Abstract
The organic cation transporter, OCT1, is a major hepatic transporter that mediates the uptake of many organic cations from the blood into the liver where the compounds may be metabolized or secreted into the bile. Because OCT1 interacts with a variety of structurally diverse organic cations, including clinically used drugs as well as toxic substances (e.g., N-methylpyridinium, MPP+), it is an important determinant of systemic exposure to many xenobiotics. To understand the genetic basis of extensive interindividual differences in xenobiotic disposition, we functionally characterized 15 protein-altering variants of the human liver organic cation transporter, OCT1, in Xenopus oocytes. All variants that reduced or eliminated function (OCT1-R61C, OCT1-P341L, OCT1-G220V, OCT1-G401S, and OCT1-G465R) altered evolutionarily conserved amino acid residues. In general, variants with decreased function had amino acid substitutions that resulted in more radical chemical changes (higher Grantham values) and were less evolutionarily favorable (lower blosum62 values) than variants that maintained function. A variant with increased function (OCT1-S14F) changed an amino acid residue such that the human protein matched the consensus of the OCT1 mammalian orthologs. Our results indicate that changes at evolutionarily conserved positions of OCT1 are strong predictors of decreased function and suggest that a combination of evolutionary conservation and chemical change might be a stronger predictor of function.
Interindividual differences in response to xenobiotics, which include many clinically used drugs, are extensive and represent a major problem in rational therapeutics. Such differences in many cases may be caused by inherited differences in enzymes and transporters which function in drug elimination in the liver (1). The organic cation transporter, OCT1, is a major transporter located in the sinusoidal membrane of the liver that mediates the uptake of many organic cations from the blood into hepatocytes. These organic cations include clinically used drugs (e.g., metformin), endogenous compounds (e.g., dopamine), as well as toxic substances (e.g., MPP+) (2–6). Although rare mutations in liver transporters (e.g., MRP2 and BSEP) have been associated with Mendelian diseases such as Dubin–Johnson syndrome (7–9), little is known about the contribution of common variants of these transporters to variation in hepatic drug disposition and disease.
To understand the genetic basis of extensive interindividual differences in xenobiotic disposition, we have screened for variants in 24 different membrane transporters, including OCT1, in 247 ethnically diverse DNA samples (10). Genetic variants of OCT1 identified in the screening study include 14 nonsynonymous single-nucleotide polymorphisms (SNPs), and one 3-bp deletion that leads to deletion of a methionine residue. In the companion paper, we used allele frequency distributions to assess two predictors of function, evolutionary conservation among close orthologs and chemical relatedness (10). Here we experimentally evaluate these predictors by characterizing the function of the 15 protein-altering variants of OCT1.
Materials and Methods
Construction of Variants and Functional Characterization in Oocytes.
OCT1 cDNA with the reference sequence (GenBank accession nos. U77086 and NM_003057) was subcloned into expression vectors pEXO and pEGFP. The Stratagene QuikChange site-directed mutagenesis kit was used to construct mutant cDNA following the manufacturer's protocols. The variants were confirmed by DNA sequencing. Healthy stage V and VI Xenopus laevis oocytes were injected with 50 nl of diethylpyrocarbonate-treated water or 25 ng of capped cRNA transcribed in vitro with T7 RNA polymerase (mCAP RNA Capping kit; Stratagene) from SpeI-linearized pEXO plasmids containing the reference or mutant OCT1 cDNA inserts. Before injection, an aliquot of the cRNA was run on an agarose gel to verify that it was not degraded; RNA concentrations were determined by spectrophotometry. The injected oocytes were maintained in modified Barth's solution at 18°C before uptake studies. Uptake experiments with the injected oocytes were conducted 2–4 days after injection as described (4). Groups of seven to nine oocytes were incubated in buffer containing 0.1 μM 3H-MPP+ (78 Ci/mmol; 1 Ci = 37 GBq) at room temperature for 1 h. Uptake was stopped by washing the oocytes five times with 3 ml of ice-cold Na+ buffer. Oocytes were then lysed individually with 100 μl of 10% SDS, and the radioactivity associated with each oocyte determined. For kinetic and inhibition studies, unlabeled substrate or inhibitors were added to the incubation mixture as needed. Each data point was determined in duplicate or triplicate for each experiment unless indicated. Data are presented as mean ± standard deviation unless indicated. All experiments were repeated at least once using different batches of oocytes or different cell passages. Water-injected oocytes and oocytes injected with reference OCT1 RNA served as controls within each batch of oocytes.
