Abstract
The cellular and molecular mechanisms that underlie age-dependent osteoporosis, the most common disease in the Western Hemisphere, are poorly understood in part due to the lack of appropriate animal models in which to study disease progression. Here, we present a model that shows many similarities to the human disease. Sca-1, well known for its expression on hematopoietic stem cells, is present on a subset of bone marrow stromal cells, which potentially include mesenchymal stem cells. Longitudinal studies showed that Sca-1−/− mice undergo normal bone development but with age exhibit dramatically decreased bone mass resulting in brittle bones. In vivo and in vitro analyses demonstrated that Sca-1 is required directly for the self-renewal of mesenchymal progenitors and indirectly for the regulation of osteoclast differentiation. Thus, defective mesenchymal stem or progenitor cell self-renewal may represent a previously uncharacterized mechanism of age-dependent osteoporosis in humans.
Osteoporosis is a multifactorial, age-related metabolic bone disease characterized by low bone mineral density (BMD) and the deterioration of the microarchitecture of cancellous bone, leading to enhanced bone fragility and increased risk of fracture (1). Type I osteoporosis, also called postmenopausal osteoporosis because it primarily affects postmenopausal women, is characterized by increased bone turnover and accelerated cancellous bone loss, increasing the risk of vertebral fracture. Type II osteoporosis, or age-related/dependent osteoporosis, affects older women and men and its origins are far less understood than postmenopausal osteoporosis. Although type II osteoporosis is not accompanied by increased bone turnover, it leads to increased risk of hip fracture and has a greater mortality and morbidity than type I osteoporosis.
Type I and II osteoporosis are thought to result from dysregulated bone remodeling during aging. Normal bone remodeling requires an exquisite balance between bone resorption by osteoclasts and bone formation by osteoblasts. Osteoblasts are mesenchymal cells that originate from a multipotential precursor, often referred to as the mesenchymal stem cell (MSC), which also gives rise to other lineages such as adipocytes, chondrocytes, and muscle (2, 3). By contrast, osteoclasts differentiate from hematopoietic monocyte/macrophage precursors (4).
Stem cell antigen 1 (Sca-1; also known as Ly-6A) is an 18-kDa glycosyl phosphatidylinositol-anchored cell surface protein of the Ly-6 gene family expressed by hematopoietic stem cells (HSCs), skeletal muscle stem cells, mammary epithelial stem cells, subsets of hematopoietic progenitors, lymphocytes and macrophages, and a subpopulation of bone marrow (BM) stromal cells including osteoblasts (5–11). Sca-1−/− mice exhibit defects in T cell signaling (12) and HSC self-renewal (13).
Here, we report that Sca-1−/− mice also model human age-related (type II) osteoporosis with reduced BMD and increased susceptibility to fractures. Unlike type I osteoporosis, which results from an imbalance toward bone resorption (14–17), Sca-1−/− adult mice have both decreased bone formation because of a deficiency in osteoprogenitors and decreased bone resorption as a consequence of the osteoprogenitor deficiency. The decrease in Sca-1−/− osteoprogenitors during aging is not due to a direct defect in osteoblastogenesis, but rather due to decreased self-renewal activity of multilineage mesenchymal progenitors. Our data suggest that stem cells or their direct descendants are the target cell population affected by Sca-1 ablation and implicate Sca-1 as a component of the stem cell self-renewal machinery.
Materials and Methods
Animals.
All mice were maintained at Mount Sinai Hospital. Sca-1−/− mice were backcrossed 10 generations to BALB/c. Backcross 10 mice were bred to generate Sca-1+/+ control and Sca-1−/− progeny, which were subsequently independently bred.
Histomorphometric and Dual-Energy X-Ray Absorptiometry (DEXA) Analysis.
For in vivo fluorescent labeling, a s.c. injection of calcein (25 mg/kg body weight) was administered at days 0 and 7. Animals were killed at day 12 (18). Plastic bone sections were prepared as described (19). All histomorphometric parameters were measured blindly on trabecular bone of the femoral metaphysis from five animals of each genotype and time point, using a microscope equipped with a TV camera interfaced to a computer running custom-designed software for bone histomorphometry (image proplus). All histomorphometric parameters are reported in accordance with the recommended American Society for Bone and Mineral Research nomenclature (20). Bone resorption parameters were measured on bones excised from five animals of each genotype and time point by counting the number and surface of tartrate-resistant acid phosphatase (TRAP)-positive cells (21, 22). The bone mineral content (BMC) and BMD of whole mice, as well as excised lumbar (L6) vertebrae and right femurs, were determined on 10 mice from each genotype and time point by DEXA as described (ref. 23; PIXImus, GE Medical Systems).
Progenitor Assays.
