amino ketones (Béguin et al., 2003) also exhibit anticonvulsant activity in the MES test. Like lacosamide, these compounds are inactive in the PTZ model, suggesting that they act through a similar mechanism. Despite the comprehensive effort, it has not been possible to identify structures with superior properties to lacosamide.
3.2 AMPA Receptor Antagonists
AMPA receptors are key mediators of seizure spread and may also play a role in seizure-induced brain damage. Moreover, AMPA receptor antagonists have a broad spectrum of activity in animal seizure models. Thus, AMPA receptor antagonists could have broad utility in epilepsy therapy, both as conventional AEDs and also for the acute treatment of status epilepticus (Rogawski and Donevan, 1999). The first selective AMPA receptor antagonists to be identified were quinoxalinedione derivatives, such as NBQX (2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide), which block AMPA receptors through a competitive interaction at the glutamate recognition site of the receptor. Such agents are effective in the MES and PTZ tests and also in kindling models (Yamaguchi et al., 1993; Löscher et al., 1993; Löscher and Honack, 1994; Sarro et al., 2003; Barton et al., 2003). However, they tend to produce motor impairment at doses similar to those that are protective against seizures. The early quinoxalinediones had poor solubility and so that they precipitated in the kidney, leading to crystaluria and nephrotoxicity. However, newer quinoxalinedione analogs and non-quinoxalinedione congeners have been synthesized that largely overcome this problem (Löscher and Rogawski, 2002). Such newer agents include the water soluble quinoxalinedione-analog zonampanel ([2,3-dioxo-7-(1H-imidazol-1-yl)-6-nitro-1,2,3,4-tetrahydro-1-quinoxalinyl]acetic acid monohydrate; YM872) (Takahashi et al., 1998) and the phosponate quinoxalinedione compound ZK200775 [1,2,3,4-tetrahydro-7-morpholinyl-2,3-dioxo-6-(trifluoromethyl)quinoxalin-1-yl]methylphosphonate] (Turski et al., 1998), which have been studied in stroke. Unfortunately, the results were not encouraging, largely because of sedative side effects and a negative impact on the level of consciousness (Walters et al., 2005). At present, no competitive AMPA receptor antagonists are under development for epilepsy.
Some years after the development of quinoxalinedione competitive AMPA receptor antagonists, a novel class of AMPA antagonists was identified that exert their blocking action in a mechanistically distinct fashion (Rogawski, 1993). These 2,3-benzodiazepines, such as GYKI 52466, do not interact with the AMPA recognition site, but appear to block AMPA receptors in a noncompetitive fashion via an allosteric site on the receptor-channel complex (Donevan and Rogawski, 1993). Like competitive AMPA antagonists, allosteric antagonists such as GYKI 52466 have anticonvulsant activity in a broad spectrum of animal seizure models, including the kindling model (Yamaguchi et al., 1993; Löscher and Rogawski, 2002). However, as is the case for the quinoxalinediones, the protective index of 2,3-benzodiazepines is low. At present, the GYKI 52466 analog talampanel (GYKI 53773; LY 300164) is undergoing Phase III clinical trials. Talampanel is the active (R)-enantiomer of GYKI 53405, the N-acetylated derivative of GYKI 52466. Both GYKI 53405 and talampanel are protective in the MES test in mice and also in various chemoconvulsant models, but only at doses near those that cause motor side effects (Andrási, 2001). In clinical trials talampanel has been reported to be well tolerated, but sedation may occur, especially with initial dosing (Chappell et al., 2002; Langan et al., 2003; Danielsson et al., 2004).
NS1209 ([8-methyl-5-(4-(N,N-dimethylsulfamoyl)phenyl)-6,7,8,9,-tetrahydro-1H-pyrrolo[3,2-h]-iso-quinoline2,3-dione-3-O-(4-hydroxybutyric acid-2-yl)oxime]; SPD502) is a structually novel, water soluble competitive AMPA receptor antagonist with good central nervous system bioavailability (Nielsen et al., 1999). NS1209 and NBQX are of comparable potency in elevating the threshold for tonic electroshock-induced seizures in mice, but the duration of action of NS1209 is more prolonged. NS1209 is also able to effectively terminate kainate-induced status epilepticus in rats even when administered 2 h after the onset of seizures. NS1209 has been reported to be well tolerated in Phase I/II clinical trials and is being evaluated for the treatment of refractory status epilepticus under the theory that it may not only terminate seizures, but also protect against status epilepticus-induced brain damage. Since sedation may be a limiting factor in the use of AMPA receptor antagonists, the focus on the acute treatment of status epilepticus is reasonable.
3.3 Neuroactive Steroids
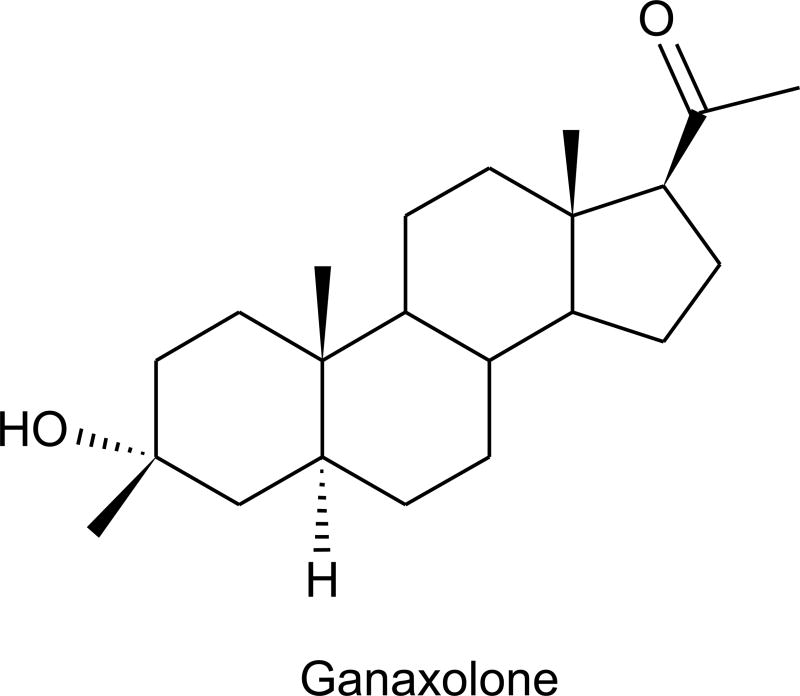
Endogenous steroid hormones including progesterone, deoxycorticosterone and testosterone, when reduced at the 5- and 3-positions of the steroid A-ring, form metabolites that lack classical hormonal activity but instead are high affinity, positive allosteric modulators of GABAA receptors. These neuroactive steroids have all of the expected properties of GABAA receptor modulators in animal models, including anticonvulsant activity (Rogawski and Reddy, 2004). The precursor hormones progesterone and deoxycorticosterone are also anticonvulsant, by virtue of their conversion to the neuroactive steroid products (Kokate et al., 1999; Reddy et al., 2002). In fact, a precursor loading strategy with progesterone has shown an indication of efficacy, at least for the treatment of catamenial epilepsy (Herzog et al., 1995). A more direct approach is to use neuroactive steroids themselves. A large number of analogs of the natural steroids have been demonstrated to be GABAA receptor modulators (Kokate et al., 1994; Hamilton, 2002). Among these analogs, ganaxolone, the 3β-methyl analog of the endogenous steroid allopregnanolone has been most extensively evaluated, and early clinical trials have been completed (Monaghan et al., 1999). Ganaxolone is a potent positive modulator of GABA receptors containing α1, α2 or α3 subunits and does not show dramatic α-subunit selectivity (Carter et al., 1997). Indeed, unlike benzodiazepines, neuroactive steroids have activity on all GABAA receptor isoforms, including those composed of α4 and α6 subunits and that lack γ2 (Lambert et al., 2003); δ-subunit containing receptors are especially sensitive to neurosteroids (Wohlfarth et al., 2002). Such δ-containing receptors are mainly localized persynaptically or extrasynaptically (Nusser et al., 1998; Farrant and Nusser, 2005) where they mediate tonic current (Stell et al., 2003), and likely represent an important target for the anticonvulsant activity of neuroactive steroids.
Ganaxolone has a profile in seizure models similar to that of other GABAA receptor modulators. It is highly active in the PTZ model and only at higher doses in the MES test (Carter et al., 1997). Ganaxolone and related neurosteroids also have activity in the 6-Hz model (Kaminski et al., 2004), against amygdala-kindled seizures (Reddy et al., 2004), and in various models of status epilepticus (Kokate et al., 1996). In contrast to benzodiazepines whose clinical utility in the chronic treatment of epilepsy is limited by tolerance, the anticonvulsant efficacy of neuroactive steroids, at least in animal models, does not diminish with chronic treatment (Kokate et al., 1998; Reddy and Rogawski, 2000). Open label clinical studies have provided indications of efficacy in infantile spasms and complex partial seizures (Monaghan et al., 1999). There is evidence from studies in an animal model of perimenstrual catamenial epilepsy that neurosteroids, including ganaxolone, have greater activity at the time of enhanced seizure susceptibility (Reddy and Rogawski, 2000; 2001). Although there is anecdotal evidence that ganaxolone may be useful in catamenial epilepsy (McAuley et al., 2001), controlled clinical trials are required.
