Abstract
CD4+ T cells expand after transfer into lymphopenic H-2b Aβ−/− mice (I-Aβ-, I-Eα-deficient mice) but not after transfer into lymphopenic MHC IIΔ/Δ mice (I-Aα-, I-Aβ-, I-Eα-, and I-Eβ-deficient mice), implying that in H-2b Aβ−/− mice, Aα chain and Eβ chain associate to form a hybrid AαEβ MHC class II molecule. In light of this unexpected result, we reexamined the MHC class II requirement in the survival and lymphopenia-induced proliferation of CD4+ T cells. Here we show that expansion, but not short-term survival, of CD4+ T cells depends on interactions with MHC class II molecules in lymphopenic mice. Nevertheless, interactions with classical MHC class II molecules are required for CD4+ T cells to survive in CD8+ T-cell-containing mice.
During the past two decades, peripheral T cell homeostasis has been studied extensively. Numerous groups have suggested that permanent interactions between T cells and self-peptide/self-MHC molecule complexes are required for T cell survival in the periphery (1–5). Together with thymic generation, such interactions would regulate the size of the peripheral T cell pool. Mice deficient for the expression of MHC class I and/or MHC class II molecules have been used to address the role of self-peptide/self-MHC molecule complexes in the viability of peripheral T cells. Adoptive transfer of mature T cells, thymus grafts, or more elegant experimental systems in which MHC molecules were transiently expressed in the thymus have been used to study this issue. Most of the studies of CD4+ T cell survival have been performed by using I-Aβ-deficient H-2b mice (Aβ−/− mice) as MHC class II-deficient hosts (4–10). Indeed, in H-2b mice, a point mutation in the I-Eα gene precluded the synthesis of the functional corresponding protein and subsequent expression of the MHC class II molecule I-E. By disrupting the I-Aβ gene in these mice, one can expect the lack of expression of conventional MHC class II molecules.
First, on the basis of the disappearance of CD4+ T cells in these mice, survival of peripheral CD4+ T cells was proposed to depend on permanent interactions with MHC class II molecules expressed on peripheral antigen-presenting cells (APCs) (4, 5, 7). Nevertheless, two different groups have recently observed that CD4+ T cells disappeared with similar kinetics in both normal and Aβ−/− mice (8, 9). On the basis of these findings, these investigators have concluded that survival of peripheral CD4+ T cells did not depend on T cell antigen receptor (TCR) signaling induced by recognition of self-peptide/self-MHC molecule complexes.
Another concept concerning naive T cells has also been challenged by new findings. The fact that naive T cells do not cycle in the periphery of normal adult mice in the absence of antigenic stimulation has been considered to result from the process of negative selection in the thymus. However, it has been shown recently that naive T cells proliferate in response to self-peptide/self-MHC molecule complexes in neonatal mice (11) and after transfer into lymphopenic hosts (12–22). Coinjection of an excess of CD4+ or CD8+ T cells inhibits the proliferation of naive CD4+ T cells in these circumstances (14, 23). These results suggest a role for intercellular competition in setting the threshold for naive T cell proliferation. The question arises as to whether such a competition also has an influence on T cell survival.
For this reason, we have studied the fate of CD4+ T cells transferred into mice lacking the expression of classical MHC class II molecules (Aβ−/− mice) as a function of the ability of recipient mice to produce endogenous T cells. In this article, we show that although CD4+ T cells transferred into Aβ−/− mice disappear, most of these cells survive and slowly cycle after transfer into CD3ɛ−/− Aβ−/− mice. Some of the transferred cells even strongly expand and convert to an effector-like phenotype. Such an expansion was not observed after transfer into lymphopenic MHC IIΔ/Δ mice, these mice lacking the expression of both α and β chains of I-A and I-E MHC class II molecules (24), implying that in Aβ−/− mice, Aα chain and Eβ chain associate to form a hybrid AαEβ MHC class II molecule. In light of this unexpected result, we reexamined the MHC class II requirement in the survival and lymphopenia-induced proliferation of CD4+ T cells.
Experimental Procedures
Mice.
C57BL/6 mice were obtained from Centre d'élevage Janvier (Le Genest Saint Isle, France), and C57BL/6 CD3ɛ-deficient mice (CD3ɛ−/− mice) (25) and C57BL/6 Aβ-deficient mice (Aβ−/− mice) (26) were from Centre de Développement des Techniques Avancées pour l'Experimentation Animale (Orléans, France). C57BL/6 Ly5.1 mice, H-2b AND TCR transgenic rag-20/0 mice (11, 21, 22, 27), C57BL/6 CD3ɛ/Aβ double-deficient mice (CD3ɛ−/− Aβ−/− mice) (11, 21), and C57BL/6 CD3ɛ/Aβ/β2m triple-deficient mice (CD3ɛ−/− Aβ−/− β2m−/− mice) (11) were maintained in our animal facilities. MHC IIΔ/Δ mice were originally purchased from The Jackson Laboratory.
Adoptive Transfer of T Cells.
Lymph node cells were depleted of macrophages, granulocytes, and CD8+ or CD4+ T cells by incubating them first with anti-CD11b (Mac-1) Ab, anti-GR1 (8C5) Ab, and anti-CD8 (Lyt-2) or CD4 (GK1.5) Ab, and then with magnetic beads coupled to anti-rat Ab (Dynal, Great Neck, NY). B cells were removed by using magnetic beads coupled to anti-mouse Ig Ab (Dynal). Purified CD4+ T cells (5 × 106) were injected i.v. into recipient mice. When indicated, CD4+ T cells were labeled with 5,6-carboxyfluorescein diacetate-succinimyl ester (CFSE) before injection. In some experiments, 7.5 × 106 purified CD8+ T cells were injected 28 days before CD4+ T cell transfer. Spleen and lymph nodes were recovered, pooled for cell preparation, and analyzed at various times after CD4+ T cell transfer. In some experiments (see Fig. 4), host mice were irradiated sublethally (650 rad) 24 h before CD4+ T cell transfer.
