Abstract
Human γδ T cells mediate innate immunity to microbes via T cell receptor-dependent recognition of unprocessed antigens with conserved molecular patterns. These nonpeptide alkylamine antigens are shared by tumor cells, bacteria, parasites, and fungi but also by edible plant products such as tea, apples, mushrooms, and wine. Here we show that priming of γδ T cells with alkylamine antigens in vitro results in a memory response to these antigens. Such priming results also in a nonmemory response to whole bacteria and to lipopolysaccharide, characterized by IL-12-dependent secretion of IFN-γ by γδ T cells and by γδ T cell proliferation. Drinking tea, which contains l-theanine, a precursor of the nonpeptide antigen ethylamine, primed peripheral blood γδ T cells to mediate a memory response on reexposure to ethylamine and to secrete IFN-γ in response to bacteria. This unique combination of innate immune response and immunologic memory shows that γδ T cells can function as a bridge between innate and acquired immunity. In addition, these data provide an explanation for the health benefits of tea.
In humans, γδ T lymphocytes comprise 2–5% of peripheral blood T cells, and the majority (Vγ2Vδ2 T cells) coexpress Vγ2 (alternatively named Vγ9) and Vδ2 T cell receptor (TCR) subunits. Found only in primates, this junctionally diverse yet functionally uniform population of T cells recognizes unprocessed, nonpeptide antigens with conserved molecular patterns such as alkylamines (1) and organophosphates (2, 3) in a TCR-dependent manner without MHC or complementarity-determining region 1 (CD1) restriction (1, 4). One such alkylamine, ethylamine (EA), is found in brewed tea as an intact molecule and in its precursor form, l-theanine (5, 6). Tea (Camellia sinensis) has been touted as a healthful and medicinal beverage for centuries, and research has focused on the antioxidant activity of the catechins (7, 8). However, no in vivo data demonstrating an immunologic effect of tea on human tissue have been published.
There is now a large body of evidence supporting the role of γδ T cells in resistance to infection. In mice, studies using specific γδ T cell mAbs and γδ T cell knockout mice provide compelling evidence for antimicrobial roles against bacterial, parasitic, and viral infection (9–13). In humans, γδ T cells expand up to 50-fold in the peripheral blood 6–10 days after infection with a wide array of bacterial, viral, and parasitic microbes (14–18). These γδ T cell expansions can be recapitulated in vitro by exposure of peripheral blood mononuclear cells (PBMCs) to purified nonpeptide microbial products (alkylamines and organophosphates) and pharmacologic agents that mimic bacterial antigens such as nitrogen-containing bisphosphonates as well as heat-killed bacteria and parasites, bacterial or parasitic extracts, and virus-infected cells (19–22). Using a severe combined immunodeficiency mouse reconstituted with human PBMCs model, we showed that the presence of human γδ T cells is necessary for survival during infection by Escherichia coli or Morganella morganii, and that protection is associated with secretion of large quantities of IFN-γ by these γδ T cells (23). The antibacterial effect of human γδ T cells in vivo is evident as early as 17 h postinfection, indicating that γδ T cell expansion is not required for an antibacterial response and suggesting that γδ T cell-mediated cytokine secretion is a crucial component of protection. PBMCs primed with alkylamine or nitrogen-containing bisphosphonate antigens such as risedronate and pamidronate, specific for Vγ2Vδ2 T cells, produced higher amounts of IFN-γ and provided more protection against infection than unprimed PBMCs (23).
Here we show that antigen-priming of γδ T cells in vitro results in their IFN-γ production in response to nonpeptide antigens, whole bacteria, and lipopolysaccharide (LPS). In a small study using human subjects, we show that tea drinking primes γδ T cells to mediate memory cytokine responses to antigens shared by tea and bacteria and also nonmemory cytokine responses to whole bacteria. Such priming may enhance innate immunity to bacteria and other microbes as well as tumors that share nonpeptide antigens with bacteria.
Materials and Methods
Stimulation of PBMCs with Alkylamine Antigens or Dead Bacteria in Vitro.
The alkylamines EA and iso-butylamine (IBA) were purchased from Sigma and prepared in RPMI medium 1640 at desired concentrations (1 mM) and brought to pH 7.4 before use. The phosphate antigen monoethyl phosphate was obtained from Craig Morita (University of Iowa Medical School, Iowa City). The PMBCs were cultured with the alkylamines in the enzyme-linked immunospot (ELISPOT) assay to the frequency of IFN-γ-producing cells.
