Abstract
To understand the range of competence of embryonic stem (ES) cell-derived neural precursors, we have examined in vitro differentiation of mouse and primate ES cells into the dorsal- (neural crest) and ventralmost (floor plate) cells of the neural axis. Stromal cell-derived inducing activity (SDIA; accumulated on PA6 stromal cells) induces cocultured ES cells to differentiate into rostral CNS tissues containing both ventral and dorsal cells. Although early exposure of SDIA-treated ES cells to bone morphogenetic protein (BMP)4 suppresses neural differentiation and promotes epidermogenesis, late BMP4 exposure after the fourth day of coculture causes differentiation of neural crest cells and dorsalmost CNS cells, with autonomic system and sensory lineages induced preferentially by high and low BMP4 concentrations, respectively. In contrast, Sonic hedgehog (Shh) suppresses differentiation of neural crest lineages and promotes that of ventral CNS tissues such as motor neurons. Notably, high concentrations of Shh efficiently promote differentiation of HNF3β+ floor plate cells with axonal guidance activities. Thus, SDIA-treated ES cells generate naïve precursors that have the competence of differentiating into the “full” dorsal–ventral range of neuroectodermal derivatives in response to patterning signals.
We have recently established conditions that induce efficient neural differentiation of embryonic stem (ES) cells by using PA6 stromal cells as an inducer source (1, 2). When cultured on PA6 cells under serum-free conditions, mouse ES cells differentiate into neural cells at a >90% efficiency. After 3–5 days of coculture on PA6 cells, the majority of ES cells differentiate into nestin+ neuroectodermal precursor cells. TuJ1+ postmitotic neurons appear on coculture days 5–6, and tyrosine hydroxylase (TH)+ dopaminergic neurons accumulate during days 7–9. The inducing activity present on the surface of PA6 cells was named stromal cell-derived inducing activity (SDIA; ref. 1).
Recent reports on ES cell differentiation have suggested the possibility that information on in vivo neurogenesis might be systematically linked to stem cell technology (3, 4). However, it remains to be known whether ES cell-derived neural precursors generated in vitro can produce the full dorsal–ventral range of neuroectodermal derivatives in response to embryonic positional information. To address this question, we have tested in this study whether SDIA-treated ES cells have the competence of differentiating into the dorsal- (neural crest) and ventralmost (floor plate) cells under embryologically relevant conditions.
Materials and Methods
Cell Culture and Treatment with Patterning Factors.
Mouse ES cells (EB5), primate ES cells (cynomolgus monkey-derived; purchased from Asahi Technoglass, Funabashi, Japan), and PA6 cells were maintained and used for induction as described (1, 2, 5). Human bone morphogenetic protein (BMP)4 and mouse Shh-N (25–198) were purchased from R&D Systems and freshly added at each medium change. The day on which ES cells are seeded on PA6 is defined as day 0.
Immunocytochemistry, Statistics, and RT-PCR.
Cells were fixed with 4% paraformaldehyde, and immunostaining was performed with secondary antibodies conjugated with FITC, cy3, or cy5. For statistics, ≈100 colonies were observed in each experiment, and three or more experiments were performed. P values for statistical significance (t test) are described in the corresponding figure legends. The values shown in graphs represent the mean ± SD. RT-PCR was performed with ES cell colonies detached from feeder cells as described (1). The primary antibodies and primers used are described in Supporting Materials and Methods, which are published as supporting information on the PNAS web site, www.pnas.org.
Colony Isolation and Axon Guidance Assays.
The 3D collagen gel assay for axon guidance was performed by using isolated ES cell colonies (day 8; ref. 1) and the cerebellar plate region excised from embryonic day 13 Wistar rats as a responder (6).
Results
Positional Identity of Neural Tissues Induced from ES Cells by SDIA.
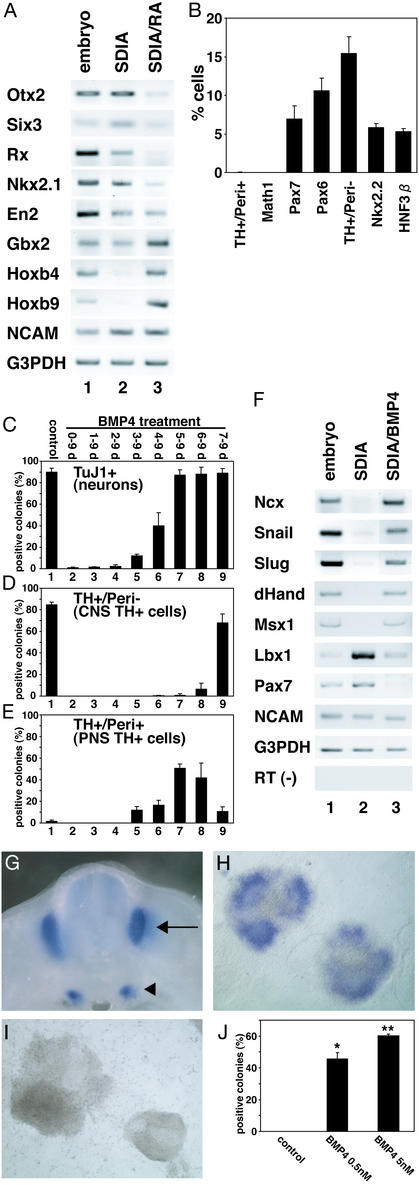
We first examined the expression of rostral–caudal CNS markers by RT-PCR (Fig. 1A). SDIA-treated mouse ES cells express the forebrain markers Otx2 and Six3, the ventral diencephalon marker Rx, the ventral forebrain marker Nkx2.1, the midbrain–hindbrain border marker En2, and the hindbrain marker Gbx2, but not the spinal cord markers Hoxb4 and b9 (lane 2). These results show that a majority of neural cells induced by SDIA express rostral neural markers. This idea is consistent with our previous report that dopaminergic neurons generated by the SDIA method are those of the midbrain type (1).