Cell Culture and Stable Transfection.
Madin–Darby canine kidney cells (passages 10–40) were maintained in MEM Eagle's with Earle's balanced salt solution supplemented with 100 units/ml penicillin, 100 μg/ml streptomycin, and 10% (vol/vol) FBS in 5% CO2/95% air. Cells were transfected with pEGFP plasmids containing the reference or mutant hOCT1 cDNA inserts, or empty vector by Effectene Transfection Reagent following the manufacture's protocols (Qiagen, Valencia, CA). Three days after transfection, stable clones were selected in media containing 0.7 mg/ml G418. After 10–14 days, individual stable clones were isolated and positive clones were further selected by immunocytochemistry. For subsequent transport studies, cells were polarized by growth on Transwell filters (0.4-μm pore size, 12-well plate, Costar, Cambridge, MA) at a confluent density for 7 days with regular media changes as described elsewhere (11).
Confocal Microscopy.
Madin–Darby canine kidney cells grown on filters for 7 days as stated above were fixed with 4% paraformaldehyde, permeablized with 0.025 (wt/vol) saponin in PBS, stained with Texas-red-conjugated phalloidin for visualization of actin, and mounted on slides in Vectashield mounting medium. Samples were analyzed by using a Bio-Rad MRC-1024 confocal microscope.
Results and Discussion
Functional Activity of hOCT1 Variants.
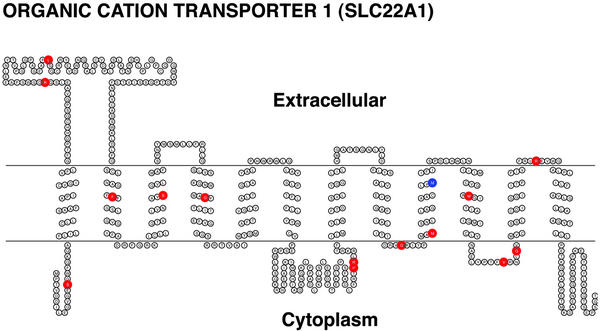
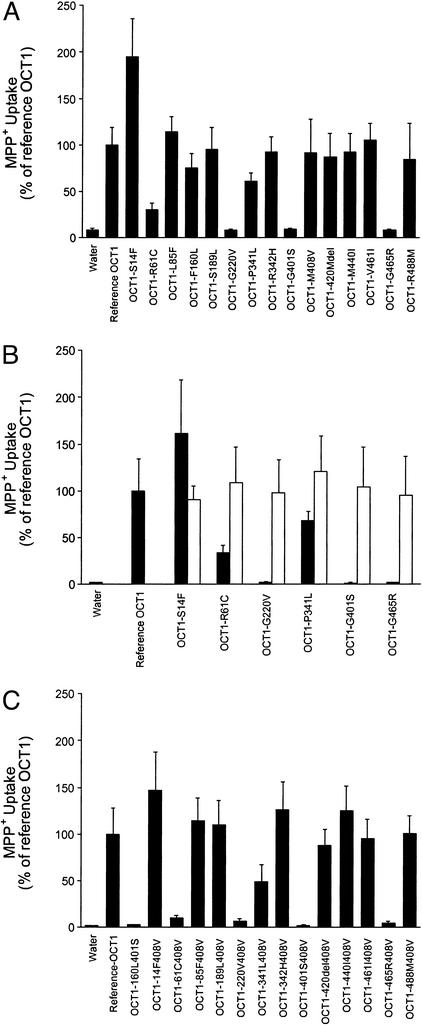
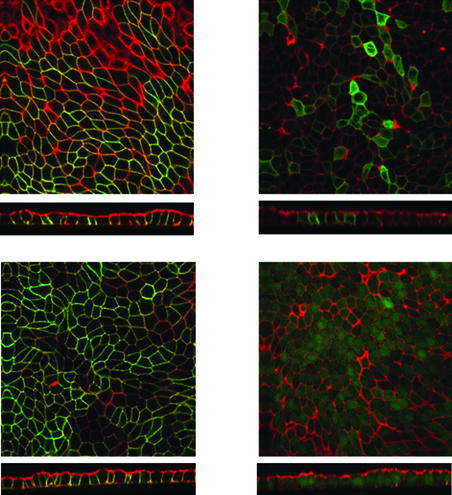
The 15 protein variants identified by Leabman et al. (10) in an ethnically diverse sample have changes in both loops (nine variants) and transmembrane domains (six variants) of OCT1 (Fig. 1). Of the 14 substitution variants of OCT1, we observed that five exhibited decreased function (OCT1-R61C, OCT1-G220V, OCT1-P341L, OCT1-G401S, and OCT1-G465R) and one had increased function (OCT1-S14F) (Table 1, Fig. 2A). MPP+ uptake was restored to normal levels after the variant sequences were changed to the reference sequence by site-directed mutagenesis (Fig. 2B), demonstrating that these