Abstract
The biological clock of Neurospora crassa includes interconnected transcriptional and translational feedback loops that cause both the transcript and protein encoded by the frequency gene (frq) to undergo the robust daily oscillations in abundance, which are essential for clock function. To understand better the mechanism generating rhythmic frq transcript, reporter constructs were used to show that the oscillation in frq message is transcriptionally regulated, and a single cis-acting element in the frq promoter, the Clock Box (C box), is both necessary and sufficient for this rhythmic transcription. Nuclear protein extracts used in binding assays revealed that a White Collar (WC)-1- and WC-2-containing complex (WCC) binds to the C box in a time-of-day-specific manner. Overexpression of an ectopic copy of FRQ or addition of in vitro-generated FRQ resulted in reduced WCC binding to the C box. These data suggest that oscillations in frq transcript result from WCC binding to the frq promoter and activating transcription with subsequent changes in FRQ levels having an inverse effect on WCC binding. In this way rhythmic expression and turnover of FRQ drives the rhythm in its own transcription.
Life on earth has evolved under the continual daily fluctuations in light and temperature. Many organisms have evolved the ability to anticipate these external changes in their environment by using endogenous “biological clocks.” In recent years, the molecular components that make up these intracellular clocks have begun to be identified, and similarities among a wide range of organisms have emerged (1–5). The clock in a fungus, fruit fly, or mammal contains a negative feedback loop in which two PAS domain-containing proteins [White Collar (WC)-2/WC-1, dCLOCK (dCLK)/CYCLE (CYC), CLOCK/BMAL1] heterodimerize and act as positive elements to activate the expression of a negative element [FREQUENCY (FRQ), PERIOD (PER)/TIMELESS (TIM), CRYPTOCHROME 1 (CRY1)/CRY2/mPERs]. The negative element(s) in turn feeds back to repress the activity of the positive elements. These transcription/translation-based negative feedback loops ultimately generate self-sustaining circadian (circa = about; dies = day) oscillations or rhythms in the level(s) of one or more of the elements within the loop. The robustness and stability of these oscillations is enhanced further by interlocking positive feedback loops (6–8) and multiple layers of regulation (2, 3, 9).
In Neurospora, a negative feedback loop comprised of the products of the frq, wc-1, and wc-2 genes is central to clock function. WC-1 and WC-2 are predominately nuclear transcription factors containing trans-activation domains and Zn-finger DNA-binding domains (10, 11). They form a WC complex (WCC) by heterodimerizing via PAS domains (12) and act as positive elements in the expression of frq (13); in a wc-1KO or wc-2KO strain, very limited, unregulated transcription of frq occurs (14–16). During the course of a day, FRQ is progressively phosphorylated and degraded (17–19), but when present FRQ acts as a negative element, repressing the levels of its own transcript (20). The mechanism by which this repression occurs is unclear, but it seems likely that the repressive role of FRQ is carried out through its physical interaction with the WCC (21–23). The interaction of FRQ, WC-1, and WC-2 relies on the constitutively expressed and more abundant WC-2 acting as a scaffold (21). The wc-1 transcript is also constitutively expressed in the dark, but interestingly WC-1 is rhythmically abundant with FRQ playing a positive role in the posttranscriptional production of WC-1 (6, 16). This positive feedback loop consequently is interconnected with the negative feedback loop controlling frq expression, setting up an essential regulatory dynamic between WC-1 and FRQ as their approximately equimolar amounts oscillate antiphasic to one another (6, 21). FRQ also plays a positive role in wc-2 expression, creating another interconnected positive feedback loop (16). The interplay of FRQ and the WCs results in the robust and rhythmic expression of both frq message and FRQ protein. Their rhythmic expression is central to the functioning of the clock such that constitutive expression of frq message results in the loss of overt rhythmicity, and step changes in frq expression reset the clock (17, 20, 24, 25).
The current model of this circadian feedback loop hinges on the positive and negative regulatory relationship among WC-1, WC-2, and FRQ at the frq promoter. Although the internal consistency of the WCC-mediated transcriptional activation of frq has led to its general acceptance, a number of critical assumptions and predictions remain untested, and a biochemical role for FRQ in the oscillator has only been inferred. Also poorly understood are the degree to which transcription drives rhythmic frq message, the mode of interaction of the WCC with the frq promoter, and whether FRQ acts on or off of its own promoter, yet these interactions lie at the core of the negative feedback that defines rhythmicity. Although no nonanimal rhythms have been examined in this detail, there are two plausible scenarios whereby FRQ might act based on precedents from animal systems. In the first, FRQ complexes with the WCC on its own promoter, directly repressing the activity of the WCC. This mode of feedback has been suggested for the mammalian circadian system with CLOCK/BMAL1 binding to DNA of mPer1 to activate transcription and PER/CRY interacting with CLOCK/BMAL1 on DNA to repress their activity (26). This model implies a constant level of heterodimer bound to promoter DNA. An alternative possibility is suggested by the report in Drosophila that PER and TIM physically interact with the dCLK/CYC heterodimer and interfere with its binding to per and tim promoter elements, thereby preventing the positive action of dCLK/CYC on per and tim transcription (27–29). This model implies a rhythmic binding of the activator complex to promoter DNA as seen recently with regulation of mCry1 (30). In this second type of scenario, the interaction of FRQ with the WCC would prevent or disrupt binding of WCC to the Clock Box (C box), indirectly repressing WCC activity. To evaluate these possibilities and understand the biochemical basis of these essential interactions, we examined in detail the cis-acting elements required for circadian transcriptional regulation of frq and the relationship among the trans-acting factors that affect this regulation. We found that oscillating frq message, the result of rhythmic transcription, is under the control of a single cis-acting element, a C box, that is both necessary and sufficient to drive cycling transcription of frq. WC-1 and WC-2 bind to the C box in a time-of-day-specific manner with a profile similar to but phase-leading the oscillations in frq transcript. FRQ negatively affects WCC binding such that increases in FRQ cause a decrease in WCC binding to the C box without affecting the levels of the WCs. In this way rhythmic expression and turnover of FRQ drives the rhythm in its own transcription.
