Abstract
Bone cell cultures were evaluated to determine if osteogenic cell populations at different skeletal sites in the horse are heterogeneous. Osteogenic cells were isolated from cortical and cancellous bone in vitro by an explant culture method. Subcultured cells were induced to differentiate into boneforming osteoblasts. The osteoblast phenotype was confirmed by immunohistochemical testing for osteocalcin and substantiated by positive staining of cells for alkaline phosphatase and the matrix materials collagen and glycosaminoglycans. Bone nodules were stained by the von Kossa method and counted. The numbers of nodules produced from osteogenic cells harvested from different skeletal sites were compared with the use of a mixed linear model. On average, cortical bone sites yielded significantly greater numbers of nodules than did cancellous bone sites. Between cortical bone sites, there was no significant difference in nodule numbers. Among cancellous sites, the radial cancellous bone yielded significantly more nodules than did the tibial cancellous bone. Among appendicular skeletal sites, tibial metaphyseal bone yielded significantly fewer nodules than did all other long bone sites. This study detected evidence of heterogeneity of equine osteogenic cell populations at various skeletal sites. Further characterization of the dissimilarities is warranted to determine the potential role heterogeneity plays in differential rates of fracture healing between skeletal sites.
Résumé
Des cultures de cellules osseuses ont été réalisées afin de déterminer si les populations de cellules ostéogéniques provenant de différents sites squelettiques chez le cheval sont hétérogènes. Les cellules ostéogéniques ont été isolées in vitro de l’os cortical et de l’os spongieux par une méthode de culture d’explant. Les cellules cultivées ont été induites à se différencier en ostéoblastes formant de l’os. Le phénotype d’ostéoblaste a été confirmé par épreuve immunohistochimique pour l’ostéocalcine et corroborer par coloration positive des cellules pour la phosphatase alcaline de même que pour le collagène et les glycosaminoglycans. Les nodules osseux ont été colorés par la méthode de von Kossa et dénombrés. Les quantités de nodules produits par les cellules ostéogéniques recueillies des différents sites squelettiques ont été comparées en utilisant un modèle linéaire mixte. En moyenne, les sites d’os cortical ont permis d’obtenir un nombre significativement plus grand de nodules que les sites d’os spongieux. Entre les sites d’os cortical, il n’y avait pas de différence significative dans le nombre de nodules. Parmi les sites d’os spongieux, le site radial a permis de recueillir plus de nodules que le site tibial. Parmi les sites squelettiques appendiculaires, l’os métaphysaire tibial a permis d’obtenir significativement moins de nodules que tous les autres sites d’os longs. La présente étude a permis de démontrer l’hétérogénéité des populations de cellules ostéogéniques à différents sites squelettiques. Une caractérisation supplémentaire des différences observées est de mise afin de déterminer le rôle potentiel joué par l’hétérogénéité dans la variabilité de la guérison des fractures entre les différents sites squelettiques.
(Traduit par Docteur Serge Messier)
Introduction
Long bone fractures in adult horses are especially difficult to repair. Although many factors, including fracture configuration, disruption of the soft tissue and blood supply, age of the animal, and stabilization technique, contribute to variability in fracture healing, heterogeneity of osteogenic cell populations of the bone tissue at various skeletal sites may also be an important factor. Cultures of bone cells derived from rats (1,2) and humans (3) from various skeletal sites show differences in osteogenic cell populations, including differences in proliferation rates (4) and osteoblast markers (5). In humans, dissimilarities between skeletal sites are also seen in the response of bone to various disease states, including hyperparathyroidism (6) and osteoporotic bone loss (7), and in the response to various therapies, including supplementation with calcium, calcitonin, sodium fluoride, and parathyroid hormone (8).
Osteogenic cell populations include osteoprogenitors, preosteoblasts, osteoblasts, osteocytes, and bone-lining cells. Osteoprogenitors differentiate into osteoblasts, the active bone-producing cells (9). Both osteoprogenitors and mature osteoblasts are crucial to the osteogenic process of callus formation during fracture healing. Cortical, trabecular, and osteonal endosteum of the local bone, periosteum, and marrow stroma adjacent to the fracture site are all major sources of osteoprogenitor cells (10).
The objective of this study was to determine if osteogenic cell populations from different skeletal sites in the horse are heterogeneous. Heterogeneity was measured by quantifying differences in bone nodule production by cell populations from different skeletal sites.
Materials and methods
Specimen collection
Eight horses of various breeds, aged 5 d to 20 y, were euthanized for reasons unrelated to this study. Two foals aged 5 and 8 d, 3 horses aged 10 mo to 2 y, 1 horse aged 4 y, and 2 horses aged 20 y were included in the study; 6 were standardbreds, 1 was a Pinto, and 1 was a Shetland pony. None were in athletic training at the time of donation or euthanasia.
Specimens of cortical bone (tibial, radial, and 3rd metacarpal diaphysis) and cancellous bone (tibial and radial proximal metaphysis and 4th coccygeal vertebra) were aseptically collected immediately post mortem and used for primary explant cultures. Consistent collection was based on specific anatomic landmarks and measurements for each skeletal site. Specimens were maintained on ice in a minimal essential medium (α -MEM; Canadian Life Technologies, Toronto, Ontario) until the cultures were established, within 2 to 24 h. All specimens from an individual were prepared for primary cultures at the same time.
