Abstract
The purpose of this study was to compare the clinical utility of the serum concentration of feline trypsin-like immunoreactivity (fTLI), the plasma and urine concentrations of trypsinogen-activation peptide (TAP), and the ratio of the urine TAP and creatinine concentrations (TAP:Cr) in the diagnosis of feline acute pancreatitis. We used 13 healthy cats and 10 cats with a diagnosis of acute pancreatitis. The mean serum fTLI and plasma TAP concentrations were significantly higher in the cats with acute pancreatitis than in the healthy cats (P < 0.05); the mean urine TAP concentrations and the median urine TAP:Cr ratios were not significantly different. Among the cats examined in this study, there was no benefit of plasma TAP over serum fTLI in the evaluation of suspected acute pancreatitis.
Résumé
L’objectif de la présente étude était de comparer l’utilité clinique du dosage radio-immunologique de la trypsine sérique féline (fTLI), les concentrations plasmatiques et urinaires du peptide d’activation du typsinogène (TAP), et le ratio des concentrations urinaires du TAP et de créatinine (TAP:Cr) pour le diagnostic de pancréatite féline aiguë. L’étude a été réalisée en utilisant 13 chats en santé et 10 chats avec un diagnostic de pancréatite aiguë. Les concentrations moyennes de fTLI sérique et de TAP plasmatique étaient significativement plus élevées chez les chats avec pancréatite aiguë que chez les chats en santé (P < 0,05); les concentrations moyennes de TAP urinaire et les médianes des ratios TAP:Cr urinaire n’étaient pas significativement différentes. Parmi les chats examinés dans cette étude, il n’y avait pas d’avantage à mesurer le TAP plasmatique par rapport au fTLI sérique pour évaluer un cas suspecté de pancréatite aiguë.
(Traduit par Docteur Serge Messier)
Acute pancreatitis is an important gastrointestinal disease in cats (1). Previously believed to be rare, pancreatitis is diagnosed during necropsy in 1.3% to 3.5% of cats (1). Feline acute pancreatitis can be difficult to diagnose owing to the nonspecific clinical signs and the poor sensitivity and specificity of routine diagnostic tests. In addition, the diagnostic tests commonly used for the diagnosis of acute pancreatitis in the dog are of no clinical value in the cat (2,3).
The serum feline trypsin-like immunoreactivity (fTLI) concentration has been shown to be useful for the diagnosis of feline acute pancreatitis but has a reported sensitivity of only 30% to 60%. Also, the serum fTLI concentration may be elevated in cats with other diseases, such as renal failure, inflammatory bowel disease, lymphosarcoma, and emaciation (1–5).
Trypsinogen-activation peptide (TAP) is a small peptide that is released when trypsinogen is activated to trypsin (6). Under physiologic conditions, activation of trypsinogen occurs in the intestinal lumen and is catalyzed by enteropeptidase (formerly known as enterokinase) (3). In the intestinal lumen, TAP is quickly degraded by peptidases of the brush border membrane. In pancreatitis, trypsinogen is prematurely activated within pancreatic acinar cells, and TAP is released into the peripheral circulation. Although TAP is quickly excreted through the kidneys, with a circulating half-life of less than 8 min, significant increases in plasma and urine TAP concentrations have been reported in canine and human patients with acute pancreatitis. An assay of TAP has been shown to be useful for the diagnosis and staging of pancreatitis in humans (7,8) and dogs (9). Also, measurement of TAP in plasma and urine has been evaluated in experimental rodent and feline models of pancreatitis and has been shown to be useful in assessing pancreatic inflammation in those species (10–13).
We compared the clinical utility of the plasma and urine TAP concentrations and the ratio of the urine TAP and creatinine concentrations (TAP:Cr) with that of the serum fTLI concentration in the diagnosis of acute pancreatitis in cats.
Acute pancreatitis was diagnosed in 10 cats on the basis of clinical signs (anorexia and depression for 1 to 7 d) in combination with either abdominal ultrasound findings suggestive of acute pancreatitis (abnormal pancreatic echogenicity with or without ascites) (in 3 cats) or histopathological changes in pancreatic biopsy specimens obtained at surgery or necropsy (in 7 cats). Ultrasonography was performed in the clinical setting, by various sonographers, but all histopathological slides were evaluated by 1 pathologist, who assessed acinar cell necrosis, fibrosis, peripancreatic fat necrosis, interlobular edema, and inflammatory infiltrates. The cats’ records were evaluated for signalment, history, clinical signs on presentation, clinicopathological findings, levels of blood urea nitrogen (BUN) and serum creatinine, and results of imaging studies. The control population consisted of 13 healthy cats that were pets of employees (5 cats) or were being anesthetized for routine dental procedures (8 cats). Signalment was recorded when known. None of the control cats had clinicopathological abnormalities, evidence of systemic disease, or a history of previous gastrointestinal disease.