Materials and Methods
Strains, Growth Conditions, and Race-Tube Assay.
The wild type (wt) strain was 87-3 (bd; frq+ a). frq promoter deletion constructs were transformed into strain 93-4 (bd; frq10 A; his-3), and hph reporter constructs were transformed into strain 87-74 (bd; frq+ a; his-3); all transformations were to the his-3 locus (31). Strains used for nuclear protein extractions were 87-3 (bd; frq+ a), 86-1 (bd; frq10), 241-6 (bd; wc-2KO), 232-4 (bd; wc-1ER45), and 87-12 (bd; his-3∷pBA50). The frqKO is also designated frq10.
General conditions for growth and race-tube experiments have been described (20, 32). Liquid culture medium consisted of 1× Vogel's salts, 2% glucose, 0.5% arginine, and 50 ng/ml biotin, and race tube medium consisted of 1× Vogel's salts, 0.1% glucose, 0.17% arginine, 50 ng/ml biotin, and 1.5% bacto-agar. Determination of conidiation density on race tubes and calculations of period length were done by using CHRONO (33).
Plasmids.
Details of the plasmids have been described (34) except for pAF52 and pAF53, which were made by annealing the following oligos together and inserting them into pAF35: pAF52 (ACF40, 5′-CAGGTTCCAGAGTTTGGCCGGACAACCAGTACGGGTGCCCGA-3′; ACF41, 5′-CTAGTCGGGCACCCGTACTGGTTGTCCGGCCAAACTCTGGAACCTGGGCC-3′) and pAF53 (ACF42, 5′-CAGTACGGGTGCCCGAGGCGTCCTGATGCCGCTGCAAGACA-3′; ACF43, 5′-CTAGTGTCTTGCAGCGGCATCAGGACGCCTCGGGCACCCGTACTGGGCC-3′).
RNA and Protein Analyses.
Western and Northern analyses were performed as described (34). Statistical differences between time points within strains of hph transcript were tested by one-factor ANOVA and subsequent post hoc Dunnett's t test where significance is set at P < 0.05.
Nuclear Protein Extract Preparation, Electrophoretic Mobility-Shift Assay (EMSA), and in Vitro Transcription/Translation.
Nucelar protein extractions and EMSAs were performed as described (34). For the time-course experiments, the cultures were inoculated at ≈5 × 105 cells per ml and grown for a total of 48 h, with staggered transfer of cultures from constant light (LL) to constant dark (DD). For the quinic acid (QA) experiments, cultures inoculated at ≈1.5 × 107 cells per ml were grown in 2% glucose medium for 23 h and then transferred into 0.25% glucose medium for 13 h, with cultures being transferred to DD 12 h before harvesting. QA was added to a final concentration of 15 mM at the indicated times. The oligonucleotides annealed for use as probe were ACF57 (5′-CGTCCTGATGCCGCTGCAAGACCGATGACGCTGCAAAATTGAGATCTA-3′) and ACF58 (5′-TAGATCTCAATTTTGCAGCGTCATCGGTCTTGCAGCGGCATCAGGACG-3′). The in vitro transcription/translation was the same as described (34). Quantitation of protein synthesis was the same as described (21).
Results
A Single cis-Acting Element Is Necessary and Sufficient for Rhythmicity of frq Message.
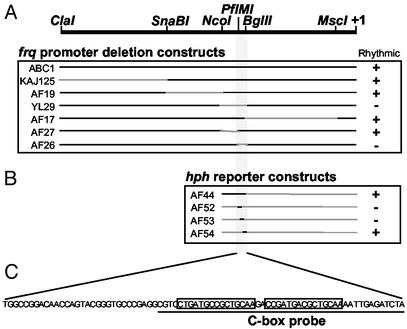
Previous work has shown that the rhythmic production of frq message is central to the function of the Neurospora circadian clock (20), but the means through which this rhythmicity is generated has not been established. To identify any cis-acting elements involved in rhythmic frq production, frq promoter deletion constructs were transformed into a frqKO strain (Fig. 1A). The use of an ≈8-kb region containing the entire frq locus in the construction of the deletion constructs allowed us to test for the necessity of specific promoter regions for the generation of rhythmicity as well as for functional rescue of the Neurospora clock (35). The most prominent output from the Neurospora clock is the rhythmic production of asexual spores or conidia (referred to as banding) that is assayed on race tubes (2). Race tubes inoculated with wt and various deletion strains were grown for 24 h under constant light and temperature and then transferred to DD. This transfer from light to dark results in the decrease in frq transcript that sets the clock to subjective dusk from which the clock then continues (36). Control ABC1 transformants, which contained the entire frq locus, banded with a period and phase similar to a frq+ strain as reported (34, 35) (Fig. 2A). The majority of the frq promoter was spanned by four deletion strains, KAJ125, AF19, YL29, and AF17 (Fig. 1), one of which (YL29) failed to band and instead conidiated down the length of the race tube. The deleted region spanned by YL29 was resected further in strains AF26 and AF27. Strain AF27 banded with a wt period and phase (data not shown), but AF26 failed to band and appeared similar to YL29 with constant conidiation down the race tube (Fig. 2A).