Cell isolation
The methods used to isolate primary osteogenic cell populations were those used in rat and human osteoblast cultures (1,11,12); they have also been described for equine bone (13). Measures were taken to reduce potential contamination of primary bone cells with soft tissue or marrow cells. Cortical and coccygeal vertebral bone specimens were stripped of all extraosseous soft tissue and washed with phosphate-buffered saline (PBS) (Canadian Life Technologies). The endosteal surface of cortical specimens was also scraped with a curette. Cancellous bone specimens were washed extensively in PBS. All specimens were cut into bone chips 1 to 3 mm3 for explants and rinsed with PBS.
The explants were placed into 25-μ L droplets of α -MEM containing 20% fetal calf serum (FCS) (Cansera International, Rexdale, Ontario), 15% citrated bovine plasma (Sigma Aldridge Canada, Oakville, Ontario), 100 μ g/mL of penicillin G (ICN Biomedicals, Costa Mesa, California, USA), 50 μ g/mL of gentamicin sulfate (Canadian Life Technologies), and 0.3 μ g/mL of the antimycotic amphotericin B (Canadian Life Technologies) in Falcon tissue-culture plastic dishes 35 mm in diameter (Fisher Scientific, Ottawa, Ontario). Primary cultures were supplemented with standard media (α -MEM, 10% FCS, 100 μ g/mL of penicillin G, 50 μ g/mL of gentamicin sulfate, and 0.3 μ g/mL of amphotericin B). Ten primary culture dishes with 8 explants per dish were established for each specimen from each horse. The explant cultures were maintained in a humidified incubator at 37°C with 5% carbon dioxide and 95% air until primary cells were confluent or until day 21. The medium was changed 3 times per week.
Cell differentiation
After confluence was achieved, or on day 21, primary osteogenic cells were obtained for subculture at standard numbers to be used for the bone nodule assay. All primary explant cultures of all donor sites from an individual horse were subcultured at the same time. Subcultures were set up as described previously (13). Briefly, primary cells were detached with 1% collagenase and 0.12% trypsin, centrifuged at 375 × g for 6 min, and resuspended in osteogenic media (α -MEM, 10% FCS, 100 μ g/mL of penicillin G, 50 μ g/mL of gentamicin sulfate, 0.3 μ g/mL of amphotericin B, 50 μ g/mL of ascorbic acid [Canadian Life Technologies], 10−8 M dexamethasone [ICN Biomedicals], and 10 mM β-glycerophosphate (βGP; Sigma Aldridge Canada]). Cell viability was determined with the use of 0.4% trypan blue (Eastman Kodak Company, Rochester, New York, USA), and viable cells were plated at 1 × 104 cells/cm2 in 35-mm diameter Falcon tissue-culture dishes. Additionally, representative primary osteogenic cells from cortical and cancellous bone were plated at 1 × 104 cells/cm2 onto 8-well glass-chamber slides (Labtek Chamber Slides; Nalgene Nunc, Naperville, Illinois, USA) and supplemented with osteogenic media. All subcultures were maintained in a humidified incubator at 37°C with 5% carbon dioxide and 95% air until colony-forming units (CFUs) that produced bone nodules were observed or until day 14. All subculture dishes of all donor sites from an individual horse were fixed and stained at the same time. The medium was changed 3 times per week.
Bone-nodule assay
Bone nodules were stained by the von Kossa method (14). One observer (L.A.M.), using phase-contrast microscopy at 100× magnification and a grid overlay, counted the von-Kossa-positive (VK+) mineralized bone nodules over a 5.3-cm2 area of culture dish.
Histochemical and immunohistochemical tests
Representative subcultures of bone nodules on chamber slides were used to evaluate CFUs and bone nodules for the presence of alkaline phosphatase (ALP), bone matrix proteins (collagen and glycosaminoglycans), and osteocalcin. All subcultures were fixed with 10% neutral buffered formalin and then encapsulated in Histogel (ESBE Scientific, Montreal, Quebec) for histologic sections. Gel and nodules were lifted off the slides with a razor blade, dehydrated, embedded in paraffin, and cut into sections 4 μ m thick, which were stained with Masson’s trichrome for collagen or alcian blue for glycosaminoglycans. For immunohistochemical evaluation of the presence of osteocalcin, fixed subcultures with bone nodules were subjected to antigen retrieval by the steamer method with citrate buffer (pH 6.0) in situ. First, endogenous peroxidases were blocked with 3% H2O2 in methanol. Then normal goat serum was applied for 30 min at room temperature. Mouse osteocalcin antibody against bovine antigen (BioGenex, San Ramon, California, USA) was applied for 24 h at 4°C. After a buffer wash, goat IgG against mouse antigen, labeled with horseradish peroxidase (Jackson Immuno Research Labs, West Grove, Pennsylvania, USA), was applied for 1 h at room temperature. The subcultures were then washed in buffer and incubated in 3,3′-diaminobenzidine tetrahydrochloride containing H2O2. For negative controls, the specific primary antibody was replaced with PBS. Bone nodules in situ were also stained routinely by the von Kossa method with the use of silver nitrate and stained for ALP (15) with the substrate naphthol AS MX-PO4 (Sigma Aldridge Canada) and Fast Blue Salt (Sigma Aldridge Canada).