To study the total number of progenitors of MSCs, osteoprogenitors, functional osteoblasts, and adipocytes in vitro, we performed colony-forming unit fibroblast (CFU-F), CFU alkaline phosphatase (CFU-ALP), CFU osteoblast (CFU-O), and CFU adipocyte (CFU-A) assays, respectively. These assays were performed in both primary calvarial and BM stroma cell cultures with a minimum of three experiments per assay. Primary murine calvarial cell cultures were prepared from 1- to 3-day-old Sca-1+/+ or Sca-1−/− mice (15 newborns from each genotype) as described (24, 25). BM was flushed from four to six animals from each genotype and time point for each experiment, and the recovered cells were counted and plated at 9 × 105 nucleated cells per cm2. For CFU-F assays, cultures were stained with Methylene blue and colonies with >20 cells were counted. For CFU-ALP and CFU-O assays, cultures were grown for 21 days, fixed, and stained by the von Kossa method (26). For the CFU-A assay, calvarial or BM cells were prepared, cultured in medium supplemented with 5 μM BRL-49653 (rosiglitazone; a generous gift from the Sankyo Company, Tokyo) for 10 days, and fixed and stained with Sudan IV to detect lipid droplets.
Osteoclast Formation Assay.
Osteoclasts in coculture were generated as described (27), fixed, and stained for TRAP (28), and TRAP+ multinucleated cells (more than three nuclei) were counted. To test resorption activity, cocultures were grown on Osteologic discs (BD Biosciences) as described by the manufacturer.
RNA Isolation and Reverse Transcription (RT).
Total RNA was isolated from cell cultures at different time points (3, 6, and 10 days). Four micrograms of total RNA was used in each RT reaction and the following primer pairs were used to amplify first strand cDNA in a semiquantitative PCR: ALP, 5′-TTAAGGGCCAGCTACACCAC-3′ and 5′-GATAGGCGATGTCCTTGCAG-3′; L32, 5′-CATGGCTGCCCTTCGGCCTC-3′ and 5′-cattctcttcgctgcgtagcc-3′; adipsin, 5′-TGTACTTCGTGGCTCTGGTG-3′ and 5′-atccggtaggatgacactcg-3′; osteopontin, 5′-AGAGGAGAAGGCGCATTACA-3′ and 5′-GCAACTGGGATGACCTTGAT-3′; and Sca-1, 5′-GGACACTTCTCACACTACAAAG-3′ and 5′-TAACACAGACTCCATCAGGGTAG-3′.
Mechanical Testing of Excised Bones.
The mechanical properties of the excised lumbar vertebral bodies (L6) and right femurs were determined by using a material testing system (Model 1011, Instron, Canton, MA) as described (29). Briefly, vertebral bodies were tested in compression (at 0.5 mm/min) and the femurs were tested in three-point bending (at 1 mm/min). Stress–strain curves were generated from the collected load-displacement data and the specimen geometry (Fig. 1 c and d). These curves were then segmented into the elastic (recoverable deformation) and plastic (permanent deformation) regions at the yield point (determined by the 0.02% offset method). Within these regions, the following bone material properties were determined: (i) elastic modulus (stiffness), (ii) yield stress (strength) and strain (deformation), (iii) failure stress and strain, (iv) postyield strain (permanent deformation), (v) toughness (energy to failure), and (vi) elastic and plastic toughness (pre- and postyield energy).
Figure 1.
BMD and mechanical analysis of Sca-1−/− (KO) and Sca-1+/+ mice (WT) demonstrate that Sca-1−/− mice exhibit age-related osteoporosis. (a) Significantly higher (P < 0.0001) whole-body BMD was observed in control mice at 12 months of age compared with Sca-1−/− animals, whereas no significant differences were observed at 2 months of age. (b) The BMD of excised femurs and L6 vertebrae at 15 months shows significantly less mineralization in Sca-1−/− femurs and vertebrae than those of WT mice, with a greater difference in the vertebrae. (c) Vertebral stress-strain response at 15 months demonstrated that vertebrae from KO were substantially weaker and more compliant than bones from WT mice. (d) Femoral stress–strain response at 15 months showed that femurs from KO were more brittle and weak than femurs from controls, although equally as stiff.
Results
Sca-1−/− Mice Exhibit Phenotypes of Age-Dependent Osteoporosis.
Sca-1−/− mice were initially generated to study the role of Sca-1 in lymphocyte and HSC function (12, 13). During the course of BM-derived hematopoietic cell isolation, we observed that Sca-1−/− bones appeared to splinter more often than bones from control animals. To determine whether Sca-1 plays a role in skeletal development, Sca-1−/− and Sca-1+/+ 14.5-dpc (days postcoitum) embryos and newborns were analyzed by alizarin red–alcian blue staining, which did not show any distinguishable differences in terms of bone development and skeletal pattern (data not shown). BMD and BMC of 2- and 12-month-old female mice were analyzed by using DEXA. No differences between WT and mutant total BMD and BMC were observed in young mice; however, by 1 year of age, Sca-1+/+ mice had dramatically increased total BMD and BMC, whereas Sca-1−/− mice had essentially the same total BMD and BMC as 10 months earlier (Fig. 1a; data not shown). Two to 3 months later, the animals were killed and the femurs and vertebrae were removed for analysis. BMC and BMD analyses performed on individual bones demonstrated that the decreased BMC and BMD of mutant 15-month-old bones were greatest in vertebrae (Fig. 1b).
