Abstract
IL-17 is a T cell-derived, proinflammatory cytokine that is suspected to be involved in the development of various inflammatory diseases. Although there are elevated levels of IL-17 in synovial fluid of patients with rheumatoid arthritis, the pathogenic role of IL-17 in the development of rheumatoid arthritis remains to be elucidated. In this report, the effects of IL-17 deficiency were examined in IL-1 receptor antagonist-deficient (IL-1Ra−/−) mice that spontaneously develop an inflammatory and destructive arthritis due to unopposed excess IL-1 signaling. IL-17 expression is greatly enhanced in IL-1Ra−/− mice, suggesting that IL-17 activity is involved in the pathogenesis of arthritis in these mice. Indeed, the spontaneous development of arthritis did not occur in IL-1Ra−/− mice also deficient in IL-17. The proliferative response of ovalbumin-specific T cells from DO11.10 mice against ovalbumin cocultured with antigen-presenting cells from either IL-1Ra−/− mice or wild-type mice was reduced by IL-17 deficiency, indicating insufficient T cell activation. Cross-linking OX40, a cosignaling molecule on CD4+ T cells that plays an important role in T cell antigen-presenting cell interaction, with anti-OX40 Ab accelerated the production of IL-17 induced by CD3 stimulation. Because OX40 is induced by IL-1 signaling, IL-17 induction is likely to be downstream of IL-1 through activation of OX40. These observations suggest that IL-17 plays a crucial role in T cell activation, downstream of IL-1, causing the development of autoimmune arthritis.
IL-17, a T cell-derived proinflammatory cytokine, is produced by TCRα/β+CD4−CD8− thymocytes, as well as by activated CD4+ and CD4+CD45RO+ memory T cells (1). IL-17 has pleiotropic activities including the induction of proinflamatory cytokines such as tumor necrosis factor α, IL-1β, and IL-6, and chemokines like IL-8 and monocyte chemoattractant protein 1 on various cell types (2, 3). In addition, IL-17 is involved in the induction of inducible nitric oxide synthase and cyclooxygenase 2 in chondrocytes (4), induction of prostaglandin E2-mediated osteoclast differentiation factor expression in osteoblasts (5), up-regulation of intracellular adhesion molecule 1 and HLA-DR expression in kerationocytes (6), promotion of stem cell factor- and granulocyte-colony stimulating factor-mediated granulopoiesis (7), promotion of tumor rejection by natural killer cell activation (8), and enhancement of allorejection via promotion of dendritic cell maturation (9).
IL-17 has been suggested to be involved in the development of rheumatoid arthritis (RA), because IL-17 is found in the synovial fluid of patients with RA (10) and is produced by T cell clones established from patients with RA (11). Actually, the incidence of arthritis can be partially reduced by the administration of an extracellular domain of IL-17R and Fc fusion protein (IL-17R:Fc), which inhibits IL-17-IL-17R binding in the elicitation phase during collagen-induced arthritis (CIA) (12). The precise role for IL-17 in the pathogenesis of RA, however, still remains to be elucidated.
IL-1 receptor antagonist (IL-1Ra) is an endogenous inhibitor of IL-1 and is believed to regulate IL-1 activity. Polyarthritis spontaneously develops in IL-1Ra−/− mice on the BALB/c background starting at 5 weeks of age, and by 12 weeks of age almost all mice are affected (13). Histopathology of the lesions closely resembles RA in humans, with marked synovial and periarticular inflammation and articular erosion caused by invasion of granulation tissue (13). High levels of auto-Abs against Ig, type II collagen (IIC), and double-stranded DNA are detectable in serum. When IL-1Ra−/− mice are crossed to scid/scid mice, the development of arthritis is completely suppressed (R.H., A. Nakajima, and Y.I., unpublished data). Furthermore, when T cells from IL-1Ra−/− mice are transferred to nu/nu mice, these mice develop arthritis (ref. 14; R.H. and Y.I., unpublished data). Thus, in this model, excess IL-1 signaling due to a deficiency in the IL-1Ra gene product causes T cell-mediated autoimmunity, resulting in joint-specific inflammation and bone destruction.