Figure 1.
(A) RT-PCR analysis of SDIA-treated ES cells (day 8). Lane 1, embryonic day (E)10.5 embryo; lanes 2 and 3, SDIA-treated ES cells without and with 0.2 μM RA treatment during days 4–8, respectively. (B) Percent immunoreactive cells/total cells are shown. (C–E) BMP4 (0.5 nM) treatment during the days indicated above. Positive colonies were defined as ones containing TuJ1+ (C), TH+/Peri− (D), and TH+/Peri+ (E) cells. Lane 1, control, no BMP4 treatment. (F) RT-PCR analysis with neural crest and neural markers. Lane 1, E10.5 whole embryos; lanes 2 and 3, SDIA-treated ES cells without or with 5 nM BMP4 treatment during days 5–8, respectively. (G) Ncx expression in E11.5 mouse by in situ hybridization. Dorsal root (arrow) and sympathetic (arrowhead) ganglia. (H) SDIA/BMP-treated ES cell colonies (day 8). (I) SDIA-treated ES cell colonies. (J) Percentages of Ncx+ colonies at 0, 0.5, and 5 nM BMP4 (n = 100, 200, and 200, respectively). *, P < 0.005 vs. control; **, P < 0.005 vs. control.
We next attempted to alter the rostral–caudal identity of SDIA-induced neural cells by the caudalizing factor retinoic acid (RA; ref. 7). RA treatment (0.2 μM all-trans RA; Fig. 1A, lane 3) suppressed the forebrain markers Otx2, Six3, Rx, and Nkx2.1, whereas it induced the hindbrain marker Gbx2 and the spinal cord markers Hoxb4 and b9. RA treatment did not significantly affect the level of neural cell adhesion molecule (NCAM) expression (Fig. 1A). This demonstrates that the differentiation potential of SDIA-treated ES cells is not limited to the rostral brain region but can also be modified in the caudal direction.
We next examined positional markers along the dorsal–ventral axis. SDIA-treated ES cells contained cells expressing a variety of dorsal-ventral neural markers (Fig. 1B), including HNF3β+ (floor plate), Nkx2.2+ (ventral CNS cells flanking the floor plate), TH+/Peripherin (Peri)− (8) (CNS dopaminergic), Pax6+ (ventral to dorsal), and Pax7+ (alar plate; i.e., dorsal CNS) cells. Interestingly, few cells expressed a phenotype of Math1+ (the dorsalmost interneuron progenitor; ref. 9) or TH+/Peri+ (peripheral autonomic neuron lineage), suggesting that SDIA-treated ES cells do not efficiently generate cells arising from the dorsalmost region of the neural tube, including neural crest derivatives.
Induction of Neural Crest and Dorsalmost CNS Differentiation in SDIA-Treated ES Cells by Late Exposure to BMP4.
The neural crest arises from the juncture of dorsal CNS and nonneural ectoderm (10). A number of BMP family factors are expressed in this dorsal region (10–12). Although BMP signals inhibit neural induction at the early gastrula stage, the same signals promote neural crest determination when applied at later developmental stages (10–12). We therefore examined the effects of BMP4 by focusing on the temporal aspect. Without BMP treatment, a high percentage of ES cell colonies contained TuJ1+ neurons after being cocultured on PA6 cells for 9 days (Fig. 1C, lane 1). Consistent with a previous study (1), TuJ1+ colonies decreased drastically when the cells were treated with BMP4 on and after day 0, 1, or 2 (Fig. 1C, lanes 2–4), whereas E-cadherin+ epidermal precursors increased (ref. 1; not shown). Similar results were obtained for the number of colonies containing TH+/Peri− CNS dopaminergic neurons (Fig. 1D, lanes 1–4). Under these conditions, few colonies contained TH+/Peri+ peripheral autonomic cells (Fig. 1E, lanes 1–4). In contrast, a number of TH+/Peri+ cells appeared in SDIA-treated ES cell colonies when BMP treatment started on days 3–7 (Fig. 1E, lanes 5–9; also see Fig. 2 E and F). Especially when SDIA-treated ES cells were incubated with BMP4 during days 5–9, an efficient induction of the TH+/Peri+ population was observed (51 ± 4% colonies, n = 264, four independent experiments; Fig. 1E, lane 7; also see Fig. 2C for percent of neurons), whereas TH+/Peri− CNS neurons were rarely seen (Fig. 1D, lane 7). These results suggest that late BMP exposure promotes differentiation of at least one type of the neural crest-derived peripheral nervous system (PNS) lineages.