Blood and spot urine samples were collected from each cat. However, only 8 of the 10 plasma samples from the cats with acute pancreatitis and 11 of the 13 serum and plasma samples from the healthy controls could be evaluated because of damage during shipping and handling. For batch analysis of TAP, by a quantitative, solid-phase immunoassay (Biotrin, Dublin, Ireland), 3 mL each of plasma and urine was frozen and stored at −4°C, a temperature at which TAP is stable (14). The minimum concentration detectable by the immunoassay is 0.56 nmol/L. The TAP concentration was measured in all the samples, the creatinine concentration was measured in the urine samples, and the urine TAP:Cr ratio was determined. The TAP assay had been validated with feline samples before this study. A recovery study showed a mean overall recovery of TAP to be 96.7%, with a mean recovery in plasma of 93.75% and in urine of 99.63%. The intra-assay coefficients of variation for plasma and urine were 7.73% and 15.2%, respectively. These results were considered appropriate for an enzyme immunoassay. One mL of serum was frozen and stored at −4°C for batch analysis of the serum fTLI concentration by an in-house enzyme-linked immunosorbent assay, at the Gastrointestinal Laboratory at Texas A&M University, College Station, Texas (15).
Descriptive statistics were used for the quantitative data. The mean serum fTLI and urine TAP concentrations in the cats with acute pancreatitis and the healthy cats were compared with the use of Student’s t-test. The mean plasma TAP concentration and the urine TAP:Cr ratio in the 2 groups of cats were compared with the use of a Mann–Whitney test, as both data sets had failed the Kolmogorov–Smirnov normality test. A P-value of less than 0.05 was considered significant. Sensitivity and specificity of the different parameters were calculated for a range of cut-off values. Correlations between test results were performed with the use of parametric calculations.
Signalment was available for all 10 of the cats with a diagnosis of acute pancreatitis. The mean age (and standard deviation) was 11.4 (4.33) y. Among the 9 domestic shorthair cats and 1 Siamese cat were 4 castrated males, 5 spayed females, and 1 intact female. Of the 13 healthy cats signalment was available for 10. The mean age was 7.7 (3.47) y, significantly lower than the mean for the cats with acute pancreatitis. The 10 healthy cats included 4 domestic shorthair, 2 American shorthair, 1 Siamese, 1 Abyssinian, 1 Himalayan, and 1 Burmese; 7 were castrated males and 3 spayed females.
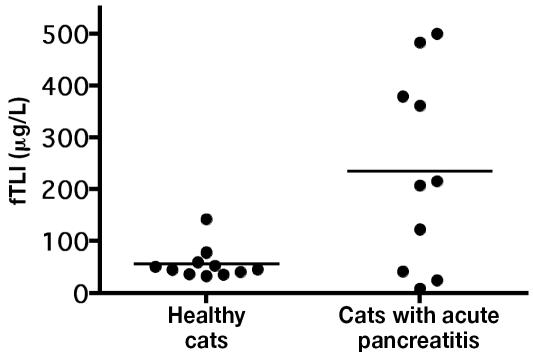
The mean serum fTLI concentration was significantly higher (P = 0.006) in the 10 cats with acute pancreatitis than in the 11 healthy cats for which the serum could be evaluated: 234.7 (standard deviation 86.5) μg/L versus 56.6 (31.08) μg/L. The values ranged from 9 to 483 μg/L and 33 to 192 μg/L, respectively (Figure 1).
Figure 1.
Serum concentrations of feline trypsin-like immunoreactivity (fTLI) in healthy control cats and cats with acute pancreatitis. The mean values (bars) are significantly different (P < 0.05).
