Abstract
Secreted protein acidic and rich in cysteine/osteonectin/BM-40 (SPARC) is a matrix-associated protein that elicits changes in cell shape, inhibits cell-cycle progression, and influences the synthesis of extracellular matrix (ECM). The absence of SPARC in mice gives rise to aberrations in the structure and composition of the ECM that result in generation of cataracts, development of severe osteopenia, and accelerated closure of dermal wounds. In this report we show that SPARC-null mice have greater deposits of s.c. fat and larger epididymal fat pads in comparison with wild-type mice. Similar to earlier studies of SPARC-null dermis, we observed a reduction in collagen I in SPARC-null fat pads in comparison with wild-type. Although elevated levels of serum leptin were observed in SPARC-null mice, their overall body weights were not significantly different from those of wild-type counterparts. The diameters of adipocytes from SPARC-null versus wild-type epididymal fat pads were 252 ± 61 and 161 ± 33 μm (means ± SD), respectively, and there was an increase in adipocyte number within SPARC-null fat pads in comparison with wild-type pads. Thus the absence of SPARC appears to result in an increase in the size of individual adipocytes as well as an increase in the number of adipocytes per fat pad. In fat pads isolated from wild-type mice, SPARC mRNA was associated with both the stromal/vascular and adipocyte fractions. We propose that SPARC limits the accumulation of adipose tissue in mice in part through its demonstrated effects on the regulation of cell shape and production of ECM.
Secreted protein acidic and rich in cysteine/osteonectin/BM-40 (SPARC) is a prototype of a group of proteins referred to as matricellular, proteins that associate with the extracellular matrix (ECM) but, unlike laminin and most collagens, probably do not contribute significantly to its structural integrity (1). SPARC consists of three modular domains: a low-affinity, high-capacity Ca2+-binding, N-terminal domain, a central region with a follistatin-like domain, and a C-terminal domain that contains a high-affinity Ca2+-binding EF hand (2). The C-terminal region of the protein has been implicated in ECM binding and cell-surface interaction. SPARC inhibits cell-cycle progression and induces cell rounding in a variety of cultured cells (3). Although expression of SPARC is high during development, adult tissues exhibit diminished levels of SPARC with the exception of those in which active turnover of the ECM is ongoing (4). For example, gut epithelia and bone display high levels of SPARC expression throughout adulthood. Processes such as angiogenesis, wound healing, and some metastases that require restructuring of the ECM are also associated with elevated production of SPARC. Thus, SPARC has been implicated as a modulator of cell-matrix interaction, particularly during matrix remodeling.
SPARC-null mice display early onset cataractogenesis and osteopenia as well as accelerated wound closure in response to dermal excisional wounding (5–8). Perturbations in the structure and composition of the ECM in the absence of SPARC have been implicated in each of these phenotypic abnormalities. For example, the SPARC-null dermal collagenous ECM is comprised of aberrant collagen fibrils, with a significant reduction in the collagen content of the skin (8). The basis for accelerated wound closure in these animals is thought to result from a dermal ECM more amenable to contraction in comparison with that of wild-type dermis. In addition, s.c.-implanted polyvinyl alcohol sponges, a model of angiogenesis in vivo, display accelerated fibrovascular invasion in SPARC-null mice in comparison with wild-type controls (9). Hence the SPARC-null dermal milieu seems to present a more favorable environment for neovascularization of implanted materials relative to that of wild-type mice.
Differentiation of mesenchymal stem cells into adipocytes is accompanied by a notable shift in the profile of ECM protein expression. Whereas stem cells or preadipocytes express primarily stromal ECM components such as fibronectin and collagen I, differentiated adipocytes synthesize primarily basement membrane-type ECM proteins such as laminin and collagen IV (10). ECM-degrading enzymes also contribute to adipogenesis. A plasma kallikrein-dependent plasminogen cascade, as well as stromelysin 1 (matrix metalloproteinase 3) have been implicated recently in the generation of adipose tissue during mammary-gland involution (11, 12). Presumably, the capacity to remodel the ECM that surrounds preadipocytes influences the differentiation of stem cells into adipocytes. The mechanism(s) by which ECM might affect adipocyte differentiation and adipose accumulation are relatively unexplored. In this report we show that the absence of the matrix-associated protein SPARC in mice resulted in increased adipose tissue without substantial increases in overall body weight. Consistent with findings in other tissues (4), the structure and composition of the ECM appear altered within the adipose tissue of mice lacking SPARC. Although the SPARC-null mouse is not a model of obesity, it seems to compensate for the loss of connective tissue (e.g., collagen I) by an accumulation of adipose tissue, especially in the dermis.
Materials and Methods
Mice.
SPARC-null mice were generated as described (5). C57Bl6/129SVJ wild-type and SPARC-null mice were housed in a modified specific pathogen-free facility and fed a standard 4% chow diet ad libitum. Mice were weighed at specified intervals. Epididymal fat pads were isolated from age-matched wild-type and SPARC-null mice at various ages and weighed as pairs. Blood was collected by cardiac puncture on anesthetized animals after 4–6 h of food deprivation. Leptin levels were assessed by Ani Lytics (Gaithersburg, MD) as part of a blood analysis that included glucose, cholesterol, high-density lipoprotein, insulin, and triglyceride levels among other metabolic indicators.