3.4 Rufinamide
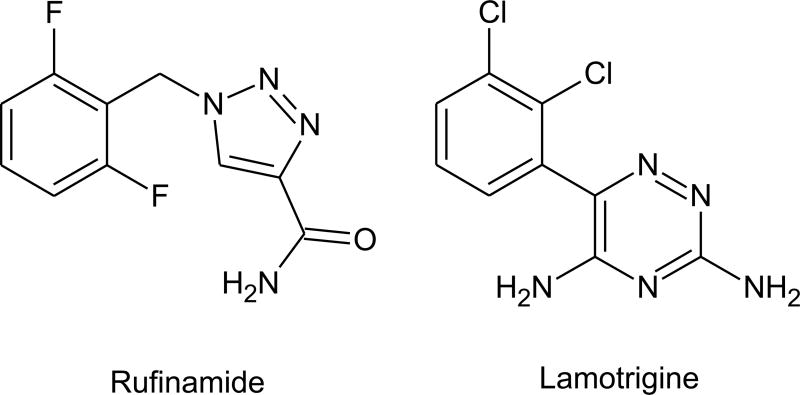
Rufinamide [1-(2,6-difluorophenyl)methyl-1H-1,2,3-triazole-4-carboxamide; E 2080, CGP 33101, RUF 331] is triazole (3 nitrogens in a 5-membered ring) compound that bears some structural similarity with lamotrigine, a triazine (3 nitrogens in a 6-membered ring). Rufinamide is effective in the mouse MES test (ED50, 15.5 mg/kg, i.p.) and at higher doses is the PTZ test (54 mg/kg) (Schmutz et al., 1993; White et al., 2005). It is ineffective against absence-like seizures in WAG/Rij rats. The drug has a very low propensity for toxicity in the rotorod test (TD50, >500 mg/kg, i.p.). Overall, rufinamide has a distinctive profile in animal testing—activity in both MES and PTZ models and a very high protective index—that differentiates it from marketed AEDs. The efficacy of rufinamide in the MES test is compatible with sodium-channel blocking activity. In fact, the drug has been reported to prolong the inactivation of sodium channels and to limit the frequency of action potential firing in cultured and acutely isolated neurons (Karolchyk and Schmidt, 2002; McLean et al., 2005). In other studies, there were no effects on sodium channels at the concentrations tested (Vickery et al., 2004). In view of its unique profile, it seems unlikely that effects on sodium channels entirely explain the anticonvulsant activity of rufinamide. To date, no alternative mechanisms have emerged: there is no evidence for interactions with GABA or glutamate systems.
Several late stage clinical trials of rufinamide have been completed in patients with partial seizures, primary generalized tonic-clonic seizures and the Lennox-Gastaut syndrome. Some of these studies reached statistically significant endpoints and applications with regulatory agencies to market the drug have been filed.
3.5 Retigabine
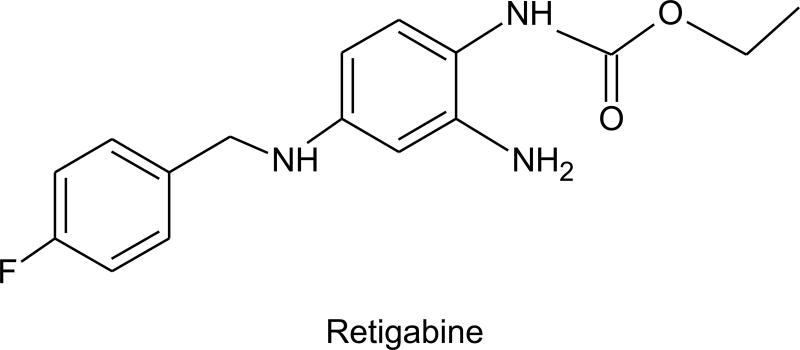
Retigabine [N-(2-amino-4-[fluorobenzylamino]-phenyl)carbamic acid; D-23129], the desaza-analog of flupirtine (a nonopiate analgesic approved in Europe for general nocioceptive pain), was originally identified as an anticonvulsant, because of its high activity in the MES test (ED50, 9.3 mg/kg, i.p., mice) (Blackburn-Munro et al., 2005). Retigabine also has activity in the PTZ test and various other epilepsy models, including seizures induced by kainate, NMDA and picrotoxin (Rostock et al., 1996); it is exceptionally potent in the amygdala kindling model (Tober et al., 1996). Retigabine has undergone clinical testing in several Phase II clinical trials, largely in patients with partial seizures with or without secondary generalization who were refractory to available therapies. Indications of efficacy were obtained in open label trials, with responder rates in the range of 35 to 44% (≥ 50% reduction in seizure frequency) (Sachdeo et al., 2005). In a multicenter randomized, double-blind, placebo controlled add-on trial, there was a dose-dependent reduction in seizure frequency with doses of 600, 900 and 1200 mg/day (divided in 3 daily doses). The maximum responder rate was 33%.
In 1997, Rundfeldt reported that retigabine could activate potassium current in various types of cultured neurons, but not in non-neuronal cells. The drug is therefore a “potassium channel opener.” Subsequently, it was found that retigabine is specific for M-type potassium current, which is carried by KCNQ (Kv7)-type potassium channels (Rundfeldt and Netzer, 2000b; Main et al., 2000; Wickenden et al., 2001). M-current is a slowly activating current whose threshold is near resting potential. The principal action of retigabine is to shift the activation of KCNQ M-current to more hyperpolarised membrane potentials, and also to slow their deactivation and accelerate their activation (Tatulian et al., 2001, 2003). The critical action of the drug is to increase potassium current near resting potential, which reduces excitability and presumably is responsible, at least in part, for the anticonvulsant effect of retigabine. There are four known neuronal KCNQ subunits (KCNQ2–5 or Kv7.2–7.5), which are homologous to KCNQ1 (KvLQT or Kv7.1), a cardiac potassium channel involved in one form of the long QT syndrome (Jentsch, 2000). Retigabine acts on all neuronal KCNQ subunits, but not on KCNQ1 which is expressed in cardiac muscle (Tatulian et al., 2001; Wickenden et al., 2001). Through the use of chimeras it has been possible to define elements of the KCNQ structure that are critical to the effects of retigabine on gating and presumably to its binding (Wuttke et al., 2005; Schenzer et al., 2005). A single tryptophan residue within the S5 segment (tryptophan 236 in KCNQ2) is critical, and is believed to be a part of a hydrophobic pocket that is created when the channel opens. Binding of retigabine to this pocket stabilizes the open, conducting state of the KCNQ channel (Delmas and Brown, 2005).
Of particular interest in view of the role of KCNQ channels as a target for retigabine is that mutations in either KCNQ2 and KCNQ3 have been associated with the epilepsy syndrome benign familal neonatal convulsions (BFNC), an autosomal dominant epilepsy of infancy (Schroeder et al., 1998). In addition, mice in which a single copy of the KCNQ2 gene was disrupted by gene targeting (KCNQ2+/−) show increased sensitivity to PTZ seizures (Watanabe et al., 2000). The combination of KCNQ2 and KCNQ3 underlies the bulk of the M-current in neurons, although KCNQ5 alone or in combination with KCNQ3 can also contribute to M-current. It is believed that M-current regulates neuronal excitability by determining the neuronal firing threshold, influencing the firing rate, and modulating neuronal responsiveness to synaptic inputs. In sum, retigabine provides the first confirmation of the concept that that a neuron-specific potassium channel opener could have anticonvulsant properties (Rogawski and Porter, 1990; Rogawski, 2000).
Although most attention has been focused on the unique ability of retigabine to activate KCNQ channels, the drug also interacts with GABA systems. A direct positive modulatory action on GABAA receptors is of particular significance (van Rijn and Willems-van Bree, 2003). Thus, retigabine enhances GABA-activated chloride current responses and GABAergic IPSCs at concentrations that are only modestly higher than those that influence KCNQ channels (Rundfeldt and Netzer, 2000a; Otto et al., 2002). The effect on GABAA receptors occurs independently of the benzodiazepine site as the benzodiazepine site antagonist flumazenil does not block the effect of retigabine on GABA-activated chloride currents (Rundfeldt and Netzer, 2000a). In view of the broad spectrum of activity of retigabine in animal seizure models, it seems unlikely that the interaction with GABA receptors fully accounts for its ability to protect against seizures. However, it is quite possible that this action could contribute, along with the effect on KCNQ channels, to the anticonvulsant activity of retigabine. Retigabine has a relatively low protective index comparing the rotarod toxicity to activity in the MES test (2.2 in mice and 1.9 in rats with i.p. administration; Blackburn-Munro et al., 2005). It seems likely that the effects of the drug on GABAA receptors could contributes to toxicity in animals and also to dose limiting central nervous system side effects—including somnolence, dizziness, ataxia, confusion, speech disorder, vertigo, tremor, amnesia and abnormal thinking—which have occurred in a dose-related fashion in human clinical trials (Abou-Khalil et al., 2004; Sachdeo et al., 2005).