amino acid changes were responsible for the altered activities. A recent screen for OCT1 variants in a European American population (n = 57) identified three variants with decreased function, OCT1-R61C and OCT1-G401S, which were also found in our study, and OCT1-C88R, a rare variant, which was not (12). The basis for the functional defects was identified for three of the variants. The two variants with reduced function (OCT1-R61C and OCT1-P341L) had an increased Km (88.7 and 24.7 μM, respectively, compared with 13.9 μM for the reference OCT1; data not shown) and a reduced Vmax (10.0 pmol per oocyte per h and 10.4 pmol per oocyte per h, respectively, compared with 18.4 pmol per oocyte per h for the reference OCT1; data not shown). The nonfunctional variant, OCT1-G465R, tagged with GFP, exhibited reduced localization at the basolateral surface whereas the reference OCT1 and OCT1-R488M, a variant that retained function, were localized to the basolateral membrane (Fig. 3) (13). It is striking that all three of the nonfunctional variants altered evolutionarily conserved glycine residues, suggesting that these residues in OCT1 may be particularly important for function (Fig. 5, which is published as supporting information on the PNAS web site, www.pnas.org). Two of them (at positions 401 and 465) are present in paralogs, OCT2 and OCT3; the other (at position 220) is present in OCT2 only, further suggesting the importance of these glycine residues in function of organic cation transporters.
Figure 1.
Secondary structure and alignment of OCT1 with coding region SNPs. The transmembrane topology diagram was rendered using the transmembrane protein display software TOPO [S. J. Johns (University of California, San Francisco) and R. C. Speth (Washington State University, Pullman), transmembrane protein display software available at the University of California, San Francisco Sequence Analysis Consulting Group web site, www.sacs.ucsf.edu/TOPO/topo.html]. Nonsynonymous amino acid changes are shown in red, and amino acid deletion is shown in blue.
Table 1.