To determine the physiological effects of decreased mineral content in Sca-1−/− bones, mechanical and structural analyses were performed on the same bones used for DEXA analysis. Consistent with the BMC and BMD data, compression testing of vertebrae and three-point bending testing of femurs revealed no differences between WT and mutant bones of 4-month-old mice. However, the vertebrae from 15-month-old Sca-1−/− mice were not as stiff and were substantially weaker and more compliant than bones from WT animals (Fig. 1c, Table 1). In contrast, the femurs from 15-month-old Sca-1−/− mice had a similar stiffness to the Sca-1+/+ bones but were weaker and considerably more brittle (Fig. 1d, Table 1).
Table 1.
Mechanical properties of excised bones at 15 months (mean ± SEM)
| Vertebrae (n = 10)
|
Femurs (n = 10)
|
|||
|---|---|---|---|---|
| WT | KO | WT | KO | |
| Elastic modulus, GPa | 0.710 ± 0.067 | 0.431 ± 0.070* | 13.4 ± 0.4 | 12.8 ± 0.6 |
| Yield stress, MPa | 21 ± 1 | 14 ± 1* | 166 ± 4 | 171 ± 12 |
| Failure stress, MPa | 21 ± 2 | 16 ± 1* | 231 ± 7 | 204 ± 6* |
| Yield strain, % | 4.2 ± 0.4 | 4.6 ± 0.6 | 1.81 ± 0.07 | 2.0 ± 0.2 |
| Failure strain, % | 5.9 ± 0.5 | 8 ± 1 | 2.8 ± 0.2 | 2.5 ± 0.2 |
| Postyield strain, % | 1.6 ± 0.3 | 3.2 ± 0.9† | 1.0 ± 0.2 | 0.5 ± 0.2† |
| Toughness (total), mJ/mm3 | 0.8 ± 0.1 | 0.8 ± 0.2 | 343 ± 44 | 248 ± 22† |
| Toughness (elastic), mJ/mm3 | 0.42 ± 0.06 | 0.30 ± 0.03† | 139 ± 5 | 154 ± 11 |
| Toughness (plastic), mJ/mm3 | 0.36 ± 0.07 | 0.5 ± 0.1 | 204 ± 45 | 93 ± 27* |
Bone mechanical properties were determined as described in Materials and Methods. Briefly, vertebral bodies (L6) were tested to failure in compression and right femurs were tested to failure in three-point bending. Resultant stress–strain curves were segmented into the elastic (recoverable) and plastic (permanent) deformation regions at the yield point. The elastic modulus, stress, strain, and toughness were determined for each of the bones from the WT and KO mice (n, the number of bones tested).
Significant difference between WT and KO (P < 0.05).
Trend between WT and KO (P < 0.1).
Sca-1−/− Mice Have a Non-Cell Autonomous Osteoclast Defect.
Type I osteoporosis in humans typically reflects an imbalance in bone remodeling such that bone resorption exceeds bone formation. As discussed above, bone resorption is the unique function of the osteoclast, a specialized multinucleated cell derived from the monocyte–macrophage lineage cell that depends on functional osteoprogenitors for its differentiation and activity. A simple explanation for osteoporosis in Sca-1−/− mice would be an increase in osteoclasts or osteoclast activity. To test the first possibility, TRAP, an enzyme specifically expressed by osteoclasts, was used to stain decalcified femoral sections, and TRAP+ cells were counted in double-blind experiments. Surprisingly, the absolute number of osteoclasts was reduced by >40% in both 2- and 9-month-old Sca-1−/− femurs compared with WT bones (Fig. 2a).
Figure 2.
Sca-1−/− mice have a non-cell autonomous osteoclast defect. (a) The absolute number of TRAP+ (osteoclasts) cells in femurs of both 2- and 9-month-old Sca-1−/− mice is <40% of that in Sca-1+/+ mice. (b) Osteoblast (OB)/splenocytes (monocyte, MO) coculture experiments. Sca-1+/+ (WT) osteoblast (2 × 104 cells per well) and splenic (1.5 × 105 cells per well) cultures were used to standardize the culture results (i.e., 100% on x axis); osteoclast formation in all other culture combinations is specified as the percentage compared with the WT cocultures. The number of TRAP+ cells in cocultures in which monocytes are derived from WT mice and osteoblasts are derived from either Sca-1−/− or WT mice is significantly different (P < 0.001). However, osteoclast formation is not significantly altered in cocultures in which OB are derived from KO and monocytes from either Sca-1−/− or WT mice (P = 0.4). (c) The number of mononucleated, binucleated, and multinucleated osteoclasts, and total number of osteoclasts (TRAP+ cells) generated in vitro by different combinations of osteoblasts and monocytes derived from WT or Sca-1−/− mice. (d) Images of TRAP+ mononucleated, binucleated, and multinucleated cells generated by a combination of monocytes and osteoblasts derived from Sca-1−/− mice in culture.