Data acquisition and evaluation
The number of bone nodules in a 5.3-cm2 area (55% of the total dish area) was determined from 5 replicate subculture dishes of each specimen from each horse when available. When 5 dishes were not available, owing to low numbers of primary osteogenic cells initially, as many dishes as possible containing 1 × 105 primary cells/35-mm dish were used. For 1 horse (10 mo old), viable primary osteogenic cells were not produced from 2 sites (radial and tibial metaphysis); thus, for that individual, those 2 sites were omitted from the analysis.
We compared bone-nodule numbers between all cortical and cancellous bone sites and between 6 individual skeletal sites using a mixed linear model with skeletal site as a fixed effect and horse and the horse-by-skeletal-site interaction as random effects. The analysis was carried out on the square-root-transformed data. Differences between sites were assessed by means of a protected least-significant-difference test (16). Estimates of experimental error and of within-horse and between-horse variability were obtained from the 3 random components in the mixed model: residual, horse-by-site interaction, and horse, respectively. All statistical tests were evaluated at the 5% level of significance, and the final results are presented as the back-transformed data.
To determine the proportion of variability associated with age, we added a regression variable to the mixed model and calculated the proportion of the between-horse variability that was accounted for by the age term.
Results
Cell isolation
Explant culture was a consistent method for isolating cells from cortical bone of immature, mature, and aged horses. All sites from all individuals consistently yielded primary outgrowth cells, which reached confluence between 14 and 21 d, depending on the individual animal. All cortical explant cultures for individual horses appeared to have similar outgrowth and proliferation rates based on time to confluence.
It was more difficult to get sufficient outgrowth of cells from cancellous bone. Cell migration typically occurred between 14 and 21 d; however, confluence was not always achieved during this period. Radial cancellous bone from 6 horses provided high outgrowth of cells and from a 2-y-old horse moderate outgrowth; there was no outgrowth from the explants of a 10-mo-old horse. Coccygeal cancellous bone provided high outgrowth of cells from all but a 2-y-old horse, whose explants provided moderate outgrowth. Tibial cancellous bone from 4 horses provided high outgrowth of cells and from 3 horses moderate outgrowth; there was no outgrowth from the explants of a 10-mo-old horse. Replicate subcultures were available for the bone-nodule assay for the horses with high or moderate outgrowth of primary cells from explant cultures. For the 10-mo-old horse who had no outgrowth cells from the radial and tibial cancellous bone explants, there were no subculture dishes available for the bone-nodule assay from these 2 skeletal sites.
Because of this variability in cell outgrowth and confluence of cell cultures, we concluded that cancellous bone explant cultures appear to have variable outgrowth and proliferation rates. The timing of subculture was determined by evaluating most culture dishes of all sites for 1 individual for confluence within the time frame of 14 to 21 d.
Additional dishes of primary cell cultures from cancellous bone explants were evaluated at points beyond 14 to 21 d for some individuals. This evaluation showed that after 14 to 21 d, when cell cultures were not confluent, additional time in culture did not result in confluence. Rather, cells would appear to lift and die. In a similar study looking at isolation of cells from cancellous bone of equine bone graft sites, we found an inconsistent ability to isolate cells with the explant method from several cancellous bone sites (13).
Cell differentiation
At subculture, viability of outgrowth cells from all specimens reached 100%. Osteogenic cells took on a polygonal shape and became grouped into sheets toward the periphery of the dishes and into discrete colonies toward the center of the dish 5 to 7 d after subculture. Clear areas of the culture dish were present between colonies. Cells did not become confluent before forming nodules. By day 7 to 14, cells piled up, forming colonies several cell layers thick (Figure 1, top). Cell colonies appeared to lay down a matrix, which then became mineralized with the addition of βGP to the medium, resulting in discrete 3-dimensional nodule formations throughout the dish. Nodules were spread across the dish by day 7 or 14 for individual horses. The time at which most dishes for all skeletal sites had nodules spread across the dish was chosen as the time for conducting the bone-nodule assay. Cell differentiation, indicated by the formation of colonies and nodules, appeared to be consistent between osteogenic cell populations of various skeletal sites within each horse.
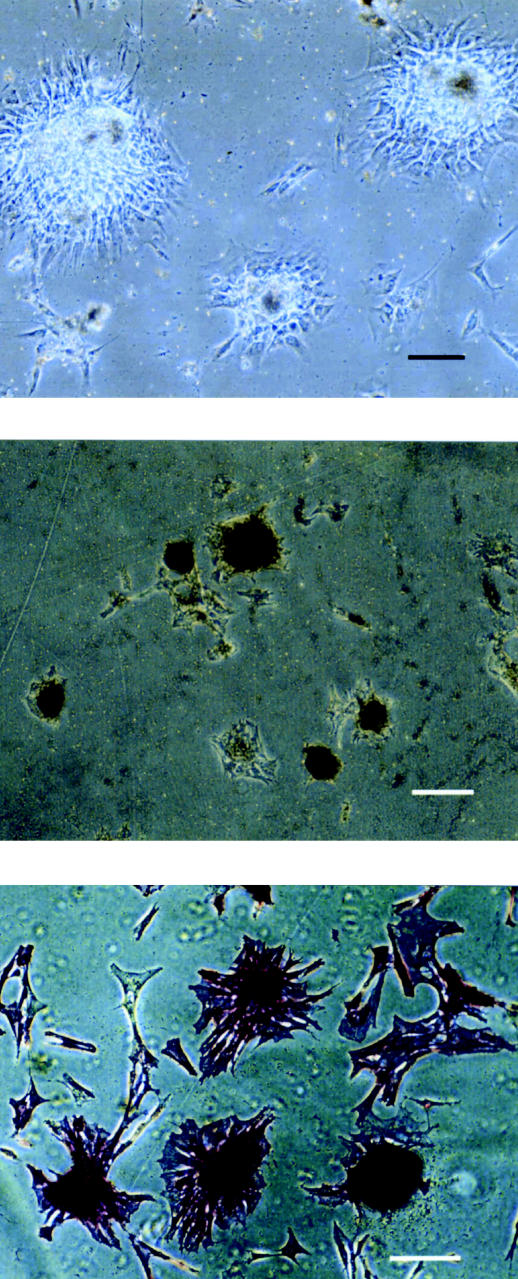
Figure 1.
