Abstract
ALS is a fatal paralytic disorder characterized by a progressive loss of spinal cord motor neurons. Herein, we show that NADPH oxidase, the main reactive oxygen species-producing enzyme during inflammation, is activated in spinal cords of ALS patients and in spinal cords in a genetic animal model of this disease. We demonstrate that inactivation of NADPH oxidase in ALS mice delays neurodegeneration and extends survival. We also show that NADPH oxidase-derived oxidant products damage proteins such as insulin-like growth factor 1 (IGF1) receptors, which are located on motor neurons. Our in vivo and in vitro data indicate that such an oxidative modification hinders the IGF1/Akt survival pathway in motor neurons. These findings suggest a non-cell-autonomous mechanism through which inflammation could hasten motor neuron death and contribute to the selective motor neuronal degeneration in ALS.
Keywords: Akt, ALS, microglia, oxidation, non-cell autonomous
ALS is the most common adult-onset paralytic disease and is characterized by a loss of motor neurons in the cerebral cortex, brainstem, and spinal cord (1). It is invariably fatal, usually within 3–5 years after onset (1). Insights into its neurodegenerative mechanisms followed the discovery that dominant mutations in the gene for superoxide dismutase 1 (SOD1) cause familial ALS (2, 3). Overexpression of SOD1 mutants in rodents emulate clinical and pathological hallmarks of ALS through a toxic gain of function (4). Development of chimeric mice provided animals with a mixture of neuronal and nonneuronal cells expressing wild-type or mutant SOD1 (5); investigation of these animals suggested that nonneuronal cells influence the fate of spinal cord motor neurons (5). Corroborating this hypothesis is the demonstration that reduction of mutant SOD1, selectively in microglia, extended survival in transgenic SOD1G37R mice (6). In light of the latter results, microglia are now thought to contribute to the non-cell-autonomous killing of motor neurons.
Among the microglia-derived mediators that could promote neurodegeneration are reactive oxygen species (ROS) produced by the enzyme NADPH oxidase complex (7). Although this ROS-generating multimeric oxidase is indispensable for protecting the host against invading microorganisms in infectious disorders (8), its inappropriate activation may be harmful in noninfectious neurodegenerative disorders. Such bystander cytotoxicity is thought to lead to the death of developing oligodendrocytes in periventricular leukomalacia (9), one of the most important causes of cerebral palsy. In light of these facts, we undertook the study of NADPH oxidase in both human ALS and one of its genetic models. Our results for both human and mouse postmortem tissues indicate that spinal cord microgliosis in ALS is accompanied with an up-regulation of NADPH oxidase. Furthermore, by using mutant deficient mice in functional NADPH oxidase as well as in neuron-like cell culture systems, we provide compelling evidence that supports the concept that this microglial ROS-generating enzymatic complex promotes spinal cord motor neuron degeneration by a mechanism involving oxidative damage to critical survival signaling pathways.
Results
NADPH Oxidase Is Up-Regulated in Inflamed Spinal Cords of ALS Mice.
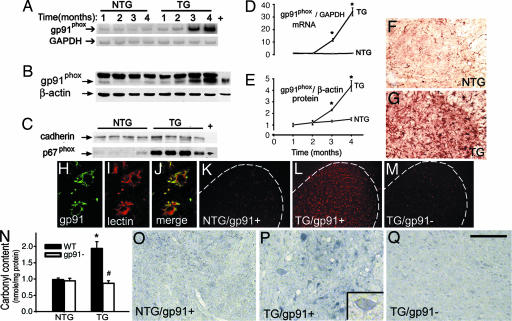
To determine the role of NADPH oxidase in motor neuron degeneration, we first evaluated its expression at different stages of the disease in transgenic mice expressing mutant human SOD1 with a substitution of glycine to alanine in position 93 (SOD1G93A), the most widely studied model of ALS. Expression of NADPH oxidase in the spinal cord, which carries the brunt of the pathology in this ALS model, was determined by analyzing its catalytic subunit, gp91phox. Both gp91phox message and protein contents in whole-tissue extracts of spinal cord rose over time in transgenic SOD1G93A mice (Fig. 1 A, B, D, and E) in concert with the development of a glial response (Fig. 6, which is published as supporting information on the PNAS web site) and the progression of the paralytic phenotype. The levels of the p67phox subunit that contains the NADPH-binding site of the NADPH oxidase complex (10) were increased in membrane fractions of spinal cord extracts from symptomatic transgenic SOD1G93A mice (Fig. 1C), indicating that this cytosolic subunit did translocate to the plasma membrane in these animals. Conversely, none of these NADPH oxidase alterations were seen in age-matched nontransgenic littermates (Fig. 1 A–E). Histological evaluation of the spinal cord of symptomatic transgenic SOD1G93A mice showed numerous gp91phox-positive cells, primarily in the gray matter of the anterior horn (Fig. 1G), whereas sparse staining was observed in the spinal cord of age-matched nontransgenic controls (Fig. 1F). Consistent with NADPH oxidase expression by professional phagocytes, confocal microscopy demonstrated the colocalization of the gp91phox subunit with a microglial marker, the ricinus communis agglutinin lectin (Fig. 1 H–J); no gp91phox colocalization was detected with the motor neuron marker, nonphosphorylated neurofilament heavy chain, or with the astrocyte marker, glial fibrillary acid protein (data not shown).
