Abstract
Siderophores are low molecular weight compounds, synthesized and secreted by microorganisms, that specifically bind ferric iron with exceptionally high affinity. Microbes capture these compounds and take up the bound iron through specific, high-affinity systems. Saccharomyces cerevisiae can take up iron bound to siderophores through the transporters of the ARN family; however, the mechanism by which the siderophore-bound iron enters the cell via these transporters is not known. Here we describe how ferrichrome, a siderophore of the hydroxamate class, is taken up by Arn1p. Arn1p exhibits two surface binding sites for ferrichrome, one that is similar in affinity to the KT for uptake and one of a much higher affinity that is specific for the metallated form of ferrichrome. Ferrichrome may gain access to the higher-affinity site through endocytosis. Tracer studies using 14C-labeled ferrichrome bound to either iron(III) or aluminum(III), a nonreducible ligand for ferrichrome, indicate that ferrichrome enters the cell as the intact metallosiderophore and accumulates in the cytosol. Both ferrichrome chelates were relatively stable within the cell, and metal-free ferrichrome did not accumulate, indicating a role for ferrichrome in intracellular iron storage. Iron stored as ferrichrome was readily mobilized to meet the metabolic needs of the cell.
Although iron is a very abundant metal in the earth's crust, the bioavailability of this essential nutrient is extremely low. Microorganisms overcome this bioavailability problem by synthesizing and secreting siderophores, small organic molecules that specifically bind ferric iron with extremely high affinity. Siderophores are secreted in the iron-free form, and can effectively solubilize ferric iron, which can then be captured by specific cellular uptake systems. Saccharomyces cerevisiae does not synthesize siderophores, but it can take up iron bound to siderophores secreted by other microbes (1, 2). Although the uptake systems for siderophores have been studied in detail in prokaryotes, much less is known about these systems in eukaryotes.
Siderophore-bound iron enters fungal cells through two distinct high-affinity systems, one that requires the reduction of the ferric iron to the ferrous form before uptake and one that does not. In S. cerevisiae, the reductive system of uptake begins with the coupled reduction and release of siderophore-bound iron and is catalyzed by plasma membrane metalloreductases encoded by FRE1, FRE2, and FRE3 (3–5). The reduced iron is then taken up by the high-affinity ferrous transport complex, which is composed of a multicopper oxidase encoded by FET3 (6) and a permease encoded by FTR1 (7).
The nonreductive system of uptake depends on a group of transport proteins that specifically recognize siderophore-iron chelates. S. cerevisiae expresses four such transport proteins, encoded by ARN1, ARN2/TAF1, ARN3/SIT1, and ARN4/ENB1 (8–13); similar proteins have recently been identified in Candida albicans and Aspergillus nidulans (14, 15). Uptake of siderophore-bound iron is augmented by a group of cell wall mannoproteins, encoded by FIT1, FIT2, and FIT3, that can increase the amount of iron associated with the cell wall (16). Budding yeast express these systems of high-affinity iron uptake during growth in iron-depleted environments. All of the genes encoding these proteins are controlled transcriptionally by Aft1p, the major iron-dependent transcription factor in yeast (17). A second iron-regulated transcription factor, Aft2p, was recently identified and may also play a role in the transcriptional response to iron deprivation (18, 19).
Individual ARN transporters exhibit specificity for siderophores of different structural classes, yet they also exhibit some overlapping specificities. Iron bound to ferrichrome (FC), a siderophore of the trihydroxamate class, is specifically taken up through Arn1p and Arn3p (12, 20). FC also influences the intracellular trafficking of Arn1p and Arn3p. At lower concentrations, FC induces the relocalization of Arn1p from the endosome to the plasma membrane, and at higher concentrations FC induces the cycling of Arn1p between the plasma membrane and the endosome (21). The mechanism by which siderophores influence the intracellular trafficking of the ARN transporters is not known. Similarly, it is not known whether siderophore-bound iron enters the cell as an intact chelate, or whether the iron is separated from the siderophore before it transits the cellular membrane, either at the surface of the cell or in an intracellular vesicle. We have investigated the mechanism of FC–iron uptake by means of the Arn1p transporter. Here we report that Arn1p has two surface binding sites with differing affinities for FC. FC entered the cell as an intact metal-chelate, accumulated in the cytosol, and decayed relatively slowly, suggesting a role for FC in the intracellular storage of iron.
Materials and Methods
Yeast Strains and Media.
The yeast strain from which all four ARN transporters had been deleted, arn1Δ arn2Δ arn3Δ arn4Δ (CWY101) (13) and the strains fre1Δ fre2Δ fre3Δ and arn1Δ arn3Δ fre1Δ fre2Δ fre3Δ were previously described (5). Complete synthetic defined medium (22) served as iron-rich medium, and iron-poor medium, containing 10 μM ferrous ammonium sulfate and 1 mM ferrozine, was prepared as described (23). Except where specifically noted in the text, FC refers to the ferric form of the siderophore.
FC-Binding and Reductase Assays and Data Analysis.