Photomicrographs showing osteogenic cells forming a multilayered colony-forming unit (CFU) that produced a bone nodule (top), mineralized bone nodules staining positive (black) by the von Kossa method (middle), and osteoblastic cells staining positive (purple) for alkaline phosphatase (bottom). Bar — 100 μ m.
Osteoblast phenotype
Primary equine osteogenic cells differentiated into osteoblasts that formed CFUs and produced mineralized bone nodules that included matrix material (collagen and glycosaminoglycans) and the bone- specific protein osteocalcin. With phase-contrast microscopy, osteoblast characteristics were confirmed by the ability of bone-derived cells from all skeletal sites to form multilayered CFUs (Figure 1, top), which then produced bone nodules. The nodules became mineralized, as indicated by VK stain uptake (Figure 1, middle). Cells of the CFU stained intensely for alkaline phosphatase (Figure 1, bottom).
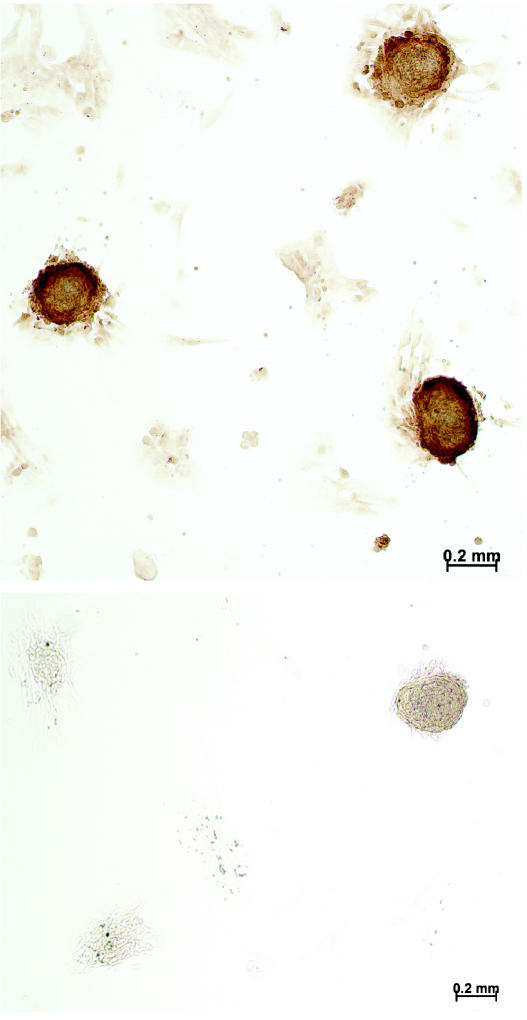
Histologic examination of cut sections of representative CFUs and bone nodules showed that the nodules were made up of cells surrounding a matrix that included collagen and glycosaminoglycans (not shown). Immunohistochemical testing of whole nodules showed the presence of osteocalcin within CFUs and bone nodules (Figure 2).
Figure 2.
Photomicrographs illustrating the results of immunohistochemical testing for osteocalcin: positive nodules (top) are intense brown, in contrast to the negative control (bottom). Bar — 200 μ m.
Bone-nodule assay
Of the 225 replicate plates, 4 plates each from different horses and sites were found to be outliers and were removed from the analysis. Table I presents the mean number of VK+ nodules and the 95% confidence limits for each skeletal site, and Figure 3 presents the least-square means for each skeletal site of each horse. Cortical bone had significantly higher numbers of nodules than cancellous bone (P = 0.0023). In comparisons of all sites individually, the tibial metaphysis and the 4th coccygeal vertebra were not significantly different and produced the fewest nodules, whereas the remaining 4 sites were not significantly different and produced the greatest number of nodules. Among the cortical bone sites there were no significant differences in nodule numbers (P = 0.4762). Among the appendicular skeletal sites, the tibial metaphysis was the only site with significantly lower numbers of nodules compared with other sites, and all P-values were less than 0.05.
Table I.