E. coli (ATCC 25922) or M. morganii (formerly known as Proteus morganii) strain 235 (National Collection of Type Cultures, London) were inactivated at 56°C for 2 h and added to PBMCs at final concentrations of 5 × 105 (E. coli) or 5 × 106 (M. morganii) colony-forming units (cfu)/ml. IBA-primed or unprimed PBMCs were also stimulated with 1 μg/ml LPS (Sigma) in the presence or absence of an mAb to IL-12 (Endogen, Cambridge, MA) at 1 μg/ml. At the indicated time point, the culture supernatant was collected for analysis of IFN-γ levels for ELISA according to manufacturer recommendations (Becton Dickinson/PharMingen, San Diego). The detection limit of the assay was 4.7 pg/ml.
Donor PBMCs.
A total of 11 healthy non-tea-drinking individuals were asked to drink five to six cups of black tea (Lipton, Englewood Cliffs, NJ), equivalent to ≈600 ml/day for either 2 or 4 weeks. Participants were instructed to steep a tea bag for 5 min in 100 ml of water that had been brought to a boil. Another 10 healthy, non-tea and non-coffee drinkers were asked to drink five to six cups per day of instant coffee (Nescafe, Nestle, Glendale, CA), a hot beverage that contains caffeine but not l-theanine. Volunteers were allowed to add sugar, lemon, or cream to suit their taste. Results from the 2- and 4-week experiments were pooled. There was one dropout in the tea group, and one in the coffee group, each in the first week; thus, after the prebleed no blood was drawn from these individuals. In this pilot study, no attempt was made to catalog age, race, sex, or other demographic parameters or dietary history, but there was a widely diverse distribution of such characteristics among the participants. Most of the volunteers worked in research laboratories or were postgraduate students. This study had the built-in control of pre- and postintervention analysis in individual volunteers. The protocol and consent forms relating to our use of human subjects were approved by the internal review board of Brigham and Women's Hospital. The standardized tea leaves yielded, when prepared in boiling water, 100 ml of brewed tea containing 2.2 mM l-theanine as determined by GC mass spectrometry, which after catabolism or acid hydrolysis yields 2.2 mM EA and an equivalent amount of glutamic acid. Based on an oral intake of 600 ml/day, this protocol thus delivered ≈1.3 mmol/day of l-theanine.
Blood was drawn before and every week after the volunteers started drinking tea or coffee for 2–4 weeks, and PBMCs were isolated by Ficoll/Paque density gradient (Amersham Pharmacia). PBMCs were frozen to be analyzed at a later time. For the in vitro assays, PBMCs were cultured in 96- or 24-well flat-bottom plates in RPMI medium 1640 containing 10% FBS, 2 mM glutamine, 1 nM 2-mercaptoethanol, and 100 units of penicillin-streptomycin solution at 37°C. On days 3 and 7, the culture was supplemented with 0.5 nM IL-2.
ELISPOT Assay.
The ELISPOT assay was performed as described (24). Briefly, 96-well nitrocellulose plates (Millipore) were coated with anti-human IFN-γ Ab (Endogen) overnight at room temperature. The wells were washed repeatedly with culture medium, and cryopreserved PMBCs were thawed and added along with alkylamine or phosphate antigens to the cells. An Epstein–Barr virus-transformed B cell line was used as a source of accessory cell in the assay. Cells were incubated for 24 h at 37°C, and the plates then were washed extensively with PBS containing 0.05% Tween 20. The wells then were incubated with biotinylated anti-human IFN-γ mAb (Endogen) overnight at 4°C and washed with PBS/Tween 20, and then peroxidase-labeled streptavidin (Kirkegaard & Perry Laboratories) was added to each well and incubated further for 1 hour. The wells were washed and the spots developed by adding substrate. The visible spots of IFN-γ-secreting cells were enumerated with a dissecting microscope. The number of ELISPOTS in the absence of antigen was less than five and was subtracted from the number of ELISPOTS in the presence of antigen. Responses to phytohemagglutinin did not depend on tea or coffee intake (data not shown).
Results
Alkylamine-Primed γδ T Cells Responded to Heat-Killed Bacteria and LPS.
Our previous studies show that, as compared with PBMCs that are unprimed, PBMCs primed with Vγ2Vδ2 T cell-specific antigens such as alkylamines or nitrogen-containing bisphosphonates have much greater antibacterial activity, and they have antibacterial activity against bacterial species that do or do not secrete alkylamines or prenyl pyrophosphates (23).