In this study, using IL-17−/− mice, we assessed the role of IL-17 in the development of arthritis in IL-1Ra−/− mouse models. We show that IL-17 production by T cells is greatly enhanced in IL-1Ra−/− mice, and IL-17 deficiency completely blocks the development of arthritis of this model. Furthermore, we demonstrate that cross-linking of OX40 on T cells enhances IL-17 production, suggesting that IL-1 enhances IL-17 production through OX40 induction.
Materials and Methods
Mice.
IL-17−/− and IL-1Ra−/− mice were generated as described (15, 16). IL-17-deficient IL-1Ra−/− mice were produced by crossing IL-17−/− mice, backcrossed three generations to BALB/cA mice, and then to IL-1Ra−/− mice on the BALB/cA background (N8). DO11.10 transgenic mice were kindly supplied by D. Y. Loh (17). These mice were kept under specific pathogen-free conditions in an environmentally controlled clean room in the Center for Experimental Medicine at the Institute of Medical Science, University of Tokyo. The experiments were conducted according to the institutional ethical guidelines for animal experiments and the safety guidelines for gene manipulation experiments.
Clinical Assessment of Arthritis.
Development of arthritis by macroscopic evaluation was determined as described (13). The histological score was evaluated independently by two individuals blind to the genotypes of the mice.
T Cell Culture.
To measure IL-17 production from lymph node (LN) cells, axillary, inguinal, and brachial LN cells were harvested from wild-type or IL-1Ra−/− mice. LN cells (2 × 105 cells per well in a 96-well flat-bottom plate) were cultured in the absence or presence of 1 μg/ml anti-CD3 mAb (145-2C11; BD Pharmingen), after which IL-17 levels were measured in the culture medium.
To purify CD4+ T cells, spleen cell suspensions were passed through a nylon wool column. Then, the flow-through fraction was incubated with anti-CD8, anti-B220, anti-Mac-1, anti-Ter119, and anti-DX5 magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) and passed through a magnetic cell sorting column (Miltenyi Biotec), and the negative fraction was collected (CD4+ T cells >95%). CD4+ T cells (2 × 105 cells per well in a 96-well flat-bottom plate) were cultured in a plate coated with an anti-CD3 mAb, a mixture of anti-CD3 mAb (0.5 μg/ml) plus recombinant IL-1α (PeproTech, Rocky Hill, NJ), or a mixture of anti-CD3 mAb (1 μg/ml) plus anti-OX40 mAb (OX86; BD Pharmingen). In the anti-CD3 plus anti-OX40 stimulation, data are shown from the time point of maximal response in IL-17 production (96 h). In the primary T cell response assay, CD4+ T cells (2.5 × 105 cells) from DO11.10 transgenic mice were cultured with antigen-presenting cells (APCs) as B220−Thy1.2− splenic adherent cells (5 × 103 cells) in the absence or presence of 0.1 μM ovalbumin peptide for 3 days as described (18).
For collagen-specific LN cell culture, mice were immunized intradermally at the base of the tail with 200 μg of bovine IIC (Cosmobio, Tokyo) in 0.02 M Tris⋅HCl/0.15 M NaCl with Freund's complete adjuvant (Difco) and killed 7 days later. LN cells (1 × 106 cells per ml; inguinal, axillary, brachial, and submaxillary) were cultured in the absence or presence of 20 μg/ml IIC for 24 h (for CD40L expression) or 72 h (for OX40 expression). Then, fluorescence-activated cell sorter analysis was carried out as described (19).
Detection of IL-17 by ELISA.
IL-17 levels were measured by ELISA as described (15). Monoclonal rat anti-mouse IL-17 and polyclonal biotinylated goat anti-mouse IL-17 Abs (DAKO) were used as capture and detection Abs, respectively. Horseradish peroxidase–avidin was obtained from PharMingen and TMB substrate (3.5′–5.5′ tetramethylbenzidine and hydrogen peroxide) was purchased from DAKO. Recombinant IL-17 as a standard reagent was obtained from Sigma.