Quantification of Adipocyte Size and Number.
Stromal/fat tissue separation was carried out by a method similar to that described by Gregoire et al. (13). Seven epididymal fat pads were isolated from age-matched SPARC-null and wild-type mice and treated with 500 units/ml bacterial collagenase type II (Sigma), in saline with 2% BSA, with agitation for 1 h at 37°C. The filtrate was centrifuged at 800 rpm for 10 min at 4°C in a Beckman GPKR centrifuge. The fat fraction was collected from the top of the centrifuge tube, and the stromal/vascular material was collected as a pellet from the bottom of the tube (see below). Equal volumes of wild-type and SPARC-null adipocyte fractions were counted by hemocytometer to determine cell number per fat pad. Four representative hemocytometer fields were photographed from each mouse for quantification of adipocyte size. Images of the fields were scanned into the NIH IMAGE software program, and the diameter of each adipocyte was measured. It was noted that very large adipocytes, primarily in the SPARC-null samples, were not able to diffuse under the glass and remained in the trough of the hemocytometer; these cells were not present in substantial numbers. In effect, however, the number of SPARC-null adipocytes was slightly underrepresented. Total DNA was isolated from fat pads (three per genotype) or adipocyte suspensions (three per genotype) by using DNAzol (Life Technologies, Rockville, MD) according to manufacturer instructions. Quantification of DNA content was determined fluorimetrically with Hoechst 33258 stain (Sigma) (14).
Analysis of Collagen I Production.
Equivalent weights of fat pads isolated from age-matched wild-type and SPARC-null mice were suspended in 0.15 M NaCl with protease inhibitors (Roche Molecular Biochemicals) in a Dounce homogenizer. Cellular material was separated from lipids by centrifugation at 3,000 × g. The pellets were extracted with cold 0.5 M acetic acid at 4°C for 18 h. Soluble material was collected after centrifugation at 12,000 × g for 15 min and subsequently lyophilized. Freeze-dried protein was resuspended in equal amounts of appropriate buffer for collagenase digestion, pepsin digestion, and/or immunoblot analysis.
RT-PCR Analysis.
Eight to 10 fat pads from both wild-type and SPARC-null adult mice were treated as described above except that digested fat pads were subjected to vigorous trituration and passed through a 100-μm mesh before separation of the fat and stromal fractions by centrifugation. Both fractions were collected into separate tubes, washed, retriturated in fresh PBS, and finally centrifuged a second time at 800 rpm for 10 min at 4°C. As described previously, the fat tissue was removed from the top of the centrifuge tube, and the stromal/vascular material was collected as a pellet. RNA was isolated from the fat and stromal/vascular fractions with TriReagent (Molecular Research Center, Cincinnati) according to manufacturer instructions. RNAs were purified further and treated with DNase by the use of RNeasy minicolumns and DNase kits (Qiagen, Valencia, CA), respectively, according to manufacturer instructions.
Two micrograms of RNA were reverse-transcribed as described (15) in a 100-μl reaction volume containing 400 units of Moloney murine leukemia virus reverse transcriptase and buffer (Promega)/1.9 μg of oligo dT15 (Invitrogen)/1 mM each dNTP (Promega). The reactions were carried out for 1 h at 42°C and stored at −20°C.
PCR was carried out as described (15). Two microliters of the reverse-transcription reactions was added to the 20-μl PCRs containing 1 unit of AmpliTAQ Gold (Applied Biosystems), 400 nM each primer, 200 μM each dNTP, and 1.5 mM MgCl2 in a buffer supplied with AmpliTAQ Gold. The standard reaction had a precycling incubation at 94°C for 12 min followed by variable cycles of 95°C for 45 sec, 65°C for 59 sec, and 72°C for 120 sec. The reactions were given a final incubation at 72°C for 8 min and maintained at 4°C. Analysis was carried out on 1.2% agarose electrophoretic gels using ethidium bromide and UV transillumination to detect the DNA bands. Images were captured by a digital imaging system (Ultra-Lum, Claremont, CA).
The PCR primers (Invitrogen) used in this study were of our own design and represented the sequences (forward and reverse) SPARC (5′-GTCCCACACTGAGCTGGC-3′ and 5′-AAGCACAGAGTCTGGGTGAGTG-3′), leptin (5′-ATGTGCTGGAGACCCCTGTG-3′ and 5′-TCAGCATTCAGGGCTAACATCC-3′), peroxisome proliferator-activated receptor γ2 (5′-GGAGATTCTCCTGTTGACCCAG-3′ and 5′-GGCACTCAATGGCCATGAG-3′), vascular endothelial cadherin (5′-GCTGATCATCCTTGCGGAG-3′ and 5′-GTAGATGTGCAGTGTGTCGTATGG-3′), and cyclophilin A (5′-GGTCAACCCCACCGTTGTT-3′ and 5′-GCTCTCCTGAGCTACAGAAGGAAT-3′).