Figure 1.
Identification of the frq promoter C box. (A) Schematic diagram of frq promoter deletion constructs. The regions deleted are indicated by gray lines. The major start site of transcription is indicated by +1. The ability to rescue rhythmic conidial production of a frqKO strain is indicated by +. (B) Schematic diagram of frq promoter reporter constructs. Black lines indicate frq promoter regions placed in the reporter. Ability to generate rhythmic levels of hph transcript is indicated by +. (C) Sequence of a 78-bp region of the frq promoter necessary and sufficient for rhythmicity. The line below indicates the sequence used as the C-box probe in EMSA experiments. Imperfect repeats are indicated by boxes.
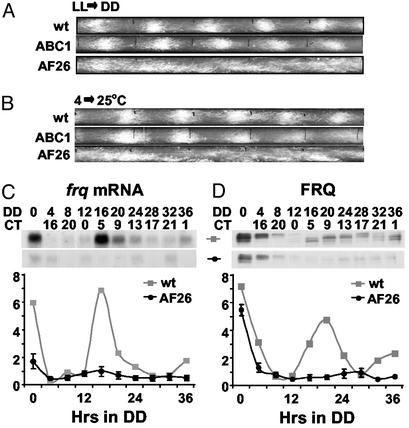
Figure 2.
The frq promoter contains a single region necessary for overt and molecular rhythmicity. (A and B) Race tube analysis showing the overt rhythmicity of wt (bd, frq+), ABC1 (bd, frqKO, pABC1), and AF26 (bd, frqKO, pAF26) strains. Construct pABC1 contains the entire frq locus, and pAF26 contains the frq locus minus a 78-bp region from the promoter. The race tubes shown are representative samples from six replicate tubes. (A) Race tubes entrained with an LL-to-DD transfer. (B) Race tubes entrained with a temperature step (4–25°C). (C and D) Molecular rhythmicity in wt and AF26 strains. A single experiment is plotted for wt (also described in ref. 17), and the average of three experiments ± SEM is plotted for AF26. (C) Densitometry and Northern blot analysis of frq transcript. (D) Densitometry and Western blot analysis of FRQ. DD0 refers to a culture kept in LL and has no CT equivalent.
The 78-bp region deleted in AF26 seemed necessary for overt rhythmicity in Neurospora, but the loss of rhythmicity could have been due to blocked light input to the clock. Light entrainment of the Neurospora clock acts by rapidly increasing frq message, and the arrhythmia of strain AF26 simply could have been the result of an asynchronous culture (36). To rule out this possibility, we used a temperature step as the entraining signal (13, 25). ABC1 and frq+ strains were entrained by the temperature step, but AF26 again was arrhythmic (Fig. 2B). The lack of overt rhythmicity of AF26 was also reflected at the molecular level, with both frq transcript and FRQ displaying low, nonrhythmic levels in the dark (Fig. 2 C and D), confirming that the 78-bp region was necessary for the generation of rhythmic frq transcript.
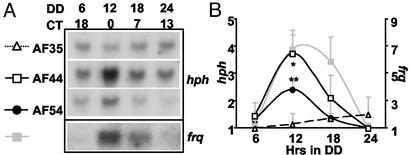
To test whether this 78-bp region, located ≈1.2 kb upstream from the major start site of transcription, was sufficient for the generation of a rhythmic transcript, the region was cloned into an hph reporter construct (pAF35) resulting in pAF44 (Fig. 1B). Transformants of frq+ strains containing either pAF44 or pAF35 were grown in DD, harvested over a 24-h period, and hph and frq transcript levels were examined by Northern blot (Fig. 3). Strain AF44 exhibited rhythmic hph levels (F3,8 = 3.88, P = 0.055) with a 3.7-fold amplitude and peak levels around DD12. Strain AF35, the hph reporter construct alone, did not exhibit rhythmic hph (F3,8 = 0.15, not siginificant). The region spanning the 78-bp deletion of AF26 then was subdivided into three overlapping 40-bp fragments that were assayed in the hph reporter construct for their ability to drive rhythmic transcription: AF52, AF53, and AF54 (Fig. 1B). One of these fragments, AF54, drove rhythmic hph levels (F3,8 = 10.635, P = 0.0036), which peaked at DD12 with a 2.4-fold amplitude (Fig. 3). Therefore, the 40-bp frq promoter region contained within AF44 and AF54 was sufficient to drive rhythmic transcription.
Figure 3.
The C box in the frq promoter region is sufficient to drive rhythmic transcription. (A) Northern analysis from timed cultures bearing minimal C box sequences driving an hph reporter show rhythmicity in reporter transcription. Representative Northern blots of hph transcript are shown from strains AF35 (bd, frq+, pAF35), AF44 (bd, frq+, pAF44), and AF54 (bd, frq+, pAF54). pAF35 is the reporter construct alone. pAF44 contains a 250-bp region of the frq promoter. pAF54 contains a 40-bp region of the frq promoter. Rhythmicity of endogenous frq message was confirmed for all strains; a single representative Northern blot of frq is shown. (B) Densitometry of hph is shown from strains AF35, AF44, and AF54. Average hph levels for each strain are plotted (n = 3) with the lowest value for each strain set to 1. *, P = 0.05, and **, P < 0.01 (one-factor ANOVA and post hoc Dunnett's t test). Error bars are the SEM. Densitometry of frq is shown for strain AF54 (n = 3).