Fluorescence-Activated Cell Sorter Analysis.
CD40L expression on CD4+ T cells was analyzed as described (20). Briefly, to determine the effect of IL-17 on CD40L expression, purified CD4+ T cells from IIC-immunized mice on 12-well plates (1 × 106 cells per well) were stimulated with plate-coated CD3 mAb with or without rIL-17 in the presence of 1 μg of biotinylated anti-mouse CD40L mAb (MR1; BD Pharmingen) or biotinylated hamster IgG (BD Pharmingen) as an isotype-matched control Ab for 24 h. OX40 expression on CD4+ T cells was examined as described (18). FITC-anti-mouse CD4 mAb (RM4–5) and phycoerythrin (PE)–streptavidin were purchased from BD Pharmingen. PE-anti-mouse OX40 mAb (OX86) was obtained from Immunotech (Luminy, France).
Immunohistochemistry.
To detect OX40 expression on synovial tissue of IL-1Ra−/− mice, immunohistochemical analysis was performed as described (21). In brief, mouse limbs were fixed in PLP solution (0.02 g/ml paraformaldehyde/0.015 M l-lysine monohydochloride, pH 7.4/0.01 M sodium perchlorate) for 6 h at 4°C. After fixing, limbs were decalcified in EDTA–glycerol solution (0.35 M dipotassium dihydrogen ethylenediaminetetraacetate/15% glycerol) for 10 days. Then, samples were embedded in OCT compound and prepared slices.
Results and Discussion
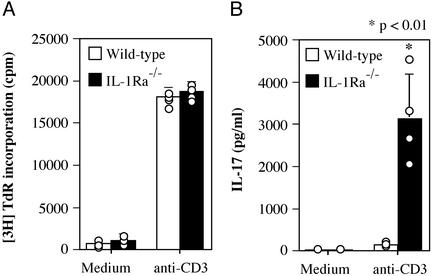
To investigate the role of IL-17 in the development of arthritis in IL-1Ra−/− mice, we examined IL-17 production in T cells from these mice. LN cells containing both T cells and APCs from arthritic IL-1Ra−/− mice were stimulated with anti-CD3 mAb, and T cell proliferation and IL-17 production were measured. Proliferative responses of IL-1Ra−/− T cells were comparable to those of wild-type T cells (Fig. 1A), indicating that the T cell antigen receptor signaling pathway underlying proliferative responses was normal in these mutant mice. In contrast, IL-17 production by IL-1Ra−/− T cells was markedly enhanced relative to wild-type T cells (Fig. 1B). Thus, excess IL-1 signaling, caused by a deficiency of IL-1Ra, induces IL-17 production, suggesting that IL-17 may be involved in the development of arthritis in IL-1Ra−/− mice.
Figure 1.
IL-17 production is markedly enhanced in T cells from IL-1Ra−/− mice. LN cells from wild-type and IL-1Ra−/− mice were cultured in the absence or presence of 1 μg/ml anti-CD3 mAb for 48 h. (A) Proliferative response was measured by [3H]thymidine incorporation. (B) IL-17 levels in the supernatants of A were determined by ELISA. Each circle represents an individual mouse, and an average and SD are shown. These results were reproducible in two independent experiments. The Student's t test was used for statistical evaluation of the results.
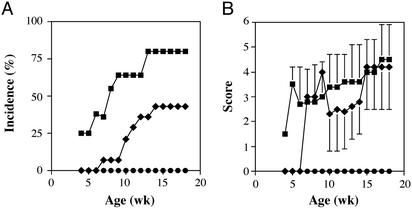
To verify this possibility, we assessed the effect of IL-17 deficiency on the development of arthritis in IL-1Ra−/− mice. IL-17−/− and IL-1Ra−/− double-deficient mice were generated by intercrossing single mutant mice, and the incidence of arthritis in IL-17−/− × IL-1Ra−/− mice was investigated. We found that the development of arthritis in IL-17−/− × IL-1Ra−/− mice was completely suppressed, as shown in Fig. 2A. The incidence of arthritis in IL-17+/− × IL-1Ra−/− mice was also significantly lower than that in IL-17+/+ × IL-1Ra−/− mice, indicating a gene dosage effect of IL-17. These results indicate that IL-17 is involved in the development of arthritis caused by excess IL-1 signaling.