To determine whether the reduction in osteoclasts is a cell-autonomous defect, coculture studies using different combinations of osteoblastic cells (derived from newborn calvaria) and osteoclast progenitors (splenocytes) from WT and Sca-1−/− mice were performed (Fig. 2b). Sca-1−/− osteoblast and splenic cocultures generated <10% of the osteoclasts found in Sca-1+/+ cultures, consistent with our in vivo findings. Although the combination of Sca-1+/+ calvaria cells and Sca-1−/− splenocytes showed a slight reduction in the number of osteoclasts compared with what was seen with Sca-1+/+ splenocytes, this was not statistically significant, suggesting that the reduction in osteoclasts in Sca-1−/− mice is not due to a hematopoietic defect. Notably, when Sca-1−/− osteoblastic cells were used with Sca-1+/+ splenocytes, markedly reduced numbers of WT osteoclasts were generated, indicating that the reduction in osteoclasts in situ is a secondary effect of a primary stromal cell defect. Furthermore, the percentage of multinucleated osteoclasts among the total osteoclasts that develop is unaltered amongst the various coculture combinations, demonstrating that there is no intrinsic defect in osteoclast maturation in the absence of Sca-1 (Fig. 2 c and d). Functional characterization of in vitro-differentiated osteoclasts by using the resorption pit assay also demonstrated that Sca-1−/− osteoclasts are capable of bone resorption (data not shown). Therefore, Sca-1−/− animals exhibit a non-cell autonomous defect in osteoclastogenesis resulting in an osteoclast cytopenia. Rather than osteoclasts contributing to osteoporosis in Sca-1-deficient mice, reduced osteoclastogenesis in these mice may actually diminish the severity of the underlying defect.
Sca-1−/− Mice Have a Reduced Number of Osteoprogenitors.
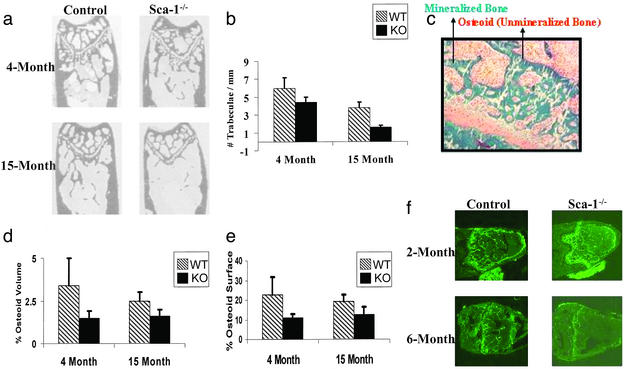
The inability of Sca-1−/− osteoblastic cells to support osteoclast development suggests that a defect in the osteoblast lineage is responsible for the reduced BMD despite our finding that Sca-1−/− animals appear to undergo normal bone formation during embryonic development. Thus, we carried out image analysis on plastic sections of femurs from 4- and 15-month-old mice (Fig. 3 a and b). Sca-1−/− femurs have 27% (P < 0.001) fewer trabeculae compared with control bones at 4 months of age and 58% (P < 0.001) compared with Sca-1+/+ bones at 15 months (Fig. 3b). Consistent with fewer trabeculae, histomorphometric analysis showed that the amount of osteoid (unmineralized bone) secreted by Sca-1−/− osteoblasts was significantly lower in both 4- and 15-month-old bones (Fig. 3 c–f), suggesting that by 4 months of age, Sca-1−/− animals already have fewer osteoblasts or less osteoblast activity. As a further assessment of osteoblast activity (i.e., bone formation), in vivo double calcein labeling of 2- and 6-month-old Sca-1−/− and Sca-1+/+ mice was performed and showed that bone formation in Sca-1−/− mice is essentially normal at 2 months of age but is dramatically reduced by 6 months of age (Fig. 3f).
Figure 3.
Progressive diminution of bone mass in Sca-1−/− mice. (a) Plastic sections of femurs from Sca-1−/− and Sca-1+/+ mice at 4 and 15 months of age used for image analysis. (b) Image analysis demonstrates that Sca-1−/− femurs have 27% (P < 0.001) fewer trabeculae compared with control bones at 4 months of age and 58% (P < 0.001) compared with Sca-1+/+ bones at 15 months of age. (c) Goldner's trichrome staining of unmineralized bone sections of femur showing mineralized bone (green), osteoid (red), and cartilage (pink) was used to measure osteoid volume and osteoid surface. (d and e) The percent osteoid volume and osteoid surface calculated from unmineralized femoral sections from 4- and 15-month-old Sca-1−/− mice are less than those of Sca-1+/+ mice. (f) Double calcein labeling of bones from Sca-1−/− and Sca-1+/+ mice at 2 and 6 months of age revealed that bone formation in Sca-1−/− mice is essentially normal at 2 months of age but is dramatically reduced by 6 months of age.