Fig. 1.
Microglial NADPH oxidase stimulates carbonylation of spinal cord motor neurons in transgenic SOD1G93A mice. (A–E) Spinal cord gp91phox mRNA (A and D) and protein (B and E) in 1-month-old (asymptomatic) to 4-month-old (end-stage) transgenic SOD1G93A (TG) and nontransgenic (NTG) mice. (C) Spinal cord p67phox protein in 4-month-old TG SOD1G93A and NTG littermates. +, positive control (mouse macrophage lysate). (F and G) Spinal cord gp91phox immunohistochemistry in NTG (F) and TG (G) mice. (H–J) Confocal analysis of a TG spinal cord showing gp91phox in green (H) and the microglial marker ricinus communis agglutinin lectin in red (I); merged image is shown in J. (K–M) Ethidium fluorescence (in red) in nontransgenic/gp91phox+ (NTG/gp91+) (K), transgenic SOD1G93A/gp91phox+ (TG/gp91+) (L), and transgenic SOD1G93A/gp91phox− (TG/gp91−) (M) spinal cords. (N) HPLC quantification of protein carbonyls in spinal cords from all four genotypes. (O–Q) Immunohistochemistry for carbonyl adducts in NTG/gp91+ (O), TG/gp91+ (P), and TG/gp91− (Q) spinal cords by using an anti-dinitrophenylhydrazine antibody. (P Inset) Carbonylated motor neuron. Studies in F–Q are in 4-month-old mice. Values (means ± SEM; n = 4–8 mice per group) were compared by ANOVA followed by Newman–Keuls post hoc testing. ∗, P < 0.05, more than NTG mice; #, P < 0.05, lower than TG/gp91+ mice. (Scale bar in Q: F and G, 0.4 mm; H–J, 40 μm; K–M, 0.5 mm; O–Q, 0.15 mm.)
NADPH Oxidase Causes Protein Oxidation in Transgenic SOD1G93A Mice.
We further characterized the status of spinal cord NADPH oxidase in transgenic SOD1G93A mice by probing for formation of ROS and evidence of protein oxidation. In nontransgenic mice, spinal cord production of ROS, evidenced by the fluorescence emitted by ethidium, the oxidation product of hydroethidine (11), was minimal (Fig. 1K). In contrast, in symptomatic transgenic SOD1G93A mice carrying the wild-type gp91phox allele (SODG93A/gp91phox+), spinal cord ethidium fluorescence was intense (Fig. 1L) and coincided anatomically with the areas of gp91phox expression (Fig. 1G) and microglial activation (Fig. 6). In symptomatic transgenic SOD1G93A mice carrying the nonfunctional mutant allele (SODG93A/gp91phox−) (12), spinal cord ethidium fluorescence was negligible (Fig. 1M), as it was in the nontransgenic controls (Fig. 1K). A similar genotypic difference was found for the analysis of protein carbonylation (13), which is used as a general marker of protein oxidation (14). Symptomatic transgenic SOD1G93A/gp91phox+ mice, but not age-matched SOD1G93A/gp91phox− mice, had increased levels of spinal cord protein carbonyl adducts compared with nontransgenic controls expressing either wild-type or null mutant gp91phox (Fig. 1N). Immunohistochemically, the most robust labeling for protein carbonyl adducts occurred in spinal cord sections from SOD1G93A/gp91phox+ mice at the level of cells with mixed morphology, including large motor neurons (Fig. 1 O–Q).
NADPH Oxidase Induction and Neuronal Protein Carbonylation in Sporadic ALS.
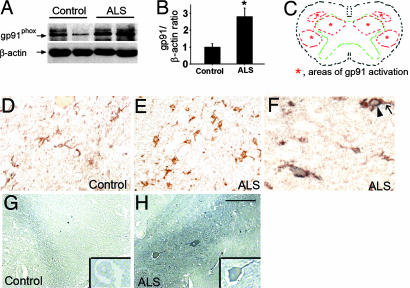
We then sought to determine whether the NADPH oxidase alterations identified in transgenic SOD1G93A mice were also present in human sporadic ALS, the most common form of the disease (1). Consistent with the mouse data, gp91phox content was low (Fig. 2 A and B) and its immunoreactivity was faint in control postmortem spinal cords (Fig. 2D), whereas gp91phox content was ≈3-fold higher and its immunoreactivity robust in sporadic ALS spinal cords (Fig. 2E). In the latter, gp91phox-positive cells colocalized with the microglial-associated antigen CD68 (Fig. 2F) and were identified in all of the typical ALS loci of neurodegeneration, including the anterior horn and the lateral corticospinal track (Fig. 2C). Also comparable with the mouse data, there was a robust labeling for protein carbonyl adducts in postmortem spinal cord sections from sporadic ALS cases, which seemed to be mainly associated with large motor neurons (Fig. 2H). On average, 6–12 of 30 counted motor neurons were positive for protein carbonyl adducts per lumbar spinal cord section in ALS patients, whereas no such immunoreactive motor neurons were seen in controls (Fig. 2G).
Fig. 2.