This 40-bp C box was shown recently to also be a light-response element, playing a role in the light-induced increase of frq message (34). The C box/light-response element therefore is essential for normal regulation of frq message both in the dark and in response to light. Not surprisingly, WC-1 and WC-2, proteins essential for both dark and light regulation of frq, were shown to bind to this cis element as a heterodimer in the dark and as a multimeric WCC in the light (34). The light-triggered multimerization of the WCs is believed to aid in the light-induced increase in frq transcription, but the question of how the WCs drive rhythmic frq transcription in the dark remains open. WC-1 and WC-2 are known to complex with FRQ (21–23) and FRQ to negatively regulate the level of its own transcript (20), but FRQ was not in the complex bound to the C box, nor was FRQ necessary for the formation of the complex (34). These data suggest that periodic time-of-day-specific formation and/or binding of this complex to the C box is the signal event driving rhythmic frq transcription.
WCC Binds to the C Box in a Time-of-Day-Specific Manner.
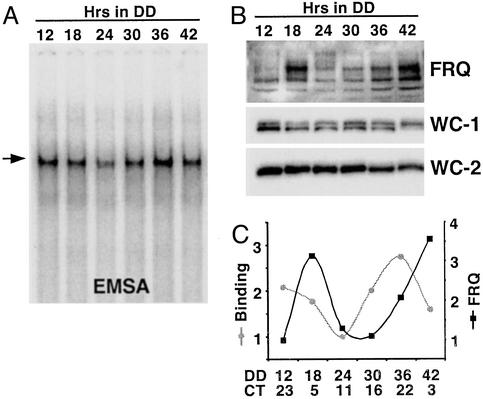
To examine temporal regulation of the complex, nuclear protein extracts were prepared from cultures harvested at different circadian times (CTs), and equal amounts of protein (3 μg) were used in a series of EMSAs. As shown in Fig. 4A, a WC-1/WC-2/C box complex was present throughout the day with no change in mobility/size. The presence of WC-1 and WC-2 in the complex at different times of day was verified by using WC-1- and WC-2-specific antisera (data not shown). Additionally, no novel complexes were seen. Although the size of the complex did not change, the amount of complex formed varied during the course of a day, with peaks in binding occurring around subjective dawn ≈CT22–24 (DD12 and DD36) (CT is used to normalize the endogenous period of an organism to a “24-h” day) and a trough in binding occurring near subjective dusk ≈CT12 (DD24) (Fig. 4 A and C). This oscillation in binding closely matches the changes in frq transcript levels, which are high shortly after subjective dawn (20, 36); this temporal correlation is appropriate given the positive role of WCs in frq transcription.
Figure 4.
The C box is rhythmically bound by the WC-1/WC-2 complex. To determine whether FRQ acts by blocking binding of the WC-1/WC-2 complex to the C box or by blocking activation by a constitutively bound complex, C-box binding by WC-1 and WC-2 was determined as a function of CT. (A) EMSA using the C-box probe and nuclear protein extracts from cultures harvested across a circadian day reveals changes in the amount of complex bound but not in its apparent size. The arrow indicates the WC-1/WC-2 complex. (B) Western blot analysis of FRQ, WC-1, and WC-2 in the nuclear protein extracts used in A confirm appropriate rhythmicities. (C) Densitometry of the C-box-bound complex and FRQ shows that the amount of complex bound to the C box changes over the course of a circadian day, peaking ≈6 h before the peak in FRQ protein. The lowest value in each group was set to 1. The experiment was repeated multiple times with similar results; representative data are shown.
To determine whether the change in binding of the WCC to the C box was due to changes in protein levels, we assessed WC-1 and WC-2 levels in the nuclear protein extracts (Fig. 4B). There was no cycling in WC-2, and WC-1 exhibited a low-amplitude rhythm peaking in the subjective night ≈CT16, similar to previous reports on both proteins (6, 16, 21). There was no apparent correlation between the WC levels and the amount of WCC bound to the C box, suggesting that the amount of binding is regulated by a factor(s) other than WC-1 or WC-2, although the rhythm in WC-1 peaking before dawn would serve to accentuate the rhythm in binding. Rhythmicity of the cultures was ensured by monitoring FRQ levels that cycled as reported (17), with the progressive phosphorylation and turnover of FRQ resulting in peak levels ≈CT4–8 (DD18 and again at DD42) (Fig. 4 B and C). These data are consistent with rhythmic WCC/DNA complex formation being the basis for rhythmic frq transcription but are mute in regards to the specific role of FRQ in bringing this about.
FRQ Inhibits Binding of the WCC to the C Box.