A benzanilide referred to as ICA-27243, which is claimed to be a more selective KCNQ opener than retigabine, is currently in early stage development. ICA-27243 is a potent activator of KCNQ2 and KCNQ2/KCNQ3 channels, but is less effective at KCNQ4 or KCNQ3/KCNQ5 channel (Wickenden et al., 2005). Unlike retigabine, ICA-27243 does not affect GABAA receptors or sodium channels. ICA-27243 has potent activity in the rat and mouse MES and PTZ tests (ED50 values, 2–3 mg/kg, p.o.) and also in the 6-Hz and amygdala kindling models (Rigdon et al., 2005). In view of its greater specificity for KCNQ channels, it will be of considerable interest to determine whether ICA-27243 has lower incidence of central nervous system side effects than does retigabine.
4. Conclusion
Largely through the use of animal screening models, it has been possible to identify a diverse portfolio of new chemical entities with potential as AEDs. The target sites and mechanism of action of these compounds are being defined through cellular electrophysiological and biochemical studies. In a few cases, drugs in the pipeline seem to act in through conventional AED mechanisms, including effects on voltage-activated sodium channels. The oxcarbazepine analog licarbazepine is in this category, and several other compounds, including rufinamide, probably act, at least in part, through this mechanism. However, in the majority of instances, the pipeline compounds exhibit a novel spectrum of activity in animal models and there is evidence for distinctive actions (or combinations of actions) on anticonvulsant targets. For example, the benzodiazepine-site ligand ELB139 acts in a similar fashion to classical benzodiazepines, but it is a partial agonist and, importantly, is selective for GABAA receptors containing α3-subunits, which confers unique properties. Similarly, the neuroactive steroid ganaxolone has distinctive properties from benzodiazepines inasmuch as neuroactive steroids act on benzodiazepine-insensitive GABAA receptor subunits (with a particular predilection for extrasynaptic δ-subunit-containing receptors) and there is no evidence that tolerance occurs to the anticonvulsant effects of neuroactive steroids. Ganaxolone could be particularly useful in hormonally-sensitive epilepsies or in infantile spasms. The levetiracetam analogs brivaracetam and seletracetam were discovered by screening against SV2A, the target for levetiracetam. Seletracetam seems to be similar to levetiracetam, although it is more potent and has an improved protective index. However, brivaracetam has a different profile and could possibly have broader clinical effectiveness than the parent levetiracetam. Other compounds, such as the AMPA receptor antagonists talampanel and NS1209, interact with entirely new AED targets and could be uniquely useful in certain clinical situations, such as in status epilepticus.
It has been the experience that each new AED introduced into the market has an individual “personality,” reflected as a distinct spectrum of activity among seizure types and epilepsy syndromes (and also non-epilepsy neurological and psychiatric conditions) (Rogawski and Löscher, 2004b; Rogawski, 2006). The new drugs also often offer better tolerability, less drug or hormonal interactions, improved pharmacokinetic characteristics, and hold the potential for greater safety during pregnancy. It is likely that the current crop of developmental stage AEDs will similarly provide new benefits for patients with epilepsy, although experience has taught us that the improvements will in most cases be incremental rather than transformative. Indeed, while the development of AEDs that are more effective and safer in preventing seizures in susceptible individuals is an important objective, we should not lose sight of the fact that the ultimate goal of epilepsy therapeutics development must be to identify approaches to reverse established epilepsy, or prevent or slow its initiation in the first place. There is now intense interest in such antiepileptogenic approaches and a paradigm shift is occurring in how we think about epilepsy therapeutic strategies, as exemplified by the work with RWJ-333369. There are major challenges in the preclinical identification of antiepileptogenic agents and even bigger challenges in validating them in the clinic (Pitkänen, 2002; Stables et al., 2002). However, the payoffs in terms of the unmet medical need provide a strong incentive to persevere. As we forge into this new frontier, a whole new set of targets and mechanisms relevant to antiepileptogenesis will surely be uncovered that are distinct from the targets that we view as relevant for seizure protection.
Fig. 1.
Structures of levetiracetam and two 4-substituted analogs with 10-fold greater SV2A binding activity.
Fig. 2.
Structures of valproate and several valproate-like agents. See text for key to abbreviations. Asymmetric centers are indicated by asterisks.
Fig. 3.
Structures of dicarbamates felbamate and fluorofelbamate and the monocarbamate RWJ-333369.
Fig. 4.
Converging metabolic pathways for carbamazepine, oxcarbazepine and S-licarbazepine acetate (BIA 2-093); the latter two compounds lead to the production licarbazepine, an active anticonvulsant.
Fig. 5.
Classical benzodiazepines with full agonist activity at the benzodiazepine recognition site of GABAA receptors and non-benzodiazepine ligands of the benzodiazepine recognition site with partial agonist activity. TPA023 and ELB139 are highly subunit-selective.
Fig. 7.
Structures of the 2,3-benzodiazepines noncompetitive AMPA receptor antagonists GYKI 52466 and talampanel and the competitive AMPA receptor antagonist NS1209. The asterisk indicates an asymmetric center. Enantiomers of NS1209 have similar properties to the racemate.
Fig. 8.
Structure of the neuroactive steroid ganaxolone.
Fig. 9.
Structures of rufinamide and lamotrigine showing the nitrogen-containing aromatic rings.
Fig. 10.
Structure of retigabine.
Acknowledgments
I thank Gerald Novak for access to unpublished data, Brian Klein for comments, and Meir Bialer for discussions. Presented in part at the workshop “New Horizons in the Development of Antiepileptic Drugs III—Innovative Strategies” (Organizers: Wolfgang Löscher and Dieter Schmidt), Washington, D.C., November 30–December 1, 2005.