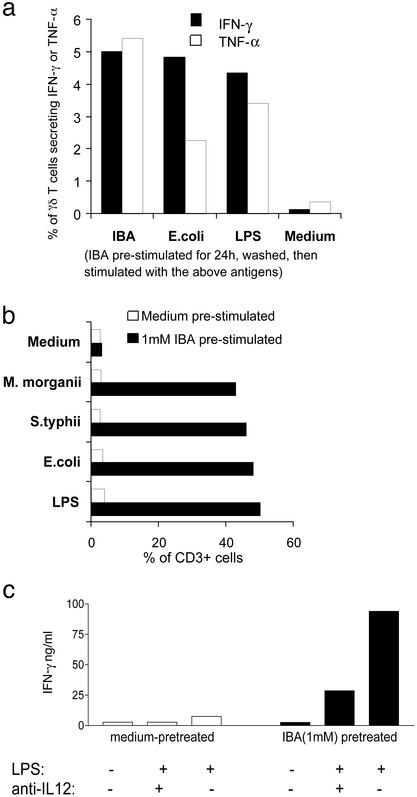
To examine the nature of the nonsecreted stimulatory bacterial antigen from killed bacterial preparations, we primed PBMCs in vitro with the alkylamine antigen IBA, washed it away, and challenged with IBA, heat-killed bacteria, or LPS. γδ T cells from unprimed PBMCs did not produce significant amounts of cytokines and did not expand. In contrast, 2–5% of γδ T cells from PBMCs primed with IBA produced IFN-γ or tumor necrosis factor α (Fig. 1a), and γδ T cells from IBA-primed PBMCs expanded up to 10-fold (Fig. 1b). The response to challenge with the priming antigen, IBA, was anticipated, but the response to heat-killed bacteria and LPS was not, because these preparations do not contain alkylamine antigens. Vγ2Vδ2 TCR transfectants that are responsive to IBA and EA could not respond to LPS or heat-killed bacteria, indicating that reactivity of the primed γδ T cells to these preparations does not depend on their TCR-mediated recognition (data not shown).
Figure 1.
Alkylamine-primed γδ T cells responded to LPS. PBMCs from a random leukopack were primed with 1 mM IBA for 24 h followed by washing. (a) These PBMCs then were challenged with medium, 1 mM IBA, 500,000 cfu/ml heat-killed E. coli, or 1 μg/ml LPS. The percentage of γδ T cells producing IFN-γ or tumor necrosis factor α (TNF-α) was enumerated after 16 h of further culture by using two-color immunofluorescence and intracellular cytokine staining. PBMCs were primed and challenged as described for a except 500,000 cfu/ml heat-killed Salmonella typhii also were used for challenge in this experiment. (b) After 10 days of incubation, the percentage of CD3+ cells that were Vδ2+ was analyzed by flow cytometry. Overall, the number of cells in the culture did not change, thus this analysis reflects an absolute expansion of γδ T cells. (c) PBMCs were primed and challenged as described for a with or without 10 μg/ml of anti-IL-12. IFN-γ production was assayed by ELISA.
IL-12, in the presence of IL-2, preferentially (as compared with αβ T cells) stimulates human γδ T cells to expand in number (25), and during bacterial infection IL-12 produced by infected monocytes provides a strong stimulus for IFN-γ production by γδ T cells (26). To determine whether the stimulatory effects of LPS on primed γδ T cells depend on IL-12, anti-IL-12 mAb was included in cultures of IBA-primed or unprimed LPS-treated PBMCs. As compared with unprimed PBMCs, IBA-primed PBMCs produced ≈10-fold more IFN-γ in response to LPS. Inclusion of anti-IL-12 mAb reduced IFN-γ production by 70%, suggesting that most of the IFN-γ production was IL-12-dependent (Fig. 1c). These data show that priming of γδ T cells with IBA, a Vγ2Vδ2 TCR-dependent alkylamine antigen, enables these γδ T cells to produce IFN-γ and to proliferate in response to heat-killed bacteria or LPS.
Tea but Not Coffee Drinking Induced a γδ T Cell Recall Response to Nonpeptide Antigens.