FC-binding assays were performed as described (21) with the following modifications. For the determinations of FC-binding sites, cells from the ARN-deletion strain were transformed with either pOE-Arn1-HA or pRS426, and were incubated with [55Fe]FC in serial 2.2-fold dilutions. Cells were washed, and the [55Fe]FC retained on the cells was measured. Data were analyzed by using prism version 3.0 for the Macintosh (GraphPad Software, San Diego). For “cold competition” analysis, cells were incubated in 100 nM [55Fe]FC mixed with 0, 0.5, 2.5, and 12.5 μM unlabeled apo-FC or Fe-FC. For latrunculin A (Lat A) treatment, the strain RH144-3D (a wild-type parent strain) (24) was grown in iron-poor medium, then resuspended in iron-poor medium containing 5% glucose at an OD600 of 2.0. Lat A (40 μM) or an equal volume of the vehicle DMSO was added, and the cells were incubated at 30°C for 20 min. FC (1 μM) was then added and the cells were incubated for an additional hour. Cells were then subjected to the FC-binding assay, using [55Fe]FC at 100 nM, or the ferric reductase assay (3), using FeCl3 at 1 mM and bathophenanthroline disulfonate at 1 mM.
Preparation of 14C-Radiolabeled Ferrichrome (*FC).
A 300-ml culture of Ustilago sphaerogena (ATCC 12421) was grown in iron-deficient medium (25) supplemented with 100 μCi (1 μCi = 37 kBq) of l-[U-14C]glutamate (0.4 μmol) for 72 hr. The conditioned medium was recovered and reduced to ≈20 ml by rotary evaporation. The 14C-labeled apo-ferrichrome (apo-*FC) was extracted, isolated, and purified by preparative electrophoresis, as previously described (26). Aqueous eluates were further purified on cellulose thin-layer chromatography plates (Aldrich) by using 1-butanol/ethanol/deionized (>17 MΩ⋅cm) water (4:1:5, vol/vol, upper layer) as the mobile phase. The apo-*FC was eluted with deionized water and stored lyophilized. Apo-*FC was quantitated spectrophotometrically (26) and by scintillation counting. The specific activity of the apo-*FC was 5.00 × 105 cpm/μmol.
Labeling with *FC and Subcellular Fractionation.
Strains were grown to midlogarithmic phase in iron-poor medium, harvested, washed twice in water, and resuspended in 5 ml of 50 mM sodium citrate, pH 6.4, with 5% glucose (citrate buffer) at a concentration of 2 × 108 cells per ml. [55Fe]*FC or Al-*FC at 10 μM was added, and the cells were incubated at 30°C for 90 min. Cells were washed with citrate buffer, then reinoculated into media or subjected to spheroplasting and hypotonic lysis (27). Lysates were precleared by centrifugation at 500 × g for 5 min, and the resulting supernatant was centrifuged at 100,000 × g for 30 min. The resulting pellets were resuspended in 100 μl of lysis buffer and proportional amounts of pellets and supernatant were subjected to SDS/PAGE and Western blotting (13) using a 1:1,000 dilution of monoclonal anti-Pep12 antibody (Molecular Probes). The remaining fractions were subjected to dual-channel scintillation counting.
Organic Extractions and Thin-Layer Chromatography.
FC was extracted from labeled cells as described (26) with the following modifications. Washed cells were resuspended in 200 μl of a saturated ammonium sulfate solution, and an equal volume of glass beads and 500 μl of a 1:1 mixture of phenol/chloroform were added. Cells were mixed on a Vortex mixer for 4 min and the phases were separated by centrifugation. The aqueous layer was removed and reserved for scintillation counting. The organic layer was collected and the remaining sample was reextracted with ammonium sulfate and phenol/chloroform. The organic phases were pooled and Vortex-mixed with 100 μl of water and 2 ml of diethyl ether. Phases were separated by centrifugation and the aqueous layer was collected. The aqueous layer was clarified by centrifugation and lyophilized or directly subjected to dual-channel scintillation counting. The lyophilized residue was resuspended in 5 μl of water and spotted on thin-layer chromatography plates, silica gel 60 on plastic backing (EM Science, Gibbstown, NJ). Chromatography was performed with the upper layer of an equilibrated mixture of 1-butanol/ethanol/water at a ratio of 4:1:5 or in 80% methanol. Images of thin-layer chromatography plates were obtained with a PhosphorImager (Molecular Dynamics).
Results
Identification of FC-Binding Sites on Arn1p.