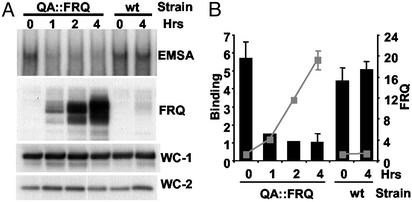
Although FRQ was not part of the complex bound to the C box (34), FRQ was a strong candidate to be involved in the regulation of WCC binding. It is well established that FRQ negatively regulates its own expression and directly interacts with WC-1 and WC-2 (20–23). Additionally, oscillations in FRQ levels are phased appropriately and suggest an attractive means of generating oscillations in WCC binding. To test the role of FRQ in WCC binding we overexpressed FRQ and looked for changes in WCC binding. We used a strain (QA∷FRQ) containing an extra copy of the FRQ ORF under the control of the inducible QA promoter (20). Identical cultures were placed in DD for 12 h, and QA inducer was added at various times before the cultures were harvested and nuclear proteins extracted. The addition of QA 1, 2, or 4 h before harvesting induced FRQ to levels 4-, 12-, and 20-fold higher than an untreated culture (Fig. 5). A series of binding reactions performed by using the QA-treated extracts showed that the increases in FRQ resulted in a strong dose-dependent reduction in WCC binding compared with an untreated culture. Over the time course of this experiment, the levels of WC-1 and WC-2 were unaffected by the addition of QA (Fig. 5A). As an additional control, nuclear protein extracts were made from a wt strain grown with or without QA added 4 h before harvesting. The QA treatment had no effect on FRQ levels or binding in the wt strain (Fig. 5). These data establish an active role for FRQ in reducing the ability of the WCC to bind to the C box.
Figure 5.
Increases in FRQ cause a decrease in WCC binding to the C box. An inducible FRQ construct was used to determine the effects in vivo of FRQ concentration on WCC binding to the C box. (A) Binding assay and Western blot analysis of QA∷FRQ and wt strains. Four identical cultures inoculated with the QA∷FRQ strain, containing a QA-inducible copy of FRQ, were grown in DD for 12 h. At DD12, FRQ levels would normally be low and complex bound to the C box high (see Fig. 4A). QA was added to three of the cultures 1, 2, or 4 h before harvesting. As a control, two cultures inoculated with a wt strain were grown under similar conditions, and QA was added 4 h before harvesting to one of the cultures. Nuclear protein extracts were made from each culture and used with the C-box probe in a series of binding reactions. Only the portion of the gel containing the WC-1/WC-2 complex is shown (Top). FRQ, WC-1, and WC-2 levels in the extracts were analyzed by Western blot (three lower panels). (B) Densitometric analysis of C-box-bound complex and FRQ. Binding is indicated by black bars, and FRQ levels are indicated by the gray line and boxes. n = 3 except for QA 1 and 2 where n = 1. Error bars are the SEM.
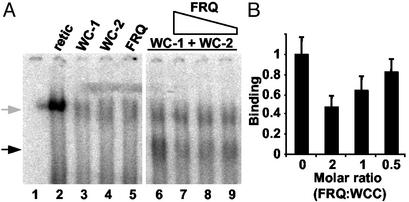
To determine whether FRQ's inhibition of WCC binding to the C box was a result of direct interaction between FRQ and the WCC, WC-1, WC-2, and FRQ were produced in vitro by using a coupled transcription/translation reticulocyte lysate system and used in a series of binding reactions (Fig. 6). WC-1 and WC-2 together (Fig. 6A, lane 6) were able to bind to the C box as a complex with mobility similar to that seen by using nuclear extracts as reported (34). This complex was not seen when either protein was used alone (Fig. 6A, lanes 3 and 4) or when unprogrammed lysate was used (Fig. 6A, lane 2). When FRQ was added to a WC-1/WC-2-containing reaction, the amount of WCC bound to the C box decreased in a dose-dependent manner; the addition of FRQ in a 1:2 molar ratio to the WCC resulted in an ≈20% decrease in binding (Fig. 6 A, lane 9, and B), a 1:1 ratio resulted in an ≈35% decrease (Fig. 6, lane 8, and B), and FRQ added in a 2:1 molar ratio resulted in an ≈55% reduction in binding (Fig. 6A, lane 7, and B). These results establish a direct role for FRQ in reducing the ability of the WCC to bind to the C box in the dark, thereby providing the molecular basis for the negative feedback of FRQ on its own expression.
Figure 6.
FRQ directly decreases WCC binding to the C box. (A) Binding assay with in vitro-synthesized WC-1, WC-2, and FRQ. Reticulocyte lysates containing 0.1 fmol each of WC-1 (lane 3), WC-2 (lane 4), FRQ (lane 5), or a mixture of WC-1 and WC-2 (lanes 6–9) were used with the C box in a series of binding reactions. Decreasing amounts of FRQ (0.2, 0.1, or 0.05 fmol) were added to the WC-1/WC-2-containing reactions (lanes 7–9). The WC-1/WC-2 complex is indicated by the black arrow. The gray arrow indicates a complex present even when using unprogrammed lysate (lane 2). Lane 1, probe alone. (B) The reactions in lanes 6–9 in A were repeated in triplicate (data not shown), and the WC-1/WC-2 complexes were quantified densitometrically. The average is plotted with the highest value set to 1 and error bars indicating ±SD.
Discussion
Existing models for eukaryotic circadian oscillators generally incorporate an important role for rhythmic transcription, and the specific biochemical mechanisms underlying this transcriptional regulation, which are of immediate interest, have heretofore been examined only in animal systems. Here we have identified a cis-acting element in the frq promoter, the C box, that is necessary and sufficient to drive rhythmic transcription of this central clock component. The C box is bound in a time-of-day-specific manner by a complex containing WC-1 and WC-2. The negative action of FRQ on its own transcript is a result of FRQ decreasing WCC binding to the C box, apparently in solution, because FRQ does not seem to be a part of the complex when bound to DNA. These results demonstrate specific interactions among WC-1, WC-2, FRQ, and the frq promoter, furthering our understanding of the mechanism underlying the complex regulation of frq expression.