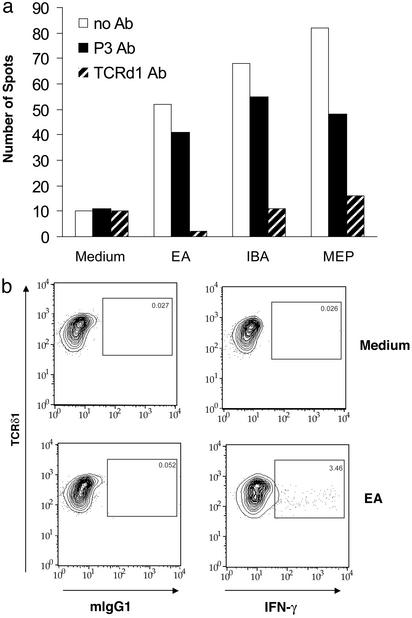
To specifically quantify peripheral blood γδ T cells responsive to alkylamine antigens, we developed an IFN-γ ELISPOT assay. PBMCs were added to wells containing EA, the alkylamine antigen derived from catabolism of l-theanine in tea, and secreted by many bacterial species (1). This analysis showed that ≈3–5% of γδ T cells formed ELISPOTS in the response to EA. Inclusion of an anti-TCR mAb decreased the numbers of spots to background levels (Fig. 2a), and intracellular cytokine analysis confirmed that γδ T cells were the source of IFN-γ (Fig. 2b).
Figure 2.
Only γδ T cells produce IFN-γ in response to EA. (a) Ab to the γδ TCR but not isotype-matched control mAb (P3) blocked the formation of IFN-γ ELISPOTS in response to EA. PBMCs from a random donor were mixed with 1 mM EA and assayed for ELISPOT formation overnight in the presence of medium, anti-TCRδ1 mAb, or control mAb. (b) Intracellular cytokine staining was performed by double staining with anti-TCRδ1 (pan-γδ T cell mAb) and anti-IFN-γ mAb, and γδ T cells were gated.
To assess whether tea drinking could augment the number of human peripheral blood γδ T cells capable of producing IFN-γ, we analyzed by ELISPOT blood samples from 11 healthy, non-tea drinkers before and 1–4 weeks after they drank 600 ml of brewed black tea containing 2.2 mM l-theanine. Lyophilized tea (iced-tea mix) reconstituted with water and brewed green or black tea, but not herbal teas, have similar concentrations (range, 2–10 mM) of l-theanine, an amino acid that comprises 50% of the dry protein weight of tea leaves (27). Evidence in humans and rats shows that l-theanine and its breakdown product, the γδ T cell antigen EA, are detected in the blood and urine after oral intake of tea (28, 29). As negative controls, 10 healthy non-tea-, non-coffee-drinking volunteers drank 600 ml of instant coffee per day, because coffee is a hot beverage that contains caffeine but no l-theanine. Eight subjects from the tea group were asked to participate for 4 weeks, and three were asked to participate for 2 weeks. For the coffee group, the numbers were four and six weeks, respectively. Data from 2- and 4-week participants were pooled.
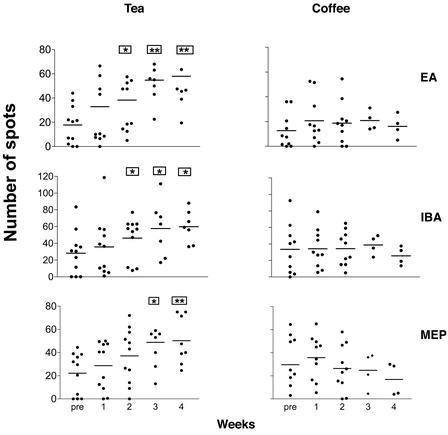
Tea drinking had no effect on the absolute numbers of γδ T cells in PBMCs (data not shown). In contrast, PBMCs analyzed 1–4 weeks after tea but not coffee drinking made 2- to 3-fold more IFN-γ ELISPOTS in response to the secreted bacterial antigens IBA and EA as well as the synthetic organophosphate antigen monoethyl phosphate (Fig. 3). Two weeks into the study, 7 of 11 (P < 0.01) volunteers showed an enhanced γδ T cell response after drinking tea; in contrast, only 1 of 10 volunteers showed an enhanced γδ T cell response after drinking coffee (Table 1). These data show that ingestion of tea, containing l-theanine, a precursor of the Vγ2Vδ2 T cell-specific antigen EA, can boost the capacity of peripheral blood γδ T cells to secrete IFN-γ after in vitro restimulation with this alkylamine antigen shared by tea and bacteria.
Figure 3.