Frequencies and characteristics of 15 protein-altering SNPs in OCT1
| Amino acid change* | Allele frequency†
|
Function‡ | Scoring systems for nonsynonymous variants§
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 494) | AA (n = 200) | EA (n = 200) | AS (n = 60) | ME (n = 20) | PA (n = 14) | Grantham | EC/EU | BLOSUM62 | SIFT¶ | ||
| S14F | 0.013 | 0.031 | 0 | 0 | 0 | 0 | ++ | 155 | EU | −2 | 1.00 |
| R61C | 0.031 | 0 | 0.072 | 0 | 0.056 | 0 | +/− | 180 | EC | −3 | 0.00 |
| L85F | 0.004 | 0.01 | 0 | 0 | 0 | 0 | + | 22 | EU | 0 | 0.00 |
| F160L | 0.032 | 0.005 | 0.065 | 0.017 | 0.05 | 0 | + | 22 | EC | 0 | 0.00 |
| S189L | 0.002 | 0 | 0.005 | 0 | 0 | 0 | + | 145 | EC | −2 | 0.00 |
| G220V | 0.002 | 0.005 | 0 | 0 | 0 | 0 | − | 109 | EC | −3 | 0.00 |
| P341L | 0.047 | 0.082 | 0 | 0.117 | 0 | 0 | +/− | 98 | EC | −3 | 0.00 |
| R342H | 0.012 | 0.031 | 0 | 0 | 0 | 0 | + | 29 | EU | 0 | 0.16 |
| G401S | 0.008 | 0.007 | 0.011 | 0 | 0 | 0 | − | 56 | EC | 0 | 0.00 |
| M408V | 0.682 | 0.735 | 0.598 | 0.762 | 0.786 | 0.929 | + | 21 | EU | 1 | 0.08 |
| M420del | 0.105 | 0.029 | 0.185 | 0 | 0.214 | 0 | + | NA | NA | NA | NA |
| M440I | 0.002 | 0.005 | 0 | 0 | 0 | 0 | + | 10 | EC | 1 | 0.00 |
| V461I | 0.004 | 0.01 | 0 | 0 | 0 | 0 | + | 29 | EU | 3 | 1.00 |
| G465R | 0.016 | 0 | 0.04 | 0 | 0 | 0 | − | 125 | EC | −2 | 0.00 |
| R488M | 0.02 | 0.05 | 0 | 0 | 0 | 0 | + | 91 | EU | −1 | 0.35 |
Variants that exhibited decreased function are indicated in bold. NA, not available.
Positions are relative to the ATG start site and are based on the cDNA sequence from GenBank accession no. NM_003057. Changes shown in bold result in decreased function (as measured by MPP+ uptake) compared to the reference OCT1.
Some samples contained amplicons that could not be sequenced. Allele frequencies were based on actual DNA samples sequenced. Total, entire sample; AA, African American; EA, European American; AS, Asian American; ME, Mexican American; PA, Pacific Islander; n is the number of chromosomes.
++, increased function relative to the reference OCT1; +, function similar to that of reference OCT1; +/−, reduced function; −, no function.
Grantham values range from 5 to 215, in which low values indicate chemical similarity and high values indicate radical differences; EC/EU indicates classification of nonsynonymous variants as EC (evolutionarily conserved) or EU (evolutionarily unconserved) based on sequence alignments with mammalian orthologs (10); blosum62 values range from −4 to +3, where negative values indicate less acceptable and non-negative values indicate more acceptable substitutions; SIFT values range from 0 to 1, where values close to 0 represent less tolerated and those near 1 represent more tolerated substitutions.
SIFT scores were assigned as described (10).
Figure 2.
Functional characterization of natural variants of OCT1. (A) Uptake of MPP+ in oocytes expressing the reference OCT1 and each of the 15 protein-altering OCT1 variants. (B) Recovery of function for six OCT1 variants with reduced or increased function. Dark-shaded columns represent uptake by variants; lighter-shaded columns represent uptake by variants changed to the OCT1 reference sequence at a single nucleotide position by site-directed mutagenesis. (C) Effect of double mutations on transporter function. X. laevis oocytes were injected with ≈50 ng of RNA. Uptake of MPP+ (0.9 μM unlabeled MPP+, 0.1 μM 3H-MPP+) was measured at room temperature after incubation for 1 h. Data are representative of experiments carried out with three different batches of oocytes. Each value represents mean ± SD from seven to nine oocytes.
Figure 3.