Figure 4.
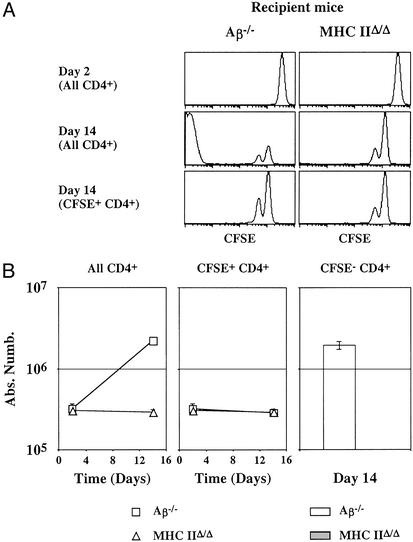
CD4+ T cells are interacting with AαEβ hybrid MHC class II molecule after transfer into lymphopenic Aβ−/− mice. Five million CFSE-labeled lymph node CD4+ T cells from C57BL/6 Ly5.1 mice were injected into irradiated Aβ−/− mice and into irradiated MHC IIΔ/Δ mice (Ly5.2). (A) CFSE fluorescence histograms of Ly5.1+ CD4+ T cells 2 and 14 days after transfer. Bottom histograms are gated on CFSE+ CD4+ cells. (B) The absolute numbers of total, CFSE+, and CFSE− donor Ly5.1+ CD4+ T cells are shown as a function of time after transfer.
Adoptive Transfer of Bone Marrow Cells.
Six- to 8-week-old CD3ɛ−/− Aβ−/− β2m−/− mice were irradiated (1,000 rad) and injected i.v. with 5 × 106 bone marrow cells from CD3ɛ−/− Aβ−/− mice or with 5 × 106 bone marrow cells from CD3ɛ−/− Aβ−/− mice + 5 × 106 bone marrow cells from CD3ɛ−/− Aβ−/− β2m−/− mice. Five weeks after bone marrow cell transfer, some mice were injected with 7.5 × 106 lymph node CD8+ T cells. All mice were injected with 5 × 106 CFSE-labeled lymph node CD4+ T cells 28 days later.
Cell Surface Staining and Flow Cytometry.
Lymph nodes and spleen were pooled, homogenized with a nylon cell strainer (Falcon) in PBS/5% FCS/0.2% NaN3, and then distributed in 96-well U-bottom microplates (8 × 106 cells per well). Staining was performed on ice for 30 min per step.
Antibodies were purchased from PharMingen unless otherwise indicated. The following antibody combinations were used: for four-color analysis, phycoerythrin anti-TCRβ, fluorescein isothiocyanate anti-CD8, PercP anti-CD4, and biotinylated anti-CD44 or anti-CD69 with allophycocyanin-streptavidin development (PharMingen); for characterization of transferred CFSE-labeled lymph node CD4+ T cells, PercP anti-CD4, and biotinylated anti-TCRβ, anti-CD5, anti-CD44, anti-CD62L, anti-CD69 or anti-Ly5.1 with allophycocyanin-streptavidin development (PharMingen).
Four-color immunofluorescence was analyzed by using a FACSCalibur cytometer (Becton Dickinson). List-mode data files were analyzed by using CELLQUEST software (Becton Dickinson).
Results
Peripheral CD4+ T Cells Divide Extensively and Convert to Effector-Like Cells After Transfer into Mice Lacking both Production of Endogenous T Cells and Expression of Classical MHC Class II Molecules.
We decided to study the fate of CD4+ T cells transferred into mice lacking the expression of classical MHC class II molecules as a function of the ability of recipient mice to produce endogenous T cells. Five million CFSE-labeled CD4+ T cells from normal C57BL/6 Ly5.1 mice were transferred into Aβ−/− mice and into CD3ɛ−/− Aβ−/− mice (Ly5.2). The initial recovery of transferred cells from lymphoid organs was similar in these two hosts (day 2 after transfer; Fig. 1A). The absolute number of donor CD4+ T cells was strongly diminished in Aβ−/− mice 14 days after transfer when compared with the number seen immediately after transfer to these hosts (Fig. 1A). Thus, similar to other groups, we found that CD4+ T cells do not sustain their numbers after transfer to Aβ−/− mice.
Figure 1.
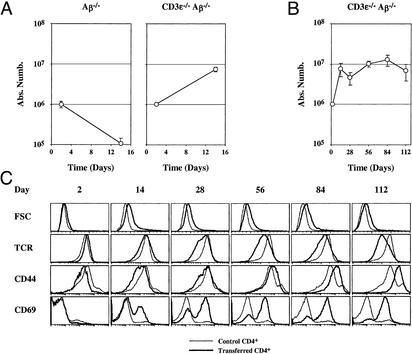
CD4+ T cells rapidly expand and convert to effector-like cells after transfer to CD3ɛ−/− Aβ−/− mice. Five million lymph node CD4+ T cells from C57BL/6 Ly5.1 mice were injected into Aβ−/− mice and into CD3ɛ−/− Aβ−/− mice (Ly5.2). At different times after transfer, lymph nodes and spleen were recovered and pooled, and single-cell suspensions were prepared. The absolute number of recovered donor Ly5.1+ CD4+ T cells was then determined (A). Five million lymph node CD4+ T cells were injected into CD3ɛ−/− Aβ−/− mice. The absolute number (B) and phenotype (C) of recovered donor CD4+ TCRhi cells were determined at different times after transfer. FSC, forward light scatter.