NADPH oxidase is up-regulated and associated with motor neuron carbonylation in the spinal cord of sporadic ALS patients. (A and B) Immunoblots and bar graph for gp91phox using spinal cord extracts from six normal controls and six age-matched ALS patients. Values in B are means ± SEM and were compared by Student's t test. ∗, P < 0.05, higher than normal controls. (C) Drawing of a spinal cord transversal plan showing the gray matter (area delineated by the green dashed line) and the loci of degeneration in ALS (areas delineated by the red dashed line). (D and E) Spinal cord gp91phox immunohistochemistry in tissue sections from controls (D) and ALS patients (E). (F) In ALS patient samples, gp91phox-positive cells (arrow) exhibit a brown membrane labeling, which colocalizes with the blue-gray cytosol labeling for the microglial marker CD68 (arrowhead). (G and H) Immunohistochemical detection of carbonyl adducts in spinal cord sections of normal controls and age-matched ALS patients obtained by using the same technique as in Fig. 1 O–Q. (Scale bar in H: D, E, G, and H, 0.2 mm; F, 0.5 mm.)
Deletion of gp91phox Mitigates the Disease Phenotype in Transgenic SOD1G93A Mice.
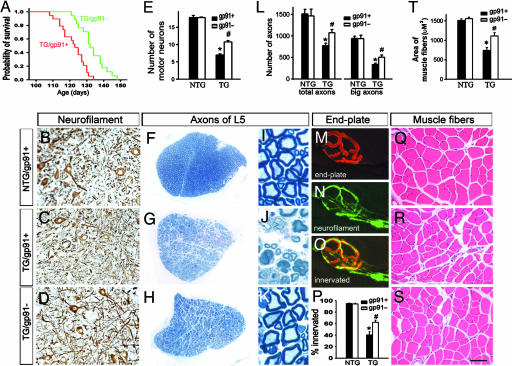
Next, we explored the contribution of NADPH oxidase activation on the disease phenotype in the SOD1G93A mouse model of ALS. Transgenic SOD1G93A/gp91phox− mice reached end-stage paralysis (defined as a loss of the righting reflex) later than their transgenic SOD1G93A/gp91phox+ counterparts (Fig. 3A), which resulted in a longer lifespan of transgenic SOD1G93A/gp91phox− mice (log-rank test = 15.3; P < 0.001). In addition to the prolonged survival, inactivation of NADPH oxidase did mitigate neurodegeneration. Compared with end-stage transgenic SOD1G93A/gp91phox+ mice, age-matched transgenic SOD1G93A/gp91phox− mice had ≈50% more anterior horn motor neurons in the spinal cord (Fig. 3 B–E) and myelinated axons in the fifth lumbar anterior roots (Fig. 3 F–L) as well as significantly more innervated endplates (Fig. 3 M–P) and larger muscle fibers in the fibularis and peroneus longus muscles (Fig. 3 Q–T). Spinal cord microgliosis, evidenced by macrophage antigen complex 1 immunostaining, and levels of the glial cytokine IL-1β did not differ between age-matched transgenic SOD1G93A/gp91phox+ mice and SOD1G93A/gp91phox− mice (Fig. 7, which is published as supporting information on the PNAS web site). Also not affected by the deficit of gp91phox were the levels of human SOD1 in transgenic SOD1G93A mice (Fig. 7) or the size of muscle fibers in the fibularis and peroneus longus muscles in nontransgenic mice (Fig. 3T). The selected muscles are mainly composed of fast-twitch fibers, and by immunostaining for myosin heavy chain, we did not observe any obvious alteration in the makeup of muscle fiber types among the different mouse groups (Fig. 7).
Fig. 3.
Deletion of gp91phox increases lifespan and lessens neurodegeneration in transgenic SOD1G93A mice. (A) Survival comparison of transgenic SOD1G93A/gp91phox+ mice (red) (122.0 ± 1.7 days; n = 19) and transgenic SOD1G93A/gp91phox− littermates (green) (135.2 ± 1.9 days; n = 17) (log-rank test = 15.3; P < 0.001). (B–D) Immunohistochemistry for nonphosphorylated heavy-chain neurofilament in the spinal cord. (E) Quantification of large (≈25 μm) motor neurons per 14-μm-thick section from the fourth to fifth lumbar spinal cord segments. (F–K) Toluidine blue-stained L5 anterior root sections. (L) Quantification of myelinated axons in fifth-lumbar (L5) anterior roots. (M–P) Immunolabeling of the muscular acetylcholine receptor by an anti-α-bungarotoxin antibody (red) (M) and of the nerve terminals by an anti-heavy-chain neurofilament (green) (N) in the fibularis and peroneus longus muscles; the merged image (O) shows a normally innervated end plate. (P) Quantification of innervated end plates. (Q–S) H&E-stained fibularis and peroneus longus muscle sections. (T) Quantification of muscle fiber size. See the legend of Fig. 1 for abbreviations. Except for the survival analysis, mice were all 115 days old. In quantifications, values (means ± SEM; n = 4–6 mice per group) were compared by using ANOVA followed by Newman–Keuls post hoc testing. ∗, P < 0.05, lower than nontransgenic controls; #, P < 0.05, higher than transgenic SOD1G93A/gp91phox+ mice. (Scale bar in S: B–D and F–H, 80 μm; I–K, 8 μm; M–O, 15 μm; Q–S, 30 μm.)