Localization of GFP-tagged reference OCT1, OCT1-G465R, and OCT1-R488M in Madin–Darby canine kidney (MDCK) cells. MDCK cells were transfected with plasmids eGFP-OCT1, eGFP-OCT1-G465R, eGFP-OCT1-R488M, and eGFP, respectively. Stable clones were selected with G418. Cells were polarized by growth on filters for 7 days. The cells were fixed, permeabilized, stained for actin with Texas-red-conjugated X phalloidin, and visualized by confocal fluorescence microscopy. A horizontal section (Upper) and a vertical section with the apical membrane on top (Lower) are shown for each set of transfected cells. (Upper Left) eGFP-OCT1. (Upper Right) eGFP-OCT1-G465R. (Lower Left) eGFP-OCT1-R488M. (Lower Right) eGFP.
We next examined the phenotype of haplotypes containing two nonsynonymous changes and measured their MPP+ uptake (Fig. 2C). M408V had an allele frequency of 68.2% in the 494 chromosomes and often occurred with other nonsynonymous mutations in haplotypes identified in this study (14). Thus, the phenotype of variants with two alterations (M408V and each of the other 14 aa changes) was examined. These doubly altered variants exhibited phenotypes like those of the 14 variants with the single amino acid changes; in other words, M408V did not alter function. We also examined the activity of a variant OCT1-F160L G401S, which occurs in a predicted haplotype. Like OCT1-G401S, OCT1-F160L G401S exhibited no MPP+ uptake.
Strikingly, OCT1-S14F displayed increased MPP+ uptake (Fig. 2A) which was reversed when the variant phenylalanine was restored to serine. Kinetic studies revealed that OCT1-S14F had a somewhat lower Km (8.2 μM) and higher Vmax (25.5 pmol/oocyte/hr) than the reference OCT1 (although the difference was not statistically significant). The ratio of Vmax to Km for OCT1-S14F was ≈2-fold greater than for the reference. Amino acid variants with increased activity are observed infrequently but are of biological interest because they provide information about residues that govern protein activity and perhaps specificity. The phenylalanine residue at codon 14 is highly conserved among mammalian orthologs of OCT1, including rat, rabbit, mouse, and chimpanzee (Fig. 5), and is also found at the corresponding position in human OCT2 and OCT3. The human S14F variant, found exclusively in the African American population sample (at 3.1% allele frequency), restores evolutionary conservation at this position. Prior functional studies with human OCT1 have shown that it has lower transport activity than rat, rabbit, and mouse orthologs (15). Whether the phenylalanine residue in the rat, rabbit or mouse OCT1 that corresponds to human position 14 contributes to their increased activity relative to human OCT1 remains to be determined. These observations suggest that high OCT1 activity may be less optimal for fitness of humans than for other mammals. Of the 155 nonsynonymous variants identified by Leabman et al., a total of eight in addition to OCT1-S14F changed the human protein to match the consensus of the other mammalian orthologs (10). It will be interesting to see whether they also affect transporter activity, in particular, whether they increase activity.
Prediction of Function by Evolutionary Conservation and Chemical Change.