References
- Abou-Khalil B, Porter RJ, Nohria V. Safety and tolerability of different titration rates of the novel AED retigabine. Epilepsia. 2004;45(Suppl 7):311. (2.356) [Google Scholar]
- Almeida L, Falcao A, Maia J, Mazur D, Gellert M, Soares-da-Silva P. Single-dose and steady-state pharmacokinetics of eslicarbazepine acetate (BIA 2-093) in healthy elderly and young subjects. J Clin Pharmacol. 2005;45:1062–1066. doi: 10.1177/0091270005279364. [DOI] [PubMed] [Google Scholar]
- Almeida L, Soares-da-Silva P. Safety, tolerability, and pharmacokinetic profile of BIA 2-093, a novel putative antiepileptic, in a rising multiple-dose study in young healthy humans. J Clin Pharmacol. 2004;44:906–918. doi: 10.1177/0091270004267591. [DOI] [PubMed] [Google Scholar]
- Ambrósio AF, Silva AP, Malva JO, Soares-da-Silva P, Carvalho AP, Carvalho CM. Inhibition of glutamate release by BIA 2-093 and BIA 2-024, two novel derivatives of carbamazepine, due to blockade of sodium but not calcium channels. Biochem Pharmacol. 2001;61:1271–1275. doi: 10.1016/s0006-2952(01)00584-6. [DOI] [PubMed] [Google Scholar]
- Andrási F. Talampanel. Drugs Fut. 2001;26:754–756. [Google Scholar]
- Asano T, Mori T, Shimoda T, Shinagawa R, Satoh S, Yada N, Katsumata S, Matsuda S, Kagamiishi Y, Tateishi N. Arundic acid (ONO-2506) ameliorates delayed ischemic brain damage by preventing astrocytic overproduction of S100B. Curr. Drug Targets CNS Neurol Disord. 2005;4:127–142. doi: 10.2174/1568007053544084. [DOI] [PubMed] [Google Scholar]
- Atack JR, Wafford K, Tye SJ, Cook S, Sohal B, Pike A, Sur C, Melillo D, Bristow L, Bromidge F, Ragan I, Kirby J, Street L, Carling R, Castro L, Whiting P, Dawson G, McKernan R. TPA023, An agonist selective for α2- and α3-containing GABAA receptors, is a non-sedating anxiolytic in rodents and primates. J Pharmacol Exp Ther. 2006;316:410–422. doi: 10.1124/jpet.105.089920. [DOI] [PubMed] [Google Scholar]
- Barel S, Yagen B, Schurig V, Soback S, Pisani F, Perucca E, Bialer M. Stereoselective pharmacokinetic analysis of valnoctamide in healthy subjects and in patients with epilepsy. Clin Pharmacol Ther. 1997;61:442–449. doi: 10.1016/S0009-9236(97)90194-6. [DOI] [PubMed] [Google Scholar]
- Barton ME, Klein BD, Wolf HH, White HS. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001;47:217–227. doi: 10.1016/s0920-1211(01)00302-3. [DOI] [PubMed] [Google Scholar]
- Barton ME, Peters SC, Shannon HE. Comparison of the effect of glutamate receptor modulators in the 6 Hz and maximal electroshock seizure models. Epilepsy Res. 2003;56:17–26. doi: 10.1016/j.eplepsyres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Béguin C, Andurkar SV, Jin AY, Stables JP, Weaver DF, Kohn H. Functionalized amido ketones: new anticonvulsant agents. Bioorg Med Chem. 2003;11:4275–4285. doi: 10.1016/s0968-0896(03)00434-6. [DOI] [PubMed] [Google Scholar]
- Béguin C, LeTiran A, Stables JP, Voyksner RD, Kohn H. N-Substituted amino acid N’-benzylamides: synthesis, anticonvulsant, and metabolic activities. Bioorg Med Chem. 2004;12:3079–3096. doi: 10.1016/j.bmc.2004.02.043. [DOI] [PubMed] [Google Scholar]
- Benes J, Parada A, Figueiredo AA, Alves PC, Freitas AP, Learmonth DA, Cunha RA, Garrett J, Soares-da-Silva P. Anticonvulsant and sodium channel-blocking properties of novel 10,11-dihydro-5H-dibenz[b,f]azepine-5-carboxamide derivatives. J Med Chem. 1999;42:2582–2587. doi: 10.1021/jm980627g. [DOI] [PubMed] [Google Scholar]
- Bialer M. Clinical pharmacology of valpromide. Clin Pharmacokinet. 1991;20:114–122. doi: 10.2165/00003088-199120020-00003. [DOI] [PubMed] [Google Scholar]
- Bialer M, Haj-Yehia A, Barzaghi N, Pisani F, Perucca E. Pharmacokinetics of a valpromide isomer, valnoctamide, in healthy subjects. Eur J Clin Pharmacol. 1990;38:289–291. doi: 10.1007/BF00315032. [DOI] [PubMed] [Google Scholar]
- Bialer M, Johannessen SI, Kupferberg HJ, Levy RH, Loiseau P, Perucca E. Progress report on new antiepileptic drugs: a summary of the Fifth Eilat Conference (EILAT V) Epilepsy Res. 2001;43:11–58. doi: 10.1016/s0920-1211(00)00171-6. [DOI] [PubMed] [Google Scholar]
- Bialer M, Johannessen SI, Kupferberg HJ, Levy RH, Perucca E, Tomson T. Progress report on new antiepileptic drugs: a summary of the Seventh Eilat Conference (EILAT VII) Epilepsy Res. 2004;61:1–48. doi: 10.1016/j.eplepsyres.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Blackburn-Munro G, Dalby-Brown W, Mirza NR, Mikkelsen JD, Blackburn-Munro RE. Retigabine: chemical synthesis to clinical application. CNS Drug Rev. 2005;11:1–20. doi: 10.1111/j.1527-3458.2005.tb00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blotnick S, Bergman F, Bialer M. Disposition of valpromide, valproic acid and valnoctamide in the brain, liver, plasma and urine of rats. Drug Metab Dispos. 1996;24:560–564. [PubMed] [Google Scholar]
- Bonifácio MJ, Sheridan RD, Parada A, Cunha RA, Patmore L, Soares-da-Silva P. Interaction of the novel anticonvulsant, BIA 2-093, with voltage-gated sodium channels: comparison with carbamazepine. Epilepsia. 2001;42:600–608. doi: 10.1046/j.1528-1157.2001.43600.x. [DOI] [PubMed] [Google Scholar]
- Carter RB, Wood PL, Wieland S, Hawkinson JE, Belelli D, Lambert JJ, White HS, Wolf HH, Mirsadeghi S, Tahir SH, Bolger MB, Lan NC, Gee KW. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3α-hydroxy-3β-methyl-5α-pregnan-20-one), a selective, high-affinity, steroid modulator of the γ-aminobutyric acidA receptor. J Pharmacol Exp Ther. 1997;280:1284–1295. [PubMed] [Google Scholar]
- Chappell AS, Sander JW, Brodie MJ, Chadwick D, Lledo A, Zhang D, Bjerke J, Kiesler GM, Arroyo S. A crossover, add-on trial of talampanel in patients with refractory partial seizures. Neurology. 2002;58:1680–1682. doi: 10.1212/wnl.58.11.1680. [DOI] [PubMed] [Google Scholar]
- Choi D, Stables JP, Kohn H. Synthesis and anticonvulsant activities of N-Benzyl-2-acetamidopropionamide derivatives. J Med Chem. 1996;39:1907–1916. doi: 10.1021/jm9508705. [DOI] [PubMed] [Google Scholar]
- Choi, Y.M., Kim, M.W., Park, J., 1997. Halogen substituted carbamate compounds from 2-phenyl-1, 2-ethanediol. U.S. Patent 5,698,588 (December 16, 1997).
- Choi, Y.M., Kim, M.W., Park. J., 2000. Halogen substituted carbamate compounds from 2-phenyl-1, 2-ethanediol. U.S. Patent 6,103,759 (August 15, 2000).
- Conley JD, Kohn H. Functionalized DL-amino acid derivatives. Potent new agents for the treatment of epilepsy. J Med Chem. 1987;30:567–574. doi: 10.1021/jm00386a021. [DOI] [PubMed] [Google Scholar]
- Cortes S, Liao ZK, Watson D, Kohn H. Effect of structural modification of the hydantoin ring on anticonvulsant activity. J Med Chem. 1985;28:601–606. doi: 10.1021/jm50001a012. [DOI] [PubMed] [Google Scholar]
- Costa E, Guidotti A. Benzodiazepines on trial: a research strategy for their rehabilitation. Trends Pharmacol Sci. 1996;17:192–200. doi: 10.1016/0165-6147(96)10015-8. [DOI] [PubMed] [Google Scholar]
- Crestani F, Martin JR, Möhler H, Rudolph U. Mechanism of action of the hypnotic zolpidem in vivo. Br J Pharmacol. 2000;131:1251–1254. doi: 10.1038/sj.bjp.0703717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson I, Su K, Kauer L, Barnette L, Reeves-Tyer P, Kelley K, Theodore WH, Wassermann E, Rogawski MA. Talampanel and human cortical excitability: EEG and TMS. Epilepsia. 2004;45(Suppl 7):120. 1.314. [Google Scholar]
- de Paulis T. ONO-2506. Curr Opin Investig Drugs. 2003;4:863–867. [PubMed] [Google Scholar]
- Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci. 2005;6:850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- Depoortere H, Zivkovic B, Lloyd KG, Sanger DJ, Perrault G, Langer SZ, Bartholini G. Zolpidem, a novel nonbenzodiazepine hypnotic. I Neuropharmacological and behavioral effects. J Pharmacol Exp Ther. 1986;237:649–658. [PubMed] [Google Scholar]
- Donevan SD, Rogawski MA. GYKI 52466, a 2,3-benzodiazepine, is a highly selective, noncompetitive antagonist of AMPA/kainate receptor responses. Neuron. 1993;10:51–59. doi: 10.1016/0896-6273(93)90241-i. [DOI] [PubMed] [Google Scholar]
- Dost, R., Langen, B., Rundfeldt, C., 2005. The α-3 subunit selective benzodiazepine agonist ELB139 does not induce tolerance in animal models for anxiety and epilepsy. Soc. Neurosci. Abst., Program No. 678.1.