NADPH Oxidase Impairs the Insulin-Like Growth Factor 1 (IGF1)/Akt Pathway in Transgenic SOD1G93A Mice.
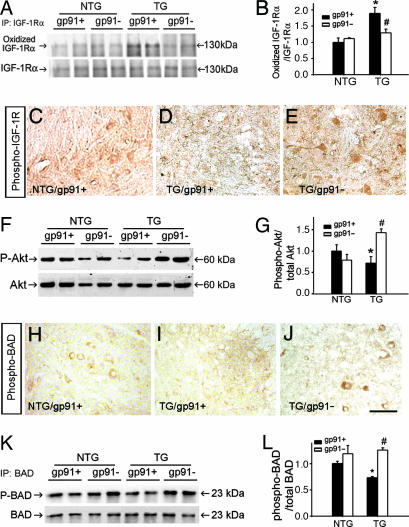
We then explored whether NADPH oxidase-mediated protein modifications might promote neurodegeneration in ALS by damaging essential surviving pathways for motor neurons such as IGF1. After IGF1 was immunoprecipitated from spinal cord extracts, it was probed for evidence of carbonylation. This approach failed to reveal evidence of IGF1 oxidation in any of the studied mouse genotypes (data not shown). However, protein carbonyl adducts were evident in the α-chain of the IGF1 tyrosine kinase cognate receptor in the spinal cord of symptomatic transgenic SOD1G93A/gp91phox+ mice (Fig. 4 A and B); similar results were obtained for the β-chain of IGF1 receptor (data not shown). This finding might be quite significant because IGF1 receptors in mouse spinal cords were detected almost exclusively on motor neurons (Fig. 8, which is published as supporting information on the PNAS web site). Contrasting with the IGF1 receptor findings, oxidation indices in the intracellular serine/threonine kinase Akt, which transduces IGF1 receptor signaling (15), did not differ between symptomatic transgenic SOD1G93A mice and their nontransgenic littermates. These results suggest that the entire IGF1 molecular pathway is not oxidatively modified by inflammation in this ALS model.
Fig. 4.
Modulation of the IGF1/Akt pathway by NADPH oxidase-derived ROS. (A) Immunoprecipitation of IGF1 receptor α-chain followed by OxyBlot (upper blot) and immunoblot for spinal cord IGF1 receptor α-chain (lower blot). (B) Bar graph showing carbonylated/total α-chain ratios in the four genotypes shown in A. (C–E) Immunostaining for spinal cord phospho-IGF1 receptor in the different mouse groups. (F) Spinal cord phospho-Akt (P-Akt) (upper blot) and total Akt (lower blot) immunoblots. (G) Bar graph showing P-Akt/total Akt ratios in the four genotypes in F. (H–J) Immunostaining for spinal cord phospho-BAD in the different mouse groups. (K) Immunoprecipitation of spinal cord BAD followed by immunoblot for phospho-BAD (P-BAD) (upper blot) and total BAD (lower blot). (L) Bar graph showing P-BAD/total BAD ratios in the four genotypes in K. The mice were all 115 days old. In the quantifications, values (means ± SEM; n = 4–8 mice per group) were compared by ANOVA followed by Newman–Keuls post hoc testing. ∗, P < 0.05, different from nontransgenic controls; #, P < 0.05, different from transgenic SOD1G93A/gp91phox+ mice. (Scale bar in J: C–E and H–J, 0.2 mm.)
Next, we compared selected IGF1 transduction events among the different mouse groups. Although mutant SOD1 is expressed in all cells, markers of IGF1 transduction such as phospho-IGF1 receptor, phospho-Akt (data not shown), and phospho-BAD were identified by immunocytochemistry mainly in large cells with a motor neuron-like appearance and to a much lesser extent in smaller glia-like cells in spinal cord sections of symptomatic transgenic SOD1G93A mice. However, there were fewer phospho-IGF1 receptor-immunoreactive cells in spinal cord sections from symptomatic transgenic SOD1G93A/gp91phox+ mice than from age-matched SOD1G93A/gp91phox− mice (Fig. 4 C–E). There were also smaller phospho-Akt:total Akt ratios (Fig. 4 F and G) as well as fewer cells that were immunoreactive for a downstream target of Akt, phospho-BAD (Fig. 4 H–J), and smaller phospho-BAD:total BAD ratios (Fig. 4 K and L) in symptomatic transgenic SOD1G93A/gp91phox+ mice compared with their age-matched SOD1G93A/gp91phox− counterparts. These data further support the idea that oxidative modification of IGF1 receptor in symptomatic transgenic SOD1G93A/gp91phox+ mice is associated with a range of molecular perturbations.
Microglial-Derived ROS Recapitulate the IGF1/Akt Pathway Defect in Vitro.