Mean number of nodules in cell cultures of bone samples from various skeletal sites in 8 horses
| Site | Mean (and 95% confidence limits) |
|---|---|
| Cortical bone | |
| Tibial diaphysis | 456a (255, 716) |
| Radial diaphysis | 450a (250, 708) |
| Third metacarpal diaphysis | 357ab (182, 591) |
| Cancellous bone | |
| Tibial metaphysis | 185c (65, 368) |
| Radial metaphysis | 382ab (195, 631) |
| Fourth coccygeal vertebra | 228bc (94, 420) |
Different superscripts indicate significant differences (P < 0.05)
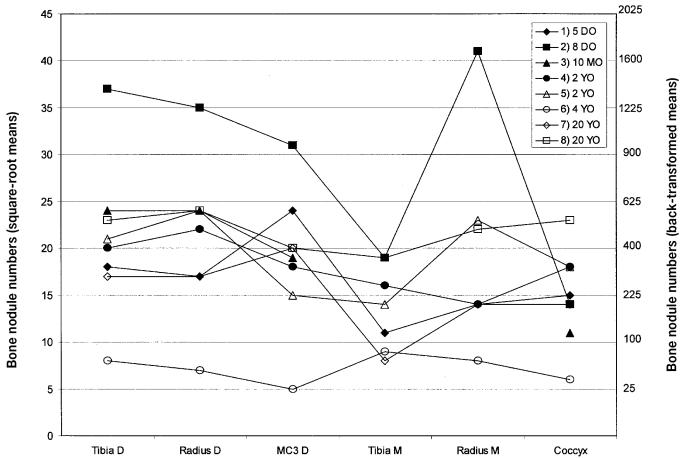
Figure 3.
Scatter plot of the mean number of bone nodules for each skeletal site for each of the 8 horses. D — diaphysis; MC3 — 3rd metacarpal; M — metaphysis; DO — days old; MO — months old; YO — years old.
Variability due to experimental error, within-horse variability, and between-horse variability were 4.5%, 33.1%, and 62.4%, respectively.
Discussion
We were primarily interested in determining if equine osteogenic cell populations isolated from various skeletal sites were heterogeneous enough to play a role in dissimilarities in fracture healing among sites. Since this was our first study isolating osteogenic cells from equine bone, we used basic methods. Differences in production of bone nodules were used in determining heterogeneity of osteogenic cell populations. The results indicate that some heterogeneity of equine osteogenic cell populations from various skeletal sites does exist.
Future characterization of the cell populations could include more sophisticated methods, such as normalizing the data to osteogenic markers. Further characterization of the differences among the populations may indicate whether dissimilarities in bone-nodule production are the result of the following: 1) osteogenic cell populations with mature osteoblasts that truly vary in their ability to produce bone nodules; 2) osteogenic cell populations with different ratios of cell types within the osteoblastic lineage, such that different numbers of bone nodules are produced; or 3) osteogenic cell populations with different ratios of osteogenic to fibroblastic or mesenchymal cell types, such that different numbers of bone nodules are produced. Further characterization of these osteogenic cell populations may also elucidate the role that heterogeneity of the populations has in the variability of equine-fracture healing.
Equine osteogenic cells were successfully isolated from bone specimens of 6 skeletal sites by the primary explant culture technique. This method was more consistent for cortical bone explants than for cancellous bone explants. Cancellous bone was quite variable in character between donor sites.
The most inconsistent explants were from a young horse (10 mo old) rather than from 1 of the 2 geriatric horses (20 y old). Differences in cell isolation and proliferation may be associated with differences in the ability of osteogenic cells to migrate and proliferate from bone explants or with variable contamination of cell cultures with adipocytes, fibroblasts, or cells of other lineages that somehow inhibit migration and proliferation of the cells in the explants. Conversely, the explants observed to have high outgrowth may have had high outgrowth of other cell types along with the osteogenic cells. Characterization of outgrowth cells in primary cultures before subculture would be required to attempt to differentiate the outgrowth cell lineages.
While collecting bone specimens from various skeletal sites, we noted obvious gross infiltration of cancellous bone from certain sites with red and yellow marrow components. Horses older than 8 d had abundant marrow fat associated with cancellous bone of the tibia and radius. Coccygeal cancellous bone had minimal to moderate fat infiltration. Cortical bone had no associated fat. There was also evidence of red marrow components infiltrating the trabeculae of radial and tibial cancellous bone in all individuals, with a higher amount present in neonates.
Cancellous bone specimens were washed extensively with PBS to clear the bone of fat and blood and to reduce contamination by cells from the bone marrow. This technique is used commonly with the explant method of cell culture (5,17–22). Some researchers believe that this is not sufficient to remove contaminating marrow cells and use collagenase treatment of bone explants, which is thought to provide a more pure population of osteogenic cells (23). However, Jonsson and colleagues (24) compared 3 common osteogenic cell culture methods and showed that there was no difference in the cells’ expression of the osteoblast phenotype when the cells were isolated from washed explants versus collagenase-treated explants. It was concluded that marrow stromal cells, remaining in the bone fragments despite extensive washing, rarely dominate the explant cultures.
Enzyme digestion, the other major technique for isolating bone-derived cells, was not used in this study because skeletal sites of interest included cortical bone sites, which would not effectively yield primary cells by the enzyme-digestion technique because of the extremely compact structure of cortical bone. Other concerns with the enzyme-digestion technique include its effect of decreasing cell proliferation (21).
It seems unlikely that extensive washing of the cancellous bone specimens to remove fat and blood would alter the numbers of cells that migrate out of the explants. Bone-lining cells, which are thought to be the cells that migrate from the trabecular surfaces, would not likely wash off with physiologic saline solution, especially since specimens treated with collagenase are still seen to provide outgrowth cells in explant cultures. However, it remains a possibility that differences in bone-nodule production could be the result of contamination of some osteogenic cell populations with fibroblasts or other cell lines present in the bone marrow that infiltrated the trabeculae of cancellous bone and were not removed by saline washing.