Deletion analysis in the context of the entire frq locus allowed us to isolate a small region in the frq promoter that when deleted resulted in the loss of rhythmic frq mRNA as well as overt rhythmicity. The ability of this frq promoter region to drive rhythmic transcription of a heterologous reporter confirmed that oscillations in frq message are transcriptionally driven. The 40-bp C box-driven reporter peaked at DD12 (CT0, subjective dawn), slightly phase-leading the peak of the endogenous frq transcript, suggesting that additional promoter elements and/or posttranscriptional regulation may also be involved in the control of endogenous frq levels. Amplitude differences between the reporter and endogenous frq also support a role for additional regulation of endogenous frq transcript. Similar patterns of regulation are seen for mper1 in mammals (26, 30, 37–39) and for per and tim in Drosophila where transcripts are rhythmically expressed with E-box promoter elements playing central roles but wt rhythmic regulation requiring additional promoter elements, intronic DNA sequences, and posttranscriptional regulation (5, 26, 40–46).
The presence of two GATG-sequence repeats in the C box (Fig. 1C and ref. 34) is consistent with the idea that the WCs bind as a heterodimer with the Zn finger of each WC interacting with one GATG. Heterodimers are also central to both Drosophila and mammalian clocks where the heterodimeric complex, dCLK/CYC or CLOCK/BMAL, respectively, activates transcription by binding to E boxes through basic helix–loop–helix domains (instead of Zn fingers as in the WCC) with one basic helix–loop–helix found in each partner (37, 47–50); the specificity and function of clock gene E-box elements are still not understood entirely (51). Such context dependence is apparent in the frq promoter in that the proximal light-response element, which is also bound by WCC (34) during light regulation, is not essential as a clock box in the dark. In Arabidopsis, an evening promoter element required for rhythmicity, has been identified recently for the clock gene TIMING OF LAB 1 (TOC1) and shown to be bound by the MYB-containing clock components CIRCADIAN CLOCK-ASSOCIATED 1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY) (52, 53); specificity and function for this 9-bp evening element (EE) and the nature of the factors bound in vivo are also not well understood.
We interpret the positive role of the WCs on frq transcript levels as a direct result of their association with the C box, and the data presented here also serve to explain the negative action of FRQ on its own transcription. Several lines of evidence support FRQ interacting with the WCC and interfering with C-box binding. First, FRQ was not found to be associated with the WCC when bound to the C box in vitro (34). Second, the amount of WCC bound to the C box fluctuated in a time-of-day-specific manner without a corresponding change in WC levels, suggesting that WCC binding indeed is regulated. Third, the size of the complex bound to the C box did not change during the course of a day, suggesting that no factors were gained or lost from the complex that could have regulated WCC activity when bound to the C box. Because FRQ acts as a dimer (22), binding of FRQ to the WCC would increase its apparent size greatly. Fourth and most importantly, overexpression of FRQ in vivo or use of in vitro-synthesized proteins demonstrated a decrease in WCC bound to the C box with increasing levels of FRQ. Taken together, this evidence suggests that FRQ acts globally to repress its own transcription by interacting directly with the WCC and preventing or disrupting binding to the frq promoter. At the molecular scale, however, we have not determined whether FRQ can interact with a DNA-bound WCC causing it to dissociate, or rather whether FRQ interacts only with soluble WCC, thereby shifting the equilibrium of free to DNA-bound. The former scenario would leave open the possibility that FRQ might be found transiently associated with DNA-bound WCC in vivo and that this interaction could also contribute to repression.
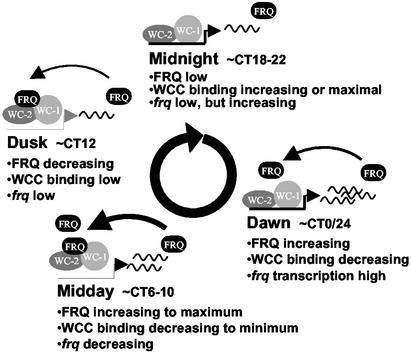
These data have closed the autoregulatory feedback loop at the center of the clock, providing a clearer and more detailed description of the molecular events that lie at the core of the Neurospora circadian system (Fig. 7). Late at night, the majority of FRQ has been degraded, WCC is able to bind to the C box, and frq levels begin to rise. FRQ levels lag behind the transcript but gradually increase by early morning. WCC levels are still in excess of FRQ, and frq transcript continues its ≈10-h rise. By midday FRQ has dimerized and reached sufficient levels to begin titrating the WCC off of the C box, and frq transcript levels are in decline. Ongoing translation continues to drive increasing levels of FRQ, with the crest reached sometime before dusk. More and more WCC is bound by FRQ, the C box is left depleted, and frq levels are reaching their low point. Progressive phosphorylation of FRQ ultimately leads to precipitous turnover; this loss of FRQ releases the WCC, allowing the cycle to begin anew.
Figure 7.
Daily changes in FRQ levels regulate WCC binding to the C box. A schematic model illustrating the dynamic interactions of FRQ, WC-1, and WC-2 at the frq promoter is shown. Starting around midnight, FRQ levels are low, and WC-1 and WC-2 (WCC) are free to bind to the frq promoter and activate transcription. By dawn, FRQ levels are beginning to increase but are still too low to prevent WCC binding; maximal rate of frq transcription is achieved. FRQ reaches levels sufficient to cause a decrease in WCC binding by midday, frq transcription is minimal, and frq message levels decline. By dusk, WCC binding and frq message levels have reached a low, and FRQ levels are beginning to decline as a result of phosphorylation-mediated turnover. FRQ levels reach a low point around midnight, allowing increasing WCC binding and activating frq transcription once again.