Tea drinking but not coffee drinking enhanced the response of γδ T cells to nonpeptide antigens in a 1-day ELISPOT assay for IFN-γ. A total of 11 healthy non-tea-drinking individuals were asked to drink five to six cups of black tea (Lipton), equivalent to ≈600 ml/day, for either 2 or 4 weeks. Participants were instructed to steep a tea bag for 5 min in 100 ml of water that had been brought to a boil. Another 10 healthy, non-tea and non-coffee drinkers were asked to drink five to six cups per day of instant coffee (Nescafe, Nestle), a hot beverage that contains caffeine but not l-theanine. PBMCs taken before and 1–4 weeks after 600 ml/day brewed tea or coffee drinking were cultured in the presence or absence of alkylamine or organophosphate antigens for 1 day in an IFN-γ ELISPOT assay. Pre, prebleed; MEP, monoethyl phosphate. The data are presented as the mean of spots ± SE. *, P < 0.05, and **, P < 0.005, using a paired t test comparing the weeks post-tea or -coffee drinking to before tea or coffee drinking.
Table 1.
Effect of tea or coffee drinking on γδ T cell response to nonpeptide antigens (expressed as responders/total)
| Weeks | Tea
|
Coffee
|
||||
|---|---|---|---|---|---|---|
| EA | IBA | MEP | EA | IBA | MEP | |
| 1 | 3/11 | 3/11 | 0/11 | 0/10 | 0/10 | 2/10 |
| 2 | 6/11* | 7/11** | 6/11* | 0/10 | 1/10 | 1/10 |
| 3 | 6/8** | 4/7 | 4/8 | 0/4 | 1/4 | 0/4 |
| 4 | 4/8 | 5/7* | 4/8 | 0/4 | 0/4 | 0/4 |
Volunteers who drank tea or coffee for either 2 or 4 weeks donated PBMCs weekly, which were assayed by ELISPOT for responses to the γδ T cell antigens, EA, IBA, and monoethyl phosphate (MEP). Data from 2- or 4-week volunteers were pooled. Responders were defined as having at least 19 more ELISPOTS 1–4 weeks after drinking tea or coffee as compared to before drinking these beverages. P values were calculated by using a two-tailed Fisher's exact test and compared to responders/total volunteers before and 1–4 weeks after drinking tea or coffee.
, P < 0.05;
, P < 0.01.
Tea Drinking Enhanced the γδ T Cell-Dependent Response to Heat-Killed Bacteria.
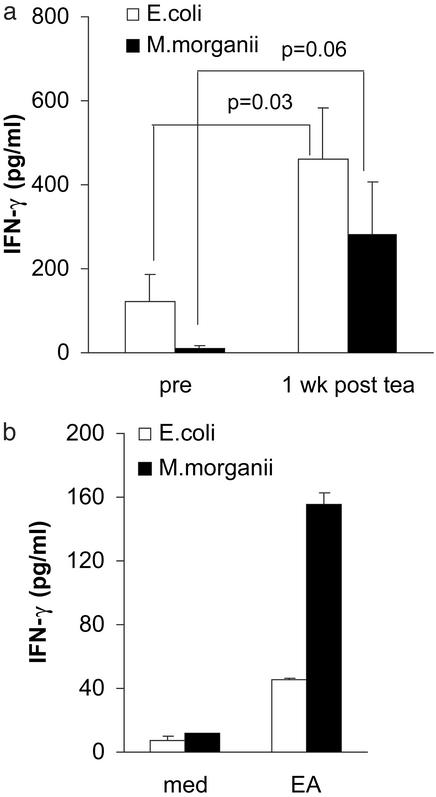
To determine whether tea drinking could prime the human immune system to secrete higher levels of IFN-γ after exposure to whole bacteria, we stimulated, with heat-killed bacteria, PBMCs taken from individuals before and 1 week after drinking tea and performed ELISA to determine IFN-γ titers. PBMCs analyzed 1 week after drinking tea secreted 2- to 4-fold higher IFN-γ titers than PBMCs analyzed before drinking tea (Fig. 4a), showing that in vivo priming can enhance the responses to two different killed bacterial preparations (E. coli and M. morganii). This in vivo priming could be recapitulated in vitro by pretreating PBMCs with EA (but not medium) and then washing it away, followed by stimulation with killed bacteria (Fig. 4b). Depletion of γδ T cells before exposure to bacteria totally abrogated the response (data not shown). These data show that ingestion of tea or in vitro treatment with γδ T cell antigens results in a markedly enhanced innate immune response to bacteria that is γδ T cell-dependent.
Figure 4.
Ingestion of tea containing EA causes PBMCs to produce IFN-γ in response to heat-killed bacteria in vivo. (a) PBMCs from each of five volunteers were stimulated with 500,000 cfu/ml heat-killed E. coli or 5 million cfu/ml heat-killed M. morganii (5 million cfu/ml). After 20 h, culture supernatants were analyzed for IFN-γ. PBMCs challenged with medium only made undetectable amounts of IFN-γ (<4.7 pg/ml). (b) PBMCs from a random donor leukopack were primed in vitro with medium as a negative control or 1 mM EA for 24 h followed by washing and challenged with heat-killed E. coli (500,000 cfu/ml) or M. morganii (5 million cfu/ml). After 20 h, culture supernatants were analyzed for IFN-γ. PBMCs challenged with medium only made undetectable amounts of IFN-γ (<4.7 pg/ml). The data depicted are representative of three independent experiments.