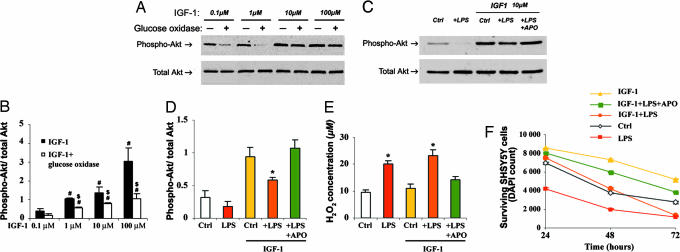
To test the idea that NADPH oxidase-derived ROS could impair IGF1 pathway function, an in vitro cell system using the neuron-like cell lines SH-SY5Y and IMR32 was used. First, cells were briefly incubated with 0.1–100 μM human recombinant IGF1 in the presence of overnight-preconditioned serum-free medium supplemented with or without 0.1 milliunits/ml glucose oxidase to provide a constant flux of H2O2; IGF1 pathway responsiveness was monitored by Akt phosphorylation. Exposure to IGF1 caused a dose-dependent phosphorylation of Akt in SH-SY5Y cells (Fig. 5 A and B) and IMR32 cells (data not shown) in the presence of conditioned medium lacking glucose oxidase. Conversely, IGF1 barely increased Akt phosphorylation in the neuroblastoma cell lines that were exposed to conditioned medium containing 0.1 milliunits/ml glucose oxidase, generating an average stable concentration of 75 μM H2O2 (Fig. 5E); this effect was abolished by adding 1,000 units/ml catalase to the medium (data not shown). Next, SH-SY5Y cells were incubated with or without conditioned medium from LPS-activated BV2 microglial cells. Akin to the glucose oxidase experiments, brief exposure to LPS-activated microglial-conditioned medium, which contained increased levels of H2O2 (Fig. 5E), attenuated IGF1-mediated Akt phosphorylation in the neuroblastoma cell line (Fig. 5 C and D). Upon longer exposure to LPS-activated microglial-conditioned medium, the Akt phosphorylation response to the IGF1 recombinant remained depressed, and, at 72 h, a reduction of cell viability, indistinguishable from the condition without IGF1, was observed (Fig. 5F). However, both the alteration of IGF1-mediated Akt phosphorylation and the loss of cell viability mediated by LPS-activated microglia were counteracted by the specific NADPH oxidase inhibitor apocynin (Fig. 5 C, D, and F).
Fig. 5.
Glucose oxidase- and microglial-derived ROS impair the IGF1 Akt pathway in vitro. (A) Phospho-Akt (upper blot) and total Akt (lower blot) immunoblots of IGF1-treated SH-SY5Y cells exposed or not exposed to 75 μM glucose oxidase-generated H2O2. (B) Bar graph showing phospho-Akt/total Akt ratios for the different IGF1 doses. #, P < 0.05, higher than 0.1 μM IGF1; $, P < 0.05, lower than the same IGF1 concentration without glucose oxidase. (C) Phospho-Akt (upper blot) and total Akt (lower blot) immunoblots of SH-SY5Y cells exposed to various BV2 serum-free conditioned media supplemented or not supplemented with 10 μM IGF1. (D) Bar graph showing phospho-Akt/total Akt ratios. ∗, P < 0.05, lower than control and +LPS +APO in the IGF1-treated group. (E) Bar graph showing the H2O2 medium concentrations for the different conditions. ∗, P < 0.05, higher H2O2 concentration than controls and +LPS +APO. (F) SH-SY5Y survival kinetic over 72-h exposure to various BV2 serum-free conditioned media supplemented or not supplemented with 10 μM IGF1. APO, apocynin. Cell survival was assessed by counting DAPI-labeled normal nuclei and confirmed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay (data not shown). Data are averages of three or more independent experiments and were compared by ANOVA or repeated-measures ANOVA for the survival kinetic, followed by Newman–Keuls post hoc testing. For the survival kinetic, only LPS and IGF1 + LPS within the 72-h time point are not significantly different from each other (P = 0.386).
Discussion
Experimental evidence supports a model for ALS neurodegeneration in which nonneuronal cells such as microglia contribute to the demise of motor neurons (6, 16, 17). Germane to the molecular basis of this deleterious effect on motor neurons is our finding that virtually all spinal cord microglial cells express the gp91phox subunit of the oxidant-producing enzyme NADPH oxidase (Fig. 1). Agreeing with the fact that in nonactivated phagocytes NADPH oxidase is quiescent (7), gp91phox-positive cells in spinal cords from 1- to 4-month-old nontransgenic mice had a morphology of resting microglia and did not seem to produce ROS (Figs. 1 and 6). Conversely, in transgenic SOD1G93A mice, paralleling the worsening of the ALS phenotype there was an intensification of spinal cord microgliosis accompanied by an up-regulation and activation of NADPH oxidase. These results suggest that over the course of the disease, spinal cord microglial cells become activated and acquire the capacity of oxidatively damaging nearby macromolecules and cells homed within inflamed ALS tissues. Corroborating this view are the levels of protein carbonyls, which were markedly elevated in spinal cord extracts of symptomatic transgenic SOD1G93A mice, for the most part in a NADPH oxidase-dependent manner (Fig. 1). Evidence of microgliosis, NADPH oxidase up-regulation, and protein carbonylation was also found in postmortem spinal cords from human sporadic ALS cases (Fig. 2), supporting the conclusion that the occurrence of inflammation-mediated oxidative damage is not restricted to familial ALS caused by SOD1 mutations but is also a pathological hallmark of the prevalent nonfamilial, sporadic form of ALS.