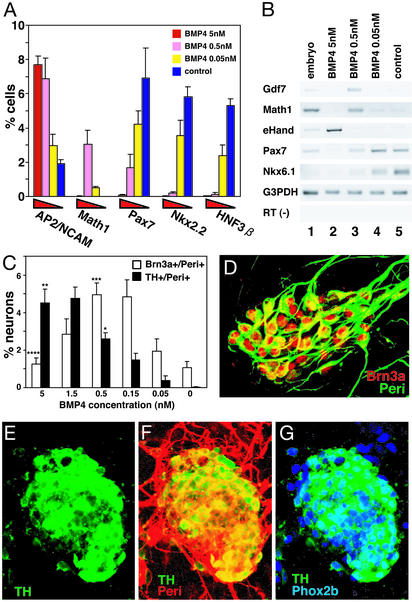
Figure 2.
(A and C) Percent marker-positive cells in the colony (BMP4, days 5–9). Neurons were defined as TuJ1+ cells. *, P < 0.001 vs. 0 nM; **, P < 0.05 vs. 0.5 nM; ***, P < 0.001 vs. 0 nM; ****, P < 0.005 vs. 0.5 nM. (B) RT-PCR analysis of neural and neural crest markers. (D) Sensory lineage markers induced at a low BMP4 concentration [0.5 nM; immunostained with anti-Brn3a (red) and anti-Peri (green) antibodies]. (E–G) Cells with autonomic-lineage markers induced at a high BMP4 concentration (5 nM). Anti-TH (E–G, green); anti-Peri (F, red); anti-Phox2b (G, blue) antibodies.
RT-PCR analysis showed that BMP treatment (from day 5 onward; 5 nM) induced the neural crest markers (10) Ncx (13), Snail, Slug, dHand, and Msx1 (Fig. 1F) in SDIA-treated ES cells (lane 3; lane 2 shows control SDIA-treated ES cells without BMP4). The CNS markers Lbx-1 (14) and Pax7 were suppressed, whereas the panneural marker NCAM was largely unaffected (lanes 2 and 3). In situ hybridization analyses showed that a significant portion of SDIA/BMP-treated ES cell colonies expressed the neural crest marker Ncx-1 (Fig. 1 G, H, and J), but not in control SDIA-treated ES cell colonies (n = 100; Fig. 1 I and J). Neither SDIA/BMP- nor SDIA-treated ES cells expressed a detectable level of the mesodermal markers Brachyury (axial), Paraxis (paraxial; ref. 15), or Foxf1 (lateral plate; ref. 16), or of the endodermal marker Sox17 (17) (not shown). These results suggest that BMP treatment at the right time can promote specification of the neural crest lineage in SDIA-treated ES cells.
We next examined the dose-dependent effect of BMP4 (from day 5 onward) quantitatively on the fates of SDIA-treated ES cells (Fig. 2 A and B). Immunostaining showed that BMP4 significantly decreased the number of HNF3β+, Nkx2.2+, and Pax7+ CNS cells in a dose-dependent manner. Math1+ cells were significantly increased at 0.5 nM BMP4 but not at 5 nM. A similar biphasic induction of Math1 and the roof plate marker Gdf7 (18) ≈0.5 nM was also observed by RT-PCR analysis (Fig. 2B).
One current obstacle in the study of mammalian neural crest determination is the lack of appropriate panneural crest markers. Therefore, we first counted the number of AP2+ cells (19), which are expressed in both neural crest cells (NCAM+; Fig. 6 A–F, which is published as supporting information on the PNAS web site) and in epidermal cells (NCAM−). BMP4 increased the number of AP2+/NCAM+ cells in a dose-dependent manner (Fig. 2A). The sensory ganglion neurons are the major population of cells that are Brn3a+/Peri+ in early neurogenesis (refs. 8, 20, and 21; Fig. 6 G–J). Dose-response analysis (Fig. 2C) showed that BMP4 induced the differentiation of Brn3a+/Peri+ neurons (Fig. 2D) at a lower concentration (0.5 nM) and less efficiently at 5 nM. In contrast, TH+/Peri+ cells were induced more efficiently at 5 nM (Fig. 2C). TH+/Peri+ cells have a round-shaped cell body and are positive for Phox2b (Fig. 2 E–G; characteristic for the autonomic lineage; ref. 22). Consistently, the autonomic lineage marker eHand (10) was preferentially induced at 5 nM (Fig. 2B). This differential requirement of BMP concentrations for sensory and autonomic cells is reminiscent of a previous report that high BMP concentrations preferentially promote differentiation of autonomic neurons from rat-dissociated neural tube explants, whereas low concentrations induce differentiation of sensory neurons (21).