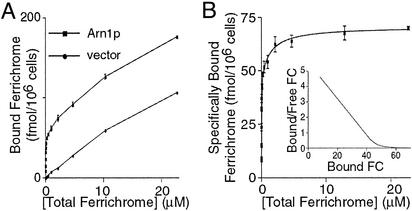
Previous kinetic studies have indicated that Arn1p-dependent transport of FC-bound iron exhibits half-maximal saturation (KT) at a FC concentration of 0.9 μM (12); therefore, Arn1p might be expected to contain a FC-binding site that exhibits half-maximal saturation (Kd) at a similar concentration. However, recent studies indicate that low-nanomolar concentrations of FC can lead to the relocalization of Arn1p from endosomes to the plasma membrane (21). These findings suggest the existence of a FC receptor with nanomolar affinity, and we considered that Arn1p itself might be functioning as this receptor. We therefore developed an assay to measure surface binding of FC in whole cells. A strain from which all four ARN transporters have been deleted was transformed with either the empty parent vector or a high-copy-number plasmid designed to give 20- to 50-fold overexpression of Arn1p (21). By indirect immunofluorescence, overexpression of Arn1p results in mislocalization of a portion of the transporter to the plasma membrane, even in the absence of FC, where Arn1p is then available for FC binding. These strains were cultured in iron-poor medium to induce the expression of Arn1p, then all transport and membrane trafficking activity was stopped by incubating the cells on ice with sodium azide and potassium fluoride. Washed cells were then incubated on ice with 55Fe-labeled FC at concentrations from 1.8 nM to 50 μM, and the amount of FC bound to the cells after washing was measured by scintillation counting. The strain transformed with the vector alone exhibited lower levels of FC binding that increased linearly with the total FC concentration and were not saturable over the concentration range tested (Fig. 1A). This nonspecific binding was subtracted from the total FC bound by the Arn1p-expressing strain to yield the specifically bound FC (Fig. 1B). These data were examined by nonlinear regression analysis (prism version 3.0) for both single-site binding and two-site binding and found to conform best to a two-site binding curve (P < 0.0001) with an R2 value of 0.985. A Scatchard plot (Fig. 1B Inset) of these data transformed after nonlinear regression analysis clearly demonstrates a curve with two slopes. The numerical results of the nonlinear regression analysis are presented in Table 1. The higher-affinity binding site has a dissociation constant of 8.1 nM, which is similar to the concentrations of FC that lead to the localization of Arn1p to the plasma membrane (10–100 nM) (21). The lower-affinity site has a dissociation constant of 1.2 μM, which is similar to the previously determined KT (12). The theoretical maximum number of binding sites for the higher-affinity site, 45 fmol per 106 cells, is roughly twice that of the lower-affinity site, 25 fmol per 106 cells, raising the possibility that each Arn1p transporter contains two higher-affinity and one lower-affinity site for FC binding. Although it is formally possible that two populations of Arn1p, each with a single binding site of either higher or lower affinity, are present, perhaps as a consequence of overexpression, we think it unlikely. In the absence of overexpression, we detect high-affinity FC binding both in a VPS1 mutant strain in which Arn1p is missorted to the plasma membrane and in a wild-type strain after treatment with nanomolar FC (21).
Figure 1.
Identification of two FC-binding sites on Arn1p. (A) The arn1Δ arn2Δ arn3Δ arn4Δ strain was transformed with either pOE-Arn1-HA (Arn1p), for overexpression of Arn1p, or pRS426 (vector), and the FC-binding assay was performed. (B) Nonlinear regression analysis of specifically bound FC. To determine the amount of [55Fe]FC bound specifically to Arn1p, the iron bound to the vector-transformed strain was subtracted from the iron bound to the Arn1p-overexpressing strain. Nonlinear regression analysis indicated the data best fit a two-site binding curve (P < 0.0001). (Inset) Scatchard plot of data transformed from nonlinear regression analysis. Two slopes are clearly seen. Duplicate samples were used in each experiment and the experiments were replicated twice. Error bars indicate the standard deviation.
Table 1.
FC-binding sites on Arn1p
| Site | Kd | 95% C.I. | Bmax, fmol/106 cells | 95% C.I., fmol/106 cells |
|---|---|---|---|---|
| 1 | 8.1 nM | 5.9–10.3 nM | 45 | 42–49 |
| 2 | 1.2 μM | 0.4–2.1 μM | 25 | 21–29 |
Kd, dissociation constant; C.I., confidence interval; Bmax, maximal binding sites.
In previous studies, the addition of both apo-FC and holo-FC to growth medium led to relocalization of Arn1p (21). However, apo-FC is rapidly converted to holo-FC in growth media and therefore it is not clear whether apo-FC is active in signaling. We tested whether the higher-affinity binding site for FC could also recognize apo-FC by measuring the binding of [55Fe]FC in the presence of increasing amounts of unlabeled apo-FC or holo-FC added as competitive inhibitors (Fig. 2). The addition of unlabeled apo-FC did not interfere with the binding of [55Fe]FC, even when present at 125-fold excess, indicating that the high-affinity binding site was specific for Fe-FC (Fig. 2A). In contrast, the addition of unlabeled holo-FC caused the expected decrease in bound [55Fe]FC (Fig. 2B).
Figure 2.
Specificity of the higher-affinity binding site for holo-FC. Strains were grown and treated as in Fig. 1 and [55Fe]FC binding was measured in the presence of 100 nM [55Fe]FC with increasing amounts of unlabeled apo-FC (Apo-ferrichrome, A) or unlabeled ferric-FC (Holo-ferrichrome, B). Specifically bound FC was calculated as in Fig. 1. Duplicate samples were used in each experiment and the experiments were replicated twice. Error bars indicate the standard deviation.
Role of Endocytosis in FC Signaling.