Acknowledgments
We thank members of our laboratory for many helpful discussions and critical reading of the manuscript. We are especially grateful to Yi Liu for use of the YL29 strain as well as experimental help and advice. This work was supported by National Institutes of Health Grants R37GM34985 (to J.C.D.) and MH44651 (to J.C.D. and J.J.L.), National Science Foundation Grant MC-BOX-0084509 (to J.J.L.), and the Norris Cotton Cancer Center Core Grant at Dartmouth Medical School.
Abbreviations
- WC
White Collar
- FRQ
FREQUENCY
- WCC
WC complex
- C box
Clock Box
- wt
wild type
- EMSA
electrophoretic mobility-shift assay
- LL
constant light
- DD
constant dark
- QA
quinic acid
- CT
circadian time
- PER
PERIOD
- TIM
TIMELESS
- dCLK
dCLOCK
- CYC
CYCLE
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Dunlap J C. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 2.Loros J J, Dunlap J C. Annu Rev Physiol. 2001;63:757–794. doi: 10.1146/annurev.physiol.63.1.757. [DOI] [PubMed] [Google Scholar]
- 3.Cermakian N, Sassone-Corsi P. Nat Rev Mol Cell Biol. 2000;1:59–67. doi: 10.1038/35036078. [DOI] [PubMed] [Google Scholar]
- 4.Allada R, Emery P, Takahashi J S, Rosbash M. Annu Rev Neurosci. 2001;24:1091–1119. doi: 10.1146/annurev.neuro.24.1.1091. [DOI] [PubMed] [Google Scholar]
- 5.Edery I. Chronobiol Int. 1999;16:377–414. doi: 10.3109/07420529908998716. [DOI] [PubMed] [Google Scholar]
- 6.Lee K, Loros J J, Dunlap J C. Science. 2000;289:107–110. doi: 10.1126/science.289.5476.107. [DOI] [PubMed] [Google Scholar]
- 7.Glossup N J R, Lyons L C, Hardin P E. Science. 1999;286:766–769. doi: 10.1126/science.286.5440.766. [DOI] [PubMed] [Google Scholar]
- 8.Shearman L, Sriram S, Weaver D, Maywood E, Chaves I, Zheng B, Kume K, Lee C, van der Horst G, Hastings M, Reppert S. Science. 2000;288:1013–1019. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- 9.Harmer S L, Panda S, Kay S A. Annu Rev Cell Dev Biol. 2001;17:215–253. doi: 10.1146/annurev.cellbio.17.1.215. [DOI] [PubMed] [Google Scholar]
- 10.Ballario P, Vittorioso P, Magrelli A, Talora C, Cabibbo A, Macino G. EMBO J. 1996;15:1650–1657. [PMC free article] [PubMed] [Google Scholar]
- 11.Linden H, Macino G. EMBO J. 1997;16:98–109. doi: 10.1093/emboj/16.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ballario P, Talora C, Galli D, Linden H, Macino G. Mol Microbiol. 1998;29:719–729. doi: 10.1046/j.1365-2958.1998.00955.x. [DOI] [PubMed] [Google Scholar]
- 13.Crosthwaite S C, Dunlap J C, Loros J J. Science. 1997;276:763–769. doi: 10.1126/science.276.5313.763. [DOI] [PubMed] [Google Scholar]
- 14.Lee K, Loros J J, Dunlap J C. Genetics. 2003;163:103–114. doi: 10.1093/genetics/163.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collett M A, Garceau N, Dunlap J C, Loros J J. Genetics. 2002;160:149–158. doi: 10.1093/genetics/160.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng P, Yang Y, Liu Y. Proc Natl Acad Sci USA. 2001;98:7408–7413. doi: 10.1073/pnas.121170298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garceau N, Liu Y, Loros J J, Dunlap J C. Cell. 1997;89:469–476. doi: 10.1016/s0092-8674(00)80227-5. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Loros J, Dunlap J C. Proc Natl Acad Sci USA. 2000;97:234–239. doi: 10.1073/pnas.97.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorl M, Merrow M, Huttner B, Johnson J, Roenneberg T, Brunner M. EMBO J. 2001;20:7074–7084. doi: 10.1093/emboj/20.24.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aronson B, Johnson K, Loros J J, Dunlap J C. Science. 1994;263:1578–1584. doi: 10.1126/science.8128244. [DOI] [PubMed] [Google Scholar]
- 21.Denault D L, Loros J J, Dunlap J C. EMBO J. 2001;20:109–117. doi: 10.1093/emboj/20.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng P, Yang Y, Heintzen C, Liu Y. EMBO J. 2001;20:101–108. doi: 10.1093/emboj/20.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merrow M, Franchi L, Dragovic Z, Gorl M, Johnson J, Brunner M, Macino G, Roenneberg T. EMBO J. 2001;20:307–315. doi: 10.1093/emboj/20.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merrow M, Garceau N, Dunlap J C. Proc Natl Acad Sci USA. 1997;94:3877–3882. doi: 10.1073/pnas.94.8.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Merrow M, Loros J J, Dunlap J C. Science. 1998;281:825–829. doi: 10.1126/science.281.5378.825. [DOI] [PubMed] [Google Scholar]