At both high and low BMP concentrations, no cells positive for the smooth muscle cell marker SMA (23), the pigment cell marker Trp-2 (24), or the Schwann cell marker P0 (25) were observed (n = 100 colonies each; not shown). To ask whether SDIA/BMP-treated ES cells also have the competence of differentiating into nonneural derivatives, we performed an in vitro study using culture on a matrix substrate. SDIA/BMP-treated ES cell colonies were isolated on day 8 by papain digestion, replated on fibronectin-coated dishes, and cultured without BMP4 in a medium containing chicken embryo extracts (26). Neuronal (Peri, TuJ, Brn3a) and glial (glial fibrillary acidic protein; positive in <10% colonies) markers were found only inside of the colonies or in their vicinities (not shown). In contrast, numerous SMA+ cells were found within the population of cells migrating from the ES cell colonies (Fig. 7, which is published as supporting information on the PNAS web site; SMA+ cells were found around 85 ± 7% of 158 colonies, four independent experiments; In such colonies, SMA+ cells occupied 84 ± 6% of the migratory cells). Control SDIA-treated ES cells cultured under the same conditions generated SMA+ cells in only 4.5 ± 3.5% colonies (n = 120, four independent experiments). In contrast, Trp-2+ cells or P0+ cells were not detected in SDIA/BMP-treated ES cells under any of the conditions tested above or in medium containing ET-3 and SCF (27) or GGF (26), even after long culture up to 24 days (not shown).
Collectively, these results demonstrate that BMP treatment, as it does in vivo (11, 12), causes dorsalization of ES cell-derived neural tissues and induces generation of neural crest progenitors, which differentiate into, at least, the autonomic, sensory, and smooth muscle cell lineages in vitro.
Shh Suppresses Neural Crest Differentiation and Induces Ventral CNS Differentiation.
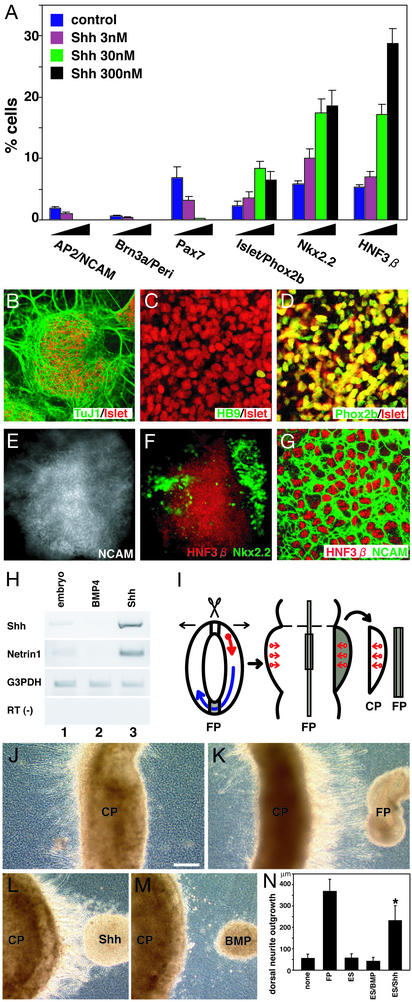
Shh suppresses the development of dorsal tissues and promotes the differentiation of ventral CNS tissues (11). Consistent with this activity, when applied to SDIA-treated ES cells, Shh treatment (days 4–9) suppressed the appearance of AP2+/NCAM+ and Brn3a+/Peri+ cells in a dose-dependent manner (Fig. 3A). Nkx2.2+ and HNF3β+ ventral cells were significantly increased by Shh treatment, whereas the alar plate marker Pax7 was suppressed. In addition, a high proportion of Islet1/2+ neurons (28) were found among TuJ1+ neurons (26 ± 2%, n = 227 colonies, 30 nM Shh during days 4–9, four independent experiments; Fig. 3B). They exhibited a marker phenotype of HB9−, Phox2b+ (Fig. 3 B–D), choline acetyletransferase+ (ChAT), Peri+, Lim3−, AP2−, and Brn3a− (not shown). This is consistent with the marker characteristics for somatic motor neurons of the midbrain–rostral hindbrain and branchiomotor/visceral motor neurons, which are located in the brainstem basal plate (i.e., brainstem-type motor neurons in the cranial nerve nuclei III, IV, V, VII, IX, X, and XI; refs. 22 and 28–31). Consistent with this idea, differentiation of HB9−/Phox2b+/Islet1/2+ neurons was suppressed by additional RA treatment, whereas it induced efficient differentiation of neurons displaying the characteristics of somatic motor neurons of the spinal cord and caudal hindbrain (Islet1/2+, HB9+, Phox2b−; Lim3+, ChAT+; refs. 22 and 28–31; Fig. 8 A–L, which is published as supporting information on the PNAS web site, and data not shown). These findings demonstrate that Shh promotes differentiation of ventral CNS tissues and suppresses that of dorsal CNS and neural crest cells.
Figure 3.
(A) Effects of Shh (days 4–9) on neural crest and dorsal–ventral neural markers. (B–D) HB9−/Phox2b+/Islet1/2+ motor neurons induced by Shh (30 nM, days 4–9). (E and F) Double staining of Nkx2.2 (green) and HNF3β (red) in an SDIA/Shh-treated ES cell colony. The NCAM staining in E indicates the colony area. (G) A high-magnification view. NCAM (green) and HNF3β (red). (H) RT-PCR analysis of Shh and Netrin expression. Lane 1, control embryo; lane 2, SDIA/BMP-treated; and lane 3, SDIA/Shh-treated ES cells. (I) A flat whole- mount preparation of embryonic rat brain and the explant coculture. Arrows, axonal direction. (J) Control CP explant; dorsal (right). (K) A floor plate explant showing chemoattraction activities on CP. (L) CP cocultured with SDIA/Shh-treated ES cells (Shh 300 nM). (M) CP cocultured with SDIA/BMP-treated ES cells (BMP4 5 nM). (N) Quantitative analysis of dorsally extending axon length from CP explants. Mean lengths of dorsal neurite outgrowth (micrometers) with CP alone (none, n = 12), FP (n = 12), undifferentiated ES (ES, n = 14), SDIA/BMP-treated ES (ES/BMP, n = 24), and SDIA/Shh-treated ES cells (ES/Shh, n = 28). *, P < 0.001 vs. none, ES, and ES/BMP.
