Abstract
HIV infection induces a wide array of B cell dysfunctions. We have characterized the effect of plasma viremia on the responsiveness of B cells to CD4+ T cell help in HIV-infected patients. In HIV-negative donors, B cell proliferation correlated with CD154 expression on activated CD4+ T cells and with the availability of IL-2, whereas in HIV-infected viremic patients, reduced B cell proliferation was observed despite normal CD154 expression on activated CD4+ T cells. Reduced triggering of B cells by activated CD4+ T cells was clearly observed in HIV-infected viremic patients compared with aviremic patients with comparable CD4+ T cell counts, and a dramatic improvement in B cell function was observed in patients whose plasma viremia was controlled by effective antiretroviral therapy. The degree of B cell dysfunction in viremic patients correlated strongly with the inability of B cells to express CD25 in response to activated CD4+ T cells, resulting in an inability to mount a normal proliferative response to IL-2. Similar defects in responsiveness to IL-2 were observed in the B cells of HIV-infected viremic patients in the context of B cell receptor stimulation. These data provide new insight into the mechanisms associated with ineffective humoral responses in HIV disease.
The scope of B cell abnormalities in HIV-infected patients, first described almost 20 years ago (1), has come to define a pervasive paradigm in HIV infection, namely that of immunodeficiency in the setting of virus-driven immune activation. Hallmarks of B cell abnormalities in HIV disease include hypergammaglobulinemia (2–4), increased expression of activation markers (5), increased levels of autoantibodies (6, 7), increased risk of developing B cell lymphomas (8), and decreased responsiveness to in vivo vaccination and ex vivo stimulation (9, 10). The range of defects in B cell responses includes both T cell-dependent and -independent pathways (1, 11, 12), suggesting that along with defects in CD4+ T cell help, HIV infection causes intrinsic B cell defects. Recent studies have shown that the majority of these abnormalities are attributable to ongoing viral replication and are reversible by the suppression of plasma viremia with effective antiretroviral therapy (2, 6, 13–15). In light of similar abnormalities described in the CD4+ and CD8+ T cell compartments of HIV-infected individuals (16–18), there is clear evidence that immune activation brought on by unchecked HIV-1 replication has widespread deleterious effects on the immune system.
Insight into the apparent paradox that in vivo B cell hyperactivity translates into poor response to immunogens and ex vivo stimuli has remained elusive for almost two decades. However, with the availability of antiretroviral therapies that can effectively sustain the suppression of virus replication, it has become apparent that B cell hyperactivity and poor responsiveness to stimulation are directly linked to ongoing virus replication by mechanisms that may include cytokine deregulation, loss of memory B cells, and induction of terminal differentiation (15, 19, 20). However, most studies on B cell function have been based primarily, if not exclusively, on responsiveness of B cells to exogenous surrogates of antigenic stimulation, such as soluble anti-Ig antibodies and trimeric CD40 ligand (CD154), triggers of the B cell receptor (BCR) and CD40, respectively.
The initiation of a humoral immune response to T cell-dependent antigens requires cognate interactions between antigen-specific B cells and activated antigen-specific CD4+ T cells involving both cell-contact interactions and soluble factors. One of the most important contact-mediated interactions between B cells and CD4+ T cells involves CD40 expressed constitutively on B cells and its membrane-bound ligand CD154 expressed on activated CD4+ T cells (21). The expression of CD154 on activated CD4+ T cells is induced after T cell receptor triggering by antigen-presenting cells, likely dendritic cells, and requires key costimulatory interactions between CD80/CD86 on mature antigen-presenting cells and CD28 on CD4+ T cells (22). These interactions also induce CD4+ T cells to secrete cytokines, which, together with expression of CD154, provide CD4+ T cells with full helper function. T helper (Th) cells can be divided into Th1 and Th2 subsets, depending on which cytokines are produced during activation. B cells can respond to either subset, although Th2 responses involving IL-4, IL-10, and IL-13 have been more widely described among B cells than Th1 responses involving IL-2, IFN-γ, and IL-12 (23). However, recent evidence from both in vivo (24) and in vitro (25) studies suggests that both Th1 and Th2 CD4+ T cells provide help to B cells.