- 26.Lee C, Etchegaray J, Cagampang F R A, Loudon A S I, Reppert S M. Cell. 2001;107:855–867. doi: 10.1016/s0092-8674(01)00610-9. [DOI] [PubMed] [Google Scholar]
- 27.Lee C, Bae K, Edery I. Neuron. 1998;21:857–867. doi: 10.1016/s0896-6273(00)80601-7. [DOI] [PubMed] [Google Scholar]
- 28.Lee C, Bae K, Edery I. Mol Cell Biol. 1999;19:5316–5325. doi: 10.1128/mcb.19.8.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bae K, Lee C, Hardin P E, Edery I. J Neurosci. 2000;20:1746–1753. doi: 10.1523/JNEUROSCI.20-05-01746.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Etchegaray J P, Lee C, Wade P A, Reppert S M. Nature. 2003;421:177–182. doi: 10.1038/nature01314. [DOI] [PubMed] [Google Scholar]
- 31.Bell-Pedersen D, Dunlap J C, Loros J J. Mol Cell Biol. 1996;16:513–521. doi: 10.1128/mcb.16.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis R L, deSerres D. Methods Enzymol. 1970;27A:79–143. [Google Scholar]
- 33.Roenneberg T, Taylor W. Methods Enzymol. 2000;305:104–119. doi: 10.1016/s0076-6879(00)05481-1. [DOI] [PubMed] [Google Scholar]
- 34.Froehlich A C, Liu Y, Loros J J, Dunlap J C. Science. 2002;297:815–819. doi: 10.1126/science.1073681. [DOI] [PubMed] [Google Scholar]
- 35.McClung C R, Fox B A, Dunlap J C. Nature. 1989;339:558–562. doi: 10.1038/339558a0. [DOI] [PubMed] [Google Scholar]
- 36.Crosthwaite S C, Loros J J, Dunlap J C. Cell. 1995;81:1003–1012. doi: 10.1016/s0092-8674(05)80005-4. [DOI] [PubMed] [Google Scholar]
- 37.Gekakis N, Stankis D, Nguyen H B, Davis F C, Wilsbacher L D, King D P, Takahashi J S, Weitz C J. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi S, Mitsui S, Miyake S, Yan L, Onishi H, Yagita K, Suzuki M, Shibata S, Kobayashi M, Okamura H. Curr Biol. 2000;10:873–876. doi: 10.1016/s0960-9822(00)00602-3. [DOI] [PubMed] [Google Scholar]
- 39.Yamaguchi S, Mitsui S, Yan L, Yagita K, Miyake S, Okamura H. Mol Cell Biol. 2000;20:4773–4781. doi: 10.1128/mcb.20.13.4773-4781.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hardin P E, Hall J C, Rosbash M. Proc Natl Acad Sci USA. 1992;89:11711–11715. doi: 10.1073/pnas.89.24.11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stanewsky R, Jamison C, Plautz J, Kay S, Hall J C. EMBO J. 1997;16:5006–5018. doi: 10.1093/emboj/16.16.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDonald M J, Rosbash M, Emery P. Mol Cell Biol. 2001;21:1207–1217. doi: 10.1128/MCB.21.4.1207-1217.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang G K, Ousley A, Darlington T K, Chen D, Chen Y, Fu W, Hickman L J, Kay S A, Sehgal A. J Neurobiol. 2001;47:161–175. doi: 10.1002/neu.1024. [DOI] [PubMed] [Google Scholar]
- 44.So W, Rosbash M. EMBO J. 1997;16:7146–7155. doi: 10.1093/emboj/16.23.7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Darlington T K, Lyons L C, Hardin P E, Kay S A. J Biol Rhythms. 2000;15:462–471. doi: 10.1177/074873040001500603. [DOI] [PubMed] [Google Scholar]
- 46.Lyons L C, Darlinton T K, Hao H, Houl J, Kay S A, Hardin P A. J Biol Rhythms. 2000;15:472–482. doi: 10.1177/074873040001500604. [DOI] [PubMed] [Google Scholar]
- 47.Allada R, White N E, So W V, Hall J C, Rosbash M. Cell. 1998;93:805–814. doi: 10.1016/s0092-8674(00)81440-3. [DOI] [PubMed] [Google Scholar]
- 48.Rutila J E, Suri V, Le M, So W V, Rosbash M, Hall J C. Cell. 1998;93:805–813. doi: 10.1016/s0092-8674(00)81441-5. [DOI] [PubMed] [Google Scholar]
- 49.Hogenesch J B, Gu Y-Z, Jain S, Bradfield C A. Proc Natl Acad Sci USA. 1998;95:5474–5479. doi: 10.1073/pnas.95.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Darlington T K, Wager-Smith K, Ceriani M F, Stankis D, Gekakis N, Steeves T, Weitz C J, Takahashi J, Kay S A. Science. 1998;280:1599–1603. doi: 10.1126/science.280.5369.1599. [DOI] [PubMed] [Google Scholar]
- 51.Kyriacou C P, Rosato E. J Biol Rhythms. 2000;15:483–490. doi: 10.1177/074873040001500605. [DOI] [PubMed] [Google Scholar]
- 52.Alabadi D, Oyama T, Yanovsky M J, Harmon F G, Mas P, Kay S A. Science. 2001;293:880–883. doi: 10.1126/science.1061320. [DOI] [PubMed] [Google Scholar]
- 53.Wang Z Y, Tobin E M. Cell. 1998;93:1207–1217. doi: 10.1016/s0092-8674(00)81464-6. [DOI] [PubMed] [Google Scholar]