Results
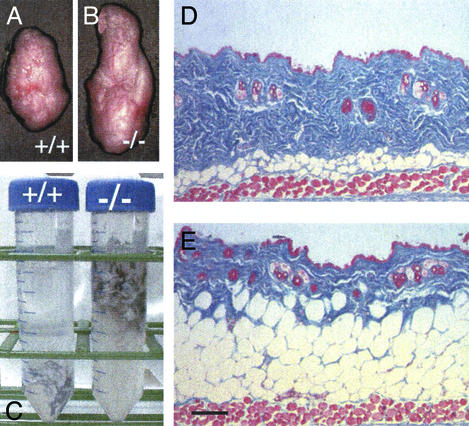
Our initial observation that SPARC-null skin had an increased adipose component was made when skin was excised from the mice and placed in dissection solution containing trypsin for isolation of dermal fibroblasts. As shown in Fig. 1C, the skin from SPARC-null mice floated in solution, whereas the skin removed from wild-type age-matched counterparts did not. Sections of skin from 5-month-old SPARC-null mice revealed a substantial increase in the subdermal adipose layer (Fig. 1E) in comparison with that of wild-type animals (Fig. 1D). To determine whether the increased fat deposition observed in SPARC-null animals was confined to skin, we isolated and weighed epididymal fat pads from male mice. There was a significant increase in the size of SPARC-null fat pads in comparison with those of wild-type animals (Fig. 1 B and A, respectively). However, histological examination of major organs including liver, lung, and kidney did not reveal differences in fat deposition relative to wild-type controls (data not shown).
Figure 1.
SPARC-null mice display increased accumulation of adipose tissue. Epididymal fat pads removed from 7-month-old wild-type (A) and SPARC-null mice (B) are shown. Although the size of the fat pad was enlarged in the absence of SPARC, the overall body weights of the mice were not significantly different (wild type, 29 g; SPARC null, 30 g). (C) An increased adipose component was also observed in skin. Fragments of skin isolated from 6-month-old SPARC-null (−/−) animals floated in solution, whereas those from wild-type (+/+) mice did not. Skin was removed from three separate animals of each genotype. The skins of SPARC-null animals 5 months old and older floated consistently when wild-type counterparts did not in >10 separate experiments. Sections from 5-month-old wild-type (D) and SPARC-null (E) mice stained with Masson's trichrome reagent are shown also. (Bar, 100 μm.)
The differences observed in fat deposition (primarily dermal and interorgan) were not reflected in the overall body weight of SPARC-null mice. For example, the fat pads shown in Fig. 1 were isolated from mice of similar age and weight (7 months of age; SPARC null, 30 g; wild type, 29 g). The compensation in body weight in SPARC-null mice reflects in part in the substantial loss of bone that has been reported in these animals (7). Another potential target for compensatory weight loss in SPARC-null mice is muscle (A.D.B., T. Nagy, and E.H.S., unpublished experiments). In addition, the decreased mass of connective tissue in SPARC-null skin (Fig. 1 D versus E, stained blue) compensates in part for the increased mass of s.c. fat.
Levels of collagen I, a primary component of connective tissue, were measured in fat pads from wild-type and SPARC-null mice of comparable ages. Shown in Fig. 2 are acetic acid-soluble proteins extracted from equal amounts of wild-type (lane 1) and SPARC-null (lanes 2 and 3) fat pads that were subjected to pepsin digestion. The arrows indicate protein bands designated as collagen α1(I) and α2(I) based on their mobility on SDS/PAGE, sensitivity to bacterial collagenase, resistance to pepsin, and immunoreactivity with anti-collagen I antibodies (data not shown). We observed a decrease in the amount of collagen I present in SPARC-null versus wild-type fat pads, whereas equivalent amounts of total protein were present in both extracts (as assessed by Coomassie blue stain of control samples and immunoblot analysis of tubulin, not shown). Thus the increased size of epididymal fat pads in SPARC-null mice was associated with decreased levels of collagen I relative to those measured in wild-type pads.
Figure 2.
SPARC-null fat pads contain less collagen I than equivalent amounts of wild-type fat pads. Equal amounts of wild-type and SPARC-null fat tissue isolated from 6-month-old animals were homogenized and extracted with acetic acid (see Materials and Methods). Equal volumes of wild-type (WT) and SPARC-null extracts were treated with pepsin, and the products were separated by SDS/PAGE. Wild-type extracts (lane 1) contained higher amounts of collagen α1(I) and α2(I) (arrows) in comparison with SPARC-null extracts (lanes 2 and 3) (shown are two separate extractions from SPARC-null pads). The experiment shown is representative of four separate extractions. Equivalent amounts of total protein were present in wild-type and SPARC-null homogenates as determined by Coomassie blue stain of control samples (without pepsin) and immunoblot analysis of tubulin (not shown).