Surprisingly, CD4+ T cells did not rapidly disappear after transfer into CD3ɛ−/− Aβ−/− mice (Fig. 1A). Indeed, the number of recovered CD4+ T cells increased 10-fold during the first 2 weeks after transfer to reach a plateau (Fig. 1B). Consistent with this evidence for cell division, most CD4+ T cells increased in size as assessed by forward cell scatter 2 weeks after transfer (Fig. 1C). Two days after transfer, the CD44 expression on recovered CD4+ T cells was lower than before injection, but the surface level of this molecule slowly increased with time to the level typical of effector CD4+ T cells. Such a slow up-regulation did not fit with a preferential expansion of preexisting memory cells within the injected CD4+ T cell populations. Indeed, transferred CD4+ T cell numbers increased rapidly during the first 2 weeks after transfer, whereas CD44 up-regulation occurred progressively and was maximum only after 4 months in the recipient animals. Concomitant with this increase in CD44, transferred CD4+ T cells progressively down-regulated their cell surface TCR level and up-regulated the early activation marker CD69 (Fig. 1C).
Taken together, these findings indicate that CD4+ T cells in CD3ɛ−/− Aβ−/− mice proliferate while converting to a phenotype characteristic of conventional effector T cells. Altogether, these results suggested that injected cells received TCR signals even in the absence of expression of classical MHC class II molecules. Moreover, the maintenance of CD4+ T cells observed in CD3ɛ−/− Aβ−/− double-deficient mice is severely impaired in Aβ−/− mice, suggesting that endogenous T cells from Aβ−/− mice interfere with the survival/expansion of transferred CD4+ T cells.
CD4+ T Cells Transferred into Lymphopenic Mice Can Be Subdivided into Two Subsets on the Basis of Their Proliferative Behavior.
Purified lymph node CD4+ T cells from control C57BL/6 mice were labeled with CFSE and injected into CD3ɛ−/− mice and into CD3ɛ−/− Aβ−/− mice. The proliferation of injected cells was studied during the first month after transfer. No cells underwent division for the first 2 days after transfer (Fig. 2A). Surprisingly, 5 days later, in both recipient mice, half of recovered cells had completely diluted the intracytoplasmic dye, whereas the others had undergone either no or only a single-cell division.
Figure 2.
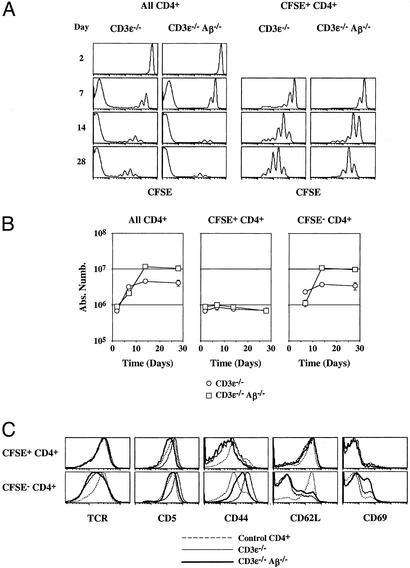
CD4+ T cells transferred into lymphopenic mice can be divided into two subsets based on their proliferative behavior. Five million CFSE-labeled lymph node CD4+ T cells were injected into CD3ɛ−/− mice and into CD3ɛ−/− Aβ−/− mice. At different times after transfer, lymph nodes and spleen were recovered and pooled, and single-cell suspensions were prepared. (A) CFSE fluorescence histograms of CD4+ TCRhi cells as a function of time after transfer. Histograms on the right are gated on CFSE+ CD4+ TCRhi cells. (B) The absolute numbers of total, CFSE+, and CFSE− donor CD4+ TCRhi cells are shown as a function of time after transfer. (C) TCR, CD5, CD44, CD62L, and CD69 fluorescence histograms of CFSE+ and CFSE− CD4+ T cells recovered 28 days after CD4 T cell transfer are shown in comparison with TCR, CD5, CD44, CD62L, and CD69 fluorescence histograms of control CD4+ T cells from normal C57BL/6 mice.
Absolute numbers of recovered CD4+ T cells then were calculated as a function of their CFSE-labeling status (Fig. 2B). In both recipient mice, the absolute number of CD4+ T cells increased strongly during the first 2 weeks to reach a plateau. This increase was clearly due to the rapid proliferation of a minority of injected cells, whereas the bulk of transferred CD4+ T cells underwent no or a limited number of cell divisions and maintained a nearly constant number. Thus, CD4+ T cells from normal C57BL/6 mice can be subdivided in two subsets with respect to their behavior after transfer into both CD3ɛ−/− mice and CD3ɛ−/− Aβ−/− mice. The first subset (CFSE+ CD4+ T cells) corresponds to the vast majority of CD4+ T cells, survives independently of classical MHC class II molecule expression, but only slowly cycles. Although CFSE+ CD4+ T cells slowly cycled, their absolute number did not increase with time suggesting a balancing cell death of proliferating cells. The second subset (CFSE− CD4+ T cells) derives from the strong expansion of few of the initially injected CD4+ T cells.
The phenotype of CD4+ T cells recovered 28 days after transfer was analyzed (Fig. 2C). The expression of CD69, the up-regulation of CD44, and concomitant TCR and CD62L down-regulation were restricted to CFSE− CD4+ T cells. Thus, in both CD3ɛ−/− mice and CD3ɛ−/− Aβ−/− mice, it is only the progeny of the few CD4+ T cells that undergo rapid and strong proliferation that convert to an effector-like phenotype. This conversion contrasts with the bulk of injected cells that slowly cycle, survive, and retain a naive phenotype.
CFSE− CD4+ T Cell Generation Strictly Depends on Interactions with MHC Class II Molecules.