Our results also show that abrogation of the gp91phox subunit of NADPH oxidase in transgenic SOD1G93A mice eliminates the production of microglial-derived ROS (Fig. 1M) and, importantly, prolongs survival and retards neurodegeneration in this ALS model (Fig. 3). Deletion of gp91phox in transgenic SOD1G93A mice did not alter the spinal cord microglial response or the expression of human SOD1 in transgenic SOD1G93A mice (Fig. 7), which is a known determinant of disease severity in this ALS model (18). Consequently, the attenuated phenotype seen in transgenic SOD1G93A/gp91phox− mice is attributable to the lack of NADPH oxidase activity and not to either an impaired microglial response or expression of the human SOD1G93A transgene. These data provide compelling evidence that NADPH oxidase contributes to the degeneration of motor neurons in ALS. They also underscore the significance of inflammatory-mediated oxidative stress in the pathogenesis of chronic, noninfectious pathological conditions such as ALS. However, the magnitude of benefit afforded by gp91phox deletion in transgenic SOD1G93A mice argues that targeting neuroinflammation by inhibiting just one of its mediators, such as NADPH oxidase, may not be sufficient to produce robust and lasting neuroprotection in ALS patients.
All cells, not motor neurons only, which are located in the vicinity of activated microglia, may indiscriminately have their plasma membrane proteins and lipids damaged by NADPH oxidase-derived ROS. However, the chronic nature of ALS suggests that neuroinflammation is likely protracted and not as strong as in acute encephalitis. Accordingly, two hypotheses that are not mutually exclusive can be formulated to reconcile the expected nonselective nature of the oxidative stress with the selective demise of motor neurons in ALS. First, at that lower level of ROS production, oxidative stress may not kill cells but instead may accelerate the demise of those already compromised, as motor neurons probably are in ALS. Second, in this paralytic disease, microglial-derived oxidants may preferentially worsen motor neuron degeneration by damaging proteins that are specifically important for their survival. Relevant to the latter scenario are our results for IGF1, a trophic factor that is known to promote motor neuron survival (19–21).
In this study, we indeed found that receptors for IGF1 were primarily expressed on motor neurons in mouse spinal cords (Fig. 8) and that the IGF1 signaling pathway was impaired by a NADPH oxidase-dependent mechanism in symptomatic transgenic SOD1G93A mice (Fig. 4). Although IGF1 per se did not seem to be damaged by inflammation, NADPH oxidase did stimulate the oxidative modification of IGF1 receptors (Fig. 4). The ligand-dependent kinase activation of IGF1 receptor relies on its arrangement into a heterotetrameric 2α/2β-chain complex (22). It may thus be predicted that oxidation of the IGF1 receptor main extracellular domains (i.e., the α-chains) could distort its proper assembly and transduction activity. Our in vivo and in vitro results (Figs. 4 and 5), in aggregate, show that several molecular events that are normally elicited by ligation of the IGF1 receptor, including autophosphorylation and Akt phosphorylation, were indeed abated by ROS in a microglial NADPH oxidase-dependent manner.
Our data also show that microglial NADPH oxidase, by impairing the IGF1 signaling pathway, renders SH-SY5Y cells in our in vitro system more prone to die upon exposure to a hostile environment such as that emulated by LPS-activated microglial-conditioned medium (Fig. 5). These results thus suggest that reduction in vital surviving pathways may be one mechanism by which inflammation attenuates the capability of motor neurons to withstand the toxicity of etiologic agents such as mutant SOD1. Muscle-specific expression of IGF1 stabilizes neuromuscular junctions, reduces inflammation in the spinal cord, and enhances motor neuronal survival in transgenic SOD1G93A mice (23). In our study, however, we did not find any evidence that the rescue of the IGF1 pathway by abrogating NADPH oxidase was associated with muscle hypertrophy (Fig. 3). Nevertheless, whether transgenic SOD1G93A mice carrying the gp91phox null mutation reach end-stage paralysis later and exhibit an attenuated neurodegenerative process because of some effects at the skeletal muscle level is an interesting possibility that cannot be excluded.
Injection of transgenic SOD1G93A mice with an adeno-associated virus carrying an IGF1 gene prolongs survival in these animals (20, 24). Together with our data, these previous studies suggest that inflammation may not abrogate, but instead may blunt, the motor neuron survival response to IGF1 in ALS. Perhaps the modest change in ALS progression that is seen in patients treated with human recombinant IGF1 (25) may be related to the issue raised above. It may thus be argued that optimal therapeutic response to IGF1 in diseases such as ALS may rely on a concomitant administration of this trophic factor and an antiinflammatory agent.
Materials and Methods
Animals.
Transgenic SOD1G93A mice [C57BL/6J-TgN(SOD1-G93A)1Gurdl] were crossed with gp91phox-deficient mice (B6.129S6-Cybbtm1din). Both lines were obtained from The Jackson Laboratory (Bar Harbor, ME) and maintained in the C57BL/6J strain. Progeny were genotyped by PCR as described in refs. 26 and 27. Refer to Supporting Text, which is published as supporting information on the PNAS web site, for details about the timeline of behavioral abnormalities in transgenic SOD1G93A mice.
RNA Extraction and RT-PCR.
Total RNA was extracted as described in ref. 28. Primer sequences for gp91phox, glial fibrillary acidic protein, macrophage antigen complex 1, and GAPDH and PCR conditions are presented in Supporting Text. Products were quantified with a phosphorimager (Bio-Rad, Hercules, CA).