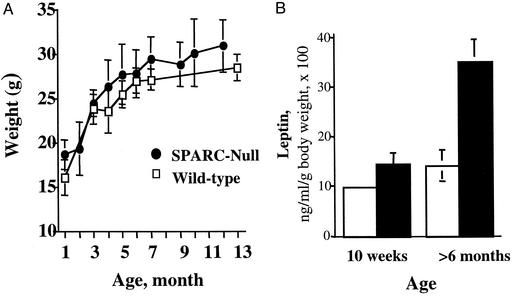
Overall body weights were determined as a function of increasing age. Shown in Fig. 3A are data from male mice given a standard 4% fat chow diet ad libitum. A weight-gain curve generated for female mice reflected similar results. The SPARC-null mice did not show significant differences in weight, although a slight increase in weight over the wild-type mice was observed at each age. In general, the overall weight of the SPARC-null mice was more variable at each time point; therefore, there was an increase in the standard deviation at each age. At no time did we observe any incidence of obesity in these mice. The largest SPARC-null male mouse to date weighed <35 g (the colony has been maintained >5 years). We also did not observe differences in the quantity of food consumed by the SPARC-null animals in comparison with wild type.
Figure 3.
SPARC-null and wild-type mice do not exhibit significant differences in overall body weight but do show increased levels of serum leptin with advancing age. (A) SPARC-null (●) and wild-type (□) mice housed under identical conditions and fed a 4% fat chow diet ad libitum did not show differences in overall weight gain over time. Ten mice or more were used to generate each time point. Error bars represent the standard deviation of the mean. (B) SPARC-null mice show increased levels of serum leptin with advancing age. Wild-type (open bars) and SPARC-null (black bars) mice were subjected to cardiac puncture 4–6 h after food deprivation. Whereas insulin, glucose, cholesterol, high-density lipoprotein, and triglyceride levels showed no significant differences between genotypes, leptin levels at older ages were increased substantially over those of age-matched, wild-type controls. Leptin levels (ng/ml) were normalized to individual body weights and are expressed as percentages of total body weight. The average weights of the mice at 10 weeks were 22.4 g (±1) (wild type) and 22.8 g (±1.4) (SPARC-null) and at >6 months 22.9 g (±3) (wild type) and 24.7 g (±3.4) (SPARC-null). Four mice for each genotype at 10 weeks and at least seven mice at >6 months contributed to the results. Error bars represent the standard error of the mean. *, P = 0.003 for >6 months.
Whereas significant differences in the overall weights of SPARC-null versus wild-type mice were not observed, the accumulation of adipose tissue in the skin and fat pads appeared to increase with age. Shown in Table 1 are comparisons of epididymal fat-pad weights as a percentage of overall body weight. As the male mice aged, more substantial differences in the size and weight of the fat pad were observed. At 4 months the ratio of fat pad to total body weight in the SPARC-null mice (expressed as a percentage) was 1.7% as opposed to 1.2% for wild-type males. However, at 6 months the SPARC-null value increased to 3.7%, whereas the wild-type mice increased only minimally (to 1.5%). Similar results were observed with s.c. adipose deposition in that the thickness of the subdermal fat layer became more substantial in SPARC-null mice over time, whereas only subtle differences were seen in the thickness of the wild-type fat layer over the same period (data not shown).
Table 1.
SPARC-null mice exhibit increased fat accumulation
| Age | Body weight, g | Epididymal fat pad weight, g | Fat pad, % of body weight* |
|---|---|---|---|
| 4 months | |||
| Wild type | 24.3 (±1.4)† | 0.29 (±0.08)† | 1.2 (±0.003)†‡ |
| SPARC null | 24.5 (±3.3) | 0.42 (±0.11) | 1.7 (±0.004) |
| 5 months | |||
| Wild type | 26.4 (±1.4) | 0.3 (±0.05) | 1.1 (±0.002)‡ |
| SPARC null | 29.2 (±3.6) | 0.8 (±0.03) | 2.6 (±0.009) |
| 6 months | |||
| Wild type | 29.3 (±3.5) | 0.4 (±0.2) | 1.5 (±0.006)‡ |
| SPARC null | 30 (±3.7) | 1.6 (±0.6) | 3.7 (±0.01) |
Epididymal fat pads were removed from age-matched wild-type and SPARC-null mice and weighed in pairs.
Ratio of pad weight to body weight × 100.
Mean ± SD.
P values: 4 months, 0.001; 5 months, 0.0002; 6 months, 0.006.
To determine whether serum indicators of increased adiposity were altered in SPARC-null mice, we subjected blood from 26 animals to laboratory analysis for specific proteins and metabolites. Although no differences were noted in the circulating amounts of glucose, insulin, cholesterol, high-density lipoprotein, and triglycerides, there was a significant increase in the level of serum leptin in the SPARC-null animals at 6–8 months of age (Fig. 3B). In accordance with the age-dependent accumulation of fat, serum leptin levels at early ages (10 weeks) did not exhibit significant differences between the two genotypes. Because leptin is synthesized and secreted primarily by differentiated adipocytes, the increase in serum leptin levels in SPARC-null animals most likely reflected the increased fat deposits of these mice in comparison with wild-type animals (16).