Although the cancellous bone explants appeared grossly clear of marrow components after washing, fat globules could be seen microscopically throughout the primary culture period in some tibial and radial cancellous culture dishes. The fat in the cultures may have contributed to inconsistency in primary cell isolation from tibial and radial cancellous bone explant cultures. In humans, it has been shown in vivo that the loss in cancellous bone that occurs with aging is associated with reduced osteoblastic bone formation and an increased volume of marrow adipose (25). An in vitro study using a coculture of adipocytes and trabecularbone-derived osteoblasts showed that mature adipocytes inhibited osteoblast proliferation (26). Further evaluation of the effect of marrow fat on equine cancellous bone cells would require identification and quantification of adipocytes in the cell cultures.
Osteogenic cells obtained from bone specimens are recognized as a heterogeneous population of cells of the osteoblastic lineage (25,26). Populations of osteogenic cells are expected to contain osteoprogenitors capable of differentiating into CFUs of osteoblasts that have the ability to deposit an organic matrix that can be mineralized (14). Osteoblasts in vitro are characterized by the ability to produce VK+ mineralized nodules in culture (12,27–29) and by high alkaline phosphatase activity (12,14,26,27,30) as well as production of osteocalcin, type 1 collagen, osteonectin, and response to parathyroid hormone (9). Although these characteristics are not all unique to the osteoblast, they represent major components of osteoblast expression and development. Currently, osteocalcin is considered the most specific osteoblast marker, and production of mineralized nodules is considered a reliable and unambiguous parameter for characterizing a cell type as osteoblastic (31,32).
In this study, equine osteoblastic cells from all skeletal sites were characterized by the production of VK+ mineralized nodules and the presence of alkaline phosphatase. Von Kossa stain consists of silver nitrate, which binds to calcium salts and stains mineralized bone nodules black (14). Alkaline phosphatase expression has been shown to be present in early osteoblasts, peak in mature osteoblasts, and possibly fade in late osteoblastic cells or osteocytes (33).
Immunohistochemical testing of representative bone nodules from cortical and cancellous bone revealed the presence of osteocalcin, confirming that osteogenic cells isolated from equine cortical and cancellous bone were of osteoblast lineage. Histochemical staining confirmed that the cells were forming a matrix rich in collagen and glycosaminoglycans; however, chemical staining of these matrix proteins is not specific to bone. Further immunohistochemical analysis with primary antibodies for collagen type I would be more specific for bone matrix.
The mean number of VK+ nodules produced by osteogenic cell populations was significantly different among cancellous but not cortical skeletal sites. These results indicate that there may be more variability in osteogenic cell populations between cancellous bone of various skeletal sites than cortical bone. Significant differences in bone-nodule numbers have also been seen in comparisons of other equine cancellous bone sites (13).
In the rat fetal calvarial system, it has been established, from limiting-dilution analysis and clonal assays, that 1 bone nodule represents a colony of osteoblasts developed from 1 osteoprogenitor (25). Therefore, in the case of the rat calvarial osteoblast system, the bone-nodule assay can be used to determine and compare numbers of osteoprogenitors in a population of osteogenic cells. Neither a limiting-dilution analysis nor a clonal assay has been conducted in the adult equine osteoblast cell culture system to confirm that 1 bone nodule arises from 1 osteoprogenitor. For this reason we conducted a bone-nodule assay rather than an osteoprogenitor assay. It is likely that each equine bone nodule also represents 1 osteoprogenitor, but this would have to be proven.
Three sources of variability were examined: donor variability (variability between horses), variability within a donor but between sites, and variability within sites within a donor. Most of the variability was due to differences between donors (62.4%) and between sites within donors (33.1%), only a small amount being due to experimental error (4.5%). Even in the presence of this variation, significant differences existed among skeletal sites.
Donor variability is a disadvantage of using primary nontransformed bone cells rather than transformed cell lines to study bone cells, and it makes comparisons more difficult. Conversely, a major advantage of using primary nontransformed cells is the ability to analyze the characteristics of lineage-restricted early osteoprogenitors located in various skeletal sites in vivo (11). In research using human bone or bone-marrow-derived cell populations, much variability between individuals has also been observed with respect to osteoblast characteristics (11,24,30,34). This variability has not been consistently correlated with donor age, sex, or isolation site.
In this study, age accounted for 36% of the between-horse variability. Although it is possible that this reflects a real result, it is also possible that this is due to the large gap in age between the younger horses (5 d to 4 y) and the geriatric horses (20 y). Further studies would be required to elucidate the relationship between age and the viability of harvested osteogenic cells.
Research with human-derived osteogenic cells has shown that donor age can contribute to variability in cell isolation (24), proliferation (3–5,21,24), and numbers of osteoprogenitors present (35), increasing donor age reducing isolation, proliferation, and numbers of osteoprogenitors. Research with rat-derived osteogenic cells has also shown that donor age can affect cell differentiation and bone formation, increasing age resulting in less cell differentiation and bone formation (36–38).
In conclusion, equine bone explants from horses of a wide age range can be used for isolation of osteoblasts. Isolation of osteoblasts and bone-nodule production was more reliable from cortical bone sites than from cancellous bone sites. This study had some limitations, which can be readily addressed in the future. The equine in vitro primary osteogenic cell cultures will be useful for further studies into variability between osteoblast populations and studies of equine bone healing.