Figure 2.
Development of arthritis in IL-1Ra−/− mice is completely suppressed by a deficiency of IL-17. (A) Incidence of arthritis that developed in IL-1Ra−/− mice on the BALB/c background. The incidences were compared among IL-17−/−, IL-17+/−, and IL-17+/+ mice. (B) Severity score of the mice. Average scores of the affected mice are shown and bars represent the SD. Squares, IL-17+/+ × IL-1Ra−/− (n = 10); diamonds, IL-17+/− × IL-1Ra−/− mice (n = 14); circles, IL-17−/− × IL-1Ra−/− mice (n = 10).
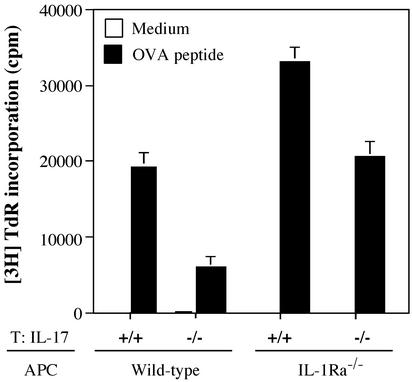
Interestingly, the severity score of arthritis in IL-17 heterozygous mice was similar to that of wild-type mice, even though the incidence was significantly suppressed (Fig. 2B). These results suggest that IL-17 plays a role in the sensitization phase rather than in the elicitation phase. To verify this possibility, we examined the effect of IL-17 on antigen-specific T cell activation by using DO11.10 mice that carry an ovalbumin (OVA)-specific T cell receptor transgene. The proliferative response of CD4+ T cells from IL-17−/− DO11.10 transgenic mice cocultured with IL-1Ra−/− APCs directed against OVA was decreased to the level of DO11.10 CD4+ T cells cocultured with wild-type APCs. In contrast, a greater proliferative response was detected in IL-17+/+ DO11.10 T cells cocultured with IL-1Ra−/− APCs (Fig. 3). These results clearly indicate that IL-17 is required for activating naive T cells in an antigen-specific manner, and that the activation of T cells by IL-1 is mediated via IL-17.
Figure 3.
IL-17 is required for antigen-specific T cell priming. Proliferative responses of DO11.10 T cells against the ovalbumin 323–339 peptide in the presence of APCs from wild-type or IL-1Ra−/− mice were assessed by measuring the incorporation of [3H]thymidine after 3 days of culture. Effects of IL-17 deficiency were evaluated by using IL-17−/− DO11.10 T cells and IL-1Ra−/− APCs. Data shows an average ± SD of three wells. These results were reproducible in two independent experiments.