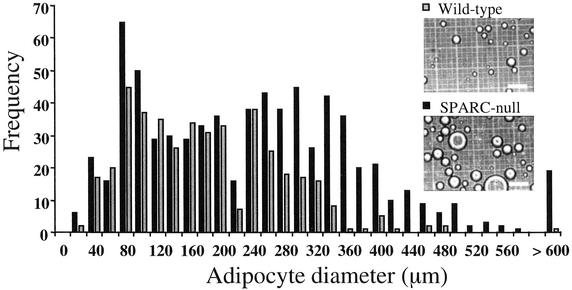
The absence of SPARC was predicted to influence the size and/or number of adipocytes. Accordingly, we digested epididymal pads from 6-month-old SPARC-null and wild-type mice with collagenase and isolated adipocytes for quantification. Although the total number of adipocytes per fat pad was increased in SPARC-null over wild-type mice (25,280 vs. 10,080, respectively), the number of fat cells per gram of tissue was decreased (28,000 vs. 49,830, respectively) (Tables 2 and 3). Moreover, the average diameter of SPARC-null adipocytes was 252 ± 61 μm, whereas the average diameter of wild-type cells was 161 ± 33 μm. The increase in diameter of the SPARC-null adipocytes translates to an ≈4-fold increase in volume over that of wild-type adipocytes (8.4 × 106 vs. 2.2 × 106 μm3). Thus an equal portion of a SPARC-null fat pad contained fewer adipocytes of greater size, in comparison with a wild-type fat pad. A comparison of the size distribution of isolated adipocytes is shown in Fig. 4. Whereas smaller adipocytes (20–200 μm in diameter) were represented similarly in wild-type and SPARC-null pads, larger adipocytes (200–500 μm in diameter) were more prevalent in extracts from SPARC-null tissue. Few very large adipocytes (>500 μm in diameter) were found in wild-type fat digests, whereas ≈5% of the cells in SPARC-null preparations were of this size. Consistent with an increase in the size of SPARC-null adipocytes over that of wild-type, quantification of DNA from fat pads and isolated adipocytes revealed more DNA per gram of tissue in wild-type relative to SPARC-null tissue and cells (18–34% lower DNA levels in null fat pads per gram of tissue, ≈13% lower DNA levels in null adipocytes per gram of tissue). Whereas the number of adipocytes in SPARC-null fat pads was ≈2.5 times that in wild-type pads, the difference in mass was ≈4-fold for the subset of 6-month-old fat pads used for quantification of adipocyte number (Tables 2 and 3). Hence we conclude that the substantial increase observed in SPARC-null relative to wild-type adipose tissue results from an increase in adipocyte size coupled with an increase in the overall number of adipocytes in the fat pad.
Table 2.
Quantification of wild-type adipocyte number and size
| WT* | Pad weight†, g | Average diameter, μm | No. of adipocytes, × 10−3 | No. of adipocytes per gram of tissue, × 10−3 |
|---|---|---|---|---|
| 4292 | 0.15 | 139 | 9.5 | 63.0 |
| 4283 | 0.15 | 139 | 7.8 | 52.0 |
| 4290 | 0.17 | 154 | 10.5 | 62.0 |
| 4291 | 0.17 | 172 | 8.0 | 47.0 |
| 4284 | 0.2 | 139 | 7.8 | 39.0 |
| 4271 | 0.47 | 222 | 1.7 | 36.0 |
| Mean ± SD | 0.22 ± 12 | 161 ± 33‡ | 10.1 ± 3.2 | 49.8 ± 1.1 |
| Volume§, μm3 | 2.20 × 106 |
Six-month-old mice were used for quantification. The column indicates animal identification number. WT, wild type.
One fat pad per animal was used for quantification.
P = 0.03.
Volume = 4/3π r3.
Table 3.
Quantification of SPARC-null adipose number and size
| Null* | Pad weight†, g | Average diameter, μm | No. of adipocytes, × 10−3 | No. of adipocytes per gram of tissue, × 10−3 |
|---|---|---|---|---|
| 4723 | 0.67 | 223 | 22.1 | 33.0 |
| 4727 | 0.74 | 221 | 22.2 | 30.0 |
| 4725 | 0.79 | 283 | 18.2 | 23.0 |
| 4718 | 0.99 | 187 | 31.7 | 32.0 |
| 4728 | 1.1 | 360 | 27.5 | 25.0 |
| 4720 | 1.2 | 238 | 30.0 | 25.0 |
| Mean ± SD | 0.92 ± 0.21 | 252 ± 61‡ | 25.3 ± 5.3 | 28.0 ± 4.2 |
| Volume§, μm3 | 8.40 × 106 |
Six-month-old mice were used for quantification. The column indicates animal identification number.