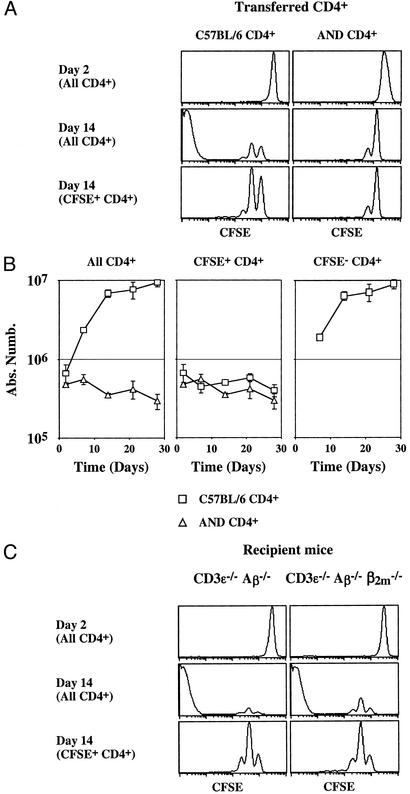
We compared the behavior of monoclonal CD4+ T cells (AND TCR transgenic CD4+ T cells) versus polyclonal CD4+ T cells after transfer into CD3ɛ−/− Aβ−/− mice (Fig. 3 A and B). All AND TCR transgenic CD4+ T cells survived, proliferated weakly, and retained a naive phenotype after transfer into CD3ɛ−/− Aβ−/− mice (Fig. 3; ref. 21). No AND TCR transgenic CFSE− CD4+ T cells were detected at any time after transfer. Thus, CFSE− CD4+ T cell generation depends on the TCR specificity of injected T cells. These results suggest the existence of a MHC molecule in CD3ɛ−/− Aβ−/− mice with which some of the transferred polyclonal CD4+ T cells need to interact to strongly expand.
Figure 3.
CFSE− CD4+ T cell generation depends on the TCR specificity of injected T cells. (A and B) Five million CFSE-labeled lymph node CD4+ T cells from AND TCR transgenic mice or from C57BL/6 mice were injected into CD3ɛ−/− Aβ−/− mice. At different times after transfer, lymph nodes and spleen were recovered and pooled, and single-cell suspensions were prepared. (A) CFSE fluorescence histograms of CD4+ TCRhi cells 2 and 14 days after transfer. Bottom histograms are gated on CFSE+ CD4+ cells. (B) The absolute numbers of total, CFSE+, and CFSE− donor CD4+ TCRhi cells are shown as a function of time after transfer. (C) Five million CFSE-labeled lymph node CD4+ T cells were injected into CD3ɛ−/− Aβ−/− mice and into CD3ɛ−/− Aβ−/− β2m−/− mice. Shown are CFSE fluorescence histograms of CD4+ TCRhi cells 2 and 14 days after transfer.
Given the presumed lack of surface MHC class II molecules in Aβ−/− mice, we examined whether MHC class I molecules might be influencing the behavior of CD4+ T cells transferred into CD3ɛ−/− Aβ−/− mice. CFSE-labeled CD4+ T cells from normal C57BL/6 mice were transferred into CD3ɛ−/− Aβ−/− mice and into CD3ɛ−/− Aβ−/− β2m−/− mice. (These mice are also deficient for the expression of MHC class I-like molecules such as CD1, the surface expression of which depends on β2m.) As expected, we found that the absence of MHC class I molecule expression did not affect the proliferation of injected CD4+ T cells (Fig. 3C).
Because MHC class I molecules were not involved in the proliferation of CD4+ T cells transferred into CD3ɛ−/− Aβ−/− mice, our results argue for the existence of a previously unrecognized MHC molecule with which transferred CD4+ T cells would be interacting. The most logical hypothesis would be that in Aβ−/− mice, Eβ chain and Aα chain associate to form a hybrid MHC class II molecule. Therefore, 5 million CFSE-labeled lymph node CD4+ T cells from C57BL/6 Ly5.1 mice were injected into irradiated Aβ−/− mice and into irradiated MHC IIΔ/Δ mice (Ly5.2), the latter mice lacking the expression of both α and β chains of I-A and I-E MHC class II molecules (24) (Fig. 4). Fourteen days after transfer into MHC IIΔ/Δ mice, no CFSE− CD4+ T cells could be detected, implying the expression of a hybrid AαEβ MHC class II molecule in Aβ−/− mice that explained the strong proliferation and expansion of few of the initially injected CD4+ T cells in CD3ɛ−/− Aβ−/− mice (Fig. 2) or in irradiated Aβ−/− mice (Fig. 4). By contrast, both proliferation and survival of CFSE+ CD4+ T cells did not differ between both types of recipient mice. These results suggest that the expression of MHC class II molecules is not a prerequisite for short-term survival and slow proliferation of the bulk of CD4+ T cells transferred to lymphopenic hosts (CFSE+ CD4+ T cells).
The Fate of CFSE+ CD4+ T Cells Depends on MHC Class II Molecule Expression in CD8+ T Cell-Containing Mice.