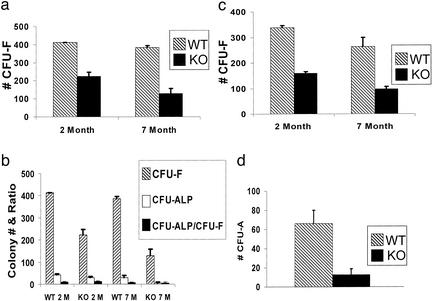
To evaluate the cellular basis of decreased bone formation in Sca-1−/− mice, in vitro analysis of osteoprogenitor frequency and differentiation capacity [i.e., CFU assays] was performed. CFU-ALP assays demonstrated that Sca-1−/− BM has reduced osteoprogenitors by 2 months of age (Fig. 4a). Furthermore, CFU-O (in vitro bone nodule formation) were reduced by more than half in Sca-1−/− cultures, consistent with decreased functional progenitors (Fig. 4b). To determine whether committed osteoprogenitors proliferate at a normal rate, primary calvaria cultures were grown and differentiated in vitro, and ALP-expressing (ALP+) cells were counted at different time points (Fig. 4c). Sca-1−/− cultures generated 45–60% fewer ALP+ cells at all time points, suggesting that CFU-ALP was reduced in Sca-1−/− mice, although ALP+ cells proliferated at the same rate as Sca-1+/+ cells. Based on the increase in ALP+ cells between 2 and 4 days in culture, the doubling time for WT and mutant ALP+ cells was found to be 49.0 and 52.5 h, respectively. The 7% increase in doubling time by Sca-1−/− cells cannot account for the 45–60% reduction in ALP+ cells at all time points assayed (Fig. 4c). Furthermore, the colony size of CFU-ALP was found to be similar in WT and Sca-1−/− cultures, again suggesting that there is no intrinsic defect in proliferation by Sca-1−/− progenitors (data not shown). Molecular analysis confirmed that Sca-1 expression increases during osteogenesis and that the osteoprogenitor differentiation pathway is intact in Sca-1−/− mice (Fig. 4d). For example, semiquantitative RT-PCR analysis of ALP, osteopontin, and bone sialoprotein mRNA expression did not show significant differences between Sca-1−/− and Sca-1+/+ cultures undergoing differentiation (Fig. 4d).
Figure 4.
In vitro analysis of the cellular and molecular basis of the bone formation deficiency in Sca-1−/− mice. (a) The numbers of CFU-F and CFU-ALP generated in BM cultures derived from 2-month-old Sca-1−/− mice are <50% of those derived from Sca-1+/+ mice. (b) The number of CFU-O formed from Sca-1−/− cultures is ≈50% of CFU-O from Sca-1+/+ cultures. (c) The number of ALP+ cells in calvarial cultures from Sca-1−/− at different time points is consistently less than that of Sca-1+/+ mice. The cultures were initiated with 10,000 cells. (d) RT-PCR analysis of osteoblast- and adipocyte-associated genes during in vitro differentiation at 3, 6, and 10 days excludes the possible disturbances of osteoblast and/or adipocyte pathways in Sca-1−/− mice. Expression of adipsin (mADP), alkaline phosphatase (ALP), osteopontin (OPN), Sca-1, and bone sialoprotein (BSP) genes were examined, and L32 expression was used as a control for relative mRNA levels.
Sca-1−/− Mice Exhibit a Defect in Mesenchymal Progenitors.
To determine the origin of the osteoprogenitor deficiency, equal numbers of nucleated BM cells from 2- and 7-month-old Sca-1−/− and Sca-1+/+ mice were cultured. The number of CFU-F, a measure of total mesenchymal precursors including stem cells and committed progenitors (30), formed in Sca-1−/− compared with Sca-1+/+ cultures was reduced in 2- and 7-month-old mice by 45% and 67%, respectively (Fig. 5a). These data demonstrate that mesenchymal precursors (potentially stem cells) are fewer and tend to lose their capability of self-renewal faster with advancing age in Sca-1−/− compared with WT mice. Furthermore, CFU-ALP was also decreased in Sca-1−/− cultures (Fig. 5b) but the percentage of osteoprogenitors among stromal progenitors (i.e., CFU-ALP and CFU-F) in cultures from Sca-1−/− and Sca-1+/+ mice was constant (≈10%), indicating that the observed deficiency in osteoprogenitors is a consequence of an overall decline in stromal progenitors. The decrease in CFU-F with aging suggests that Sca-1 is required for self-renewal of the MSC or an early progenitor in vivo. In an attempt to test this hypothesis in vitro, replicate BM stromal cultures were put through secondary and tertiary passaging by plating equal numbers of Sca-1−/− or WT cells at each passage. One set of cultures was stained for CFU-F analysis and the second set was harvested for replating. In each passage, the CFU-F in Sca-1−/− cultures was reduced by more than half, consistent with defective MSC self-renewal. Specifically, an average reduction of 53% and 63%, respectively, was observed in CFU-F from mutant cultures in the secondary passages of stroma from 2- and 7-month mice (Fig. 5c). Too few cells were recovered from 7-month-old mutant mice for tertiary passaging; however, we observed a 57% loss of CFU-F from cultures derived from 2-month-old Sca-1−/− mice (data not shown). Annexin V staining and viability analysis demonstrated that under the conditions used for these assays, neither WT nor Sca-1−/− cells undergo significant apoptosis (data not shown). Furthermore, examination of plastic sections of femurs did not show any difference in cell viability in vivo between WT and mutant bone cells (data not shown).