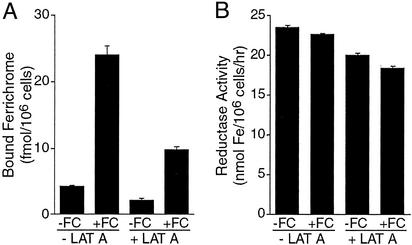
A requirement of the model, in which intracellular Arn1p acts as the high-affinity receptor for extracellular FC, is that the two species must occupy the same compartment to interact. Recent data indicate that, in the absence of FC, Arn1p cycles between the early and late endosomes, but does not cycle to the plasma membrane. We therefore hypothesized that FC might gain access to the early endosome through fluid phase endocytosis. Both fluid-phase and receptor-mediated endocytosis are actin-dependent processes, and treatment of cells with the actin-depolymerizing drug Lat A results in an inhibition of endocytosis (28). We inhibited endocytosis by treating cells with Lat A, then we tested the capacity of FC to induce relocalization of Arn proteins to the plasma membrane by measuring the binding of FC to Arn proteins on the cell surface. Wild-type cells were pretreated with either vehicle or Lat A to inhibit endocytosis. Cells were then exposed to FC for 1 hr to allow Arn protein relocalization to occur, then cells were harvested and washed, and the surface expression of Arn proteins was quantitated by binding of [55Fe]FC (Fig. 3A). In the absence of Lat A, FC induced a dramatic increase in the surface expression of Arn proteins as measured by the surface binding of [55Fe]FC. In contrast, pretreatment of cells with Lat A inhibited the capacity of FC to induce the surface expression of Arn proteins, with the surface expression of Arn proteins in Lat A-treated cells less than half of that of untreated cells. We confirmed that the loss of Arn protein relocalization in Lat-A-treated cells was not due to nonspecific effects on the activity of proteins at the plasma membrane by measuring the activity of the plasma membrane metalloreductases in identically treated cells (Fig. 3B). These data suggest that endocytosis is important in the FC signaling process and suggest that FC reaches the Arn-containing endosomes through an endocytic mechanism.
Figure 3.
Dependence on endocytosis for FC-induced Arn protein surface relocalization. (A) Wild-type cells were grown to midlogarithmic phase in iron-poor medium to induce expression of Arn proteins. Cells were pretreated with Lat A (+LAT A) or an equal volume of DMSO (−LAT A) for 20 min. Cells were then grown in the presence (+FC) or absence (−FC) of FC for 1 hr. Cultures were harvested, and the FC-binding assay was performed. Samples were analyzed in triplicate, and the experiment was replicated twice. (B) Lack of effect of Lat A on surface reductase activity. Cells were grown and treated as in A, then plasma membrane ferric reductase activity was measured as described in the text. Error bars indicate standard deviation.
Transport of FC into the Cytosol.
Previous studies of siderophore–iron uptake in S. cerevisiae have used iron as the radiolabeled ligand. A limitation of these studies is that they do not distinguish between an uptake mechanism that involves the release of iron from the siderophore before uptake and a mechanism that involves uptake of the intact siderophore–iron chelate. Variations of both mechanisms have been described in fungi (29). We examined the mechanism of FC uptake by using 14C-labeled FC (*FC) bound to either 55Fe(III) or aluminum(III). Alumichrome (Al–FC) chelates are stable and recognized by FC-specific transport systems (30), but the metal is nonreducible and the chelate would not be active in uptake systems that involve reduction of the metal ligand. To prevent the reduction of Fe–*FC before uptake, we performed these assays in strains in which the plasma membrane reductases encoded by FRE1, FRE2, and FRE3 had been deleted. Two versions of these strains were used, one that expressed all four ARN transporters (ARN+) and one in which the FC transporters, Arn1p and Arn3p, had been deleted (arn1Δ arn3Δ). These strains were incubated in either [55Fe]*FC or Al–*FC. After FC loading, the cells were washed, spheroplasted, and lysed by Dounce homogenization. Lysates were cleared of unbroken cells and cell wall debris (500 × g pellet) and then were subjected to differential centrifugation designed to separate intracellular membrane-bound vesicles (100,000 × g pellet) from cytosol (100,000 × g supernatant). The resulting fractions were analyzed by scintillation counting and Western blotting (Fig. 4). Both [55Fe]*FC and Al–*FC were taken up by the ARN+ strain and found to accumulate in the cytosolic fraction, with almost no accumulation in the vesicular fraction (Fig. 4A). Accumulation of [55Fe]*FC or Al-*FC was very low in the arn1Δ arn3Δ strain, indicating that the observed accumulation of FC depended on the FC transporters, Arn1p and Arn3p (Fig. 4B). Western blotting of the individual fractions with an antibody directed against Pep12p, a resident protein of the late endosome (31), confirmed the separation of vesicles from cytosol. [55Fe]*FC and Al-*FC accumulated in the cell at essentially the same rate, indicating that both chelates were recognized by the FC transport system and that reduction of the chelate was not likely to be required for uptake. Iron taken up with the [55Fe]*FC was also detected in the cytosol and not in the vesicular fraction, which contains vacuoles and mitochondria, sites of iron storage and utilization, respectively (32). Quantitation of 55Fe and 14C in the conditioned media after growth indicated that neither 55Fe nor *FC was excreted by the cells after loading (data not shown).