- Dubinsky B, Vaidya AH, Rosenthal DI, Hochman C, Crooke JJ, DeLuca S, DeVine A, Cheo-Isaacs CT, Carter AR, Jordan AD, Reitz AB, Shank RP. 5-ethoxymethyl-7-fluoro-3-oxo-1,2,3,5-tetrahydrobenzo[4,5]imidazo[1,2a]pyridine-4-N-(2-fluorophenyl)carboxamide (RWJ-51204), a new nonbenzodiazepine anxiolytic. J Pharmacol Exp Ther. 2002;303:777–790. doi: 10.1124/jpet.102.036954. [DOI] [PubMed] [Google Scholar]
- Eadie MJ. Could valerian have been the first anticonvulsant? Epilepsia. 2004;45:133–1343. doi: 10.1111/j.0013-9580.2004.27904.x. [DOI] [PubMed] [Google Scholar]
- Faigle JW, Menge GP. Metabolic characteristics of oxcarbazepine and their clinical significance: comparison with carbamazepine. Behav Neurol. 1990;3 (suppl 1):21–30. doi: 10.3233/BEN-1990-31S104. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Francois J, Ferrandon A, Koning E, Nehlig A. A new drug RWJ 333369 protects limbic areas in the lithium-pilocarpine model (li-pilo) of epilepsy and delays or prevents the occurrence of spontaneous seizures. Epilepsia. 2005;46(Suppl 8):269–270. C.04. [Google Scholar]
- Gower AJ, Hirsch E, Boehrer A, Noyer M, Marescaux C. Effects of levetiracetam, a novel antiepileptic drug, on convulsant activity in two genetic rat models of epilepsy. Epilepsy Res. 1995;22:207–213. doi: 10.1016/0920-1211(95)00077-1. [DOI] [PubMed] [Google Scholar]
- Gower AJ, Noyer M, Verloes R, Gobert J, Wulfert E. ucb L059, a novel anti-convulsant drug: pharmacological profile in animals. Eur J Pharmacol. 1992;222:193–203. doi: 10.1016/0014-2999(92)90855-x. [DOI] [PubMed] [Google Scholar]
- Grabenstatter HL, Dudek FE. The use of chronic models in antiepileptic drug discovery: the effect of RWJ-333369 on spontaneous motor seizures in rats with kainate-induced epilepsy. Epilepsia. 2004;45(Suppl 7):197. doi: 10.1111/j.0013-9580.2005.13404.x. (2.016) [DOI] [PubMed] [Google Scholar]
- Hamilton NM. Interaction of steroids with the GABAA receptor. Curr Top Med Chem. 2002;2:887–902. doi: 10.2174/1568026023393570. [DOI] [PubMed] [Google Scholar]
- Harty TP, Rogawski MA. Felbamate block of recombinant N-methyl-D-aspartate receptors: selectivity for the NR2B subunit. Epilepsy Res. 2000;39:47–55. doi: 10.1016/s0920-1211(99)00108-4. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Progesterone therapy in women with complex partial and secondary generalized seizures. Neurology. 1995;45:1660–1662. doi: 10.1212/wnl.45.9.1660. [DOI] [PubMed] [Google Scholar]
- Holmes, G.L., 2002. Carbamazepine. Adverse effects. In: Levy, R.H., Mattson, R.H., Meldrum, B.S., Perucca, E., eds. Antiepileptic drugs. Philadelphia: Lippincott-Raven, 284–297.
- Hovinga CA. SPM-927 (Schwarz Pharma) IDrugs. 2003;6:479–485. [PubMed] [Google Scholar]
- Hovinga CA. Valrocemide (Teva/Acorda) Curr Opin Investig Drugs. 2004;5:101–106. [PubMed] [Google Scholar]
- Isoherranen N, White HS, Klein BD, Roeder M, Woodhead JH, Schurig V, Yagen B, Bialer M. Pharmacokinetic-pharmacodynamic relationships of (2S,3S)-valnoctamide and its stereoisomer (2R,3S)-valnoctamide in rodent models of epilepsy. Pharm Res. 2003a;20:1293–1301. doi: 10.1023/a:1025069519218. [DOI] [PubMed] [Google Scholar]
- Isoherranen N, Woodhead JH, White HS, Bialer M. Anticonvulsant profile of valrocemide (TV1901): a new antiepileptic drug. Epilepsia. 2001;42:831–836. doi: 10.1046/j.1528-1157.2001.042007831.x. [DOI] [PubMed] [Google Scholar]
- Isoherranen N, Yagen B, Bialer M. New CNS-active drugs which are second-generation valproic acid: can they lead to the development of a magic bullet? Curr Opin Neurol. 2003b;16:203–211. doi: 10.1097/01.wco.0000063774.81810.30. [DOI] [PubMed] [Google Scholar]
- Isoherranen N, Yagen B, Woodhead JH, Spiegelstein O, Blotnik S, Wilcox KS, Finnell RH, Bennett GD, White HS, Bialer M. Characterization of the anticonvulsant profile and enantioselective pharmacokinetics of the chiral valproylamide propylisopropyl acetamide in rodents. Br J Pharmacol. 2003c;138:602–613. doi: 10.1038/sj.bjp.0705076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz R, Goda Y, Geppert M, Missler M, Südhof TC. SV2A and SV2B function as redundant Ca2+ regulators in neurotransmitter release. Neuron. 1999;24:1003–1016. doi: 10.1016/s0896-6273(00)81046-6. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci. 2000;1:21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- Johnstone TB, Hogenkamp DJ, Coyne L, Su J, Halliwell RF, Tran MB, Yoshimura RF, Li WY, Wang J, Gee KW. Modifying quinolone antibiotics yields new anxiolytics. Nat Med. 2004;10:31–32. doi: 10.1038/nm967. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Livingood MR, Rogawski MA. Allopregnanolone analogs that positively modulate GABA receptors protect against partial seizures induced by 6-Hz electrical stimulation in mice. Epilepsia. 2004;45:864–867. doi: 10.1111/j.0013-9580.2004.04504.x. [DOI] [PubMed] [Google Scholar]
- Karolychyk, M.A., Schmidt, D., 2002. Drugs in development. Rufinamide. In: Levy, R.H., Mattson, R.H., Meldrum, B.S., Perucca, E., eds. Antiepileptic drugs. Philadelphia: Lippincott-Raven, 906–912.
- Kasteleijn-Nolst Trenitè DG, Parain D, Masnou P, Genton P, Steinhoff BJ, Hirsch E. Proof of principle in the new AED UCB 34714: Use of the photosensitivity model. Epilepsia. 2004;45(Suppl 7):309. (2.349) [Google Scholar]
- Kenda BM, Matagne AC, Talaga PE, Pasau PM, Differding E, Lallemand BI, Frycia AM, Moureau FG, Klitgaard HV, Gillard MR, Fuks B, Michel P. Discovery of 4-substituted pyrrolidone butanamides as new agents with significant antiepileptic activity. J Med Chem. 2004;47:530–549. doi: 10.1021/jm030913e. [DOI] [PubMed] [Google Scholar]
- Kleckner NW, Glazewski JC, Chen CC, Moscrip TD. Subtype-selective antagonism of N-methyl-D-aspartate receptors by felbamate: insights into the mechanism of action. J Pharmacol Exp Ther. 1999;289:886–894. [PubMed] [Google Scholar]
- Kleckner NW, Glazewski JC, Chen CC, Moscrip TD. Subtype-selective antagonism of N-methyl-D-aspartate receptors by felbamate: insights into the mechanism of action. J Pharmacol Exp Ther. 1999;289:886–894. [PubMed] [Google Scholar]
- Klitgaard H. Levetiracetam: the preclinical profile of a new class of antiepileptic drugs? Epilepsia. 2001;42(Suppl 4):13–18. [PubMed] [Google Scholar]
- Klitgaard H, Matagne A, Gobert J, Wulfert E. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur J Pharmacol. 1998;353:191–206. doi: 10.1016/s0014-2999(98)00410-5. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Banks MK, Magee T, Yamaguchi S, Rogawski MA. Finasteride, a 5α-reductase inhibitor, blocks the anticonvulsant activity of progesterone in mice. J Pharmacol Exp Ther. 1999;288:679–684. [PubMed] [Google Scholar]
- Kokate TG, Cohen AL, Karp E, Rogawski MA. Neuroactive steroids protect against pilocarpine- and kainic acid-induced limbic seizures and status epilepticus in mice. Neuropharmacology. 1996;35:1049–1056. doi: 10.1016/s0028-3908(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: correlation with γ-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther. 1994;270:1223–1229. [PubMed] [Google Scholar]
- Kokate TG, Yamaguchi S, Pannell LK, Rajamani U, Carroll DM, Grossman AB, Rogawski MA. Lack of anticonvulsant tolerance to the neuroactive steroid pregnanolone in mice. J Pharmacol Exp Ther. 1988;287:553–558. [PubMed] [Google Scholar]
- Kralic JE, Korpi ER, O’Buckley TK, Homanics GE, Morrow AL. Molecular and pharmacological characterization of GABAA receptor α1 subunit knockout mice. J Pharmacol Exp Ther. 2002;302:1037–1045. doi: 10.1124/jpet.102.036665. [DOI] [PubMed] [Google Scholar]
- Kume A, Greenfield LJ, Jr, Macdonald RL, Albin RL. Felbamate inhibits [3H]t-butylbicycloorthobenzoate (TBOB) binding and enhances Cl– current at the γ-aminobutyric acidA (GABAA) receptor. J Pharmacol Exp Ther. 1996;277:1784–1792. [PubMed] [Google Scholar]
- Kuo CC, Lin BJ, Chang HR, Hsieh CP. Use-dependent inhibition of the N-methyl-D-aspartate currents by felbamate: a gating modifier with selective binding to the desensitized channels. Mol Pharmacol. 2004;65:370–380. doi: 10.1124/mol.65.2.370. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Harney SC, Peters JA, Frenguelli BG. Modulation of native and recombinant GABAA receptors by endogenous and synthetic neuroactive steroids. Brain Res Brain Res Rev. 2001;37:68–80. doi: 10.1016/s0165-0173(01)00124-2. [DOI] [PubMed] [Google Scholar]
- Langan YM, Lucas R, Jewell H, Toublanc N, Schaefer H, Sander JW, Patsalos PN. Talampanel, a new antiepileptic drug: single- and multiple-dose pharmacokinetics and initial 1-week experience in patients with chronic intractable epilepsy. Epilepsia. 2003;44:46–53. doi: 10.1046/j.1528-1157.2003.128902.x. [DOI] [PubMed] [Google Scholar]
- Langen B, Egerland U, Bernoster K, Dost R, Unverferth K, Rundfeldt C. Characterization in rats of the anxiolytic potential of ELB139 [1-(4-chlorophenyl)-4-piperidin-1-yl-1,5-dihydro-imidazol-2-on], a new agonist at the benzodiazepine binding site of the GABAA receptor. J Pharmacol Exp Ther. 2005;314:717–724. doi: 10.1124/jpet.105.084681. [DOI] [PubMed] [Google Scholar]
- Langen, B., Rundfeldt, C., Dost, R., Luddens, H., Rabe, H., 2004. Method of treating or preventing central nervous system disorders with compounds having selectivity for the alpha3 subunit of the benzodiazepine receptor. U.S. Patent Application 20050032863.