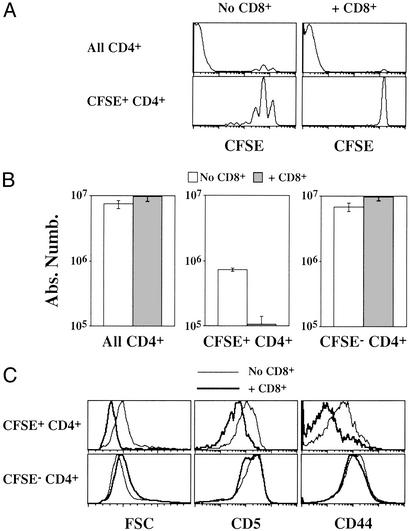
CD4+ T cells rapidly disappeared after transfer to Aβ−/− mice containing endogenous T cells (Fig. 1A), whereas the majority of these cells (CFSE+ CD4+ T cells) survived independently of the expression of MHC class II molecules in hosts lacking T cells (Fig. 4B). We therefore investigated whether the presence of T cells (particularly CD8+ T cells) in Aβ−/− mice was responsible for the failure of transferred CD4+ T cells to maintain their numbers in these recipients. Five million CFSE-labeled CD4+ T cells from normal C57BL/6 mice were transferred into CD3ɛ−/− Aβ−/− mice (AαEβ-expressing mice). Half of recipient mice had been previously injected with purified CD8+ T cells. Proliferation and survival of CD4+ T cells were assessed 14 days after their transfer. Proliferation and subsequent expansion of the minor CD4+ T cell subset that produces CFSE− CD4+ T cells were not affected by the presence of CD8+ T cells (Fig. 5 A and B). Thus, CD8+ T cells are not involved in controlling proliferation of the few CD4+ T cells that give rise to the CFSE− population. Accordingly, the phenotype of the resulting CFSE− CD4+ T cells was the same as observed after transfer to CD3ɛ−/− Aβ−/− mice (Fig. 5C).
Figure 5.
CD8+ T cells inhibit survival and proliferation of slowly cycling but not of rapidly dividing CD4+ T cells in CD3ɛ−/− Aβ−/− mice (AαEβ-expressing mice). Five million CFSE-labeled lymph node CD4+ T cells were injected into CD3ɛ−/− Aβ−/− mice. In some cases, host mice were coinjected with 7.5 × 106 lymph node CD8+ T cells 28 days before CD4+ T cells. (A) CFSE fluorescence histograms of CD4+ TCRhi cells 14 days after transfer. Histograms in the lower part are gated on CFSE+ CD4+ TCRhi cells. (B) Fourteen days after transfer, the absolute numbers of total, CFSE+, and CFSE− CD4+ TCRhi were calculated. (C) Forward light scatter (FSC), CD5, and CD44 fluorescence histograms of CFSE+ and CFSE− CD4+ T cells recovered 14 days after CD4+ T cell transfer to CD3ɛ−/− Aβ−/− mice.
By contrast, in CD8+ T cell-containing mice, the bulk of transferred CD4+ T cells (CFSE+ CD4+ T cells) did not proliferate (Fig. 5A) or sustain their numbers (Fig. 5B). According to their nonproliferation, CFSE+ CD4+ T cells did not increase in cell size. Moreover, the expression of both CD5 and CD44 on CFSE+ CD4+ T cells was decreased in the presence of CD8+ T cells (Fig. 5C).
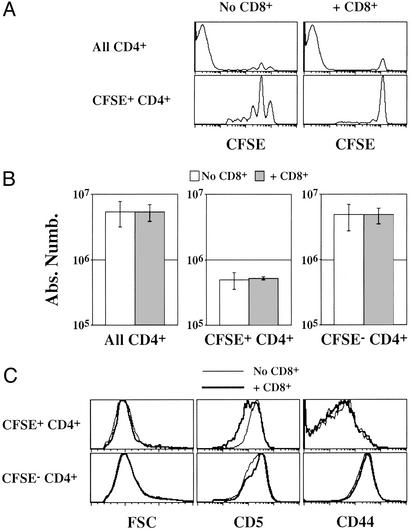
A similar experiment was conducted in mice expressing classical MHC class II molecules (CD3ɛ−/− mice: AαAβ-expressing mice, Fig. 6). As in CD3ɛ−/− Aβ−/− mice, CD8+ T cells did not affect the behavior of the few CD4+ T cells that underwent extensive division and gave rise to CFSE− CD4+ T cells and still inhibited the slow proliferation of the bulk of transferred CD4+ T cells (CFSE+ CD4+ T cells; Fig. 6A). By contrast, the absolute number and phenotype of CFSE+ CD4+ T cells were not affected by the presence of CD8+ T lymphocytes (Fig. 6 B and C). Such results demonstrate a role of MHC class II molecules in the peripheral maintenance of CD4+ T cells in a nonlymphopenic environment.
Figure 6.
CD8+ T cells do not inhibit survival of slowly cycling CD4+ T cells in CD3ɛ−/− mice (AαAβ-expressing mice). Five million CFSE-labeled lymph node CD4+ T cells were injected into CD3ɛ−/− mice. In some cases, host mice were coinjected with 7.5 × 106 lymph node CD8+ T cells 28 days before CD4+ T cells. (A) CFSE fluorescence histograms of CD4+ TCRhi cells 14 days after transfer. Histograms in the lower part are gated on CFSE+ CD4+ TCRhi cells. (B) Fourteen days after transfer, the absolute numbers of total, CFSE+, and CFSE−, CD4+ TCRhi were calculated. (C) Forward light scatter (FSC), CD5, and CD44 fluorescence histograms of CFSE+ and CFSE− CD4+ T cells recovered 14 days after CD4+ T cell transfer to CD3ɛ−/− mice.
CD8+ T Lymphocytes Set Up the Threshold for CD4+ T Cell Survival by Limiting the Accessibility to APCs.