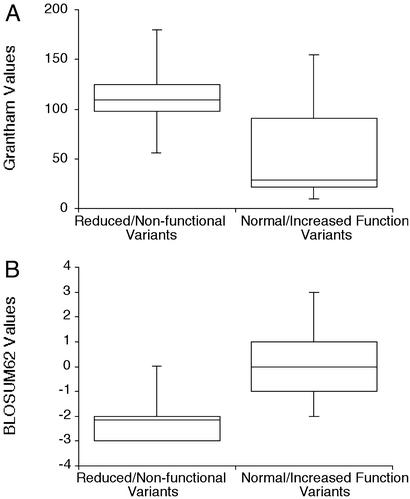
One important reason for developing algorithms and criteria to predict the function of nonsynonymous variants is that it informs the choice of which SNPs to choose for genetic association studies. We have therefore evaluated the changes in OCT1 variants by different criteria (chemical change, evolutionary conservation, and amino acid substitution scoring matrices such as BLOSUM62 and SIFT) (16, 17). In general, the variants with decreased function had larger chemical changes (greater Grantham values) than variants that did not reduce function (Table 1; Fig. 4A), suggesting that the nature of the amino acid change may be a useful predictor of function (18). For example, of the seven variants with Grantham values ≤ 56, six (with values from 10–29) exhibited normal function, whereas one (with a value of 56) was nonfunctional. The usefulness of Grantham values to predict function was weaker for more radical amino acid substitutions. Of the seven variants with Grantham values >90, four (with values from 98–180) exhibited reduced function, two (with values of 91 and 145 exhibited normal function, and one (with a value of 155) exhibited increased function. The three completely nonfunctional variants, which have substitutions of glycine residues, had Grantham values of 109 (G220V), 56 (G401S), and 125 (G465R). We constructed a variant with a more modest chemical change at position 465, OCT1-G465A (GV 61) and observed that it exhibited normal activity (data not shown), indicating that radical change at this position is responsible for reduced function. The evolutionary conservation of G401 and the low Grantham value of the G401S variant suggest that this position has a particularly stringent requirement for glycine.
Figure 4.
Grantham and BLOSUM62 values for OCT1 variants. (A) Grantham values for OCT1 variants with or without decreased function. Mean ± SE values for the variants with decreased function are 114 ± 20 versus 58.2 ± 19 for the variants with increased or normal function, respectively (P < 0.1). (B) BLOSUM62 values for OCT1 variants with or without decreased function. Mean ± SE values for the variants with decreased function are −2.2 ± 0.57 versus 0.0 ± 0.53 for the variants with increased or normal function, respectively (P < 0.02).
The analysis presented by Leabman et al. showed that evolutionary conservation is a strong predictor of allele frequency, indicating that substitutions at evolutionarily conserved (EC) positions are more deleterious than those at evolutionarily unconserved (EU) positions (10). Our analysis of the 14 amino acid substitution variants of OCT1 made it possible to determine whether this prediction is experimentally validated. As before, we defined EC residues as those that were identical in all members of a set of mammalian OCT1 orthologs; EU residues are those in which there is not a consensus (10). Five of eight variants that affected evolutionarily conserved residues exhibited decreased function whereas none of the six variants that altered evolutionarily unconserved residues decreased function (Table 1, χ2 = 5.83, P < 0.05).
blosum62, an amino acid substitution matrix, is derived from amino acid changes in an unselected protein set (17) and has been used to infer protein function (19). BLOSUM62 scores of nonsynonymous SNPs did not predict allele frequency distribution and therefore were not indicators of function for the set of 24 transporters taken as a whole (10). To experimentally determine whether BLOSUM62 values predicted function for OCT1 variants, we compared BLOSUM62 values for the variants with decreased function with those for the variants that retained function. We observed that the values for the decreased-function variants were significantly more negative (evolutionarily unfavorable) than values for the variants that retained function (−2.2 ± 0.57 versus 0.0 ± 0.53, P < 0.02) (Fig. 4B). In particular, six of seven variants with non-negative BLOSUM62 scores (deemed evolutionarily acceptable) exhibited normal OCT1 function. The relationship between negative BLOSUM62 scores was less apparent: four of seven variants with negative BLOSUM62 scores exhibited reduced activity, two exhibited normal activity, and one exhibited increased activity. Overall, these observations on OCT1 support the use of BLOSUM62 scores to predict protein function (10, 19).