Figure 4.
Accumulation of *FC in the cytosol. An Arn-expressing strain (ARN+, A) and a strain in which the FC transporters Arn1p and Arn3p had been deleted (arn1Δ arn3Δ, B) were grown to midlogarithmic phase in iron-poor medium. Cells were incubated in either [55Fe]*FC or Al–*FC for 90 min, washed, spheroplasted, and lysed by Dounce homogenization in hypotonic buffer. Unbroken cells and cellular debris were collected by centrifugation at 500 × g (500 g pellet), and the 500 × g supernatant was subjected to centrifugation at 100,000 × g. The resulting pellet, containing intracellular vesicles (100K g pellet), and the supernatant, containing the cytosol (100K g sup), were collected and analyzed by scintillation counting (bar graph) and Western blotting for the endosomal marker protein Pep12 (Lower). The experiment was replicated three times with essentially identical results; data from a representative experiment are shown.
Detection of Intact Holo-FC in the Cytosol.
The data presented in Fig. 4 indicated that [55Fe]*FC and Al–*FC were taken up as intact chelates and that both the metal and the siderophore ligands accumulated in the cytosolic compartment. However, these experiments did not indicate whether the metal remained bound to the siderophore, or whether the siderophore was intact or had undergone degradation. To address these questions, we labeled cells with either [55Fe]*FC or Al–*FC and prepared whole-cell organic extracts that were enriched for FC. We then subjected these extracts to thin-layer chromatography designed to differentiate apo-*FC, [55Fe]*FC, and Al–*FC (Fig. 5). In vitro, free ferric iron can rapidly replace iron or aluminum bound to FC in a process called ligand exchange. In this extraction method, free or loosely bound iron immediately partitions into the aqueous phase, whereas apo-*FC, [55Fe]*FC, and Al–*FC partition into the organic phase, thereby minimizing the potential for ligand exchange during purification (ref. 26 and data not shown). Extracts were prepared from cells labeled with [55Fe]*FC or Al–*FC (Labeled Cell Extract, lanes 4 and 5 in Fig. 5) and from unlabeled cells (Unlabeled Extract, lanes 1–3) to which apo-*FC, [55Fe]*FC, and Al–*FC were added directly as a control. As additional controls, apo-*FC, [55Fe]*FC, Al–*FC, and 55FeCl3 were directly spotted onto the plate (No Extract, lanes 6–9). Duplicate plates were spotted, and either 80% methanol (MeOH) or the upper layer of an equilibrated 1-butanol/ethanol/water mixture (BuOH/EtOH/H2O) was used as the mobile phase. The chromatography plates were dried and the radiolabeled species were detected by phosphorimaging.
Figure 5.
Accumulation of intact holo-FC in the cytosol. The ARN+ fre1–3Δ strain was labeled with either [55Fe]*FC or Al–*FC as in Fig. 4. FC was extracted from the cells and spotted in duplicate onto silica plates (lanes 4 and 5). Apo-*FC, [55Fe]*FC, or Al–*FC was added as a control to extracts prepared from identically treated, unlabeled cells immediately before spotting (lanes 1–3). As an additional control, apo-*FC, [55Fe]*FC, Al–*FC, or 55FeCl3 was spotted directly on the plate (lanes 6–9). Duplicate plates were prepared and subjected to thin-layer chromatography with either the upper phase of a 1-butanol/ethanol/water mixture (BuOH/EtOH/H2O) or a methanol/water mixture (MeOH) as the mobile phase. Radiolabeled species were detected by phosphorimage analysis. The origin is indicated by filled arrowheads and holo-FC is indicated by open arrowheads. Bars mark the migration of Fe–FC vs. Al–FC. This experiment was repeated three times with essentially the same result; data from a representative experiment are shown.
Extracts from cells labeled with [55Fe]*FC and Al–*FC contained radiolabeled species that migrated in patterns identical to those of the holo forms of both siderophores, indicating that FC was present in cells as the intact iron- or aluminum-chelate. A smaller amount of material with a less distinct migration pattern was also detected; this migration pattern was similar to that of apo-*FC, suggesting that a small amount of apo-*FC was also present. When BuOH/EtOH/H2O was used as the mobile phase, [55Fe]*FC migrated slightly ahead of Al–*FC, and this pattern of migration was observed both in the control samples and in the extracts from labeled cells. This observation suggested that the Al–FC had not undergone ligand exchange with iron while in the cytosol.
Intracellular Metabolism of FC.