Figure 5.
Mesenchymal progenitor self-renewal deficiency in Sca-1−/− mice. (a) Total number of CFU-F formed in Sca-1−/− compared with Sca-1+/+ cultures is reduced 45% and 67%, respectively, in 2- and 7-month-old mice. (b) The total numbers of CFU-F and CFU-ALP are decreased in primary BM stromal cultures derived from 2- and 7-month-old Sca-1−/− cultures; however, the ratio of CFU-ALP/CFU-F is consistent (≈10%) between KO and WT cultures, indicating that the decrease in osteoprogenitors is due to a reduced total number of stroma progenitors in Sca-1−/− mice. (c) Total number of CFU-F in secondary BM stromal cultures derived from 2- and 7-month-old Sca-1−/− mice is ≈50% of those derived from Sca-1+/+ mice, suggesting a self-renewal deficiency by Sca-1−/− stromal progenitors. (d) Reduced CFU-A (adipocyte) was generated in BM stromal cultures derived from Sca-1−/− mice.
Diminished self-renewal of early mesenchymal progenitors in Sca-1−/− mice suggested that other mesenchymal lineages might be affected in a manner analogous to osteoprogenitors; therefore, we studied adipogenesis in vitro. Consistent with the observed decrease in total stromal progenitors and osteoprogenitors, Sca-1−/− BM stroma contains significantly fewer adipocyte progenitors (CFU-A) than present in Sca-1+/+ mice (Fig. 5d), but those present appeared to mature normally, as shown by morphological and gene expression analysis (Fig. 4d; data not shown).
Discussion
We have shown that mice deficient in the glycosyl phosphatidylinositol-anchored cell surface protein Sca-1 develop age-related osteoporosis, representing a unique genetic model of the most common degenerative disease affecting the Western Hemisphere. The imbalance between bone formation and resorption in these mutant animals is due to a primary defect in the self-renewal capacity of an early mesenchymal precursor, possibly the MSC, leading to decreased osteoprogenitor development and failure of the mice to attain peak bone mass during skeletal growth, a critical risk factor for human osteoporotic syndromes. Although all of the data presented in this manuscript were obtained by using females, BMD and structural analysis of young and old male mice recapitulates the phenotypes observed in females (S.D.W. and M.D.G., unpublished results), suggesting that Sca-1−/− mice provide a unique model of type II osteoporosis.
Osteoporosis-like phenotypes have been reported in a variety of transgenic or knockout mouse models, including ones in which cytokines or growth factors have been overexpressed or ablated. However, in each case the osteoporotic phenotypes result from developmental defects in mesenchymal or hematopoietic cells or accompany pleiotropic phenotypes (15–17, 31–36). Thus, although a number of mouse mutants have elucidated critical bone developmental and homeostatic pathways, Sca-1−/− mice provide a unique model because they have phenotypically normal bones into adulthood and the age-related osteoporosis is due to defective self-renewal capacity of mesenchymal progenitors or stem cells, which becomes more severe as the animals age. Furthermore, the cellular hallmark of type II osteoporosis is a decline in osteoblast numbers and a decrease in bone formation activity leading to a slow loss in bone mass (37), which is consistent with the Sca-1−/− phenotype and the self-renewal defect of primitive mesenchymal progenitors underlying the phenotype.
In addition to contributing an important tool for dissecting the molecular mechanisms that underlie this disease and testing treatments, our work leads to two fundamental hypotheses. First, although calvaria osteoblasts arise developmentally from a different pool of precursors than do those in the axial and appendicular skeleton, the defects in Sca-1−/− mice are consistent throughout the skeletal system, suggesting that the MSCs that seed these tissues in postnatal life may have the same origin or that Sca-1 function is conserved throughout the skeletal system. This also suggests that well established site-specific differences in bone architecture and make-up, responsiveness to hormones, and heterogeneity of osteoblast phenotype (38) arise downstream of the MSCs that contribute to the precursor and mature cell pools at these sites.