Discussion
Vγ2Vδ2 T cells are part of the adaptive immune system in that they have a memory phenotype, junctionally diverse TCRs that require gene rearrangement for their cell-surface expression, and the ability to become either anergic or expanded depending on the availability of costimulation (1, 30). On the other hand, Vγ2Vδ2 T cells also are part of the innate immune response, because their frequently paired TCR variable (V) region genes Vγ2 and Vδ2 reflect limited germ-line gene diversity. This V-gene pairing enables each Vγ2Vδ2 TCR to recognize families of unprocessed antigens with conserved molecular patterns, such as the alkylamines and prenyl pyrophosphates. This pattern recognition by the Vγ2Vδ2 TCR allows cytokine secretion by a large number of memory γδ T cells in response to a diverse array of bacterial species. These memory T cells that are capable of responding within 2 h to alkylamine and organophosphate antigens produced by microbes thus bridge the gap between innate and adaptive immune responses.
The present study shows that exposure to alkylamine antigens in vivo by ingestion of tea or in vitro by brief exposure to these antigens initiates classic immunologic memory, because reexposure to these same antigens results in a secondary response. In addition, our data show that γδ T cells that are exposed to these alkylamines in concentrations and duration insufficient for cytokine secretion or proliferation can mount such responses after subsequent exposure to heat-killed bacteria or LPS. This nonmemory cytokine and proliferative response to LPS, which does not depend on Vγ2Vδ2 TCR-mediated recognition of LPS, may explain why primed Vγ2Vδ2 T cells can mediate in vivo resistance to Gram-negative bacteria that do not secrete alkylamine antigens (23). LPS-mediated activation of primed γδ T cells may be accomplished indirectly through monocytes wherein LPS-activated monocytes produce IL-12, which in turn stimulates the primed γδ T cells. IFN-γ and tumor necrosis factor α are two cytokines that are required for monocyte-mediated bacterial killing, in particular, IFN-γ. Prior work from our laboratory has demonstrated that the augmentation by γδ T cells of monocyte-mediated in vitro bactericide can be replaced completely by the addition of exogenous IFN-γ and tumor necrosis factor α (23).
Alkylamines are ubiquitous molecules found in the blood, urine, breast milk, vaginal secretions, and amniotic fluid of healthy individuals (29, 31, 32). They are secreted by commensal and pathogenic bacteria but also are found at high concentrations in tea (mostly in precursor form) and at lower concentrations in other edible plant products such as mushrooms, apples, and wine (33, 34). Although these antigens, on occasion, can achieve in vivo the millimolar concentrations necessary for γδ T cell proliferation, alkylamine antigens are found more frequently at micromolar concentrations, providing an explanation for why γδ T cells do not constitutively proliferate and secrete cytokines. These low levels of antigen exposure may serve to maintain γδ T cells in a ready state, quickly able to respond to pathogens. After exposure to bacterial LPS, on occasions when pathogenic or opportunistic bacteria secrete high levels of alkylamines (1), or when pathogenic bacteria produce foreign and potent prenyl pyrophosphate antigens (22) and induce costimulatory molecules (30), these primed γδ T cells then may secrete large quantities of antimicrobial cytokines within hours (23, 35) and expand 50-fold or more between 6 and 10 days postinfection. These cytokines act within 24 h to abrogate bacterial infections, and the expanded γδ T cells may have either an antiinflammatory or antibacterial role later in the infection (23, 36).
Our data also show that ingestion of tea that contains EA can prime γδ T cells for a recall response to EA and other Vγ2Vδ2 TCR-dependent antigens. These observations are consistent with monkey data showing that i.v. administration of phosphoantigens can boost the capacity of γδ T cells to expand in response to phosphoantigen rechallenge in vitro (37), and that prior infection with Mycobacterium bovis Bacille Calmette–Guerin (bacillus Calmette–Guérin) enhances in vivo expansion of γδ T cells after reexposure to bacillus Calmette–Guérin or primary infection with Mycobacterium tuberculosis (38). These data thus confirm with γδ T cells and nonpeptide antigens what we have long known about αβ T cells and peptide antigens: Prior specific antigen exposure results in a recall response.