- Lees G, Stohr T, Errington AC. Stereoselective effects of the novel anticonvulsant lacosamide against 4-AP induced epileptiform activity in rat visual cortex in vitro. Neuropharmacology. 2006;50:98–110. doi: 10.1016/j.neuropharm.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Löscher, W., 2002. Valproic acid. Mechanisms of action. In: Levy, R.H., Mattson, R.H., Meldrum, B.S., Perucca, E., eds. Antiepileptic drugs. Philadelphia: Lippincott-Raven, 768–779.
- Löscher W, Hönack D. Profile of ucb L059, a novel anticonvulsant drug, in models of partial and generalized epilepsy in mice and rats. Eur J Pharmacol. 1993;232:147–158. doi: 10.1016/0014-2999(93)90768-d. [DOI] [PubMed] [Google Scholar]
- Löscher W, Honack D. Effects of the non-NMDA antagonists NBQX and the 2,3-benzodiazepine GYKI 52466 on different seizure types in mice: comparison with diazepam and interactions with flumazenil. Br J Pharmacol. 1994;113:1349–1357. doi: 10.1111/j.1476-5381.1994.tb17146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löscher W, Honack D, Rundfeldt C. Antiepileptogenic effects of the novel anticonvulsant levetiracetam (ucb L059) in the kindling model of temporal lobe epilepsy. J Pharmacol Exp Ther. 1998;284:474–479. [PubMed] [Google Scholar]
- Löscher W, Nau H. Pharmacological evaluation of various metabolites and analogues of valproic acid. Anticonvulsant and toxic potencies in mice. Neuropharmacology. 1985;24:427–435. doi: 10.1016/0028-3908(85)90028-0. [DOI] [PubMed] [Google Scholar]
- Löscher W, Nolting B. The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. IV Protective indices. Epilepsy Res. 1991;9:1–10. doi: 10.1016/0920-1211(91)90041-d. [DOI] [PubMed] [Google Scholar]
- Löscher, W., Rogawski, M.A., 2002. Epilepsy. In, Ionotropic Glutamate Receptors as Therapeutic Targets (D. Lodge, W. Danysz and C.G. Parsons, eds.), F.P. Graham Publishing Co., Johnson City, TN, pp. 91–132.
- Löscher W, Rundfeldt C, Honack D. Low doses of NMDA receptor antagonists synergistically increase the anticonvulsant effect of the AMPA receptor antagonist NBQX in the kindling model of epilepsy. Eur J Neurosci. 1993;5:1545–1550. doi: 10.1111/j.1460-9568.1993.tb00224.x. [DOI] [PubMed] [Google Scholar]
- Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, Fuks B. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA. 2004;101:9861–9866. doi: 10.1073/pnas.0308208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main MJ, Cryan JE, Dupere JR, Cox B, Clare JJ, Burbidge SA. Modulation of KCNQ2/3 potassium channels by the novel anticonvulsant retigabine. Mol Pharmacol. 2000;58:253–262. doi: 10.1124/mol.58.2.253. [DOI] [PubMed] [Google Scholar]
- Marescaux C, Vergnes M, Depaulis A. Genetic absence epilepsy in rats from Strasbourg—a review. J Neural Transm Suppl. 1992;35:37–69. doi: 10.1007/978-3-7091-9206-1_4. [DOI] [PubMed] [Google Scholar]
- McAuley JW, Moore JL, Reeves AL, Flyak J, Monaghan EP, Data J. A pilot study of the neurosteroid ganaxolone in catamenial epilepsy: Clinical experience in two patients. Epilepsia. 2001;42(Suppl 7):85. [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor α1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- McLean MJ, Schmutz M, Pozza M, Wamil A. The influence of rufinamide on sodium currents and action potential firing in rodent neurons. Epilepsia. 2005;46(Suppl 8):296. (3.062) [Google Scholar]
- Monaghan EP, McAuley JW, Data JL. Ganaxolone: a novel positive allosteric modulator of the GABAA receptor complex for the treatment of epilepsy. Expert Opin Investig Drugs. 1999;8:1663–1671. doi: 10.1517/13543784.8.10.1663. [DOI] [PubMed] [Google Scholar]
- Nau H, Löscher W. Pharmacologic evaluation of various metabolites and analogs of valproic acid: teratogenic potencies in mice. Fundam Appl Toxicol. 1986;6:669–676. doi: 10.1016/0272-0590(86)90180-6. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Rigoulot M-A, Boehrer A. A new drug, RWJ 333369 displays potent antiepileptic properties in genetic models of absence and audiogenic epilepsy. Epilepsia. 2005;46(Suppl 8):215. (2.368) [Google Scholar]
- Nielsen EØ, Varming T, Mathiesen C, Jensen LH, Moller A, Gouliaev AH, Watjen F, Drejer J. SPD 502: a water-soluble and in vivo long-lasting AMPA antagonist with neuroprotective activity. J Pharmacol Exp Ther. 1999;289:1492–1501. [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto JF, Kimball MM, Wilcox KS. Effects of the anticonvulsant retigabine on cultured cortical neurons: changes in electroresponsive properties and synaptic transmission. Mol Pharmacol. 2002;61:921–927. doi: 10.1124/mol.61.4.921. [DOI] [PubMed] [Google Scholar]
- Parada A, Soares-da-Silva P. The novel anticonvulsant BIA 2-093 inhibits transmitter release during opening of voltage-gated sodium channels: a comparison with carbamazepine and oxcarbazepine. Neurochem Int. 2002;40:435–440. doi: 10.1016/s0197-0186(01)00101-2. [DOI] [PubMed] [Google Scholar]
- Perucca E. Pharmacological and therapeutic properties of valproate: a summary after 35 years of clinical experience. CNS Drugs. 2002;16:695–714. doi: 10.2165/00023210-200216100-00004. [DOI] [PubMed] [Google Scholar]
- Petersen EN. DMCM: a potent convulsive benzodiazepine receptor ligand. Eur J Pharmacol. 1983;94:117–124. doi: 10.1016/0014-2999(83)90448-x. [DOI] [PubMed] [Google Scholar]
- Pisani F, Fazio A, Artesi C, Oteri G, Spina E, Tomson T, Perucca E. Impairment of carbamazepine-10,11-epoxide elimination by valnoctamide, a valpromide isomer, in healthy subjects. Br J Clin Pharmacol. 1992;34:85–87. doi: 10.1111/j.1365-2125.1992.tb04114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani F, Haj-Yehia A, Fazio A, Artesi C, Oteri G, Perucca E, Kroetz DL, Levy RH, Bialer M. Carbamazepine-valnoctamide interaction in epileptic patients: in vitro/in vivo correlation. Epilepsia. 1993;34:954–959. doi: 10.1111/j.1528-1157.1993.tb02117.x. [DOI] [PubMed] [Google Scholar]
- Pitkänen A. Drug-mediated neuroprotection and antiepileptogenesis: animal data. Neurology. 2002;59 (9 Suppl 5):S27–33. doi: 10.1212/wnl.59.9_suppl_5.s27. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Kharatishvili I, Narkilahti S, Lukasiuk K, Nissinen J. Administration of diazepam during status epilepticus reduces development and severity of epilepsy in rat. Epilepsy Res. 2005;63:27–42. doi: 10.1016/j.eplepsyres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Pugliese AM, Passani MB, Pepeu G, Corradetti R. Felbamate decreases synaptic transmission in the CA1 region of rat hippocampal slices. J Pharmacol Exp Ther. 1996;279:1100–1108. [PubMed] [Google Scholar]