Fe(III) that is bound to FC could be mobilized for the cells' metabolic needs either by reduction to Fe(II) and release from FC or by degradation of the peptide backbone of FC. We considered that, although [55Fe]*FC and Al–*FC remained intact in the cytosol immediately after loading, these species might be metabolized during a period of growth after loading. We therefore loaded cells with [55Fe]*FC and Al–*FC and then divided the washed cells into aliquots for immediate extraction of iron and FC, or into aliquots for growth in either iron-poor medium or iron-rich medium before extraction of iron and FC. The extracts were again subjected to thin-layer chromatography and phosphorimaging (Fig. 6A). Again, immediately after loading, both [55Fe]*FC and Al–*FC (lanes 1 and 4) were detected largely as intact species in whole-cell extracts, with a smaller amount of indistinctly migrating apo-*FC also present. After 4 hr of growth in iron-poor medium (lanes 2 and 5), most of the [55Fe]*FC and Al–*FC remained intact in the cell. When the FC-loaded cells were grown in iron-rich medium, however (lanes 3 and 6), there appeared to be a significant loss of signal from the [55Fe]*FC and Al–*FC.
Figure 6.
Stability and iron-dependent degradation of holo-FC. The ARN+ fre1–3Δ strain was labeled with [55Fe]*-FC or Al–*FC as in Fig. 4, washed, and divided into aliquots for immediate extraction of FC (time 0, lanes 1 and 4), for 4 hr of growth in iron-poor medium (time 4, −Fe, lanes 2 and 5), or for 4 hr of growth in iron-rich medium (time 4, +Fe, lanes 3 and 6). (A) Stability of holo-FC in growing cells. Extracts were prepared and thin-layer chromatography was performed as in Fig. 5. Filled arrows, origin; open arrows, holo-FC. (B) Slow decay of FC in growing cells. Extracts identical to those in A were quantitated by scintillation counting. Black bars indicate [55Fe]*FC, white bars indicate apo-*FC, and gray bars indicate mixed apo-/Al–*FC. (C) Uncomplexed iron pools in growing cells. 55Fe that was neither bound to FC nor tightly bound to protein was isolated in the initial aqueous phase of extracts of cells labeled in A and was quantitated by scintillation counting. Experiments were replicated twice and results from a representative experiment are shown.
To quantitatively examine the metabolism of intracellular FC, we measured by scintillation counting the 55Fe and *FC content of a set of whole-cell extracts identical to those examined in Fig. 6A (Fig. 6B). By comparing the molar ratios of 55Fe and *FC in the FC-containing extracts, we determined that 39% of the intracellular FC was present in the apo form, and that similar proportions of apo-FC were present after growth in iron-poor and iron-rich media (Fig. 6B, white bars). Greater than 85% of the original [55Fe]*FC and Al–*FC was still present in the cells after 4 hr of growth in iron-poor medium, whereas 44% of the [55Fe]*FC and 53% of the Al–*FC was present after 4 hr of growth in iron-rich medium. Quantitative analysis of the chromatogram presented in Fig. 6A yielded similar results. The whole-cell extraction procedure also allowed us to isolate and quantitate the uncomplexed 55Fe in the cells (Fig. 6C), and the amount of this species remained relatively constant during the growth period. 55Fe and 14C-labeled amino acids from the degradation of *FC would be incorporated into proteins during the growth period, and proteins were not recovered by the extraction procedure. This incorporation likely accounts for the decrease in total labeled species after growth. Together, these results suggested that (i) much of the holo-FC was retained intact in the cell during growth and (ii) apo-FC did not accumulate after the metabolism of holo-FC.
The slow decay of Fe–FC in cells growing in iron-poor medium was paradoxical, as cells need to mobilize stored iron for growth in iron-poor medium. We tested whether the quantity or the molecular form (Fe–FC vs. other iron species) of the stored iron affected the capacity of cells to metabolize iron stores and grow in iron-poor medium. No difference in growth rate was observed between cells loaded with Fe–FC and Fe(II), and no difference was observed between cells with larger and smaller iron stores (data not shown). These data suggested that the intracellular pool of Fe–FC was degraded slowly because it was large compared with the metabolic needs of the cell. We also determined that cells loaded with Fe–FC or Fe(II) exhibited identical growth rates when reinoculated into either iron-poor or iron-rich media (data not shown). This observation suggested that mobilization of either type of stored iron met the cells' metabolic needs as well as iron that was newly acquired from the environment.
Discussion
Here we have demonstrated that Arn1p exhibits two surface binding sites for FC: a site with low-nanomolar affinity and a site with low-micromolar affinity. The detection of the lower-affinity site was expected, as the affinity of this site is similar to the KT. The detection of a second, higher-affinity, site was somewhat unexpected, however, and suggests that Arn1p itself acts as a FC receptor. We propose a model in which FC enters the early endosome through fluid phase endocytosis and binds to a high-affinity site on Arn1p, causing a conformational change in Arn1p. This conformational change leads to exocytosis of Arn1p and redistribution to the plasma membrane. The demonstration here of both a requirement for endocytosis and the existence of a high-affinity FC-binding site strengthens this model. Additionally, preliminary data suggest that mutations in Arn1p that affect FC binding also affect the intracellular localization of Arn1p (Y.K. and C.C.P., unpublished observations). Binding of FC to the lower-affinity site may also affect the trafficking of Arn1p. Not only was the Kd of the lower-affinity FC-binding site similar to the KT, but it was similar to the concentrations at which FC induces the internalization of Arn1p from the plasma membrane back to the endosome (1–10 μM). This result suggests that the binding of a second molecule of FC to the lower-affinity site leads to a second conformational change that triggers the internalization of Arn1p.