SIFT is an algorithm that assigns scores to amino acid changes using alignments of orthologs of the protein of interest (16). Because SIFT incorporates phylogenetic information specific to OCT1, we anticipated that it might be a particularly strong predictor of function of the OCT1 variants. We did not, however, detect a significant difference in the SIFT scores of the variants with decreased function and the variants that retained function. The poor correlation between SIFT score and function resulted primarily from the fact that three variants with alterations at evolutionarily conserved positions, which were assigned a SIFT score of 0 (indicating functional intolerance), exhibited normal function. It is notable that of these three variants, two had only modest chemical changes: OCT1-F160L and OCT1-M440I had Grantham values of 22 and 10, respectively. One other variant (OCT1-L85F) was also assigned a SIFT score of 0 and exhibited normal function. It, too, had a low Grantham value (Grantham value was 22). Leabman et al. also noted that alleles present at high frequency that altered evolutionarily conserved residues have low Grantham values, indicating that this change was tolerated even though it affects an evolutionarily conserved residue (10). Our observations on OCT1 reinforce the proposal of Leabman et al. that combining evolutionary conservation (e.g., SIFT) with Grantham values may be particularly useful for optimizing algorithms to predict function.
Relationship Between Allele Frequency and Functional Activity of OCT1 Variants.
The two most common variants of OCT1, OCT1-M408V and OCT1-M420del (which have allele frequencies >10%; Table 1), both exhibited normal function, consistent with the premise that common variants are less likely to exhibit altered function than are rare variants. A similar trend was observed in our recent study of natural variants of OCT2, in which the most common nonsynonymous variant, OCT2-A270S, exhibited transport properties similar to that of the reference OCT2 (20). In contrast, all three nonfunctional variants of OCT1 were present at overall allele frequencies of <2% (Table 1). It is worth noting, however, that two of these variants exhibited frequencies of 1.1% and 4% in European Americans and that the two variants with reduced function were present at substantial frequencies, 7.2% in European Americans (R61C) and 8.2% in African Americans (P341L).
Possible Consequences of Variation in OCT1.
The high variability of OCT1 may have implications both for human disease and drug response, given that the transporter interacts with a variety of structurally diverse compounds and controls access to drug metabolizing enzymes in the liver (2–6). Genetic variation in OCT1 could also contribute to neurodegenerative diseases because OCT1 appears to govern hepatic uptake and elimination of 1-methy-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which is responsible for a Parkinsonian syndrome (21, 22). Reduced-activity variants of OCT1 may lead to enhanced exposure to endogenous and environmental toxins and contribute to neurodegenerative disease. Variation in OCT1 may influence drug response by altering hepatic drug clearance. Dramatic differences in the liver distribution of the anticancer drug MIBG and the antidiabetic drug metformin have been observed in Oct1 knockout mice compared with wild-type mice (23, 24). Given that OCT1 is primarily expressed in the liver, we expect to see similar differences between normal individuals and those with variants that have reduced OCT1 function (4, 5).
Supplementary Material
Acknowledgments
We thank Richard Kim and John Pritchard for comments on the manuscript. This work was supported by National Institutes of Health Grant GM 61390. Data are available at www.pharmgkb.org and www.pharmacogenetics.ucsf.edu. Y.S. is a recipient of a Merck Sharp and Dohme International Fellowship in Clinical Pharmacology.
Abbreviation
- SNP
single-nucleotide polymorphism
References