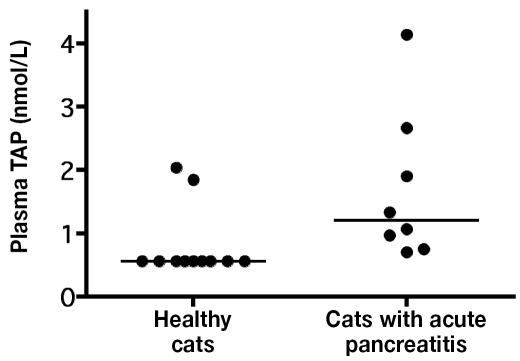
The median plasma TAP concentration was significantly higher (P = 0.007) in the 8 cats with acute pancreatitis than in the 11 healthy cats for which the plasma could be evaluated: 1.20 (range 0.7 to 4.14) nmol/L versus 0.56 (0.86 to 2.76) nmol/L (Figure 2).
Figure 2.
Plasma concentrations of trypsinogen-activation peptide (TAP) in healthy control cats and cats with acute pancreatitis. The mean values (bars) are significantly different (P < 0.05).
The mean urine TAP concentration did not differ significantly (P = 0.62) between the 10 cats with acute pancreatitis and the 13 healthy cats, at 37.06 (standard deviation 61.8) nmol/L and 46.95 (31.61) nmol/L, respectively; the ranges were 0.56 to 69.9 nmol/L and 2.86 to 107.5 nmol/L, respectively. The median urine TAP:Cr ratio also did not differ significantly (P = 0.09) between the 2 groups of cats, at 4.07 (range 0.18 to 48) and 1.88 (range 0.48 to 3.60), respectively.
There was a significant correlation between the serum fTLI and plasma TAP concentrations in all the cats and in the cats with acute pancreatitis, but there was no significant correlation between the serum fTLI and the urine TAP concentrations or between the urine and plasma TAP concentrations in either group (Table I).
Table I.
Correlation between concentrations of serum feline trypsin-like immunoreactivity (fTLI), plasma trypsinogen-activation peptide (TAP), and urine TAP in cats with acute pancreatitis and healthy control cats
| Variables correlated
|
|||
|---|---|---|---|
| Group | fTLI and plasma TAP | fTLI and urine TAP | Urine and plasma TAP |
| All cats | r =0.739, P = 0.0006 | r = −0.22, P = 0.33 | r = −0.13, P = 0.58 |
| Cats with acute Pancreatitis | r = 0.808, P = 0.0084 | r = −0.20, P = 0.56 | r = −0.17, P = 0.66 |
For the serum fTLI concentration, the sensitivity in diagnosing acute pancreatitis was 63.6% and the specificity 90.9% for cut-off values of 80, 100, and 120 μg/L. For the plasma TAP concentration, optimal sensitivity of 100% and specificity of 81.8% were obtained with a cut-off value of 0.60 nmol/L.
Serum BUN and creatinine concentrations were measured in 9 of the 10 cats with acute pancreatitis, the exception being a cat that was euthanized shortly after admission. The BUN concentration ranged from 14 to 119 mg/dL, with 4 values being outside the normal range of 15 to 34 mg/dL. The creatinine concentration ranged from 0.6 to 4.8 mg/dL, with 4 values being outside the normal range of 0.8 to 2.3 mg/dL. Although correlation was not performed, on cursory evaluation the cats with the highest BUN and creatinine concentrations also had the highest plasma TAP and serum fTLI concentrations.
In this study, both serum fTLI and plasma TAP concentrations were significantly increased in cats with acute pancreatitis. The plasma TAP concentration had a higher diagnostic sensitivity than the serum fTLI concentration but a lower specificity, with approximately 20% of results being false-positive. The plasma TAP concentration may be more sensitive because it remains elevated for longer after the development of pancreatitis than does the serum fTLI concentration. The reason the serum fTLI concentration is more specific is less clear. There is likely a small amount of TAP excreted into the circulation in healthy cats. It is possible that this secretion is episodic and associated with pancreatic secretions. Thus, at times of pancreatic secretion the plasma TAP concentration would be higher, leading to a false-positive result. Also, it is possible that the test detects other proteins that are not associated with TAP but are similar, leading to a false-positive result. In addition, despite the significant difference in mean plasma TAP concentration between the 2 groups of cats, there was an overlap of values, such that a cat with acute pancreatitis could be considered healthy.
Despite the differences in specificity and sensitivity, there was a significant correlation between the fTLI and plasma TAP concentrations. Considering the limited availability and high costs of the TAP enzyme immunoassay, determining the plasma TAP concentration may not provide enough advantage over measuring the serum fTLI concentration for the diagnosis of acute pancreatitis in cats in a clinical setting.