In the present study we have investigated the responsiveness of B cells of HIV-infected patients to CD4+ T cell help in an autologous and physiologic system. We examined the impact of plasma viremia, both in cross-sectional and longitudinal settings, on the capacity of activated CD4+ T cells to induce B cells to proliferate. We observed that although plasma viremia had no impact on the capacity of CD4+ T cells to express CD154 upon activation, B cells of viremic patients showed poor proliferative responses that were found to correlate with reduced induction of CD25 on the surface of responding B cells.
Materials and Methods
Patients.
Study subjects included patients chronically infected with HIV-1 and HIV-negative healthy donors (Table 1). Initial observations were made by comparing HIV-viremic patients to HIV-negative donors. The effect of plasma viremia then was considered in cross-sectional analyses by pairing HIV-viremic patients with HIV-aviremic patients who had similar CD4+ T cell counts. Finally, the cross-sectional analyses were extended to longitudinal analyses by comparing HIV-infected patients before and after reduction of plasma viremia. HIV-infected patients with high plasma viremia were antiretroviral drug-naive, not fully compliant with their antiretroviral regimens, or failing therapy, whereas those with low plasma viremia were receiving effective antiretroviral regimens. Leukapheresis was conducted in accordance with protocols approved by the Institutional Review Board of the National Institute of Allergy and Infectious Diseases.
Table 1.
Profiles of patients studied
| Group/description | Patient no. | Plasma HIV RNA copies per ml* | CD4 count per μl | % CD154 on CD4+ T cells† | IL-2 secretion,† ng per 106 cells |
|---|---|---|---|---|---|
| 1, HIV-viremic (V) versus HIV-negative (N) | V1 | 12,221 | 248 | 69.1 | 15.6 |
| V2 | 103,836 | 450 | 58.8 | 6.6 | |
| V3 | 210,433 | 153 | 44.8 | 10.3 | |
| V4 | 755,760 | 108 | 47.8 | 11.9 | |
| V5 | 27,659 | 320 | 41.3 | 1.1 | |
| Median | 103,836 | 248 | 47.8 | 10.3 | |
| N1 | NA | ND | 62.2 | 5.3 | |
| N2 | NA | ND | 48.2 | ND | |
| N3 | NA | ND | 82.6 | 2.5 | |
| N4 | NA | ND | 48.6 | 5.7 | |
| N5 | NA | ND | 49.2 | 1.8 | |
| Median | NA | ND | 49.2 | 3.9 | |
| 2, Cross-sectional HIV-viremic (V) versus HIV-aviremic (A) | V6 | 23,031 | 476 | 60 | 3.4 |
| V7 | 91,473 | 743 | 40.1 | 9.8 | |
| V8 | 183,361 | 314 | 63.5 | 5.9 | |
| V9 | 35,533 | 309 | 60.5 | 16.7 | |
| V10 | 162,467 | 145 | 61.8 | 3.4 | |
| Median | 91,473 | 314 | 60.5 | 5.9 | |
| A6 | <50 | 482 | 55.1 | 4.9 | |
| A7 | 67 | 724 | 52.3 | 4.9 | |
| A8 | <50 | 338 | 66.5 | 6.4 | |
| A9 | <50 | 338 | 62.6 | 25.9 | |
| A10 | <50 | 106 | 61.6 | ND | |
| Median | <50 | 338 | 61.6 | 5.6 | |
| 3, Longitudinal HIV-viremic (V) versus HIV-aviremic (A) | V11 | 72,513 | 300 | 43.9 | 2.5 |
| V12 | 891,850 | 158 | 87.3 | 3.1 | |
| V13 | 251,000 | 248 | 67.5 | 4.7 | |
| V14 | 84,093 | 412 | 71 | 0.7 | |
| V15 | 183,361 | 314 | 63.5 | 5.9 | |
| Median | 183,361 | 300 | 67.5 | 3.1 | |
| A11 | <50 | 462 | 66.4 | 3.5 | |
| A12 | <50 | 356 | 55.9 | 4.1 | |
| A13 | <50 | 482 | 55.1 | 4.9 | |
| A14 | <50 | 497 | 75.4 | 1.3 | |
| A15 | 299 | 586 | 52.4 | ND | |
| Median | <50 | 482 | 55.9 | 3.4 |
ND, not done; NA, not applicable.