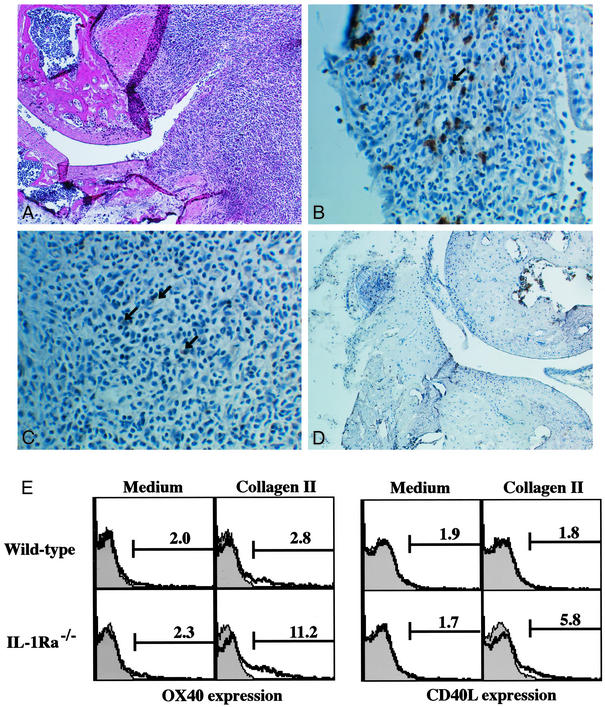
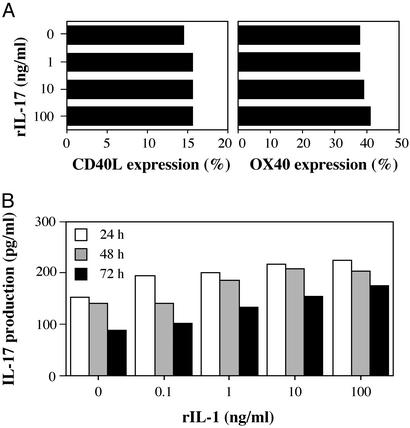
We recently showed that IL-1 produced by APCs induced CD40L and OX40 expression on CD4+ T cells (18). CD40L and OX40 act as costimulatory molecules in T cell–APC interaction, and these molecules are suggested to be involved in the development of arthritis (22–25). Consistent with this notion, the blockade of CD40L–CD40 or OX40–OX40L interaction suppressed the development of arthritis in IL-1Ra−/− mice (ref. 14; R.H. and Y.I., unpublished data). Actually, OX40+ cells were detected in the follicular structure resembling secondary lymphoid follicles in the synovial tissue (Fig. 4 A and C), where CD4+ cells were accumulated (Fig. 4B). Similar follicular structures are formed in synovial tissues of patients with RA (26). On the other hand, CD40L expression could not be detected histologically in synovial tissues of IL-1Ra−/− mice. With regard to this, it is known that CD40L expression on cell surface is very low because of down-modulation of the expression by CD40 cross-linking (27, 28). Then, we examined CD40L and OX40 expression on T cells from IL-1Ra−/− mice after stimulation with IIC, which was known as one of autoantigens in the synovial tissues (13). As shown in Fig. 4E, the expression of CD40L and OX40 on CD4+ T cells in IL-1Ra−/− mice was augmented compared with that in wild-type mice. These observations suggest that CD40L and OX40 expression on CD4+ T cells in synovial tissues is enhanced in IL-1Ra−/− mice. This enhancement, however, was not induced by IL-17, because treatment with rIL-17 did not induce CD40L or OX40 expression on CD4+ T cells (Fig. 5A).
Figure 4.
Enhanced OX40 and CD40L expression on T cells in IL-1Ra−/− mice. CD4+ and OX40+ cell infiltration was observed in synovial tissues of IL-1Ra−/− mice (A–C) but not in wild-type mice (D). Both OX40 and CD40L expression on CD4+ T cells was enhanced in IL-1Ra−/− mice compared with wild-type mice after immunization with IIC (E). (A) Synovial tissues of IL-1Ra−/− mice; hematoxylin/eosin (H&E) staining (magnification, ×25). (B) Synovial tissues of IL-1Ra−/− mice stained with anti-CD4 mAb (magnification, ×100). Arrow shows positive cells. (C) Synovial tissues of IL-1Ra−/− mice stained with anti-OX40 mAb (magnification, ×100). Arrows show positive cells. (D) Synovial tissues of wild-type mice; H&E staining (magnification, ×25). (E) Seven days after immunization with IIC, LN cells were cultured in the absence or presence of 20 μg/ml IIC for 24 (CD40L expression) or 72 h (OX40 expression). Then, CD40L and OX40 expression in CD4+ population was analyzed by flow cytometry. Shaded area shows an isotype-matched control Ig staining.
Figure 5.