- Radatz M, Ehlers K, Yagen B, Bialer M, Nau H. Valnoctamide, valpromide and valnoctic acid are much less teratogenic in mice than valproic acid. Epilepsy Res. 1998;30:41–48. doi: 10.1016/s0920-1211(97)00095-8. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Castaneda DC, O’Malley BW, Rogawski MA. Anticonvulsant activity of progesterone and neurosteroids in progesterone receptor knockout mice. J Pharmacol Exp Ther. 2004;310:230–239. doi: 10.1124/jpet.104.065268. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. J Pharmacol Exp Ther. 2000;295:1241–1248. [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of ganaxolone after neurosteroid withdrawal in a rat model of catamenial epilepsy. J Pharmacol Exp Ther. 2000;294:909–915. [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia. 2001;42:337–344. doi: 10.1046/j.1528-1157.2001.10200.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Stress-induced deoxycorticosterone-derived neurosteroids modulate GABAA receptor function and seizure susceptibility. J Neurosci. 2002;22:3795–805. doi: 10.1523/JNEUROSCI.22-09-03795.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho JM, Donevan SD, Rogawski MA. Mechanism of action of the anticonvulsant felbamate: opposing effects on N-methyl-D-aspartate and γ-aminobutyric acidA receptors. Ann Neurol. 1994;35:229–234. doi: 10.1002/ana.410350216. [DOI] [PubMed] [Google Scholar]
- Rigdon, G.C., Roeloffs, R., Wickenden, A.D., McNaughton-Smith, G., Jones, L., Harrison, W., Ghodadra, N., McNamara, J.O., 2005. In vivo profile of ICA-27243, a potent and selective KCNQ2/3 activator in rodent anticonvulsant models. Soc. Neurosci. Abst. Prog. No. 153.15. [DOI] [PubMed]
- Rogawski, A.M., 1998. Mechanism-specific pathways for new antiepileptic drug discovery. Antiepileptic Drug Development. Advances in Neurology, Vol. 76 (French, J., Leppik, I., Dichter, M.A., eds.) Lippincott-Raven Publishers, pp. 11–27, Philadelphia. [PubMed]
- Rogawski MA. Therapeutic potential of excitatory amino acid antagonists: channel blockers and 2,3-benzodiazepines. Trends Pharmacol Sci. 1993;14:325–331. doi: 10.1016/0165-6147(93)90005-5. [DOI] [PubMed] [Google Scholar]
- Rogawski, M.A., 1996. Epilepsy. In: Neurotherapeutics: Emerging Strategies. (L. Pullan, J. Patel, eds.) Humana Press, Totowa, NJ, pp. 193–273.
- Rogawski MA. KCNQ2/KCNQ3 K+ channels and the molecular pathogenesis of epilepsy: implications for therapy. Trends Neurosci. 2000;23:393–398. doi: 10.1016/s0166-2236(00)01629-5. [DOI] [PubMed] [Google Scholar]
- Rogawski MA. Molecular targets versus models for new antiepileptic drug discovery. Epilepsy Res. 2006;68:22–28. doi: 10.1016/j.eplepsyres.2005.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA, Donevan SD. AMPA receptors in epilepsy and as targets for antiepileptic drugs. Adv Neurol. 1999;79:947–963. [PubMed] [Google Scholar]
- Rogawski MA, Kurzman PS, Yamaguchi SI, Li H. Role of AMPA and GluR5 kainate receptors in the development and expression of amygdala kindling in the mouse. Neuropharmacology. 2001;40:28–35. doi: 10.1016/s0028-3908(00)00112-x. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Löscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. 2004a;5:553–564. doi: 10.1038/nrn1430. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Löscher W. The neurobiology of antiepileptic drugs for the treatment of nonepileptic conditions. Nat Med. 2004b;10:685–692. doi: 10.1038/nm1074. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Porter RJ. Antiepileptic drugs: pharmacological mechanisms and clinical efficacy with consideration of promising developmental stage compounds. Pharmacol Rev. 1990;42:223–286. [PubMed] [Google Scholar]
- Rogawski, M.A., Reddy, D.S., 2004. Neurosteroids: Endogenous modulators of seizure susceptibility. In, Epilepsy: Scientific Foundations of Clinical Practice (J.M. Rho, R. Sankar and J. Cavazos, eds.), Marcel Dekker, New York, pp. 319–355.
- Rogawski MA, Taylor CP. Calcium channel α2–δ subunit, a new antiepileptic drug target, in: Löscher W, Schmidt D. New horizons in the development of antiepileptic drugs: innovative strategies. Epilepsy Res. 2006;69:183–272. doi: 10.1016/j.eplepsyres.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostock A, Tober C, Rundfeldt C, Bartsch R, Engel J, Polymeropoulos EE, Kutscher B, Loscher W, Honack D, White HS, Wolf HH. D-23129: a new anticonvulsant with a broad spectrum activity in animal models of epileptic seizures. Epilepsy Res. 1996;23:211–223. doi: 10.1016/0920-1211(95)00101-8. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Möhler H. Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Rundfeldt C. The new anticonvulsant retigabine (D-23129) acts as an opener of K+ channels in neuronal cells. Eur J Pharmacol. 1997;336:243–249. doi: 10.1016/s0014-2999(97)01249-1. [DOI] [PubMed] [Google Scholar]
- Rundfeldt C, Netzer R. Investigations into the mechanism of action of the new anticonvulsant retigabine. Interaction with GABAergic and glutamatergic neurotransmission and with voltage gated ion channels. Arzneimittelforschung. 2000a;50:1063–1070. doi: 10.1055/s-0031-1300346. [DOI] [PubMed] [Google Scholar]
- Rundfeldt C, Netzer R. The novel anticonvulsant retigabine activates M-currents in Chinese hamster ovary-cells tranfected with human KCNQ2/3 subunits. Neurosci Lett. 2000b;282:73–76. doi: 10.1016/s0304-3940(00)00866-1. [DOI] [PubMed] [Google Scholar]
- Sachdeo R, Porter R, Biton V, Rosenfeld W, Alves W, Nohria V. Dose finding study of retigabine (a novel AED) in patients with epilepsy. Epilepsia. 2005;46(Suppl 8):185. (2.280) [Google Scholar]
- Sarro G, Ferreri G, Gareri P, Russo E, De Sarro A, Gitto R, Chimirri A. Comparative anticonvulsant activity of some 2,3-benzodiazepine derivatives in rodents. Pharmacol Biochem Behav. 2003;74:595–602. doi: 10.1016/s0091-3057(02)01040-7. [DOI] [PubMed] [Google Scholar]
- Schenzer A, Friedrich T, Pusch M, Saftig P, Jentsch TJ, Grotzinger J, Schwake M. Molecular determinants of KCNQ (Kv7) K+ channel sensitivity to the anticonvulsant retigabine. J Neurosci. 2005;25:5051–5060. doi: 10.1523/JNEUROSCI.0128-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D, Rogawski MA. New strategies for the identification of drugs to prevent the development or progression of epilepsy. Epilepsy Res. 2002;50:71–78. doi: 10.1016/s0920-1211(02)00070-0. [DOI] [PubMed] [Google Scholar]
- Schmutz M, Allgeier H, Jeker A, Klebs K, McLean MJ, Mondadori C, Portet Ch, Vassout A, Wamil AW, Meier R. Anticonvulsant profile of CGP 33101 in animals. Epilepsia 34. 1993;2:122. [Google Scholar]
- Schroeder BC, Kubisch C, Stein V, Jentsch TJ. Moderate loss of function of cyclic-AMP-modulated KCNQ2/KCNQ3 K+ channels causes epilepsy. Nature. 1998;396:687–90. doi: 10.1038/25367. [DOI] [PubMed] [Google Scholar]
- Shen M, Béguin C, Golbraikh A, Stables JP, Kohn H, Tropsha A. Application of predictive QSAR models to database mining: identification and experimental validation of novel anticonvulsant compounds. J Med Chem. 2004;47:2356–2364. doi: 10.1021/jm030584q. [DOI] [PubMed] [Google Scholar]
- Simeone TA, McClellan AM. Felbamate demonstrates subunit selective activity in recombinant GABA-A receptors. Epilepsia. 2000;41(Suppl 7):36. [Google Scholar]
- Sobol E, Bialer M, Yagen B. Tetramethylcyclopropyl analogue of a leading antiepileptic drug, valproic acid. Synthesis and evaluation of anticonvulsant activity of its amide derivatives. J Med Chem. 2004;47:4316–4326. doi: 10.1021/jm0498351. [DOI] [PubMed] [Google Scholar]
- Sobol E, Yagen B, Winkler I, Britzi M, Gibson D, Bialer M. Pharmacokinetics and metabolism of a new potent antiepileptic drug, 2,2,3,3-tetramethycyclopropanecarbonylurea, in rats. Drug Metab Dispos. 2005;33:1538–1546. doi: 10.1124/dmd.105.005637. [DOI] [PubMed] [Google Scholar]