Measured by ultrasensitive bDNA assay with a detection limit of 50 copies per ml of plasma.
IL-2 secretion and CD154 expression were measured on CD4+ T cells activated with anti-CD3 and anti-CD28.
Cell Preparation and Culture Conditions.
CD4+ T cells were isolated from leukapheresis-derived fresh peripheral blood mononuclear cells (PBMCs) by using an immunomagnetic column-based negative selection technique (StemCell Technologies, Vancouver) as described (26). The same technique was used to isolate B cells from cryopreserved PBMCs. Purity of each cell population (>95%) was confirmed by flow cytometry. Cultures of 4 × 106 CD4+ T cells per well in 24-well plates coated with anti-CD3 mAb (BD Biosciences PharMingen) were established in the presence or absence of soluble anti-CD28 mAb (1 μg/ml, BD Biosciences PharMingen), human cytokine IL-2 (20 units/ml, Roche Diagnostics), and IL-12 (1 ng/ml, R & D Systems). After 40 h of stimulation, the cells were collected, washed, irradiated with 5,000 rad (1 rad = 0.01 Gy), and cocultured at 1 × 105 cells per well in 96-well plates with the same number of autologous B cells. Cocultures were performed either in anti-CD3-coated plates (BioCoat, BD Biosciences) or uncoated plasticware in the presence of 20 units/ml IL-2. After an additional 72 h of incubation, proliferation of B cells was measured by [3H]thymidine uptake during an additional 16 h of incubation. Negative controls for proliferation included incubation of irradiated CD4+ T cells alone and B cells cocultured with unstimulated CD4+ T cells. Culture supernatant from 40-h-activated CD4+ T cells was collected and assayed for IL-2 secretion by using a commercial ELISA kit (R & D Systems).
Inhibition experiments consisted of stimulating B cells with activated CD4+ T cells or phorbol 12-myristate 13-acetate (PMA) in the presence or absence of 10 μg/ml neutralizing mAbs against CD154 (Ancell, Bayport, MN), IL-2, IL-4, IL-10, or IL-12 (all anti-cytokines were purchased from BD Biosciences PharMingen). B cell monocultures were performed as described (15), with anti-IgM in the presence or absence of 20 units/ml IL-2.
Flow Cytometry.
Cell surface levels of CD154 on 40-h-activated CD4+ T cells were measured with phycoerythrin (PE)-conjugated anti-human CD154 and mouse IgG1 isotype control (BD Biosciences). Cell surface levels of CD25 and CD80 on 48-h-stimulated B cells were measured with allophycocyanin- conjugated anti-human CD19, PE-conjugated anti-human CD80, FITC-conjugated anti-human CD25, and appropriate mouse Ig isotype controls (BD Biosciences). Stained cells were analyzed with a FACSCalibur flow cytometer (BD Biosciences).
Statistical Analyses.
Statistical associations between B cell proliferation and other variables were determined by the Spearman rank test for correlation.
Results
Determinants of B Cell Responsiveness to Activated CD4+ T Cells.
Most studies on B cell function in HIV disease have focused on the response of B cells to surrogates of T cell-dependent and -independent antigenic stimulation. We and others have shown that B cells of viremic HIV-infected patients respond poorly to B cell stimuli such as BCR- or CD40-triggering agents (10, 15). However, there is growing evidence that these in vitro surrogates of immunologic stimulation may not adequately reflect cognate interactions that occur in vivo between B cells and CD4+ T cells (25, 27). In addition, little is known about how HIV disease affects B cell responsiveness to CD4+ T cell help. In an attempt to delineate B cell responses to CD4+ T cell help in a physiologic context, we established an ex vivo autologous activation system with PBMCs derived from HIV-negative and HIV-positive donors as described in Table 1. A fraction of the fresh PBMCs was used to isolate CD4+ T cells, whereas another fraction was cryopreserved for subsequent isolation of B cells. The CD4+ T cells were activated by using immobilized anti-CD3 mAbs alone or in the presence of a variety of stimuli that enhance the expression of CD154, including soluble anti-CD28 mAbs and cytokines IL-2 and IL-12. After 40 h, the activated CD4+ T cells were analyzed for surface expression of CD154 and irradiated before addition of B cells purified from the cryopreserved PBMCs. In addition to CD154, proliferation of B cells in this system required the presence of cytokines obtained either by restimulation of irradiated CD4+ T cells with anti-CD3 (25) or by addition of exogenous cytokines.