Directed Differentiation of Functional Floor Plate Cells.
Shh exerted its ventralizing activity on ES cell-derived neural precursors. A similar conclusion has been reported in the study using the Shh/RA-treated embryoid bodies (EBs) (4). Interestingly, there is a clear qualitative difference between the observations in two studies. In the study using the Shh/RA treatment of EBs, the ventralmost CNS markers such as HNF3β were not significantly induced even at a very high concentration of Shh (4). Thus, it seems that the neural progenitors induced in the EB-RA method can be only partially ventralized even by strong Shh signaling. In contrast, Shh treatment (300 nM) showed strong ventralizing effects and resulted in production of numerous Nkx2.2+ and HNF3β+ cells (19%, n = 291 and 29%, n = 279, respectively; Fig. 3 A, E, and F) in SDIA-treated ES cells.
This difference led us to ask whether HNF3β+ cells generated in SDIA/Shh-treated ES cells had additional features of floor plate cells. Most of the HNF3β+ cells were NCAM+ and were found in tightly packed cell clusters (Fig. 3 E–G). RT-PCR analyses showed that SDIA/Shh-treated ES cells expressed significant levels of the floor plate factors Shh and Netrin1 (Fig. 3H, lane 3), whereas SDIA/BMP-treated cells did not (lane 2).
We next tested whether SDIA/Shh-induced tissues possessed axon guidance activities similar to floor plate tissues by using a 3D coculture assay (Fig. 3I). In accordance with previous reports (6, 32), when metencephalic alar plate [cerebellar plate (CP)] explants were cultured alone in collagen gels, virtually no neurites were induced from dorsal surface (Fig. 3J); however, when cocultured with floor plate explants at a distance, neurites were induced to grow dorsally toward the floor plate explants (in the right direction, Fig. 3K). Similarly, when cocultured with SDIA/Shh-treated ES cells, many neurites extended from CP explants toward the ES cell colony located dorsally (Fig. 3 L and N). SDIA/BMP-treated ES cell colonies did not show significant axon growth inducing activities on CP explants (Fig. 3 M and N). These results indicate that Shh treatment induces differentiation of functional floor plate cells in SDIA-treated ES cells.
Differentiation of Neural Crest Derivatives, CNS Neurons, and Floor Plate Cells from Primate ES Cells.
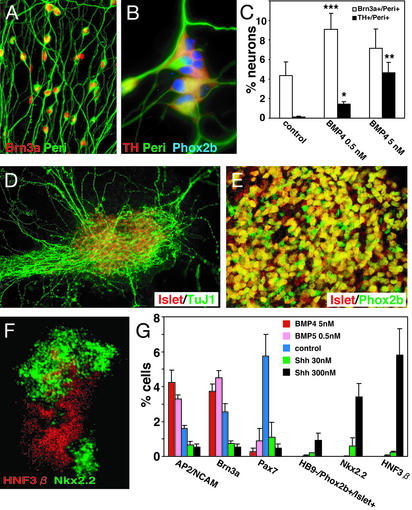
Finally, we examined the differentiation competence of SDIA-treated primate ES cells (2, 5). Primate ES cells differentiated into Brn3a+/Peri+ (sensory lineage) and TH+/Phox2b+/Peri+ (autonomic lineage) neurons (Fig. 4 A–C) under the same conditions used in the mouse cell experiments (Fig. 2), i.e., at low and high BMP doses, respectively. SDIA/Shh-treated primate ES cells differentiated into cells with the brainstem-type motor neuron phenotype (Islet1/2+, HB9−, Phox2b+, choline acetyletransferase (ChAT)+, and Lim3−; Fig. 4 D and E and not shown), whereas SDIA/Shh/RA treatment induced neurons with caudal somatic motor neuron markers (Islet1/2+, HB9+, Phox2b−, ChAT+, and Lim3+; Fig. 9, which is published as supporting information on the PNAS web site). SDIA/Shh treatment also induced a significant number of Nkx2.2+ and HNF3β+ cells from primate ES cells (Fig. 4 F and G). These results show that SDIA-treated primate ES cells can generate the full range of dorsal and ventral neural tissues, as mouse cells.
Figure 4.
(A–C) At low and high concentrations, BMP4 induced sensory bipolar neurons and autonomic neurons, respectively. Cells were treated with 0, 0.5, and 5 nM BMP4 during days 7–13 and analyzed on day 13 for Brn3a/Peri and TH/Peri staining, respectively. Neurons were defined as TuJ1+ cells. *, P < 0.001 vs. control; **, P < 0.005 vs. BMP4 0.5 nM; ***, P < 0.001 vs. control. (D and E) Islet1/2+/Phox2b+ motor neurons generated by SDIA/Shh treatment. (F) Double staining of Nkx2.2 (green) and HNF3β (red) in an SDIA/Shh-treated primate ES cell colony. (G) Effects of BMP4 and Shh on expression of dorsal and ventral markers. BMP4 and Shh were added during days 7–13 and 3–13, respectively.