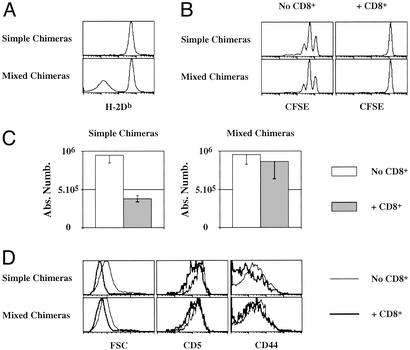
In CD3ɛ−/− Aβ−/− recipient mice (AαEβ-expressing mice), pretransferred CD8+ T cells might diminish the accessibility to APCs and thus inhibit the survival of the bulk of CD4+ T cells transferred into these mice (CFSE+ CD4+ T cells). To examine this hypothesis, we designed an experiment in which CD4+ T cell accessibility to APCs could not be completely inhibited by CD8+ T cells. Irradiated CD3ɛ−/− Aβ−/− β2m−/− mice were reconstituted with bone marrow cells either from CD3ɛ−/− Aβ−/− mice (simple chimeras) or from CD3ɛ−/− Aβ−/− mice and CD3ɛ−/− Aβ−/− β2m−/− mice (mixed chimeras). Half of the mice of each group were injected with purified CD8+ T cells 5 weeks after bone marrow reconstitution. All mice then were injected with 5 million CFSE-labeled CD4+ T cells 28 days later. In mixed chimeras, half of the APCs did not express MHC class I molecules (Fig. 7A), and therefore, CD8+ T cells would not be expected to interact with them in a TCR-dependent manner. We first verified that CD8+ T cell recovery was not significantly different between the two groups of recipients with 18 × 106 CD8+ cells recovered in simple chimeras versus 16 × 106 in mixed chimeras. As expected from previous experiments (Figs. 5 and 6), the strong proliferation and resulting expansion of some injected CD4+ T cells were not affected by the transfer of CD8+ T cells into either chimera (data not shown). Moreover, CD8+ T cells still inhibited both proliferation and survival of CFSE+ CD4+ T cells in simple chimeras (Fig. 7 B and C). By contrast, CD4+ T cell number was maintained in the presence of CD8+ T cells in mixed chimeras (Fig. 7C). The survival of CFSE+ CD4+ T cells in chimeras correlated with their cell size and surface expression of CD5 and CD44. Indeed, in the presence of CD8+ T cells, CFSE+ CD4+ T cell expression of CD5 and CD44 remained unchanged in mixed chimeras, whereas both proteins were down-regulated significantly at the cell surface of the bulk of transferred CD4+ T cells in simple chimeras (Fig. 7D).
Figure 7.
CD8+ T cells inhibit survival of slowly dividing CD4+ T cells (CFSE+ CD4+ T cells) in CD3ɛ−/− Aβ−/− mice through competition for APCs. Irradiated CD3ɛ−/− Aβ−/− β2m−/− mice were injected with 5 × 106 bone marrow cells from CD3ɛ−/− Aβ−/− mice (simple chimeras) or with 5 × 106 bone marrow cells from CD3ɛ−/− Aβ−/− mice + 5 × 106 bone marrow cells from CD3ɛ−/− Aβ−/− β2m−/− mice (mixed chimeras). Five weeks later, some chimeric mice were injected with 7.5 × 106 lymph node CD8+ T cells. Twenty-eight days later, all mice were then injected with 5 × 106 CFSE-labeled lymph node CD4+ T cells. (A) H-2Db fluorescence histograms of CD19+ cells 14 days after CD4+ T cell transfer. (B) CFSE fluorescence histograms of CFSE+ CD4+ TCRhi cells 14 days after transfer. (C) Fourteen days after transfer, the absolute number of CFSE+ CD4+ TCRhi were calculated. (D) Forward light scatter (FSC), CD5, and CD44 fluorescence histograms of CFSE+ CD4+ T cells recovered 14 days after CD4+ T cell transfer.
Discussion
In this article, we provide evidence showing the expression of a previously unrecognized MHC class II molecule in Aβ−/− mice with which transferred CD4+ T cells are interacting. Indeed, CFSE− CD4+ T cells that derived from the extensive division of few of the CD4+ T cells transferred into lymphopenic wild-type mice and into lymphopenic Aβ−/− mice were not detected in lymphopenic MHC IIΔ/Δ mice. C57BL/6 Aβ−/− mice are deficient for the expression of the α chain of I-E and β chain of I-A; MHC IIΔ/Δ mice lack the expression of both α and β chains of I-A and I-E MHC class II molecules (24). Thus, our results clearly imply that, at least in Aβ−/− mice, Eβ chain and Aα chain associate to form a hybrid AαEβ MHC class II molecule and that the generation of CFSE− CD4+ T cells in lymphopenic hosts strictly depends on interactions with MHC class II molecules. Dorfman et al. (8) have observed that the proliferation of CD4+ T cells transferred into Aβ−/− mice was abolished by treating the mice with Y3P, an antibody directed against an epitope located in the α chain of the MHC class II molecule I-A (28, 29). Such a result is also in favor of the expression of a hybrid AαEβ MHC class II molecule in Aβ−/− mice.
The bulk of CD4+ T cells transferred into lymphopenic hosts (CFSE+ CD4+ T cells) slowly proliferated. The extent of such a proliferation also depends on interactions with MHC class II molecules. Indeed, at all time points after transfer, the slow proliferation of CFSE+ CD4+ T cells was stronger in CD3ɛ−/− mice than in CD3ɛ−/− Aβ−/− mice (Fig. 2). Similarly, after transfer into CD3ɛ−/− Aβ−/− mice, the proliferation of AND TCR transgenic CD4+ T cells was less important than the proliferation of CFSE+ CD4+ T cells from normal mice (Fig. 3). Nevertheless, such a proliferation could also be observed in the complete absence of expression of MHC class II molecules (MHC IIΔ/Δ mice, Fig. 4). These results suggest that other stimuli that TCR signaling could mediate the proliferation of the bulk of CD4+ T cells transferred into lymphopenic hosts. Cytokines such as IL-7 could explain these results. Indeed, several groups have recently proposed that IL-7 would be required for “homeostatic” proliferation of the bulk of CD4+ T cells (30–33).