IL-1 could not directly induce IL-17 in CD4+ T cells. (A) IL-17 could not induce CD40L and OX40 expression on CD4+ T cells. Splenic CD4+ T cells from wild-type mice were stimulated with plate-coated anti-mouse CD3 mAb (1 μg/ml) with or without rIL-17 for 24 (for CD40L expression) and 72 h (for OX40 expression). These results were reproducible in two independent experiments. (B) Splenic CD4+ T cells from wild-type mice were stimulated with or without rIL-1α together with plate-coated anti-mouse CD3 mAb for 24, 48, and 72 h. Then, IL-17 levels in culture supernatants were determined by ELISA. Data show a pooled supernatant from three wells. These results were reproducible in two independent experiments.
We next analyzed the mechanism of IL-17 induction. Because the previous results indicate that IL-17 functions downstream of IL-1, we examined the possibility that IL-1 directly induces IL-17 production in T cells. When purified CD4+ T cells from LN cells were treated with varying concentrations of IL-1, together with stimulation by plate-coated anti-CD3 mAb (0.5 μg/ml) for 24, 48, and 72 h, essentially no stimulation of IL-17 production was observed (Fig. 5B). Thus, overproduction of IL-17 in IL-1Ra−/− LN cells is not a result of direct effects of IL-1 on CD4+ T cells.
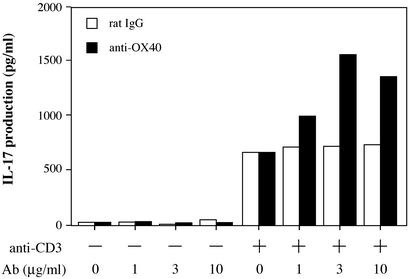
We next examined the possibility that OX40 signaling might induce IL-17 production, because OX40 expression on T cells is augmented in IL-1Ra−/− mice. As shown in Fig. 6, IL-17 production in plate-coated anti-CD3 mAb-stimulated CD4+ T cells was enhanced by stimulation with plate-coated anti-OX40 mAb. High concentrations (10–100 μg/ml) of anti-OX40 mAb, however, inhibited IL-17 production and T cell proliferation (Fig. 6; data not shown). IL-17 production was not observed with anti-OX40 mAb alone, without anti-CD3 mAb stimulation. These results indicate that OX40 signaling enhances IL-17 production induced by T cell antigen receptor stimulation in CD4+ T cells.
Figure 6.
Cross-linking of OX40 on CD4+ T cells promotes IL-17 production. Cross-linking of OX40 could promote IL-17 production by CD4+ T cells. Splenic CD4+ T cells from wild-type mice were stimulated with plate-coated anti-mouse CD3 mAb (1 μg/ml) plus varying amounts of plate-coated anti-OX40 mAb or isotype control rat IgG for 96 h. Then, IL-17 levels in culture supernatants were determined by ELISA. Data show a pooled supernatant from three wells. These results were reproducible in three independent experiments.
In this report, we have shown that IL-17 production is enhanced by excess IL-1 signaling through activation of OX40 in IL-1Ra−/− mice, resulting in the development of autoimmunity and arthritis. It was reported that systemic or local overproduction of IL-17 with an adenoviral vector accelerated the development of CIA, and inhibition of IL-17 signaling by an IL-17R:Fc fusion protein suppressed the development of CIA in mice (12), indicating an important role for IL-17 in the development of CIA. Moreover, this aggravated CIA could not be suppressed by administration of an anti-IL-1α/β Ab and IL-17-induced exaggeration of bacterial cell wall-induced arthritis was not diminished in IL-1β−/− mice, suggesting an IL-1-independent role of IL-17 (12). These observations are consistent with our notion that IL-17 acts downstream of IL-1.
Because IL-17 is produced by activated T cells and can induce various proinflammatory cytokines, chemokines, and cell adhesion molecules (2, 3, 6), it is implied that this cytokine plays an important role in the development of inflammation at the elicitation phase. In this report, however, we have shown that IL-17 plays a crucial role in the activation of T cells at the sensitization phase in the development of arthritis. With regard to this, Yao et al. (29) reported that T cell proliferation and IL-2 production induced by phytohemagglutinin, Con A, and anti-T cell antigen receptor mAb were inhibited by soluble IL-17R, indicating that IL-17 is involved in T cell activation. We also observed that T cell sensitization is impaired in IL-17−/− mice after induction of contact hypersensitivity, delayed-type hypersensitivity, and airway hypersensitivity responses (15). The molecular mechanisms by which naive T cells are activated by IL-17, however, remain to be elucidated.