Azotemia was evident in 4 of the cats with acute pancreatitis. Azotemia has been shown to elevate the fLTI concentration in cats (16) and the plasma TAP concentration in dogs (14). In another study (unpublished data), cats with renal failure had a mean plasma TAP concentration of 7.17 (standard deviation 10.59), urine TAP concentration of 22.14 (24.1), and urine TAP:Cr ratio of 4.24 (5.43); the plasma TAP concentration and urine TAP:Cr ratio were significantly greater in the cats with renal failure than in cats with acute pancreatitis (Caroline Mansfield, University College, Dublin, Ireland: personal communication, 2004). In our study, the 4 cats with azotemia and acute pancreatitis may have had falsely elevated fTLI and plasma TAP values. In the clinical setting, the plasma TAP concentration would most likely be measured in cats within several hours of admission. If azotemia leads to falsely elevated plasma TAP values, then the clinical utility of the assay would be reduced considerably. Further research needs to be done to see if azotemia truly affects the plasma TAP concentration in cats.
Plasma and urine TAP concentrations were measurable in healthy cats in our study, which goes against the theory that TAP is exclusively secreted into the small intestine in healthy animals; TAP may leak into the abdomen and subsequently the circulation even in healthy cats. Also, we did not obtain pancreas specimens for histopathological study from the healthy cats, and there could have been some underlying subclinical pancreatic disease, although this seems unlikely, considering the cats’ clinical history.
The healthy control cats were significantly younger than the cats with acute pancreatitis in our study, although this could be because it is difficult to find suitable age-matched healthy controls. Many older cats have renal insufficiency, which may affect the plasma TAP measurement. Thus, evaluating older control cats may be warranted.
There were more purebred cats in our control population than in the group of cats with acute pancreatitis. This may be because acute pancreatitis is more prevalent in domestic shorthair cats or, more likely, because the control cats were recruited in part from cats that had been presented for routine dental care, and owners of purebred cats are more likely to seek preventive veterinary care. However, the number of cats in the study was small, and general assumptions cannot be made about either group of cats.
In human patients with acute pancreatitis, the urine TAP concentration is thought to be superior to the plasma TAP concentration because TAP is concentrated in the urine and because urinary excretion of TAP persists 5 h longer than the elevation in the plasma TAP concentration (17). A previous study showed that urine TAP concentrations in cats with experimentally induced pancreatitis were significantly different from baseline concentrations (11). In dogs, the urine TAP:Cr ratio has been shown to be the most useful test for diagnosing pancreatitis and assessing its severity. Interestingly, in our study the urine TAP concentration and the urine TAP:Cr ratio were determined not to be useful clinically, and there was poor correlation between the urine TAP and plasma TAP concentrations and between the urine TAP and serum fTLI concentrations. Many, if not most, of the cats with acute pancreatitis had active disease for several days before collection of the samples; therefore, the TAP concentrations may have been falsely lowered. Also, the urine TAP concentration was measured in spot urine samples. Thus, the urine concentration may have altered the clinical utility of the urine TAP measurement. However, urine production should not alter the clinical utility of the urine TAP:Cr ratio. Another possible explanation for the lack of clinical utility of the urine TAP concentration and the urine TAP:Cr ratio is that TAP may be unstable in cat urine. However, this hypothesis needs to be addressed in further studies.
In conclusion, the serum fTLI and plasma TAP concentrations were significantly increased in the cats with acute pancreatitis that we studied. The plasma TAP concentration had a higher sensitivity but a lower specificity for feline acute pancreatitis than the serum fTLI concentration. Also, the cost of the TAP assay is high and the availability limited. Thus, this study failed to identify any advantage of measuring the plasma TAP concentration over measuring the serum fTLI concentration for the diagnosis of acute pancreatitis in cats.
Acknowledgments
We thank Chris Demarco and Michele Cohen for their assistance with this project and Dr. James Walberg for his assistance with the pathological studies. The project was supported in part by a grant from the Winn Feline Foundation, Manasquan, New Jersey, USA. The TAP work was supported by Ralston Purina, Mississauga, Ontario, and Biotrin, Dublin, Ireland.
Footnotes
This study was presented in part as a research abstract at the American College of Veterinary Internal Medicine Forum, Charlotte, North Carolina, USA, June 2003.
Dr. Allen’s current address is Hope Center for Advanced Veterinary Medicine, 416 Maple Avenue West, Vienna, Virginia 22180, USA.