- 1.Evans W E, Relling M V. Science. 1999;286:487–491. doi: 10.1126/science.286.5439.487. [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Schaner M E, Giacomini K M. J Pharmacol Exp Ther. 1998;286:354–361. [PubMed] [Google Scholar]
- 3.Zhang L, Brett C M, Giacomini K M. Annu Rev Pharmacol Toxicol. 1998;38:431–460. doi: 10.1146/annurev.pharmtox.38.1.431. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Dresser M J, Gray A T, Yost S C, Terashita S, Giacomini K M. Mol Pharmacol. 1997;51:913–921. doi: 10.1124/mol.51.6.913. [DOI] [PubMed] [Google Scholar]
- 5.Gorboulev V, Ulzheimer J C, Akhoundova A, Ulzheimer-Teuber I, Karbach U, Quester S, Baumann C, Lang F, Busch A E, Koepsell H. DNA Cell Biol. 1997;16:871–881. doi: 10.1089/dna.1997.16.871. [DOI] [PubMed] [Google Scholar]
- 6.Dresser M J, Leabman M K, Giacomini K M. J Pharm Sci. 2001;90:397–421. doi: 10.1002/1520-6017(200104)90:4<397::aid-jps1000>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.Strautnieks S S, Bull L N, Knisely A S, Kocoshis S A, Dahl N, Arnell H, Sokal E, Dahan K, Childs S, Ling V, et al. Nat Genet. 1998;20:233–238. doi: 10.1038/3034. [DOI] [PubMed] [Google Scholar]
- 8.Bull L N, van Eijk M J, Pawlikowska L, DeYoung J A, Juijn J A, Liao M, Klomp L W, Lomri N, Berger R, Scharschmidt B F, et al. Nat Genet. 1998;18:219–224. doi: 10.1038/ng0398-219. [DOI] [PubMed] [Google Scholar]
- 9.Toh S, Wada M, Uchiumi T, Inokuchi A, Makino Y, Horie Y, Adachi Y, Sakisaka S, Kuwano M. Am J Hum Genet. 1999;64:739–746. doi: 10.1086/302292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leabman M K, Huang C C, DeYoung J, Carlson E J, Taylor T R, de la Cruz M, Johns S J, Stryke D, Kawamoto M, Urban T J, et al. Proc Natl Acad Sci. 2003;100:5896–5901. doi: 10.1073/pnas.0730857100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mangravite L M, Lipschutz J H, Mostov K E, Giacomini K M. Am J Physiol. 2001;280:F879–F885. doi: 10.1152/ajprenal.2001.280.5.F879. [DOI] [PubMed] [Google Scholar]
- 12.Kerb R, Brinkmann U, Chatskaia N, Gorbunov D, Gorboulev V, Mornhinweg E, Keil A, Eichelbaum M, Koepsell H. Pharmacogenetics. 2002;12:591–595. doi: 10.1097/00008571-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Meyer-Wentrup F, Karbach U, Gorboulev V, Arndt P, Koepsell H. Biochem Biophys Res Commun. 1998;248:673–678. doi: 10.1006/bbrc.1998.9034. [DOI] [PubMed] [Google Scholar]
- 14.Stephens M, Smith N J, Donnelly P. Am J Hum Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dresser M J, Gray A T, Giacomini K M. J Pharmacol Exp Ther. 2000;292:1146–1152. [PubMed] [Google Scholar]
- 16.Ng P C, Henikoff S. Genome Res. 2001;11:863–874. doi: 10.1101/gr.176601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henikoff S, Henikoff J G. Proc Natl Acad Sci USA. 1992;89:10915–10919. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grantham R. Science. 1974;185:862–864. doi: 10.1126/science.185.4154.862. [DOI] [PubMed] [Google Scholar]
- 19.Cargill M, Altshuler D, Ireland J, Sklar P, Ardlie K, Patil N, Shaw N, Lane C R, Lim E P, Kalyanaraman N, et al. Nat Genet. 1999;22:231–238. doi: 10.1038/10290. [DOI] [PubMed] [Google Scholar]
- 20.Leabman M K, Huang C C, Kawamoto M, Johns S J, Stryke D, Ferrin T E, DeYoung J, Taylor T, Clark A G, Herskowitz I, Giacomini K M. Pharmacogenetics. 2002;12:395–405. doi: 10.1097/00008571-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Langston J W, Ballard P, Tetrud J W, Irwin I. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 22.Yang M C, McLean A J, Le Couteur D G. Biochem Biophys Res Commun. 2001;289:130–136. doi: 10.1006/bbrc.2001.5954. [DOI] [PubMed] [Google Scholar]
- 23.Jonker J W, Wagenaar E, Mol C A, Buitelaar M, Koepsell H, Smit J W, Schinkel A H. Mol Cell Biol. 2001;21:5471–5477. doi: 10.1128/MCB.21.16.5471-5477.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D S, Jonker J W, Kato Y, Kusuhara H, Schinkel A H, Sugiyama Y. J Pharmacol Exp Ther. 2002;302:510–515. doi: 10.1124/jpet.102.034140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.