In contrast, quantitative analysis with dorsal–ventral markers revealed some intriguing species differences. Consistent with our previous study, primate ES cells differentiated into neural cells about half as efficiently as mouse cells on PA6 cells (NCAM+ at 45% efficiency and TuJ1+ at 25%; ref. 2). More importantly, without BMP or Shh treatment, SDIA-treated primate ES cell colonies expressed only dorsal markers (AP2/NCAM, Brn3a, and Pax7; Fig. 4G), whereas mouse ES cell colonies cultured on PA6 expressed both ventral (HNF3β, Nkx2.2) and dorsal (Pax7) neural markers (Fig. 1B). Primate ES cells expressed the ventral markers HNF3β and Nkx2.2 only in the presence of Shh (Fig. 4G). Thus, SDIA-treated primate ES cells (without Shh or BMP treatment) have a positional identity that is more dorsally deviated than SDIA-treated mouse ES cells. This may partly explain why primate ES cells seemed to require a higher Shh concentration (300 nM) for ventral marker induction than did mouse cells (30 nM) (Figs. 3A and 4G), and why a number of Brn3a+/Peri+ cells were generated even without BMP4 treatment (Fig. 4C) from primate ES cells.
Discussion
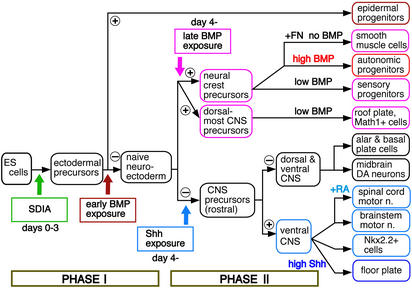
Together with our previous report (1), the present study demonstrates that systematic generation of major ectodermal categories (epidermis, neural crest, roof plate, CNS neurons, and floor plate) from ES cells in vitro is now made possible by manipulating the timing, concentration, and combination of exogenous factors. Our induction strategy and working hypothesis are summarized in Fig. 5. This systematic induction involves at least two critical phases of fate determination. In phase I (days 0–3), SDIA treatment promotes ectodermal differentiation. SDIA suppresses mesodermal differentiation even in the presence of BMP4 (1), which has been shown to have a mesoderm-inducing activity on ES cells in vitro (33, 34). Instead, when treatment of exogenous BMP4 is started during phase I (early BMP exposure), it strongly promotes epidermogenesis (1). Without early BMP exposure, SDIA-treated ES cells acquire a differentiation competence similar to naïve neuroectodermal progenitors.
Figure 5.
Strategy for systematic induction of neural crest, dorsalmost CNS, and ventralmost CNS differentiation by timely exposure to patterning factors.
Phase II (days 4–7) is critical for the acquisition of the dorsal–ventral identity of the neuroectodermal derivatives. In the absence of exogenous BMP4, SDIA-treated ES cells have the competence of differentiating into a variety of dorsal and ventral cells of the rostral CNS (e.g., midbrain dopaminergic neurons), except for the dorsalmost cells of the neural tube. Addition of Shh promotes differentiation of ventral CNS cells, including floor plate cells, Nkx2.2+ cells, and brainstem-type motor neurons, as well as caudal motor neurons with additional RA treatment. In particular, a high Shh concentration induces efficient differentiation of functional floor plate cells (Fig. 3). Motor neurons generated with SDIA/Shh are also functional; innervating C2C12-derived myotubes and causing nicotinic acetylcholine receptor-dependent muscular contraction in vitro (Fig. 8 G–L and Movies 1–3, which are published as supporting information on the PNAS web site).
Treatment of exogenous BMP4 during phase II (late BMP exposure) results in suppression of ventral neural differentiation and promotion of dorsal differentiation. SDIA-treated ES cells with late BMP4 exposure have the competence of differentiating into neural crest derivatives and dorsalmost CNS cells (Math1+ cells and roof plate) (Figs. 1 and 2). Among neural crest-derived cells, a high BMP4 concentration preferentially induces differentiation of autonomic neuron lineages, whereas a low concentration more efficiently promotes generation of sensory neurons (Fig. 2C). When SDIA/BMP4-treated ES cells are replated and cultured without exogenous BMP4 on fibronectin after phase II, smooth muscle cells are efficiently produced.
Interestingly, the timing of phases I and II would correspond well with the embryonic days of pre-/early gastrulation and neurulation, if 3.5 days (the embryonic day of the inner cell mass from which ES cells are derived) are added to the induction periods.
Questions for Future Studies
An important question to be addressed in future studies is the molecular nature of SDIA. Its elucidation is crucial for the establishment of a feeder-free induction system. As reported in our previous study (1), the SDIA activity is not detected in conditioned medium of PA6 cells but on the cell surface. This makes biochemical approaches difficult for molecular identification. The candidate approach has been unsuccessful so far (1).