Primates have evolved their γδ T cells in such a way as to take advantage of their dietary intake of plants that contain alkylamine antigens or their precursors. In contrast to the ingested foodstuffs that result in food allergies, food antigens also may favorably prime discrete subsets of immune cells, just as microbial antigens do. Our data suggest that the primary physiologic role of alkylamine antigens may be to prime γδ T cells in vitro to enable them to respond more efficiently to challenges by pathogenic and opportunistic organisms that possess more potent antigens such as the prenyl pyrophosphate antigens from M. tuberculosis (TUBag1–4) (22, 39) and LPS from Gram-negative bacteria. These primed γδ T cells may abort or abrogate subclinical microbial infections, lessen the severity of clinically apparent infections, and provide enhanced immunosurveillance against tumors, some of which share nonpeptide antigens with bacteria (40). These data provide evidence that dietary intake of tea and perhaps other vegetables and fruits containing alkylamine antigens or their precursors may prime human γδ T cells that then can provide natural resistance to microbial infections and perhaps tumors.
Acknowledgments
We thank Dr. Kelly Zhou for statistical assistance and Drs. Michael Brenner and Craig Morita for critical reading of the manuscript. This research was supported by grants from the National Institute of Allergy and Infectious Disease, the National Institute of Arthritis, Musculoskeletal, and Skin Diseases, the Office of Alternative Medicine at the National Institutes of Health, and the Arthritis Foundation.
Abbreviations
- TCR
T cell receptor
- EA
ethylamine
- PBMC
peripheral blood mononuclear cell
- LPS
lipopolysaccharide
- IBA
iso-butylamine
- ELISPOT
enzyme-linked immunospot
- cfu
colony-forming unit(s)
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Bukowski J F, Morita C T, Brenner M B. Immunity. 1999;11:57–65. doi: 10.1016/s1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka Y, Morita C T, Nieves E, Brenner M B, Bloom B R. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 3.Constant P, Davodeau F, Peyrat M A, Poquet Y, Puzo G, Bonneville M, Fournie J J. Science. 1994;264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 4.Bukowski J F, Morita C T, Tanaka Y, Bloom B R, Brenner M B, Band H. J Immunol. 1995;154:998–1006. [PubMed] [Google Scholar]
- 5.Cartwright R, Roberts E, Wood D. J Sci Food Agric. 1954;5:597–599. [Google Scholar]
- 6.Sakato Y. Nippon Nogei Kagaku Kaishi. 1949;23:262–271. [Google Scholar]
- 7.Yang C S. Nutrition. 1999;15:946–949. doi: 10.1016/s0899-9007(99)00190-2. [DOI] [PubMed] [Google Scholar]
- 8.Trevisanato S I, Kim Y I. Nutr Rev. 2000;58:1–10. doi: 10.1111/j.1753-4887.2000.tb01818.x. [DOI] [PubMed] [Google Scholar]
- 9.Mombaerts P, Arnoldi J, Russ F, Tonegawa S, Kaufmann S H E. Nature. 1993;365:53–56. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- 10.Ladel C H, Blum C, Dreher A, Reifenberg K, Kaufmann S H. Eur J Immunol. 1995;25:2877–2881. doi: 10.1002/eji.1830251025. [DOI] [PubMed] [Google Scholar]
- 11.Hiromatsu K, Yoshikai Y, Matsuzaki G, Ohga S, Muramori K, Matsumoto K, Bluestone J A, Nomoto K. J Exp Med. 1992;175:49–56. doi: 10.1084/jem.175.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sciammas R, Kokukula Q, Tang R L, Hendricks R L, Bluestone J A. J Exp Med. 1997;185:1969–1975. doi: 10.1084/jem.185.11.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuji M, Mombaerts P, Lefrancois L, Nussenzweig R S, Zavala F, Tonegawa S. Proc Natl Acad Sci USA. 1994;91:345–349. doi: 10.1073/pnas.91.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermann E, Lohse A W, Mayet W J, van der Zee R, Van Eden W, Probst P, Poralla T, Meyer zum Buschenfelde K H, Fleischer B. Clin Exp Immunol. 1992;89:427–433. doi: 10.1111/j.1365-2249.1992.tb06975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kersten C M, McCluskey R T, Boyle L A, Kurnick J T. J Immunol. 1996;157:1613–1619. [PubMed] [Google Scholar]