Immunohistochemistry and Histological Methods.
Mouse spinal cords were fixed and processed for immunostaining as described in ref. 29. All antibodies used in this study are listed in Supporting Text. For quantification of motor neurons, fixed spinal cord slices were immunostained with SMI-32 monoclonal antibody directed against the nonphosphorylated neurofilament heavy chain (Sternberger Monoclonals, Lutherville, MD). SMI-32-positive large (≈25 μm in diameter) motor neurons were counted as described in ref. 29 by using 14-μm-thick sections. Preparation and processing of L5 anterior roots and fibularis and peroneus longus muscles were also performed as described in ref. 29 with only minor modifications. Paraffin-embedded muscle sections were stained with H&E, and fiber cross-sectional areas were determined by averaging the area of 100 fibers; it was assumed that fibularis and peroneus longus muscle fibers are cylindrical.
Immunoprecipitation and Western Blots.
Total and plasma membrane proteins were prepared as described in ref. 27. All antibodies used are listed in Supporting Text. Bound primary antibody was detected by using HRP-conjugated secondary antibodies against IgG and a chemiluminescent substrate (SuperSignal Ultra; Pierce, Rockford, IL). Films were quantified by using the NIH Image analysis system.
In Situ Visualization of ROS.
In situ visualization of superoxide radicals and derived oxidants was assessed on 14-μm-thick spinal cord sections by hydroethidine histochemistry, following a method described in ref. 11.
Measurement of Protein Oxidation.
Protein carbonyls were detected after derivatization of spinal cord homogenates with 2,4-dinitrophenylhydrazine by using a modification of a method described in ref. 13.
OxyBlot on Immunoprecipitated Proteins.
Supernatants of mouse spinal cord tissue homogenized in Nonidet P-40 buffer containing 50 mM DTT were collected and incubated (at 4°C for 1 h) with rabbit anti-IGF1 receptor α (Santa Cruz Biotechnology, Santa Cruz, CA) or rabbit anti-Akt (Cell Signaling Technology, Danvers, MA). Immunoprecipitation was performed as described above, followed by an immunoblot detection of carbonyl groups introduced into proteins by oxidative reaction using the OxyBlot protein oxidation kit (Chemicon, Temecula, CA) as specified by the manufacturer. Refer to Supporting Text for more technical details. For immunohistochemistry, tissue sections were incubated with 3 μM 2,4-dinitrophenylhydrazine (DNPH) in 3% trifluoroacetic acid for 15 min at room temperature to convert carbonyl to hydrazone derivatives. Sections were then incubated with 1:500 rabbit anti-DNPH antibody and finally visualized with VECTOR SG (blue-gray).
IL-1β Measurement.
Spinal cord content of mouse IL-1β was determined as specified by the manufacturer by using an ELISA kit that is specific for this cytokine (R & D Systems, Minneapolis, MN).
Human Samples.
Age at death and interval from death to tissue processing were as follows: control group (n = 6), 60.5 ± 10.2 years and 8.0 ± 2.6 h, respectively; ALS group, (n = 6), 60.5 ± 4.2 years and 7.7 ± 1.4 h, respectively. For the ALS patients, the mean duration of disease was 19.3 ± 2.6 months. All human samples (anonymized) were kindly provided by A. Hays (Columbia University Medical Center).
In Vitro Experiments.
SH-SY5Y and IMR32 neuroblastoma cell lines were purchased from the American Type Culture Collection (Manassas, VA). The BV2 microglial cell line was kindly provided by Tong H. Joh (Burke Medical Research Institute, New York, NY). All cell culture products were obtained from Gibco/Invitrogen (Carlsbad, CA). Phosphorylation of Akt and cell viability in response to IGF1 recombinant and to H2O2 or activated BV2 cells were assessed at selected time points as described in Supporting Text.
Statistical Analysis.
Values are means ± SEM. Differences between means were tested by Student's t test, and differences among means were tested by ANOVA followed by Newman–Keuls post hoc testing. Survival statistics were obtained by Kaplan–Meyer analysis. The null hypothesis was rejected at the 0.05 level.
Supplementary Material
Acknowledgments
We thank Ms. Julia Jeon for assistance in preparing the manuscript and Drs. Lewis P. Rowland and Irwin Fridovich for editorial comments. This work was supported by the Muscular Dystrophy Association/Wings over Wall Street; National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke Grants NS42269, NS38370, and NS11766; NIH Grant AG 021617; National Institute of Environmental Health Sciences (NIEHS) Grant ES013177; U.S. Department of Defense Grant DAMD 17-03-1; the Parkinson's Disease Foundation Grant CU51523606; and a Gardner's Fellowship from the Muscular Dystrophy Association (to M.N.).