One fat pad per animal was used for quantification.
P = 0.03.
Volume = 4/3π r3.
Figure 4.
SPARC-null epididymal fat pads contain significantly more large adipocytes than wild-type fat pads. Fat pads were treated with collagenase, and adipocytes were fractionated by centrifugation. Isolated adipocytes were photographed on a hemocytometer slide ( , wild-type adipocytes; ■, SPARC-null adipocytes), and captured images were imported into nih IMAGE software for quantification. (Scale bars, 200 μm.) Wild-type (423 cells, gray bars) and SPARC-null (717 cells, black bars) adipocytes contributed to the distribution. The histogram was generated from the data reported in Tables 2 and 3.
, wild-type adipocytes; ■, SPARC-null adipocytes), and captured images were imported into nih IMAGE software for quantification. (Scale bars, 200 μm.) Wild-type (423 cells, gray bars) and SPARC-null (717 cells, black bars) adipocytes contributed to the distribution. The histogram was generated from the data reported in Tables 2 and 3.
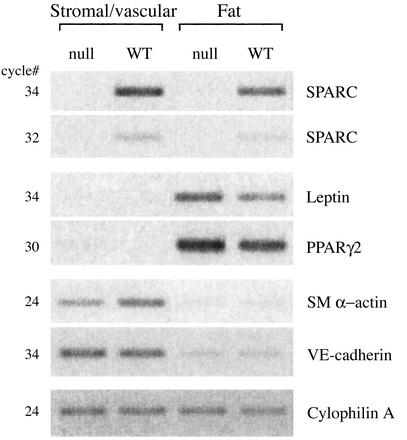
To ascertain how the absence of SPARC might affect accumulation of adipose tissue, we examined the levels of SPARC mRNA in separate fractions of epididymal fat pads. As shown in Fig. 5, total mRNA isolated from the fat fraction contained the adipose-specific mRNAs leptin and peroxisome proliferator-activated receptor γ (PPARγ), both of which were absent from the stromal/vascular fraction (16, 17). Although the stromal/vascular isolate showed little if any fat-cell contamination, the fat fraction most likely contained low levels of stroma/vasculature, because the endothelial-specific marker vascular endothelial-cadherin and smooth muscle cell marker, smooth muscle α-actin, were reduced greatly in the fat fraction but still detectable by PCR (18). Expression of SPARC was found principally in association with the stromal/vascular fraction, although lower levels of SPARC mRNA were also detectable in association with the adipocyte fraction. Although the presence of SPARC mRNA in the stromal/vascular fraction was predicted, based on previous characterization of SPARC-expression patterns (3), the production of SPARC by adipocytes was surprising. Each adipocyte synthesizes a basal lamina that invests the cell (10). The absence of SPARC expression in mice might therefore result in an altered stromal ECM that favors adipocyte differentiation, and/or the absence of SPARC in basal laminae surrounding mature adipocytes might confer a more permissive environment for fat expansion.
Figure 5.
SPARC mRNA was expressed predominantly by stromal/vascular cells in comparison with adipocytes. Fat pads were fractionated into stromal/vascular and adipocyte compartments, and RNA was isolated for RT-PCR analysis (see Materials and Methods). The purity of the stromal/vascular and adipocyte fractions was confirmed by analysis of known tissue-specific genes. The fat fraction contained mRNA encoding peroxisome proliferator-activated receptor γ (PPARγ) and leptin, whereas the stromal/vascular component contained mRNA encoding vascular endothelial (VE)-cadherin (endothelial cells) and smooth muscle (SM) α-actin (smooth muscle cells). That the stromal/vascular component was devoid of detectable fat-specific genes at the number of cycles used indicated very little, if any, fat contamination in this fraction. However, the fat fraction did contain low levels of stromal/vascular-specific mRNAs. The mRNA encoding cyclophilin A, a constitutively expressed peptidyl-prolyl isomerase (25), was used as a control to ensure equal starting amounts of mRNA from each fraction. WT, wild type.
Discussion
We have reported an increase in fat deposition in mice with a targeted deletion of the SPARC gene. Greater adipose accumulation was not accompanied by significant increases in the overall body weight, reinforcing the observation that the SPARC-null mouse is not a model of obesity. In SPARC-null dermis, maintenance of weight in the presence of fat accumulation was attributed to a loss of connective tissue, e.g., collagen I. Clearly the substantial osteopenia in these mice is also a contributing factor (7). Enhanced levels of serum leptin paralleled larger deposits of fat, both of which were augmented with age. The expansion of epididymal fat pads was attributed to an increase in the average size of SPARC-null adipocytes (a higher frequency of larger adipocytes), coupled with a greater number of adipocytes per fat pad. Although the studies reported here were carried out in C57Bl6 × 129SVJ mice, similar expansion of white-fat depots was also observed in 129SVJ SPARC-null animals. In this regard, it is interesting that another major phenotype of SPARC-null mice, cataract formation, was found to be 100% penetrant in three different strains of mice lacking SPARC (5, 6).