By using irradiated MHC IIΔ/Δ mice, we showed that short-term survival of CD4+ T cells did not depend on interactions with MHC class II molecules in lymphopenic hosts. As proposed by other groups (31, 34), in lymphopenic hosts, soluble factors such as IL-7 could be concentrated enough to allow the maintenance of the CD4+ T cell pool. Nevertheless, MHC class II molecules play a role in the peripheral maintenance of CD4+ T cells in a nonlymphopenic environment. Indeed, CD8+ T cells inhibited the survival of the bulk of CD4+ T cells (CFSE+ CD4+) transferred into CD3ɛ−/− Aβ−/− mice (AαEβ-expressing mice) but not into CD3ɛ−/− mice (AαAβ-expressing mice). CD8+ T cells might act by consuming circulating cytokines, thus limiting their availability. In these conditions, interactions with MHC class II molecules would become crucial for CD4+ T cell survival.
The inhibition of CD4+ T cell survival by CD8+ T lymphocytes in CD3ɛ−/− Aβ−/− mice required MHC class I molecule expression on APCs (Fig. 7). Nevertheless, MHC class I molecule expression by APCs was not required by itself for CD4+ T cells to survive in CD3ɛ−/− Aβ−/− mice (Fig. 3C). Thus, CD8+ T cells act not only by consuming circulating cytokines but also by surrounding APCs. Our last experiment by itself does not permit a definite conclusion on the nature of the molecule expressed by APCs with which CD4+ T cells need to interact to survive in CD8+ T cell-containing CD3ɛ−/− Aβ−/− mice. Nevertheless, as said above, interactions with MHC class II molecules are crucial for CD4+ T cell survival in the presence of CD8+ T cells (Fig. 5 versus Fig. 6). Thus, it is logical to postulate that by surrounding APCs, CD8+ T cells would reduce the ability of CD4+ T cells to interact with the self-peptide/self-MHC molecule complexes presented by APCs. Moreover, such a hypothesis would explain the strong correlation observed in our model between CD4+ T cell survival and CD5 surface expression. Indeed, CD5 expression has been shown to be adjusted to reflect TCR contact with self-peptide/self-MHC molecule complexes (35). Thus, the decreased expression of CD5 by transferred CD4+ T cells in the presence of CD8+ T cells confirmed that in these conditions, the majority of CD4+ T cells are receiving less TCR signaling than in their absence. The decreased ζ phosphorylation in CD4+ T cells 2 days after transfer into Aβ−/− mice when compared with CD3ɛ−/− Aβ−/− mice (data not shown) also accords with the idea that the decline in CD4+ T cells in CD8+ T cell containing Aβ−/− mice results from decreased TCR signaling.
CD8+ T cells did not inhibit the survival of the bulk of CD4+ T cells transferred into CD3ɛ−/− mice. One plausible hypothesis would be that the affinity of CFSE+ CD4+ T cells for the self-peptide/AαAβ MHC class II molecule complexes they are interacting with in CD3ɛ−/− mice is so high that CD8+ T cells would not be able to block their interaction with APCs. Competition for accessibility to APCs would depend on affinity for self-peptide/self-MHC molecule complexes. Such a model would also explain why in both CD3ɛ−/− and CD3ɛ−/− Aβ−/− mice, CFSE− CD4+ T cell generation is not inhibited by CD8+ T lymphocytes. Indeed, the few CD4+ T cells that underwent extensive division, converted to an effector-like phenotype, and gave rise to CFSE− CD4+ T cells in lymphopenic hosts certainly correspond to high-affinity clones, their behavior in a lymphopenic environment being quite similar to what is observed in antigen-driven proliferation. In nonlymphopenic animals, a cell subset other than CD8+ T cells (regulatory CD4 CD25+ T cells and CD4+ NK T cells in normal mice; CD4+ NK T cells in Aβ−/− mice) might prevent their reactivity.
In normal mice, intercellular competition for circulating cytokines, accessibility to APCs and for limited amounts of specific self-peptide/self-MHC molecule complexes sets up the threshold for peripheral T cell survival and “homeostatic” proliferation. Tolerance to self thus would depend on the maintenance of the size of the peripheral T cell pool. Homeostasis of the peripheral T cell pool might be not only a guarantee for preventing foreign danger but also to maintain tolerance to self. The fact that lymphopenic individuals are known to be at much higher risk in developing certain autoimmune diseases supports such a hypothesis (36–38).
Acknowledgments
We thank S. Léaument for help in generating CD3ɛ−/− Aβ−/− and CD3ɛ−/− Aβ−/− β2m−/− mice and C. Boitard, B. Faideau, R. N. Germain, A. Lehuen, and C. Pénit for critical reading of the manuscript. This work was supported by the Agence Nationale de Recherche sur le SIDA and C.B. was supported by a Ph.D. fellowship from the Fondation pour la Recherche Médicale.