Acknowledgments
This study was supported by the Animal Welfare Center, generous donations from the Sir James Dunn Foundation, and funding from the Canadian Institute of Health Research.
References
- 1.Denkovski P. Characterization of cell populations isolated from different skeletal sites of adult rats [MSc thesis]. Toronto, Ontario: University of Toronto, 1995:40–64,69–83.
- 2.Moskalewski S, Osiecka A, Malejczyx J. Comparison of bone formed intramuscularly after transplantation of scapular and calvarial osteoblasts. Bone. 1988;9:101–106. doi: 10.1016/8756-3282(88)90110-x. [DOI] [PubMed] [Google Scholar]
- 3.Pfeilschifter J, Diel I, Pilz U, et al. Mitogenic responsiveness of human bone cells in vitro to hormones and growth factors decreases with age. J Bone Miner Res. 1993;8:707–717. doi: 10.1002/jbmr.5650080609. [DOI] [PubMed] [Google Scholar]
- 4.Martinez ME, del Campo MT, Medina S, et al. Influence of skeletal site of origin and donor age on osteoblastic cell growth and differentiation. Calcif Tissue Int. 1999;64:280–286. doi: 10.1007/s002239900619. [DOI] [PubMed] [Google Scholar]
- 5.Martinez P, Esbrit P, Rodrigo A, et al. Age-related changes in parathyroid hormone-related protein and vascular endothelial growth factor in human osteoblastic cells. Osteoporos Int. 2002;13:874–881. doi: 10.1007/s001980200120. [DOI] [PubMed] [Google Scholar]
- 6.Bilezikian JP, Silverberg SJ, Shaane E, et al. Characterization and evaluation of asymptomatic primary hyperparathyroidism. J Bone Miner Res. 1991;6:S85–S89. doi: 10.1002/jbmr.5650061419. [DOI] [PubMed] [Google Scholar]
- 7.Davis JW, Ross PD, Wasnich RW. Evidence for both generalized and regional low bone mass among elderly women. J Bone Min Res. 1994;9:305–309. doi: 10.1002/jbmr.5650090303. [DOI] [PubMed] [Google Scholar]
- 8.Riggs BL, Melton LJ. The prevention and treatment of osteoporosis. N Engl J Med. 1992;327:620–627. doi: 10.1056/NEJM199208273270908. [DOI] [PubMed] [Google Scholar]
- 9.Aubin JE, Turksen K, Heersche JNM. Osteoblastic cell lineage. In: Noda M, ed. Cellular and Molecular Biology of Bone. San Diego, California: Academic Press, 1993:1–46.
- 10.Banks WJ, ed. Supportive tissues. In: Applied Veterinary Histology. Baltimore, Maryland: Williams and Wilkins, 1986:119–145.
- 11.Pei W, Yoshimine Y, Heersche JNM. Identification of dexamethasone-dependent osteoprogenitors in cell populations derived from adult human female bone. Calcif Tissue Int. 2003;72:124–134. doi: 10.1007/s00223-001-2052-4. [DOI] [PubMed] [Google Scholar]
- 12.Jia D, Heersche JNM. Insulin-like growth factor-1 and -2 stimulate osteoprogenitor proliferation and differentiation and adipocyte formation in cell populations derived from adult rat bone. Bone. 2000;27:785–794. doi: 10.1016/s8756-3282(00)00400-2. [DOI] [PubMed] [Google Scholar]
- 13.McDuffee LA, Anderson GI. In vitro comparison of equine cancellous bone graft donor sites and tibial periosteum as sources of viable osteogenic cells. Vet Surg. 2003;32:455–463. doi: 10.1053/jvet.2003.50060. [DOI] [PubMed] [Google Scholar]
- 14.Bhargava U, Bar-Lev M, Bellows CG, Aubin JE. Ultrastructural analysis of bone nodules formed in vitro by isolated rat calvaria cells. Bone. 1988;9:155–163. doi: 10.1016/8756-3282(88)90005-1. [DOI] [PubMed] [Google Scholar]
- 15.Burstone M. Histochemical observation on enzymatic processes in bone and teeth. Ann N Y Acad Sci. 1960;85:431–444. doi: 10.1111/j.1749-6632.1960.tb49972.x. [DOI] [PubMed] [Google Scholar]
- 16.Steel RGD, Torrie JH. Principles and Procedures of Statistics: a Biometric Approach. Toronto, Ontario: McGraw Hill, 1981: 176.