The number of adipocytes corrected for the weight of each fat pad was decreased in SPARC-null relative to wild-type mice (Tables 2 and 3). Interestingly, there seemed to be a reverse correlation between the average diameter of adipocytes and the cell number per fat pad. In both wild-type and SPARC-null mice, the larger fat pads generally contained fewer adipocytes per gram of tissue in comparison with the smaller fat pads, but the cells exhibited greater diameters (Tables 2 and 3). We observed a 2.5-fold increase in the number of adipocytes per SPARC-null fat pad; however, the increase in overall mass of the fat pads in the absence of SPARC was ≈4-fold at 6 months (Tables 2 and 3). The increase in diameter of the SPARC-null adipocytes resulted in a 4-fold increase in the volume of SPARC-null relative to wild-type cells. DNA quantification performed on intact fat pads as well as on isolated adipocytes supported the conclusion that the SPARC-null pads contained lower numbers of cells per gram of tissue.
The larger size of the fat pads in the absence of SPARC seems to depend less on the differentiation of preadipocytes to adipocytes than on the maturation of differentiated adipocytes. In support of this proposal, we did not observe differential expression of the preadipocyte-specific mRNA encoding pref-1 in SPARC-null versus wild-type stromal fractions by RT-PCR analysis (data not shown and ref. 19). The time course of fat-pad expansion that occurs in SPARC-null adult animals is more consistent with an effect on adipocyte maturation as opposed to significant differences in adipogenesis that would be expected in the first 1–3 months postpartum.
To date, the majority of phenotypic abnormalities reported in the SPARC-null mice are characterized by a perturbation in ECM structure and composition. In addition to the diminished collagen type I expression reported in SPARC-null mesangial cells (20), the dermis of SPARC-null mice also showed a substantial reduction in collagen type I (8). The decrease in collagen content, as determined by hydroxyproline analysis in SPARC-null skin, is progressive and at 6 months of age was found to be approximately half that of wild-type skin (8, 21). Similarly, we observed a decrease in collagen I protein per gram of tissue in SPARC-null fat pads in comparison with wild-type (Fig. 2). The similar reduction in collagen content of SPARC-null versus wild-type adipose tissue was substantiated by quantification of hydroxyproline levels in epididymal fat pads (data not shown). We predict that a decrease in the amount of fibrillar collagen in the fat pads, i.e., stromal compartmentalization, leads to a more permissive environment for adipocyte expansion over time. Recently, Lijnen et al. (22) reported that inhibition of matrix metalloproteinases in mice fed a high-fat diet led to significantly smaller gonadal fat pads. An increase in total collagen content and the presence of a distinct collagen cap were observed in animals treated with a matrix metalloproteinase inhibitor (22). Thus, increased collagen deposition was associated with smaller fat deposits. This finding is consistent with the decreased collagen content that we observed in SPARC-null mice that have larger fat pads than their wild-type counterparts.
An effect on the fibrillar collagen content of the fat pads does not preclude a function for SPARC in the organization of adipocyte basal laminae. Alterations in the distribution of both laminin and collagen IV in the basement membrane of the lens capsule in SPARC-null mice indicate that altered ECM deposition in the absence of SPARC impedes lens epithelial cell function and contributes to cataract formation (23).
SPARC is an ECM-associated protein (without proteinase activity) in which transgenic ablation of expression has led to an effect on adipose accumulation. SPARC is consistently observed in remodeling tissues in which turnover of the ECM is critical for tissue function. The differentiation of stromal stem cells to adipocytes also relies on a phase of ECM remodeling. Perhaps the absence of SPARC leads to disruption of ECM structure and composition in the connective tissue of the stromal/vascular fraction and/or in the basal lamina surrounding each adipocyte. Tartare-Deckert et al. (24) have reported an increase in SPARC mRNA in adipose tissue of ob/ob mice as well as in animals with diet- or drug-induced obesity in comparison with lean mice. SPARC apparently is not required for hypertrophic fat accumulation, because SPARC-null mice displayed increased adipose depots. Its heightened expression in the models of obesity might reflect the enhanced remodeling and synthesis of connective tissue that are typically associated with weight gain. SPARC-null animals, however, might be resistant to obesity when given a high-fat diet.
Expression of SPARC by both the stromal/vascular and adipocyte fractions of fat pads indicates that SPARC might have a dual function in adipose tissue. Perhaps the absence of SPARC influences adipocyte size in lean mice by perturbation of the surrounding ECM, whereas in obesity, expression of SPARC by adipocytes accompanies fat expansion as cells increase basal lamina deposition in association with increased adipocyte growth. Although the function of SPARC in adipose tissue is apparently complex, it is clearly able to influence adipose accretion and as such introduces an exciting area of research in the control of fat deposition by ECM and matricellular proteins.