Although early neural crest markers are induced by SDIA/BMP4 treatment (Fig. 1), this study shows the generation of PNS neurons and smooth muscle cells but not that of other neural crest lineages. Modifying culture conditions should allow us to ask whether SDIA/BMP-induced progenitors can generate other neural crest derivatives. A challenging future study is to purify neural crest progenitors from SDIA/BMP-treated ES cells by FACS (26) and examine the differentiation capacity into neuronal and nonneuronal lineages. For instance, it is worth testing whether the crest progenitors can differentiate into such cells as sensory and enteric neurons and form functional connections in vivo. It is also intriguing to test whether induced progenitors can differentiate into connective tissues, which consist of a major but less understood lineage of the head neural crest. It remains to be clarified how other signaling factors implicated in neural crest differentiation (35) may work in the SDIA system. Our preliminary study shows that neural crest differentiation is not significantly induced in BMP4-treated ES cells simply by adding fibroblast growth factor, Wnt, or RA signals without SDIA treatment.
Collectively, SDIA-based differentiation should serve as a system to bridge a gap between knowledge of neural development and application in regenerative medicine of the nervous system. The method may be used to even broader application as our recent reports indicate that SDIA-treated ES cells can differentiate into ectoderm-derived sensory tissues, such as retinal pigment epithelium (2) and lens cells (36).
Supplementary Material
Acknowledgments
We are grateful to R. Ladher, H. Enomoto, N. Osumi, and N. Rao for comments on this work; to J.-F. Brunet (Centre National de la Recherche Scientifique Unité Mixte de Recherche 8542 Ecole Normale Supérieure, France), J. E. Johnson (University of Texas Southwestern Medical Center, Dallas), J. J. Archelos (Karl-Franzens-Universität, Graz, Austria), and V. J. Hearing (National Cancer Institute, Bethesda) for antisera; to H. Niwa (RIKEN, Kobe, Japan) for GFP-expressing ES cells; to A. Sehara and Y. Tanabe for helpful discussion; to N. Sasai for help in floor plate marker analysis; and to Y. Nakano and T. Katayama for excellent technical assistance. This work was supported by grants from the Organization of Pharmaceutical Safety and Research (to Y.S.), the Japan Society for the Promotion of Science (to N.N. and K.M.), the Ministry of Education, Culture, Sports, Science, and Technology (to Y.S., N.N., and K.M.), the Ministry of Health, Labor, and Welfare (to Y.S.), and the Human Frontier Science Program Organization (to Y.S.).
Abbreviations
- BMP
bone morphogenetic protein
- ES cells
embryonic stem cells
- Peri
peripherin
- PNS
peripheral nervous system
- RA
retinoic acid
- SDIA
stromal cell-derived inducing activity
- Shh
Sonic hedgehog
- TH
tyrosine hydroxylase
- NCAM
neural cell adhesion molecule
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kawasaki H, Mizuseki K, Nishikawa S, Kaneko S, Kuwana Y, Nakanishi S, Nishikawa S-I, Sasai Y. Neuron. 2000;28:31–40. doi: 10.1016/s0896-6273(00)00083-0. [DOI] [PubMed] [Google Scholar]
- 2.Kawasaki H, Suemori H, Mizuseki K, Watanabe K, Urano F, Ichinose H, Haruta M, Takahashi M, Yoshikawa K, Nishikawa S-I, et al. Proc Natl Acad Sci USA. 2002;99:1580–1585. doi: 10.1073/pnas.032662199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee S-H, Lumelsky N, Studer L, Auerbach J M, McKay R D. Nat Biotechnol. 2000;18:675–679. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]
- 4.Wichterie H, Liberam I, Porter J A, Jessell T M. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 5.Suemori H, Tada T, Torii R, Hosoi Y, Kobayashi K, Imahie H, Kondo Y, Iritani A, Nakatsuji N. Dev Dyn. 2001;222:273–279. doi: 10.1002/dvdy.1191. [DOI] [PubMed] [Google Scholar]
- 6.Tamada A, Shirasaki R, Murakami F. Neuron. 1995;14:1083–1093. doi: 10.1016/0896-6273(95)90347-x. [DOI] [PubMed] [Google Scholar]
- 7.Durston A J, van der Wees J, Pijnappel W W, Godsave S F. Curr Top Dev Biol. 1998;40:111–175. doi: 10.1016/s0070-2153(08)60366-x. [DOI] [PubMed] [Google Scholar]