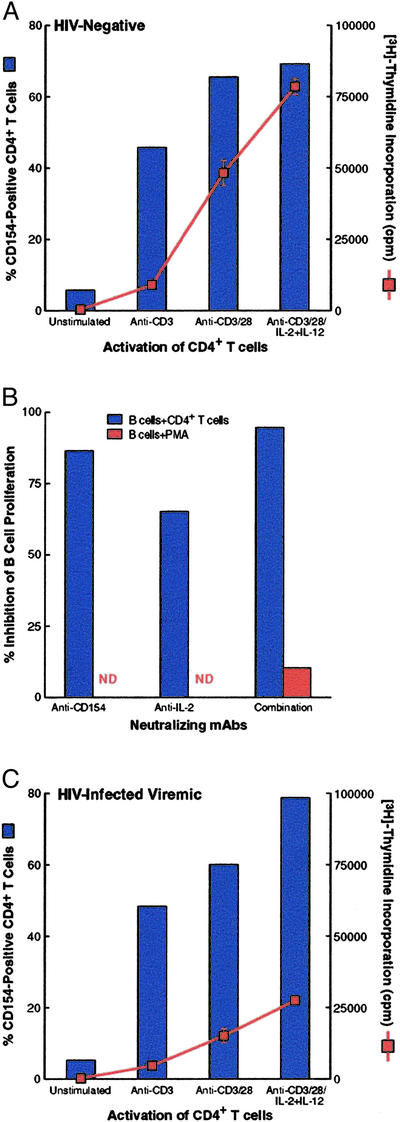
Fig. 1A depicts a typical profile of CD4+ T cell activation and corresponding B cell proliferation using cells from HIV-negative donors. Proliferation of B cells correlated with the level of surface expression of CD154 on activated CD4+ T cells. To identify the determinants of B cell proliferation in this system, various neutralizing antibodies were added to B cells that were cocultured with anti-CD3/anti-CD28-activated CD4+ T cells under conditions that did not require addition of exogenous cytokines, namely restimulation with immobilized anti-CD3. Under these conditions, B cell proliferation was inhibited almost completely by the combination of anti-CD154 and anti-IL-2 mAbs, whereas the individual Abs suppressed proliferation substantially, but in most cases less than did the combination (Fig. 1B). Furthermore, the addition of anti-IL-2 and anti-CD154 mAbs had no effect on the proliferation of B cells stimulated with PMA, a B cell mitogen that acts through a CD40-independent pathway, indicating that these antibodies were not exerting nonspecific inhibitory effects on B cells. Taken together, these data indicate that, in the system used in this study, B cell proliferation in response to CD4+ T cell help is dictated by the availability of CD154 and IL-2.
Figure 1.
B cell responses induced by activated CD4+ T cells and soluble stimuli. (A) CD4+ T cells isolated from a representative HIV-negative donor were stimulated with media or anti-CD3 ± anti-CD28 ± IL-2/IL-12 for 40 h, irradiated, and cocultured with B cells in the presence of anti-CD3. (B) Effect of neutralizing mAbs on B cells from a representative HIV-negative donor stimulated with anti-CD3/CD28-activated CD4+ T cells in the presence of anti-CD3 or B cells stimulated with phorbol 12-myristate 13-acetate (PMA)/ionomycin. (C) Effect of HIV infection and plasma viremia on B cell responsiveness to CD4+ T cells under conditions described in A; data are from a representative HIV-infected viremic patient. (See Fig. 2A for additional pairs of HIV-negative versus HIV-infected viremic individuals.) All culture conditions were performed in triplicate with standard deviation indicated by the error bars.
Effect of HIV Infection and Plasma Viremia on B Cell Response to CD4+ T Cell Help.