- 16.Skeen M J, Ziegler H K. J Exp Med. 1993;178:971–984. doi: 10.1084/jem.178.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hara T, Mizuno Y, Takaki K, Takada H, Akeda H, Aoki T, Nagata M, Ueda K, Matsuzaki G, Yoshikai Y, et al. J Clin Invest. 1992;90:204–210. doi: 10.1172/JCI115837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Paoli P, Gennari D, Martelli P, Basaglia G, Crovatto M, Battistin S, Santini G. Clin Exp Immunol. 1991;83:187–191. doi: 10.1111/j.1365-2249.1991.tb05612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bukowski J F, Morita C T, Brenner M B. J Immunol. 1994;153:5133–5140. [PubMed] [Google Scholar]
- 20.Das H, Wang L, Kamath A, Bukowski J F. Blood. 2001;98:1616–1618. doi: 10.1182/blood.v98.5.1616. [DOI] [PubMed] [Google Scholar]
- 21.Poccia F, Cipriani B, Vendetti S, Colizzi V, Poquet Y, Battistini L, Lopez-Botet M, Fournie J J, Gougeon M L. J Immunol. 1997;159:6009–6017. [PubMed] [Google Scholar]
- 22.Morita C T, Mariuzza R A, Brenner M B. Springer Semin Immunopathol. 2000;22:191–217. doi: 10.1007/s002810000042. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Kamath A, Das H, Li L, Bukowski J. J Clin Invest. 2001;108:1349–1357. doi: 10.1172/JCI13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyahira Y, Murata K, Rodriguez D, Rodriguez J R, Esteban M, Rodrigues M M, Zavala F. J Immunol Methods. 1995;181:45–54. doi: 10.1016/0022-1759(94)00327-s. [DOI] [PubMed] [Google Scholar]
- 25.Satoh M, Seki S, Hashimoto W, Ogasawara K, Kobayashi T, Kumagai K, Matsuno S, Takeda K. J Immunol. 1996;157:3886–3892. [PubMed] [Google Scholar]
- 26.Skeen M J, Ziegler H K. J Immunol. 1995;154:5832–5841. [PubMed] [Google Scholar]
- 27.Feldheim W, Yongvanit P, Cummings P. J Sci Food Agric. 1986;37:527–534. [Google Scholar]
- 28.Asatoor A M. Nature. 1966;210:1358–1360. doi: 10.1038/2101358b0. [DOI] [PubMed] [Google Scholar]
- 29.Perry T L, Shaw N F, Walker D, Redlich D. Pediatrics. 1962;30:576–584. [Google Scholar]
- 30.Das H, Groh V, Kuijl C, Sugita M, Morita C T, Spies T, Bukowski J F. Immunity. 2001;15:83–93. doi: 10.1016/s1074-7613(01)00168-6. [DOI] [PubMed] [Google Scholar]
- 31.Lichtenberger L M, Gardner J W, Barreto J C, Morriss F H., Jr J Pediatr Gastroenterol Nutr. 1991;13:342–346. doi: 10.1097/00005176-199111000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Jones B M, al-Fattani M, Gooch H. Int J STD AIDS. 1994;5:52–55. doi: 10.1177/095646249400500112. [DOI] [PubMed] [Google Scholar]
- 33.Hartmann T. Experientia. 1967;23:680–681. doi: 10.1007/BF02144201. [DOI] [PubMed] [Google Scholar]
- 34.Ibe A, Saito K, Nakazato M, Kikuchi Y, Fujinuma K, Nishima T. J Assoc Off Anal Chem. 1991;74:695–698. [PubMed] [Google Scholar]
- 35.Garcia V E, Jullien D, Song M, Uyemura K, Shuai K, Morita C T, Modlin R L. J Immunol. 1998;160:4322–4329. [PubMed] [Google Scholar]
- 36.Stenger S, Hanson D A, Teitelbaum R, Dewan P, Niazi K R, Froelich C J, Ganz T, Thoma-Uszynski S, Melian A, Bogdan C, et al. Science. 1998;282:121–125. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 37.Poccia F, Malkovsky M, Pollak A, Colizzi V, Sireci G, Salerno A, Dieli F. Mol Med. 1999;5:471–476. [PMC free article] [PubMed] [Google Scholar]
- 38.Shen Y, Zhou D, Qiu L, Lai X, Simon M, Shen L, Kou Z, Wang Q, Jiang L, Estep J, et al. Science. 2002;295:2255–2258. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Constant P, Poquet Y, Peyrat M A, Davodeau F, Bonneville M, Fournie J J. Infect Immun. 1995;63:4628–4633. doi: 10.1128/iai.63.12.4628-4633.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gober H J, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. J Exp Med. 2003;197:163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]