Finally, we hypothesize that type II osteoporosis, or some subset of cases, is a stem cell disease. Although many degenerative diseases affecting other tissues have primary or secondary stem cell defects, there has been little direct evidence for this in age-related (type II) osteoporosis. However, the observation that CFU-F colony sizes showed significant reduction with age in human populations, suggesting that a change in proliferative potential of progenitors occurs with age (39), supports this concept. We postulate that the mechanical stress of movement and dynamic bone remodeling eventually require replacement of limited lifespan bone forming osteoblasts from progenitor pools, a process that would begin with MSC division. If the MSC asymmetrically divides into a daughter stem cell as well as a daughter progenitor that proceeds along the developmental pathway to form osteoblasts, then homeostasis is preserved. However, if MSCs lack appropriate self-renewal signals or when both daughter cells differentiate because of defective cell signaling, the stem cell pool would be reduced with age, leading to fewer osteoblasts and the eventual development of osteoporosis. Simmons and colleagues (Paul Simmons, personal communication) have found that one in nine Sca-1+CD45− compact bone cells forms at least a bipotential mesenchymal colony. This highly enriched population of mesenchymal progenitors suggests that Sca-1 is a marker of the MSC or a MSC-like progenitor. Unfortunately, it is difficult to conclusively determine that Sca-1 is expressed by MSCs because the stromal cell field has not developed true stem cell assays such as the competitive repopulation assay for the HSC (13). However, we hypothesize that Sca-1 influences the cell fate decision of mesenchymal progenitors as well as other stem cells to self-renew, and its absence leads to a decrease in the stem cell/progenitor pool. Consistent with this hypothesis, our preliminary biochemical analysis of Sca-1 signaling in mast cells demonstrates that Sca-1−/− cells have altered levels of tyrosine phosphorylated proteins in response to proliferation signals (N. Ciliberti, M. Ohishi, and W.L.S., unpublished results). Furthermore, we have shown elsewhere (13) that Sca-1−/− mice exhibit defective HSC self-renewal as measured by transplantation capacity. In addition, preliminary experiments have demonstrated that muscle regeneration is impaired in Sca-1−/− mice, suggesting that skeletal muscle progenitors also require Sca-1 for normal function (M.B. and W.L.S., unpublished results). Sca-1 is expressed on additional stem cell lineages; therefore, the investigation of the role of Sca-1 in the regenerative potential of these organ systems, as well as potential Sca-1 signaling pathways, may provide new strategies to manipulate stem cells toward therapeutic and tissue engineering ends.
Acknowledgments
We thank Jim Dennis, John E. Dick, Janet Rossant, and Peter Zandstra for helpful discussions and critical reading of the manuscript, and Nadia Ciliberti, Sepideh Mansoob, Fakhree Rokhfrouz, Ben Rogers, and Usha Bhargava for excellent technical assistance. This work was supported by operating grants from the Canadian Institutes of Health Research (to J.E.A. and W.L.S.) and the Stem Cell Network (to J.E.A.), and salary awards from Canada Research Chairs (to W.L.S.), the Canadian Institutes of Health Research (to M.B.), the Leukemia and Lymphoma Society (to W.L.S., the Karyn Glick Memorial Special Fellow), and the Stem Cell Network (to M.B.). This work was also supported by infrastructure and operating grants awarded to the Centre for Modeling Human Disease (www.cmhd.ca) and through collaborations with members of the Centre for Modeling Human Disease.
Abbreviations
- ALP
alkaline phosphatase
- BM
bone marrow
- BMC
bone mineral content
- BMD
bone mineral density
- CFU
colony-forming unit
- CFU-A
CFU adipocyte
- CFU-F
CFU fibroblast
- CFU-O
CFU osteoblast
- HSC
hematopoietic stem cell
- MSC
mesenchymal stem cell
- TRAP
tartrate-resistant acid phosphatase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Wasnich R D. In: Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Favus M J, editor. Philadelphia: Lippincott Williams & Wilkins; 1996. pp. 249–251. [Google Scholar]
- 2.Bellows C G, Wang Y H, Heersche J N M, Aubin J E. Endocrinology. 1994;134:2221–2229. doi: 10.1210/endo.134.5.8156925. [DOI] [PubMed] [Google Scholar]
- 3.Aubin J E. J Cell Biochem. 1998;Suppl. 30/31:73–82. [Google Scholar]
- 4.Roodman G D. Exp Hematol. 1999;27:1229–1241. doi: 10.1016/s0301-472x(99)00061-2. [DOI] [PubMed] [Google Scholar]
- 5.Spangrude G J, Heimfeld S, Weissman I L. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 6.Gussoni E, Soneoka Y, Strickland C D, Buzney E A, Khan M K, Flint A F, Kunkel L M, Mulligan R C. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 7.Welm B E, Tepera S B, Venezia T, Graubert T A, Rosen J M, Goodell M A. Dev Biol. 2002;245:42–56. doi: 10.1006/dbio.2002.0625. [DOI] [PubMed] [Google Scholar]