To determine the effect of HIV infection on the responsiveness of B cells to CD4+ T cell help in HIV-infected patients, we performed coculture assays on cells isolated from HIV-infected viremic patients. The short activation period for CD4+ T cells followed by irradiation and coincubation with B cells was designed to minimize propagation of endogenous HIV and associated cell death. Nonetheless, to account for slight reductions in viability after activation of CD4+ T cells of some viremic patients (data not shown), equal numbers of viable cells were used in each assay. As shown in Fig. 1C in comparison with Fig. 1A, a striking dichotomy was observed between the responses of HIV-negative versus HIV-infected viremic patients. Although the level of CD154 expression was comparable between HIV-infected and HIV-negative donors, the B cells of the HIV-infected patient showed a poor proliferative response compared with the B cells of the HIV-negative donor. When these analyses were extended to five pairs of HIV-negative and HIV-infected viremic patients examined in parallel, the same dichotomous pattern of comparable CD154 induction on activated CD4+ T cells (Table 1, group 1) and poor B cell proliferation in HIV-infected patients was observed (Fig. 2A). The B cells of HIV-negative donors proliferated a median of 4.1-fold (1.9, 10.2) higher than the B cells of the viremic patients. Given that under these culture conditions the source of IL-2 was endogenous, poor B cell proliferation also could have been caused by reduced levels of IL-2 secretion by the activated CD4+ T cells of the HIV-infected viremic patients. However, this was likely not the case, because similar, if not elevated, levels of IL-2 were measured in the culture supernatant of HIV-infected viremic patients compared with that of HIV-negative donors (Table 1).
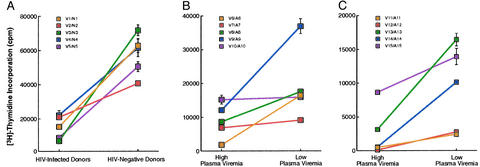
Figure 2.
Effect of plasma viremia in HIV infection on B cell proliferation in response to activated CD4+ T cells. CD4+ T cells isolated from patients described in Table 1 were stimulated for 40 h with anti-CD3/CD28 (A) or anti-CD3/CD28 + IL-2/IL-12 (B and C), irradiated, and cocultured with B cells in the presence of immobilized anti-CD3 (A) or IL-2 (B and C). Three sets of patients were compared: HIV-infected viremic versus HIV-negative (A), cross-sectional HIV-infected viremic versus HIV-infected aviremic with matched CD4+ T cell counts (B), and longitudinal HIV-infected viremic versus HIV-infected aviremic (C). Inset legends identify paired patients from Table 1, and, for sets A and B, assays on paired patients were performed in parallel on the same day. All culture conditions were performed in triplicate with standard deviation indicated by the error bars.
To address the effect of plasma viremia on B cell responsiveness to CD4+ T cell help, we studied HIV-infected patients at high and low plasma viremia in both cross-sectional and longitudinal settings (Table 1). To eliminate the potentially confounding variable of the presence of endogenous IL-2 in the B cell–CD4+ T cell cocultures (Table 1), IL-2 was added as an exogenous source of cytokine in lieu of restimulation with anti-CD3 mAb. As shown in Fig. 2B, B cells from 10 cross-sectional HIV-infected patients matched for CD4+ T cell counts were analyzed in pairs of parallel cocultures. The B cells of the aviremic patients proliferated a median of 2.0-fold (1.0, 8.7) higher than the B cells of the viremic patients. When the assay system was extended to longitudinal analyses where viremic patients were investigated before and after undergoing effective antiretroviral therapy, the differences in B cell proliferation were even more pronounced than in the cross-sectional analyses. As shown in Fig. 2C for five patients investigated at high and low plasma viremia, the B cells of patients at high plasma viremia proliferated a median of 5.2-fold (1.6, 22) lower than when the patients were at low plasma viremia. Of note, in contrast to the cross-sectional analyses, the reduction in plasma viremia in longitudinal analyses also was associated with an increase in CD4+ T cell count (Table 1). In addition, B cells showed enhanced proliferation when they were added to effector CD4+ T cells obtained at low, rather than high, plasma viremia (data not shown). Taken together, these data suggest that HIV replication, as manifested by high plasma viremia, has a detrimental effect on B cell responsiveness to CD4+ T cell help and that lower CD4+ T cell counts associated with high levels of viremia may further impair B cell–CD4+ T cell interactions.