Abbreviations
- APCs
antigen-presenting cells
- TCR
T cell antigen receptor
- CFSE
5,6-carboxyfluorescein diacetate-succinimyl ester
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Takeda S, Rodewald H R, Arakawa H, Bluethmann H, Shimizu T. Immunity. 1996;5:217–228. doi: 10.1016/s1074-7613(00)80317-9. [DOI] [PubMed] [Google Scholar]
- 2.Tanchot C, Lemonnier F A, Pérarnau B, Freitas A A, Rocha B. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 3.Kirberg J, Berns A, von Boehmer H. J Exp Med. 1997;186:1269–1275. doi: 10.1084/jem.186.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rooke R, Waltzinger C, Benoist C, Mathis D. Immunity. 1997;7:123–134. doi: 10.1016/s1074-7613(00)80515-4. [DOI] [PubMed] [Google Scholar]
- 5.Brocker T. J Exp Med. 1997;186:1223–1232. doi: 10.1084/jem.186.8.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swain S L, Hu H, Huston G. Science. 1999;286:1269–1275. doi: 10.1126/science.286.5443.1381. [DOI] [PubMed] [Google Scholar]
- 7.Witherden D, van Oers N, Waltzinger C, Weiss A, Benoist C, Mathis D. J Exp Med. 2000;191:355–364. doi: 10.1084/jem.191.2.355. [DOI] [PubMed] [Google Scholar]
- 8.Dorfman J R, Stefanova I, Yasutomo K, Germain R N. Nat Immunol. 2000;1:329–335. doi: 10.1038/79783. [DOI] [PubMed] [Google Scholar]
- 9.Clarke S R M, Rudensky A Y. J Immunol. 2000;165:2458–2464. doi: 10.4049/jimmunol.165.5.2458. [DOI] [PubMed] [Google Scholar]
- 10.Kassiotis G, Garcia S, Simpson E, Stockinger B. Nat Immunol. 2002;3:244–250. doi: 10.1038/ni766. [DOI] [PubMed] [Google Scholar]
- 11.Le Campion A, Bourgeois C, Lambolez F, Martin B, Léaument S, Dautigny N, Tanchot C, Penit C, Lucas B. Proc Natl Acad Sci USA. 2002;99:4538–4543. doi: 10.1073/pnas.062621699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruno L, von Boehmer H, Kirberg J. Eur J Immunol. 1996;26:3179–3185. doi: 10.1002/eji.1830261251. [DOI] [PubMed] [Google Scholar]
- 13.Oehen S, Brduscha-Riem K. Eur J Immunol. 1999;29:608–614. doi: 10.1002/(SICI)1521-4141(199902)29:02<608::AID-IMMU608>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 14.Ernst B, Lee D-S, Chang J M, Sprent J, Surh C D. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 15.Goldrath A W, Bevan M J. Immunity. 1999;11:183–190. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldrath A W, Bevan M J. Nature. 1999;402:255–262. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- 17.Kieper W C, Jameson S C. Proc Natl Acad Sci USA. 1999;96:13306–13311. doi: 10.1073/pnas.96.23.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreira C, Barthlott T, Garcia S, Zamoyska R, Stockinger B. J Immunol. 2000;165:3689–3694. doi: 10.4049/jimmunol.165.7.3689. [DOI] [PubMed] [Google Scholar]
- 19.Cho B K, Rao V P, Ge Q, Eisen H N, Chen J. J Exp Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murali-Krishna K, Ahmed R. J Immunol. 2000;165:1733–1737. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 21.Tanchot C, Le Campion A, Léaument S, Dautigny S, Dautigny N, Lucas B. Eur J Immunol. 2001;31:2256–2265. doi: 10.1002/1521-4141(200108)31:8<2256::aid-immu2256>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 22.Tanchot C, Le Campion A, Martin B, Léaument S, Dautigny N, Lucas B. J Immunol. 2002;168:5042–5046. doi: 10.4049/jimmunol.168.10.5042. [DOI] [PubMed] [Google Scholar]
- 23.Dummer W, Ernst B, LeRoy E, Lee D, Surh C. J Immunol. 2001;166:2460–2468. doi: 10.4049/jimmunol.166.4.2460. [DOI] [PubMed] [Google Scholar]
- 24.Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C, Mathis D, Fugger L. Proc Natl Acad Sci USA. 1999;96:10338–10343. doi: 10.1073/pnas.96.18.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malissen M, Gillet A, Ardouin L, Bouvier G, Trucy J, Ferrier P, Vivier E, Malissen B. EMBO J. 1995;14:4641–4650. doi: 10.1002/j.1460-2075.1995.tb00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, Mathis D. Cell. 1991;66:1051–1063. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 27.Le Campion A, Lucas B, Dautigny N, Léaument S, Vasseur F, Pénit C. J Immunol. 2002;168:1664–1671. doi: 10.4049/jimmunol.168.4.1664. [DOI] [PubMed] [Google Scholar]
- 28.Braunstein N S, Germain R N, Loney K, Berkowitz N. J Immunol. 1990;145:1635–1645. [PubMed] [Google Scholar]
- 29.Murphy D B, Rath S, Pizzo E, Rudensky A Y, George A, Larson J K, Janeway C A., Jr J Immunol. 1992;148:3483–3491. [PubMed] [Google Scholar]
- 30.Schluns K S, Kieper W C, Jameson S C, Lefrançois L. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 31.Tan J T, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg K I, Surh C D. Proc Natl Acad Sci USA. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fry T J, Mackall C L. Trends Immunol. 2001;22:564–571. doi: 10.1016/s1471-4906(01)02028-2. [DOI] [PubMed] [Google Scholar]
- 33.Seddon B, Zamoyska R. J Immunol. 2002;169:3752–3759. doi: 10.4049/jimmunol.169.7.3752. [DOI] [PubMed] [Google Scholar]
- 34.Lantz O, Grandjean I, Matzinger P, Di Santo J P. Nat Immunol. 2000;1:54–58. doi: 10.1038/76917. [DOI] [PubMed] [Google Scholar]
- 35.Smith K, Seddon B, Purbhoo M A, Zamoyska R, Fisher A G, Merkenschlager M. J Exp Med. 2001;194:1253–1261. doi: 10.1084/jem.194.9.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barrett S P, Toh B H, Alderuccio F, van Driel I R, Gleeson P A. Eur J Immunol. 1995;25:238–244. doi: 10.1002/eji.1830250139. [DOI] [PubMed] [Google Scholar]
- 37.Gleeson P A, Toh B H, van Driel I R. Immunol Rev. 1996;149:97–125. doi: 10.1111/j.1600-065x.1996.tb00901.x. [DOI] [PubMed] [Google Scholar]
- 38.Sherer Y, Shoenfeld Y. Bone Marrow Transplant. 1998;22:873–881. doi: 10.1038/sj.bmt.1701437. [DOI] [PubMed] [Google Scholar]