Abbreviations
- ROS
reactive oxygen species
- SOD1
superoxide dismutase 1
- IGF1
insulin-like growth factor 1
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Rowland L. P., Shneider N. A. N. Engl. J. Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- 2.Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J. P., Deng H.-X., et al. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 3.Deng H.-X., Hentati A., Tainer J. A., Iqbal Z., Cayabyab A., Hung W.-Y., Getzoff E. D., Hu P., Herzfeldt B., Roos R. P., et al. Science. 1993;261:1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- 4.Julien J. P. Cell. 2001;104:581–591. doi: 10.1016/s0092-8674(01)00244-6. [DOI] [PubMed] [Google Scholar]
- 5.Clement A. M., Nguyen M. D., Roberts E. A., Garcia M. L., Boillee S., Rule M., McMahon A. P., Doucette W., Siwek D., Ferrante R. J., et al. Science. 2003;302:113–117. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- 6.Boillee S., Yamanaka K., Lobsiger C. S., Copeland N. G., Jenkins N. A., Kassiotis G., Kollias G., Cleveland D. W. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 7.Babior B. M. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 8.Heyworth P. G., Cross A. R., Curnutte J. T. Curr. Opin. Immunol. 2003;15:578–584. doi: 10.1016/s0952-7915(03)00109-2. [DOI] [PubMed] [Google Scholar]
- 9.Li J., Baud O., Vartanian T., Volpe J. J., Rosenberg P. A. Proc. Natl. Acad. Sci. USA. 2005;102:9936–9941. doi: 10.1073/pnas.0502552102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith R. M., Connor J. A., Chen L. M., Babior B. M. J. Clin. Invest. 1996;98:977–983. doi: 10.1172/JCI118882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bindokas V. P., Jordán J., Lee C. C., Miller R. J. J. Neurosci. 1996;16:1324–1336. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollock J. D., Williams D. A., Gifford M. A., Li L. L., Du X., Fisherman J., Orkin S. H., Doerschuk C. M., Dinauer M. C. Nat. Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 13.Levine R. L., Garland D., Oliver C. N., Amici A., Climent I., Lenz A.-G., Ahn B.-W., Shaltiel S., Stadtman E. R. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 14.Dalle-Donne I., Giustarini D., Colombo R., Rossi R., Milzani A. Trends Mol. Med. 2003;9:169–176. doi: 10.1016/s1471-4914(03)00031-5. [DOI] [PubMed] [Google Scholar]
- 15.Brazil D. P., Hemmings B. A. Trends Biochem. Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen M. D., D'Aigle T., Gowing G., Julien J. P., Rivest S. J. Neurosci. 2004;24:1340–1349. doi: 10.1523/JNEUROSCI.4786-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urushitani M., Sik A., Sakurai T., Nukina N., Takahashi R., Julien J. P. Nat. Neurosci. 2006;9:108–118. doi: 10.1038/nn1603. [DOI] [PubMed] [Google Scholar]
- 18.Raoul C., Abbas-Terki T., Bensadoun J. C., Guillot S., Haase G., Szulc J., Henderson C. E., Aebischer P. Nat. Med. 2005;11:423–428. doi: 10.1038/nm1207. [DOI] [PubMed] [Google Scholar]
- 19.Rind H. B., von Bartheld C. S. Mol. Cell. Neurosci. 2002;19:58–71. doi: 10.1006/mcne.2001.1069. [DOI] [PubMed] [Google Scholar]
- 20.Kaspar B. K., Llado J., Sherkat N., Rothstein J. D., Gage F. H. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- 21.Neff N. T., Prevette D., Houenou L. J., Lewis M. E., Glicksman M. A., Yin Q. W., Oppenheim R. W. J. Neurobiol. 1993;24:1578–1588. doi: 10.1002/neu.480241203. [DOI] [PubMed] [Google Scholar]
- 22.Boni-Schnetzler M., Rubin J. B., Pilch P. F. J. Biol. Chem. 1986;261:15281–15287. [PubMed] [Google Scholar]
- 23.Dobrowolny G., Giacinti C., Pelosi L., Nicoletti C., Winn N., Barberi L., Molinaro M., Rosenthal N., Musaro A. J. Cell Biol. 2005;168:193–199. doi: 10.1083/jcb.200407021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaspar B. K., Frost L. M., Christian L., Umapathi P., Gage F. H. Ann. Neurol. 2005;57:649–655. doi: 10.1002/ana.20451. [DOI] [PubMed] [Google Scholar]
- 25.Lai E. C., Felice K. J., Festoff B. W., Gawel M. J., Gelinas D. F., Kratz R., Murphy M. F., Natter H. M., Norris F. H., Rudnicki S. A. Neurology. 1997;49:1621–1630. doi: 10.1212/wnl.49.6.1621. [DOI] [PubMed] [Google Scholar]
- 26.Kikuchi H., Almer G., Yamashita S., Guegan C., Nagai M., Xu Z., Sosunov A. A., McKhann G. M., Przedborski S. Proc. Natl. Acad. Sci. USA. 2006;103:6025–6030. doi: 10.1073/pnas.0509227103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu D. C., Teismann P., Tieu K., Vila M., Jackson-Lewis V., Ischiropoulos H., Przedborski S. Proc. Natl. Acad. Sci. USA. 2003;100:6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Almer G., Guégan C., Teismann P., Naini A., Rosoklija G., Hays A. P., Chen C., Przedborski S. Ann. Neurol. 2001;49:176–185. [PubMed] [Google Scholar]
- 29.Kostic V., Jackson-Lewis V., De Bilbao F., Dubois-Dauphin M., Przedborski S. Science. 1997;277:559–562. doi: 10.1126/science.277.5325.559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.