- 8.Troy C M, Brown K, Greene L A, Shelanski M L. Neuroscience. 1990;36:217–237. doi: 10.1016/0306-4522(90)90364-a. [DOI] [PubMed] [Google Scholar]
- 9.Helms A W, Johnson J E. Development (Cambridge, UK) 1998;125:919–928. doi: 10.1242/dev.125.5.919. [DOI] [PubMed] [Google Scholar]
- 10.Knecht A K, Bronner-Fraser M. Nat Rev Genet. 2002;3:453–461. doi: 10.1038/nrg819. [DOI] [PubMed] [Google Scholar]
- 11.Jessell T M. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 12.Sasai Y, De Robertis E M. Dev Biol. 1997;182:5–20. doi: 10.1006/dbio.1996.8445. [DOI] [PubMed] [Google Scholar]
- 13.Hatano M, Iitsuka Y, Yamamoto H, Dezawa M, Yusa S, Kohno Y, Tokuhisa T. Anat Embryol (Berlin) 1997;195:419–425. doi: 10.1007/s004290050061. [DOI] [PubMed] [Google Scholar]
- 14.Gross M K, Dottori M, Goulding M. Neuron. 2002;34:535–549. doi: 10.1016/s0896-6273(02)00690-6. [DOI] [PubMed] [Google Scholar]
- 15.Burgess R, Cserjesi P, Ligon K L, Olson E N. Dev Biol. 1995;168:296–306. doi: 10.1006/dbio.1995.1081. [DOI] [PubMed] [Google Scholar]
- 16.Peterson R S, Lim L, Ye H, Zhou H, Overdier D G, Costa R H. Mech Dev. 1997;69:53–69. doi: 10.1016/s0925-4773(97)00153-6. [DOI] [PubMed] [Google Scholar]
- 17.Kanai-Azuma M, Kanai Y, Gad J M, Tajima Y, Taya C, Kurohmaru M, Sanai Y, Yonekawa H, Yazaki K, Tam P P, et al. Development (Cambridge, UK) 2002;129:2367–2379. doi: 10.1242/dev.129.10.2367. [DOI] [PubMed] [Google Scholar]
- 18.Lee K J, Mendelsohn M, Jessell T M. Genes Dev. 1998;12:3394–3407. doi: 10.1101/gad.12.21.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell P J, Timmons P M, Hebert J M, Rigby P W, Tjian R. Genes Dev. 1991;5:105–119. doi: 10.1101/gad.5.1.105. [DOI] [PubMed] [Google Scholar]
- 20.Fedtsova N G, Turner E E. Mech Dev. 1995;53:291–304. doi: 10.1016/0925-4773(95)00435-1. [DOI] [PubMed] [Google Scholar]
- 21.Lo L, Dormand E, Greenwood A, Anderson D J. Development (Cambridge, UK) 2002;129:1553–1567. doi: 10.1242/dev.129.7.1553. [DOI] [PubMed] [Google Scholar]
- 22.Pattyn A, Morin X, Cremer H, Goridis C, Brunet J-F. Development (Cambridge, UK) 1997;124:4065–4075. doi: 10.1242/dev.124.20.4065. [DOI] [PubMed] [Google Scholar]
- 23.Owens G K. Physiol Rev. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 24.Tsukamoto K, Jackson I J, Urabe K, Montague P M, Hearing V J. EMBO J. 1992;11:519–526. doi: 10.1002/j.1460-2075.1992.tb05082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Archelos J J, Roggenbuck K, Schneider-Schaulies J, Linington C, Toyka K V, Hartung H-P. J Neurosci Res. 1993;35:46–53. doi: 10.1002/jnr.490350107. [DOI] [PubMed] [Google Scholar]
- 26.Morrison S J, White P M, Zock C, Anderson D J. Cell. 1999;96:737–749. doi: 10.1016/s0092-8674(00)80583-8. [DOI] [PubMed] [Google Scholar]
- 27.Reid K, Turnley A M, Maxwell G D, Kurihara Y, Kurihara H, Bratlett P F, Murphy M. Development (Cambridge, UK) 1996;122:3911–3919. doi: 10.1242/dev.122.12.3911. [DOI] [PubMed] [Google Scholar]
- 28.Ericson J, Thor S, Edlund T, Jessell T M, Yamada T. Science. 1992;256:1555–1560. doi: 10.1126/science.1350865. [DOI] [PubMed] [Google Scholar]
- 29.Arber S, Han B, Mendelsohn M, Smith M, Jessell T M, Sockanathan S. Neuron. 1999;23:659–674. doi: 10.1016/s0896-6273(01)80026-x. [DOI] [PubMed] [Google Scholar]
- 30.Thaler J, Harrison K, Sharma K, Lettieri K, Kehrl J, Pfaff S L. Neuron. 1999;23:675–687. doi: 10.1016/s0896-6273(01)80027-1. [DOI] [PubMed] [Google Scholar]
- 31.Varela-Echavarria A, Pfaff S L, Guthrie S. Mol Cell Neurosci. 1996;8:242–257. doi: 10.1006/mcne.1996.0061. [DOI] [PubMed] [Google Scholar]
- 32.Tessier-Lavigne M, Placzek M, Lumsden A, Dodd J, Jessell T M. Nature. 1988;336:775–778. doi: 10.1038/336775a0. [DOI] [PubMed] [Google Scholar]
- 33.Johansson B M, Wiles M V. Mol Cell Biol. 1995;15:141–151. doi: 10.1128/mcb.15.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanatsu M, Nishikawa S-I. Development (Cambridge, UK) 1996;122:823–830. doi: 10.1242/dev.122.3.823. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Castro M I, Marcelle C, Bronner-Fraser M. Science. 2002;297:848–851. doi: 10.1126/science.1070824. [DOI] [PubMed] [Google Scholar]
- 36. Ooto, S., Haruta, M., Honda, Y., Kawasaki, H., Sasai, Y. & Takahashi, M. (2003) Invest. Ophthalmol. Vis. Sci. 44, in press. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.