- Spiegelstein O, Yagen B, Levy RH, Finnell RH, Bennett GD, Roeder M, Schurig V, Bialer M. Stereoselective pharmacokinetics and pharmacodynamics of propylisopropyl acetamide, a CNS-active chiral amide analog of valproic acid. Pharm Res. 1999;16:1582–1588. doi: 10.1023/a:1018960722284. [DOI] [PubMed] [Google Scholar]
- Stables JP, Bertram EH, White HS, Coulter DA, Dichter MA, Jacobs MP, Löscher W, Lowenstein DH, Moshé SL, Noebels JL, Davis M. Models for epilepsy and epileptogenesis: report from the NIH workshop, Bethesda, Maryland. Epilepsia. 2002;43:1410–1420. doi: 10.1046/j.1528-1157.2002.06702.x. [DOI] [PubMed] [Google Scholar]
- Stefani A, Calabresi P, Pisani A, Mercuri NB, Siniscalchi A, Bernardi G. Felbamate inhibits dihydropyridine-sensitive calcium channels in central neurons. J Pharmacol Exp Ther. 1996;277:121–127. [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by γ subunit-containing GABAA receptors. Proc Natl Acad Sci USA. 2003;100:14439–4444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöhr, T., Krause, E., Selve, N., 2005. Lacosamide displays potent antinociceptive effects in animal models for inflammatory pain. Eur. J. Pain; Epub ahead of print. [DOI] [PubMed]
- Subramaniam S, Rho JM, Penix L, Donevan SD, Fielding RP, Rogawski MA. Felbamate block of the N-methyl-D-aspartate receptor. J Pharmacol Exp Ther. 1995;273:878–886. [PubMed] [Google Scholar]
- Taglialatela M, Ongini E, Brown AM, Di Renzo G, Annunziato L. Felbamate inhibits cloned voltage-dependent Na+ channels from human and rat brain. Eur J Pharmacol. 1996;316:373–377. doi: 10.1016/s0014-2999(96)00802-3. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Ni JW, Kawasaki-Yatsugi S, Toya T, Yatsugi SI, Shimizu-Sasamata M, Koshiya K, Shishikura JI, Sakamoto S, Yamaguchi T. YM872, a novel selective α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor antagonist, reduces brain damage after permanent focal cerebral ischemia in cats. J Pharmacol Exp Ther. 1998;284:467–473. [PubMed] [Google Scholar]
- Tatulian L, Brown DA. Effect of the KCNQ potassium channel opener retigabine on single KCNQ2/3 channels expressed in CHO cells. J Physiol. 2003;549 (Pt 1):57–63. doi: 10.1113/jphysiol.2003.039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatulian L, Delmas P, Abogadie FC, Brown DA. Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anticonvulsant drug retigabine. J Neurosci. 2001;21:5535–5545. doi: 10.1523/JNEUROSCI.21-15-05535.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CD, Miller TA, Barthen MT, Dieckhaus CM, Sofia RD, Macdonald TL. The synthesis, in vitro reactivity, and evidence for formation in humans of 5-phenyl-1,3-oxazinane-2,4-dione, a metabolite of felbamate. Drug Metab Dispos. 2000;28:434–439. [PubMed] [Google Scholar]
- Tober C, Rostock A, Rundfeldt C, Bartsch R. D-23129: a potent anticonvulsant in the amygdala kindling model of complex partial seizures. Eur J Pharmacol. 1996;303:163–169. doi: 10.1016/0014-2999(96)00073-8. [DOI] [PubMed] [Google Scholar]
- Troughton, G.M., Kleckner, N.M., 2005. An Investigation of the subtype-selective antagonism of N-methyl-D-aspartate receptors by new antiepileptic agent, R-2-acetamido-N-benzyl-3-methoxypropionamide, Bates College Thesis Abst.
- Turski L, Huth A, Sheardown M, McDonald F, Neuhaus R, Schneider HH, Dirnagl U, Wiegand F, Jacobsen P, Ottow E. ZK200775: a phosphonate quinoxalinedione AMPA antagonist for neuroprotection in stroke and trauma. Proc Natl Acad Sci USA. 1998;95:10960–10965. doi: 10.1073/pnas.95.18.10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn CM, Willems-van Bree E. Synergy between retigabine and GABA in modulating the convulsant site of the GABAA receptor complex. Eur J Pharmacol. 2003;464:95–100. doi: 10.1016/s0014-2999(03)01426-2. [DOI] [PubMed] [Google Scholar]
- Vickery RG, Amagasu SM, Chang R, Mai N, Kaufman E, Martin J, Hembrador J, O’Keefe MD, Gee C, Marquess D, Smith JA. Comparison of the pharmacological properties of rat NaV1.8 with rat NaV1.2a and human NaV1.5 voltage-gated sodium channel subtypes using a membrane potential sensitive dye and FLIPR. Receptors Channels. 2004;10:11–23. [PubMed] [Google Scholar]
- Volosov A, Xiaodong S, Perucca E, Yagen B, Sintov A, Bialer M. Enantioselective pharmacokinetics of 10-hydroxycarbazepine after oral administration of oxcarbazepine to healthy Chinese subjects. Clin Pharmacol Ther. 1999;66:547–553. doi: 10.1053/cp.1999.v66.103170001. [DOI] [PubMed] [Google Scholar]
- Walters MR, Kaste M, Lees KR, Diener HC, Hommel M, De Keyser J, Steiner H, Versavel M. The AMPA antagonist ZK 200775 in patients with acute ischaemic stroke: a double-blind, multicentre, placebo-controlled safety and tolerability study. Cerebrovasc Dis. 2005;20:304–309. doi: 10.1159/000087929. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Nagata E, Kosakai A, Nakamura M, Yokoyama M, Tanaka K, Sasai H. Disruption of the epilepsy KCNQ2 gene results in neural hyperexcitability. J Neurochem. 2000;75:28–33. doi: 10.1046/j.1471-4159.2000.0750028.x. [DOI] [PubMed] [Google Scholar]
- White HS, Schmutz M, Pozza M, Wolf H, Stables J, Kupferberg H. The anticonvulsant profile and tolerability of rufinamide in mice and rats. Epilepsia. 2005;46(Suppl 8):305–306. (3.088) [Google Scholar]
- White HS, Wolf HH, Swinyard EA, Skeen GA, Sofia RD. A neuropharmacological evaluation of felbamate as a novel anticonvulsant. Epilepsia. 1992;33:564–572. doi: 10.1111/j.1528-1157.1992.tb01711.x. [DOI] [PubMed] [Google Scholar]
- White, H.S., Woodhead, J.H., Wilcox, K.S., Stables, J.P., Kupferberg, H.J., Wolf, H.H., 2002. General principles. Discovery and preclinical development of antiepileptic drugs. In: Levy, R.H., Mattson, R.H., Meldrum, B.S., Perucca, E., eds. Antiepileptic drugs. Philadelphia: Lippincott-Raven, 36–48.
- Whiting PJ. The GABAA receptor gene family: new opportunities for drug development. Curr Opin Drug Discov Devel. 2003;6:648–657. [PubMed] [Google Scholar]
- Wickenden, A.D., McNaughton-Smith, G., Roeloffs, R., London, B., Clark, S., Wilson, W.A., Rigdon, G.C., Wagoner, P.K., 2005. ICA-27243: A novel, potent, and selective KCNQ2/Q3 potassium channel activator. Soc. Neurosci. Abst. Prog. No. 153.13. [DOI] [PubMed]
- Wickenden AD, Zou A, Wagoner PK, Jegla T. Characterization of KCNQ5/Q3 potassium channels expressed in mammalian cells. Br J Pharmacol. 2001;132:381–384. doi: 10.1038/sj.bjp.0703861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler I, Blotnik S, Shimshoni J, Yagen B, Devor M, Bialer M. Efficacy of antiepileptic isomers of valproic acid and valpromide in a rat model of neuropathic pain. Br J Pharmacol. 2005;146:198–208. doi: 10.1038/sj.bjp.0706310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABAA receptors containing the δ subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuttke TV, Seebohm G, Bail S, Maljevic S, Lerche H. The new anticonvulsant retigabine favors voltage-dependent opening of the Kv7.2 (KCNQ2) channel by binding to its activation gate. Mol Pharmacol. 2005;67:1009–1017. doi: 10.1124/mol.104.010793. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Donevan SD, Rogawski MA. Anticonvulsant activity of AMPA/kainate antagonists: comparison of GYKI 52466 and NBOX in maximal electroshock and chemoconvulsant seizure models. Epilepsy Res. 1993;15:179–184. doi: 10.1016/0920-1211(93)90054-b. [DOI] [PubMed] [Google Scholar]