- 8.Trevisan M, Iscove N N. J Exp Med. 1995;181:93–103. doi: 10.1084/jem.181.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gumley T P, McKenzie I F, Sandrin M S. Immunol Cell Biol. 1995;73:277–296. doi: 10.1038/icb.1995.45. [DOI] [PubMed] [Google Scholar]
- 10.Horowitz M C, Fields A, DeMeo D, Qian H Y, Bothwell A L, Trepman E. Endocrinology. 1994;135:1032–1043. doi: 10.1210/endo.135.3.7520861. [DOI] [PubMed] [Google Scholar]
- 11.Satoh M, Mioh H, Shiotsu Y, Ogawa Y, Tamaoki T. Exp Hematol. 1997;25:972–979. [PubMed] [Google Scholar]
- 12.Stanford W L, Haque S, Alexander R, Liu X, Latour A M, Snodgrass H R, Koller B H, Flood P M. J Exp Med. 1997;186:705–717. doi: 10.1084/jem.186.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito C Y, Li C Y J, Bernstein A, Dick J E, Stanford W L. Blood. 2003;101:517–523. doi: 10.1182/blood-2002-06-1918. [DOI] [PubMed] [Google Scholar]
- 14.Lewis D B, Liggitt H D, Effmann E L, Motley S T, Teitelbaum S L, Jepsen K J, Goldstein S A, Bonadio J, Carpenter J, Perlmutter R M. Proc Natl Acad Sci USA. 1993;90:11618–11622. doi: 10.1073/pnas.90.24.11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erlebacher A, Derynck R. J Cell Biol. 1996;132:195–210. doi: 10.1083/jcb.132.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi T, Wada T, Mori M, Kokai Y, Ishii S. Lab Invest. 1996;74:827–834. [PubMed] [Google Scholar]
- 17.Riggs B L, Meltion L J. N Engl J Med. 1986;314:1676–1686. doi: 10.1056/NEJM198606263142605. [DOI] [PubMed] [Google Scholar]
- 18.Vignery A, Baron R. Anat Rec. 1980;196:191–200. doi: 10.1002/ar.1091960210. [DOI] [PubMed] [Google Scholar]
- 19.Sabatakos G, Sims N A, Chen J, Aoki K, Kelz M B, Amling M, Bouali Y, Mukhopadhyay K, Ford K, Nestler E J, Baron R. Nat Med. 2000;6:985–990. doi: 10.1038/79683. [DOI] [PubMed] [Google Scholar]
- 20.Parfitt A M, Drezner M K, Glorieux F H, Kanis J A, Malluche H, Meunier P J, Ott S M, Recker R R. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 21.Boyce B F, Yoneda T, Lowe C, Soriano P, Mundy G R. J Clin Invest. 1992;90:1622–1627. doi: 10.1172/JCI116032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersson G, Ek-Rylander B. Acta Orthop Scand Suppl. 1995;266:189–194. [PubMed] [Google Scholar]
- 23.Nagy T R, Clair A L. Obesity Res. 2000;8:392–398. doi: 10.1038/oby.2000.47. [DOI] [PubMed] [Google Scholar]
- 24.Bellows C G, Heersche J N M, Aubin J E. Dev Biol. 1990;140:132–138. doi: 10.1016/0012-1606(90)90060-v. [DOI] [PubMed] [Google Scholar]
- 25.Jochum W, David J P, Elliott C, Wutz A, Plenk H, Jr, Matsuo K, Wagner E F. Nat Med. 2000;6:980–984. doi: 10.1038/79676. [DOI] [PubMed] [Google Scholar]
- 26.Baron R, Vignery A, Neff L, Silvergate A, Maria A S. Bone Histomorphometry: Technique and Interpretation. Vol. 1. Boca Raton, FL: CRC Press; 1983. pp. 13–35. [Google Scholar]
- 27.Aoki K, Didomenico E, Sims N A, Mukhopadhyay K, Neff L, Houghton A, Amling M, Levy J B, Horne W C, Baron R. Bone. 1999;25:261–267. doi: 10.1016/s8756-3282(99)00174-x. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi N, Yamana H, Yoshiki S, Roodman G D, Mundy G R, Jones S J, Boyde A, Suda T. Endocrinology. 1988;122:1373–1385. doi: 10.1210/endo-122-4-1373. [DOI] [PubMed] [Google Scholar]
- 29.Turner C H, Hsieh Y F, Muller R, Bouxsein M L, Baylink D J, Rosen C J, Grynpas M D, Donahue L R, Beamer W G. J Bone Miner Res. 2000;15:1126–1131. doi: 10.1359/jbmr.2000.15.6.1126. [DOI] [PubMed] [Google Scholar]
- 30.Prockop D J. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 31.Bucay N, Sarosi I, Dunstan C R, Morony S, Tarpley J, Capparelli C, Scully S, Tan H L, Xu W, Lacey D L, et al. Genes Dev. 1988;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, et al. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 33.Li B, Boast S, de los Santos K, Schieren I, Quiroz M, Teitelbaum S L, Tondravi M M, Goff S P. Nat Genet. 2000;24:304–308. doi: 10.1038/73542. [DOI] [PubMed] [Google Scholar]
- 34.Kato M, Patel M S, Levasseur R, Lobov I, Chang B H, Glass D A, 2nd, Hartmann C, Li L, Hwang T H, Brayton C F, et al. J Cell Biol. 2002;157:303–314. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gong Y, Slee R B, Fukai N, Rawadi G, Roman-Roman S, Reginato A M, Wang H, Cundy T, Glorieux F H, Lev D, et al. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 36.Xu T, Bianco P, Fisher L W, Longenecker G, Smith E, Goldstein S, Bonadio J, Boskey A, Heegaard A M, Sommer B, et al. Nat Genet. 1998;20:78–82. doi: 10.1038/1746. [DOI] [PubMed] [Google Scholar]
- 37.Manolagas S C, Jilka R L. N Engl J Med. 1995;332:305–311. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- 38.Aubin J E, Candeliere G A, Bonnelye E. The Endocrinologist. 1999;9:25–31. [Google Scholar]
- 39.Oreffo R O, Bord S, Triffitt J T. Clin Sci. 1998;94:549–555. doi: 10.1042/cs0940549. [DOI] [PubMed] [Google Scholar]