Dr. Mansfield’s current address is Division of Veterinary and Biomedical Sciences, Murdoch University, South Street, Murdoch, West Australia 6150, Australia.
References
- 1.Steiner JM, Williams DA. Feline pancreatitis. Compend Contin Educ Pract Vet. 1997;19:590–603. [Google Scholar]
- 2.Parent C, Washabau RJ, Williams DA, et al. Serum trypsin-like immunoreactivity, amylase, and lipase in the diagnosis of feline acute pancreatitis [abstract] J Vet Intern Med. 1995;9:194. [Google Scholar]
- 3.Swift NC, Marks SL, MacLachlan NJ, et al. Evaluation of serum feline trypsin-like immunoreactivity for the diagnosis of pancreatitis in cats. J Am Vet Med Assoc. 2000;217:37–42. doi: 10.2460/javma.2000.217.37. [DOI] [PubMed] [Google Scholar]
- 4.Steiner JM, Williams DA. Disagrees with criteria for diagnosing pancreatitis in cats. J Am Vet Med Assoc. 2000;217:816–818. doi: 10.2460/javma.2000.217.816. [DOI] [PubMed] [Google Scholar]
- 5.Gerhardt A, Steiner JM, Williams DA, et al. Comparison of the sensitivity of different diagnostic tests for pancreatitis in cats. J Vet Intern Med. 2001;15:329–333. [PubMed] [Google Scholar]
- 6.Rinderknecht H. Pancreatic secretory enzymes. In Go VW, Dimagno EP, eds. The Pancreas: Biology, Pathobiology, and Disease. New York: Raven Press, 1993:219–251.
- 7.Fernández-del Castillo C, Schmidt J, Rattner DW, et al. Generation and possible significance of trypsinogen activation peptides in experimental acute pancreatitis in the rat. Pancreas. 1992;7:263–270. doi: 10.1097/00006676-199205000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Fernández-del Castillo C, Schmidt J, Rattner DW, et al. TAP assay: a new tool for research in acute experimental pancreatitis. Pancreas. 1992:A-121. [Google Scholar]
- 9.Mansfield CS, Jones BR, Spillman T. Assessing the severity of canine pancreatitis. Res Vet Sci. 2003;74:137–144. doi: 10.1016/s0034-5288(02)00181-9. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt J, Fernandez-del Castillo C, Rattner DW, et al. Trypsinogen-activation peptides in experimental rat pancreatitis: prognostic implications and histopathologic correlates. Gastroenterology. 1992;103:1009–1016. doi: 10.1016/0016-5085(92)90036-x. [DOI] [PubMed] [Google Scholar]
- 11.Karanjia ND, Widdison AL, Jehanli A, et al. Assay of trypsinogen activation in the cat experimental model of acute pancreatitis. Pancreas. 1993;8:189–195. doi: 10.1097/00006676-199303000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Gudgeon MA, Heath DI, Hurley P. Trypsinogen activation peptide assay in the early prediction of severity of acute pancreatitis. Lancet. 1990;335:4–8. doi: 10.1016/0140-6736(90)90135-r. [DOI] [PubMed] [Google Scholar]
- 13.Mansfield C. New insights into pancreatitis in dogs. In Proc Annu Meet Eur Coll Vet Med. 2002:100–102. [Google Scholar]
- 14.Mansfield CS, Jones BR. Plasma and urinary trypsinogen activation peptide in healthy dogs, dogs with pancreatitis, and dogs with other systemic disease. Aust Vet J. 2000;78:416–422. doi: 10.1111/j.1751-0813.2000.tb11833.x. [DOI] [PubMed] [Google Scholar]
- 15.Steiner JM, Williams DA, Moeller EM, Melgarejo T. Development and validation of an enzyme-linked immunosorbent assay for feline trypsin-like immunoreactivity. Am J Vet Res. 2000;61:620–623. doi: 10.2460/ajvr.2000.61.620. [DOI] [PubMed] [Google Scholar]
- 16.Steiner JM, Finco PR, Williams DA. Serum feline trypsinogen-like immunoreactivity in cats with experimentally induced chronic renal failure. Proc Annu Meet Am Coll Vet Intern Med. 2002:816. [Google Scholar]
- 17.Hurley P, Cooke A, Jehanli A, et al. TAP assay: a novel immunoassay specific for free trypsinogen activation peptides reporting pathological trypsinogen activation. Gastroenterology. 1988;94:A198. [Google Scholar]