Our data indicated that Arn1p facilitated the uptake of FC as an intact metal-chelate into the cytosolic compartment, where the siderophore was degraded and the iron was released. This mechanism differs from the mechanisms of siderophore–iron uptake that have been described previously for yeast. Uptake of the intact ferric chelate followed by the intracellular reductive release of the iron and resecretion of the aposiderophore has been reported for the uptake of FC- and coprogen-type siderophores in Ustilago sphaerogena and Neurospora crassa (29). However, we found no evidence that S. cerevisiae excreted apo-FC, and the similar rate of decay of Fe–FC and the nonreducible Al–FC suggested that intracellular reduction of the iron ligand was not required for iron release. Small amounts of apo-FC were detected, however, and it is possible that some reduction and release of iron occurred. The apparent recovery of Al–FC from cells suggested that the cytosolic Al–FC did not undergo ligand exchange with cytosolic pools of free ferric iron. This is consistent with the conclusion of some investigators that there are no pools of free metal ions in the cytosol, as has been shown for copper and zinc (33, 34). Our data effectively rule out the “taxicab mechanism” of siderophore–iron uptake seen in Rhodotorula species, in which iron from extracellular rhodotorulate is passed across the membrane to an intracellular siderophore (35).
We found that immediately after loading and after a 4-hr period of growth, significant amounts of Fe–FC remained intact in the cell. These results indicate that FC serves as an intracellular iron storage compound in S. cerevisiae. Intracellular FC-type siderophores have been noted to be the predominant form of iron storage in N. crassa, Penicillium chrysogenum, Aspergillus nidulans, and Aspergillus ochraceus (36, 37). Neither these organisms nor S. cerevisiae produces ferritins, the predominant iron-storage molecules in higher eukaryotes and some prokaryotes, and perhaps intracellular siderophores can replace the function of ferritins in these fungi. S. cerevisiae is unique among this group, however, because it does not synthesize an intracellular siderophore and instead relies on the uptake of exogenous siderophores. Two recent reports indicate that mammalian cells express a protein of the lipocalin superfamily that specifically binds the catecholate-type siderophore enterobactin (38, 39); perhaps a functionally similar protein is expressed in budding yeast that can contribute to the stability of FC inside cells.
Acknowledgments
We thank Jerry Kaplan for his critical reading of this manuscript and Jerry Kaplan and Olga Protchenko for numerous helpful discussions.
Abbreviations
- FC
ferrichrome
- Lat A
latrunculin A
- apo-FC
metal-free FC
- holo-FC
metallated FC
- *FC
14C-labeled FC
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Byers B R, Arceneaux J E L. In: Iron Transport and Storage in Microorganisms, Plants, and Animals, Metal Ions in Biological Systems, Sigel A, Sigel H, editors. Vol. 35. New York: Dekker; 1998. pp. 37–66. [PubMed] [Google Scholar]
- 2.Neilands J B. J Biol Chem. 1995;270:26723–26726. doi: 10.1074/jbc.270.45.26723. [DOI] [PubMed] [Google Scholar]
- 3.Dancis A, Klausner R D, Hinnebusch A G, Barriocanal J G. Mol Cell Biol. 1990;10:2294–2301. doi: 10.1128/mcb.10.5.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Georgatsou E, Alexandraki D. Mol Cell Biol. 1994;14:3065–3073. doi: 10.1128/mcb.14.5.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yun C W, Bauler M, Moore R E, Klebba P E, Philpott C C. J Biol Chem. 2001;276:10218–10223. doi: 10.1074/jbc.M010065200. [DOI] [PubMed] [Google Scholar]
- 6.Askwith C, Eide D, Van Ho A, Bernard P S, Li L, Davis-Kaplan S, Sipe D M, Kaplan J. Cell. 1994;76:403–410. doi: 10.1016/0092-8674(94)90346-8. [DOI] [PubMed] [Google Scholar]
- 7.Stearman R, Yuan D S, Yamaguchi-Iwai Y, Klausner R D, Dancis A. Science. 1996;271:1552–1557. doi: 10.1126/science.271.5255.1552. [DOI] [PubMed] [Google Scholar]
- 8.Heymann P, Ernst J F, Winkelmann G. Biometals. 1999;12:301–306. doi: 10.1023/a:1009252118050. [DOI] [PubMed] [Google Scholar]
- 9.Heymann P, Ernst J F, Winkelmann G. Biometals. 2000;13:65–72. doi: 10.1023/a:1009250017785. [DOI] [PubMed] [Google Scholar]