We have shown that auto-Ab levels in sera are elevated in IL-1Ra−/− mice (13). The levels of auto-Abs against IgG (rheumatoid factor) in sera of IL-17−/− × IL-1Ra−/− mice were significantly reduced compared with that of IL-17+/+ × IL-1Ra−/− mice [0.111 ± 0.022 (n = 6) vs. 0.152 ± 0.068 (n = 10), P = 0.05], which was consistent with our observation that IL-17 was involved in Ab production (15). This reduction of auto-Ab production in IL-17−/− × IL-1Ra−/− mice, however, may not be the major reason for the suppression of the development of arthritis, because IL-1Ra−/− mouse serum transfer could not induce arthritis in wild-type mice (R.H. and Y.I., data not shown).
Taken together, we have demonstrated that a cascade of IL-1 signaling leads to autoimmunity and arthritis in IL-1Ra−/− mice. Excess IL-1 signaling caused by IL-1Ra deficiency induces excess OX40 expression on T cells, and then, OX40 signaling induces overproduction of IL-17 from CD4+ T cells, leading to the development of autoimmunity and arthritis. These findings may provide a cue for development of novel therapeutics to treat autoimmune inflammatory diseases.
Acknowledgments
We thank all of the members of the laboratory for animal care. This work was supported by grants from the Ministry of Education, Science, Sports, and Culture of Japan and the Ministry of Health and Welfare of Japan.
Abbreviations
- IL-1Ra
IL-1 receptor antagonist
- RA
rheumatoid arthritis
- CIA
collagen-induced arthritis
- LN
lymph node
- APC
antigen-presenting cell
- IIC
type II collagen
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Yao Z, Painter S L, Fanslow W C, Ulrich D, Macduff B M, Spriggs M K, Armitage R J. J Immunol. 1995;155:5483–5486. [PubMed] [Google Scholar]
- 2.Fossiez F, Banchereau J, Murray R, Van Kooten C, Garrone P, Lebecque S. Int Rev Immunol. 1998;16:541–551. doi: 10.3109/08830189809043008. [DOI] [PubMed] [Google Scholar]
- 3.Jovanovic D V, Di Battista J A, Martel-Pelletier J, Jolicoeur F C, He Y, Zhang M, Mineau F, Pelletier J P. J Immunol. 1998;160:3513–3521. [PubMed] [Google Scholar]
- 4.Shalom-Barak T, Quach J, Lotz M. J Biol Chem. 1998;273:27467–27473. doi: 10.1074/jbc.273.42.27467. [DOI] [PubMed] [Google Scholar]
- 5.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie M T, et al. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albanesi C, Scarponi C, Cavani A, Federici M, Nasorri F, Girolomoni G. J Invest Dermatol. 2000;115:81–87. doi: 10.1046/j.1523-1747.2000.00041.x. [DOI] [PubMed] [Google Scholar]
- 7.Schwarzenberger P, Huang W, Ye P, Oliver P, Manuel M, Zhang Z, Bagby G, Nelson S, Kolls J K. J Immunol. 2000;164:4783–4789. doi: 10.4049/jimmunol.164.9.4783. [DOI] [PubMed] [Google Scholar]
- 8.Hirahara N, Nio Y, Sasaki S, Takamura M, Iguchi C, Dong M, Yamasawa K, Itakura M, Tamura K. Anticancer Res. 2000;20:3137–3142. [PubMed] [Google Scholar]
- 9.Antonysamy M A, Fanslow W C, Fu F, Li W, Qian S, Troutt A B, Thomson A W. J Immunol. 1999;162:577–584. [PubMed] [Google Scholar]