- 17.Beresford JN, Gallagher JA, Russel RGG. 25-dihydroxyvitamin D3 and human bone-derived cells in vitro: effects on alkaline phosphatase, type 1 collagen and proliferation. Endocrinology. 1986;119:1776–1785. doi: 10.1210/endo-119-4-1776. [DOI] [PubMed] [Google Scholar]
- 18.Maurin AC, Chavassieux PM, Frappart L, et al. Influence of mature adipocytes on osteoblast proliferation in human primary cocultures. Bone. 2000;26:485–489. doi: 10.1016/S8756-3282(00)00252-0. [DOI] [PubMed] [Google Scholar]
- 19.McAlinden MG, Wilson DJ. Comparison of cancellous bone-derived cell proliferation in autologous human and fetal bovine serum. Cell Transplant. 2000;9:445–451. doi: 10.1177/096368970000900401. [DOI] [PubMed] [Google Scholar]
- 20.Hankey DP, McCabe RE, Doherty MJ, et al. Enhancement of human osteoblast proliferation and phenotypic expression when cultured in human serum. Acta Orthop Scand. 2001;72:395–403. doi: 10.1080/000164701753542069. [DOI] [PubMed] [Google Scholar]
- 21.Voegele TJ, Voegele-Kadletz M, Esposito V, et al. The effect of different isolation techniques on human osteoblast-like cell growth. Anticancer Res. 2000;20:3575–3582. [PubMed] [Google Scholar]
- 22.Basso N, Mirkopoulos P, Heersche JNM. Osteoprogenitor viability in cell populations isolated from rat femora is not affected by 24 h storage at 4°C. Cryobiology. 2005;50:211–215. doi: 10.1016/j.cryobiol.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Robey PG, Termine JD. Human bone cells in vitro. Calcif Tissue Int. 1985;37:453–460. [PubMed] [Google Scholar]
- 24.Jonsson KB, Frost A, Nilsson O, et al. Three isolation techniques for primary culture of human osteoblast-like cells. Acta Orthop Scand. 1999;70:365–373. doi: 10.3109/17453679908997826. [DOI] [PubMed] [Google Scholar]
- 25.Bellows CG, Aubin JE. Determination of numbers of osteoprogenitors present in isolated fetal rat calvaria cells in vitro. Dev Biol. 1989;133:8–13. doi: 10.1016/0012-1606(89)90291-1. [DOI] [PubMed] [Google Scholar]
- 26.Robey PG. Collagenase-treated trabecular bone fragments: a reproducible source of cells in the osteoblastic lineage. Calcif Tissue Int. 1995;56:S11–S12. [Google Scholar]
- 27.Cowan CM, Quarto N, Warren SM, et al. Age-related changes in the biomolecular mechanisms of calvarial osteoblast biology affect fibroblast growth factor-2 signaling and osteogenesis. J Biol Chem. 2003;278:32005–32013. doi: 10.1074/jbc.M304698200. [DOI] [PubMed] [Google Scholar]
- 28.Aubin JE. Osteoprogenitor cell frequency in rat marrow stromal populations: role of heterotypic cell–cell interactions in osteoblast differentiation. J Cell Biochem. 1999;72:396–410. [PubMed] [Google Scholar]
- 29.Purpura KA, Aubin JE, Zandstra PW. Sustained in vitro expansion of bone progenitors is cell density dependent. Stem Cells. 2004;22:39–50. doi: 10.1634/stemcells.22-1-39. [DOI] [PubMed] [Google Scholar]
- 30.Walsh S, Jefferiss C, Stewart K. TGFβ1 limits the expansion of the osteoprogenitor fraction in cultures of human bone marrow stromal cells. Cell Tissue Res. 2003;311:187–198. doi: 10.1007/s00441-002-0679-8. [DOI] [PubMed] [Google Scholar]
- 31.Bellows CG, Aubin JE, Heersche JNM, Antosz ME. Mineralized bone nodules formed in vitro from enzymatically released rat calvarial cell populations. Calcif Tissue Int. 1986;38:143–154. doi: 10.1007/BF02556874. [DOI] [PubMed] [Google Scholar]
- 32.Liu F, Malaval L, Aubin JE. The mature osteoblast phenotype is characterized by extensive plasticity. Exp Cell Res. 1997;232:97–105. doi: 10.1006/excr.1997.3501. [DOI] [PubMed] [Google Scholar]
- 33.Aubin JE, Heersche JN, Merrilees MJ, Sodek J. Isolation of bone cell clones with differences in growth, hormone responses, and extracellular matrix production. J Cell Biol. 1982;92:452–461. doi: 10.1083/jcb.92.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasperk CH, Werdegal JE, Strong DD, et al. Human bone cell phenotypes differ depending on their skeletal site of origin. J Clin Endocrinol Metab. 1995;80:2511–2517. doi: 10.1210/jcem.80.8.7629252. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H, Lewis CG, Aronow MS, et al. The effects of patient age on human osteoblasts’ response to T1-6Al-4V implants in vitro. J Orthop Res. 2004;22:30–38. doi: 10.1016/S0736-0266(03)00155-4. [DOI] [PubMed] [Google Scholar]
- 36.Tsuji T, Hughes FJ, McCulloch CA, et al. Effects of donor age on osteogenic cells of rat bone marrow in vitro. Mech Ageing Dev. 1990;51:121–132. doi: 10.1016/0047-6374(90)90094-v. [DOI] [PubMed] [Google Scholar]
- 37.Fujieda M, Kiriu M, Mizouchi S, Hagiya K, Kaneki H, Ide H. Formation of mineralized bone nodules by rat calvarial osteoblasts decreases with donor age due to a reduction in signaling through EP1 subtype of prostaglandin E2 receptor. J Cell Biochem. 1999;75:215–225. doi: 10.1002/(sici)1097-4644(19991101)75:2<215::aid-jcb4>3.3.co;2-j. [DOI] [PubMed] [Google Scholar]
- 38.Declercq H, Van den Vreken N, De Maeyer E, et al. Isolation, proliferation, and differentiation of osteoblastic cells to study cell/biomaterial interactions: comparison of different isolation techniques and source. Biomaterials. 2004;25:757–768. doi: 10.1016/s0142-9612(03)00580-5. [DOI] [PubMed] [Google Scholar]