- 10.Heymann P, Ernst J F, Winkelmann G. FEMS Microbiol Lett. 2000;186:221–227. doi: 10.1111/j.1574-6968.2000.tb09108.x. [DOI] [PubMed] [Google Scholar]
- 11.Lesuisse E, Simon-Casteras M, Labbe P. Microbiology. 1998;144:3455–3462. doi: 10.1099/00221287-144-12-3455. [DOI] [PubMed] [Google Scholar]
- 12.Yun C W, Tiedeman J S, Moore R E, Philpott C C. J Biol Chem. 2000;275:16354–16359. doi: 10.1074/jbc.M001456200. [DOI] [PubMed] [Google Scholar]
- 13.Yun C W, Ferea T, Rashford J, Ardon O, Brown P O, Botstein D, Kaplan J, Philpott C C. J Biol Chem. 2000;275:10709–10715. doi: 10.1074/jbc.275.14.10709. [DOI] [PubMed] [Google Scholar]
- 14.Ardon O, Bussey H, Philpott C, Ward D M, Davis-Kaplan S, Verroneau S, Jiang B, Kaplan J. J Biol Chem. 2001;276:43049–43055. doi: 10.1074/jbc.M108701200. [DOI] [PubMed] [Google Scholar]
- 15.Haas H, Zadra I, Stoffler G, Angermayr K. J Biol Chem. 1999;274:4613–4619. doi: 10.1074/jbc.274.8.4613. [DOI] [PubMed] [Google Scholar]
- 16.Protchenko O, Ferea T, Rashford J, Tiedeman J, Brown P O, Botstein D, Philpott C C. J Biol Chem. 2001;276:49244–49250. doi: 10.1074/jbc.M109220200. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi-Iwai Y, Dancis A, Klausner R D. EMBO J. 1995;14:1231–1239. doi: 10.1002/j.1460-2075.1995.tb07106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blaiseau P L, Lesuisse E, Camadro J M. J Biol Chem. 2001;276:34221–34226. doi: 10.1074/jbc.M104987200. [DOI] [PubMed] [Google Scholar]
- 19.Rutherford J C, Jaron S, Ray E, Brown P O, Winge D R. Proc Natl Acad Sci USA. 2001;98:14322–14327. doi: 10.1073/pnas.261381198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lesuisse E, Blaiseau P L, Dancis A, Camadro J M. Microbiology. 2001;147:289–298. doi: 10.1099/00221287-147-2-289. [DOI] [PubMed] [Google Scholar]
- 21.Kim Y, Yun C W, Philpott C C. EMBO J. 2002;21:3632–3642. doi: 10.1093/emboj/cdf382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherman F. Methods Enzymol. 1991;194:3–20. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- 23.Philpott C C, Rashford J, Yamaguchi-Iwai Y, Rouault T A, Dancis A, Klausner R D. EMBO J. 1998;17:5026–5036. doi: 10.1093/emboj/17.17.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raths S, Rohrer J, Crausaz F, Riezman H. J Cell Biol. 1993;120:55–65. doi: 10.1083/jcb.120.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garibaldi J A, Neilands J B. J Am Chem Soc. 1955;77:2429–2430. [Google Scholar]
- 26.Emery T F. Biochemistry. 1966;5:3694–3701. doi: 10.1021/bi00875a045. [DOI] [PubMed] [Google Scholar]
- 27.Holthuis J C M, Nichols B J, Pelham H R B. Mol Biol Cell. 1998;9:3383–3397. doi: 10.1091/mbc.9.12.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayscough K. Methods Enzymol. 1998;298:18–25. doi: 10.1016/s0076-6879(98)98004-1. [DOI] [PubMed] [Google Scholar]
- 29.van der Helm D, Winkelmann G. In: Metal Ions in Fungi, Mycology. Winkelmann G, Winge D R, editors. Vol. 11. New York: Dekker; 1994. pp. 39–98. [Google Scholar]
- 30.Emery T. Biochemistry. 1971;10:1483–1488. doi: 10.1021/bi00784a033. [DOI] [PubMed] [Google Scholar]
- 31.Becherer K A, Rieder S E, Emr S D, Jones E W. Mol Biol Cell. 1996;7:579–594. doi: 10.1091/mbc.7.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, Chen O S, McVey Ward D, Kaplan J. J Biol Chem. 2001;276:29515–29519. doi: 10.1074/jbc.M103944200. [DOI] [PubMed] [Google Scholar]
- 33.Outten C E, O'Halloran T V. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 34.Rae T D, Schmidt P J, Pufahl R A, Culotta V C, O'Halloran T V. Science. 1999;284:805–808. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- 35.Carrano C J, Raymond K N. J Bacteriol. 1978;136:69–74. doi: 10.1128/jb.136.1.69-74.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charlang G, Ng B, Horowitz N H, Horowitz R M. Mol Cell Biol. 1981;1:94–100. doi: 10.1128/mcb.1.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matzanke B F, Bill E, Trautwein A X, Winkelmann G. J Bacteriol. 1987;169:5873–5876. doi: 10.1128/jb.169.12.5873-5876.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang J, Goetz D, Li J Y, Wang W, Mori K, Setlik D, Du T, Erdjument-Bromage H, Tempst P, Strong R, Barasch J. Mol Cell. 2002;10:1045–1056. doi: 10.1016/s1097-2765(02)00710-4. [DOI] [PubMed] [Google Scholar]
- 39.Goetz D H, Holmes M A, Borregaard N, Bluhm M E, Raymond K N, Strong R K. Mol Cell. 2002;10:1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]