Acknowledgments
We thank the members of the Sage Laboratory for helpful discussion; Chris Ganders and John I. Clark for assistance with the blood analysis; and Juliet G. Carbon for assistance with the mice. This work was supported by National Institutes of Health Grants GM 40711 and HL 59574 (to E.H.S.), DK 07467 (to A.D.B. and D.C.G.), and AR 02220 (to A.D.B.) and National Science Foundation Grant EE 04-150.
Abbreviations
- SPARC
secreted protein acidic and rich in cysteine/osteonectin/BM-40
- ECM
extracellular matrix
References
- 1.Bornstein P. J Cell Biol. 1995;130:503–506. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hohenester E, Maurer P, Timpl R. EMBO J. 1997;16:3778–3786. doi: 10.1093/emboj/16.13.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brekken R A, Sage E H. Matrix Biol. 2000;19:569–580. doi: 10.1016/s0945-053x(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 4.Bradshaw A D, Sage E H. J Clin Invest. 2001;107:1049–1054. doi: 10.1172/JCI12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norose K, Lo W-K, Clark J I, Sage E H, Howe C C. Exp Eye Res. 2000;71:295–307. doi: 10.1006/exer.2000.0884. [DOI] [PubMed] [Google Scholar]
- 6.Gilmore D T, Lyon G J, Carlton M B L, Sanes J R, Cunningham J M, Anderson J R, Hogan B L M, Evans M J, Colledge W H. EMBO J. 1998;17:1860–1870. doi: 10.1093/emboj/17.7.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delaney A M, Amling M, Priemel M, Howe C, Baron R, Canalis E. J Clin Invest. 2000;105:915–923. doi: 10.1172/JCI7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradshaw A D, Reed M J, Sage E H. J Histochem Cytochem. 2002;50:1–10. doi: 10.1177/002215540205000101. [DOI] [PubMed] [Google Scholar]
- 9.Bradshaw A D, Reed M J, Carbon J G, Pinney E, Sage E H. Wound Repair Regen. 2001;9:522–530. doi: 10.1046/j.1524-475x.2001.00522.x. [DOI] [PubMed] [Google Scholar]
- 10.Smas C M, Sul H S. Biochem J. 1995;309:697–710. doi: 10.1042/bj3090697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selvarajan S, Lund L R, Takeuchi T, Craik C S, Werb Z. Nat Cell Biol. 2001;3:267–275. doi: 10.1038/35060059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander C M, Selvarajan S, Mudgett J, Werb Z. J Cell Biol. 2001;152:693–703. doi: 10.1083/jcb.152.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gregoire F M, Johnson P R, Greenwood M R. Int J Obes Relat Metab Disord. 1995;19:664–670. [PubMed] [Google Scholar]
- 14.Latt S A, Stetten G. J Histochem Cytochem. 1976;24:24–33. doi: 10.1177/24.1.943439. [DOI] [PubMed] [Google Scholar]
- 15.Graves D C, Yablonka-Reuveni Z. J Histochem Cytochem. 2000;48:1173–1193. doi: 10.1177/002215540004800902. [DOI] [PubMed] [Google Scholar]
- 16.Spiegelman B M, Flier J S. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 17.Rosen E D, Sarraf P, Troy A E, Bradwin G, Moore K, Milstone D S, Spiegelman B M, Mortenson R M. Mol Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 18.Breier G, Breviario F, Caveda L, Berthier R, Schnurch H, Gotsch U, Vestweber D, Risau W, Dejana E. Blood. 1996;87:630–641. [PubMed] [Google Scholar]
- 19.Zhou Y T, Wang Z W, Higa M, Newgard C B, Unger R H. Proc Natl Acad Sci USA. 1999;96:2391–2395. doi: 10.1073/pnas.96.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francki A, Bradshaw A D, Bassuk J A, Howe C C, Couser W G, Sage E H. J Biol Chem. 1999;274:32145–32152. doi: 10.1074/jbc.274.45.32145. [DOI] [PubMed] [Google Scholar]
- 21. Bradshaw, A. D., Puolakkainen, P., Dasgupta, J., Davidson, J. M., Wight, T. N. & Sage, E. H. (2003) J. Invest. Dermatol., in press. [DOI] [PubMed]
- 22.Lijnen H R, Maquoi E, Hansen L B, Van Hoef B, Frederix D C. Arterioscler Thromb Vasc Biol. 2002;22:374–379. doi: 10.1161/hq0302.104522. [DOI] [PubMed] [Google Scholar]
- 23.Yan Q, Clark J I, Wight T N, Sage E H. J Cell Sci. 2002;115:2747–2756. doi: 10.1242/jcs.115.13.2747. [DOI] [PubMed] [Google Scholar]
- 24.Tartare-Deckert S, Chavey C, Monthouel M N, Gautier N, Van Obberghen E. J Biol Chem. 2001;276:22231–22237. doi: 10.1074/jbc.M010634200. [DOI] [PubMed] [Google Scholar]
- 25.Danielson P E, Forss-Petter S, Brow M A, Calavetta L, Douglass J, Milner R J, Sutcliffe J G. DNA. 1988;7:261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]