- 10.Ziolkowska M, Koc A, Luszczykiewicz G, Ksiezopolska-Pietrzak K, Klimczak E, Chwalinska-Sadowska H, Maslinski W. J Immunol. 2000;164:2832–2838. doi: 10.4049/jimmunol.164.5.2832. [DOI] [PubMed] [Google Scholar]
- 11.Aarvak T, Chabaud M, Miossec P, Natvig J B. J Immunol. 1999;162:1246–1251. [PubMed] [Google Scholar]
- 12.Lubberts E, Joosten L A, Oppers B, van den Bersselaar L, Coenen-de Roo C J, Kolls J K, Schwarzenberger P, van de Loo F A, van den Berg W B. J Immunol. 2001;167:1004–1013. doi: 10.4049/jimmunol.167.2.1004. [DOI] [PubMed] [Google Scholar]
- 13.Horai R, Saijo S, Tanioka H, Nakae S, Sudo K, Okahara A, Ikuse T, Asano M, Iwakura Y. J Exp Med. 2000;191:313–320. doi: 10.1084/jem.191.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwakura Y. Cytokine Growth Factor Rev. 2002;13:341–355. doi: 10.1016/s1359-6101(02)00021-7. [DOI] [PubMed] [Google Scholar]
- 15.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 16.Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, Takahashi M, Iwakura Y. J Exp Med. 1998;187:1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy K M, Heimberger A B, Loh D Y. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 18.Nakae S, Asano M, Horai R, Sakaguchi N, Iwakura Y. J Immunol. 2001;167:90–97. doi: 10.4049/jimmunol.167.1.90. [DOI] [PubMed] [Google Scholar]
- 19.Saijo S, Asano M, Horai R, Yamamoto H, Iwakura Y. Arthritis Rheum. 2002;46:533–544. doi: 10.1002/art.10172. [DOI] [PubMed] [Google Scholar]
- 20.Jaiswal A I, Dubey C, Swain S L, Croft M. Int Immunol. 1996;8:275–285. doi: 10.1093/intimm/8.2.275. [DOI] [PubMed] [Google Scholar]
- 21.Mori S, Sawai T, Teshima T, Kyogoku M. J Histochem Cytochem. 1988;36:111–114. doi: 10.1177/36.1.3275709. [DOI] [PubMed] [Google Scholar]
- 22.Durie F H, Fava R A, Foy T M, Aruffo A, Ledbetter J A, Noelle R J. Science. 1993;261:1328–1330. doi: 10.1126/science.7689748. [DOI] [PubMed] [Google Scholar]
- 23.Webster E A, Khakoo A Y, Mackus W J, Karpusas M, Thomas D W, Davidson A, Christian C L, Lederman S. Arthritis Rheum. 1999;42:1291–1296. doi: 10.1002/1529-0131(199906)42:6<1291::AID-ANR29>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 24.Yoshioka T, Nakajima A, Akiba H, Ishiwata T, Asano G, Yoshino S, Yagita H, Okumura K. Eur J Immunol. 2000;30:2815–2823. doi: 10.1002/1521-4141(200010)30:10<2815::AID-IMMU2815>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Weinberg A D. Trends Immunol. 2002;23:102–109. doi: 10.1016/s1471-4906(01)02127-5. [DOI] [PubMed] [Google Scholar]
- 26.Wagner U G, Kurtin P J, Wahner A, Brackertz M, Berry D J, Goronzy J J, Weyand C M. J Immunol. 1998;161:6390–6397. [PubMed] [Google Scholar]
- 27.Yellin M J, Sippel K, Inghirami G, Covey L R, Lee J J, Sinning J, Clark E A, Chess L, Lederman S. J Immunol. 1994;152:598–608. [PubMed] [Google Scholar]
- 28.Higuchi T, Aiba Y, Nomura T, Matsuda J, Mochida K, Suzuki M, Kikutani H, Honjo T, Nishioka K, Tsubata T. J Immunol. 2002;168:9–12. doi: 10.4049/jimmunol.168.1.9. [DOI] [PubMed] [Google Scholar]
- 29.Yao Z, Fanslow W C, Seldin M F, Rousseau A M, Painter S L, Comeau M R, Cohen J I, Spriggs M K. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]