Correlates of B Cell Responsiveness to CD4+ T Cell Help.
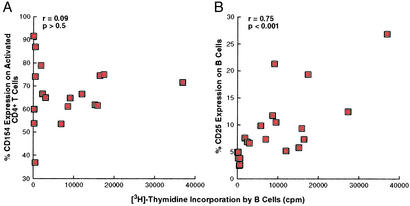
In comparing B cell responsiveness to CD4+ T cell help in HIV-infected viremic patients versus HIV-negative donors, we observed that B cells of HIV-infected viremic patients responded poorly to CD4+ T cell help despite apparently normal levels of CD154 on the surface of activated CD4+ T cells (Table 1). To further pursue this observation, we performed comparisons between proliferation of B cells and the level of CD154 expression on corresponding autologous, activated CD4+ T cells of all HIV-infected patients studied. As shown in Fig. 3A, no significant correlation was found between B cell proliferation and levels of CD154 on activated CD4+ T cells, confirming the preliminary observation that lower B cell proliferation in HIV-viremic patients was not associated with lower levels of CD154 on the stimulatory CD4+ T cells. In contrast, a strong correlation was found between B cell proliferation and the capacity of these cells to up-regulate CD25 (Fig. 3B), the inducible component of high-affinity IL-2 receptor complexes. These data suggest that the reduced levels of B cell proliferation observed in HIV-infected viremic patients were associated with a reduced capacity to up-regulate their high-affinity IL-2 receptor complexes in response to activation signals, including those provided by CD4+ T cell help.
Figure 3.
Comparisons between B cell proliferation and up-regulation of CD154 on activated CD4+ T cells and activation markers on B cells. B cell proliferation in response to anti-CD3/CD28 + IL-2/IL-12-stimulated CD4+ T cells and IL-2 was correlated with induction of CD154 on the effector CD4+ T cells (A) and induction of CD25 on the responding B cells (B). HIV-negative donors were excluded from A, and all data points represent individual patients.
B Cell Response to BCR–IL-2 Stimulation.
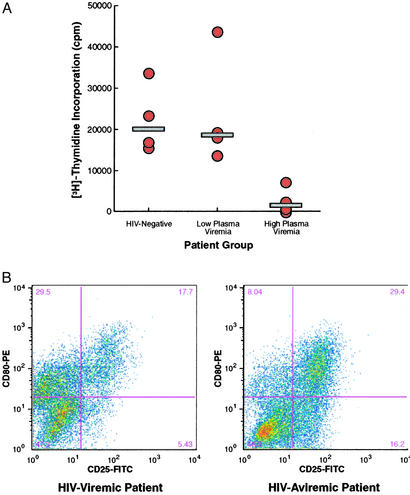
Having demonstrated that poor induction of CD25 on B cells of HIV-infected patients in response to CD4+ T cell help was contributing to the impaired proliferation of these B cells, we sought to determine whether low CD25 induction was unique to activation of B cells by CD154-expressing CD4+ T cells. Thus, we investigated the induction of CD25 and B cell proliferation in the context of BCR stimulation, i.e., in the absence of CD4+ T cell help. As shown in Fig. 4A, under these culture conditions the proliferation of B cells of HIV-infected viremic patients was reduced dramatically in comparison with B cells of HIV-uninfected and HIV-infected aviremic patients. This low level of B cell proliferation was accompanied by a low induction of CD25 in B cells of viremic versus aviremic HIV-infected patients (Fig. 4B). Thus, viremic HIV-infected individuals manifest a fundamental defect in their ability to mount a proliferative response to both CD4+ T cell-dependent and -independent activation signals, and the common denominator of this defect is an impaired ability to up-regulate CD25 expression, which is required for a normal response to IL-2.
Figure 4.
Response of B cells to BCR stimulation in the presence of IL-2. (A) B cells were isolated from four HIV-negative, four HIV-infected aviremic, and four HIV-infected viremic patients and stimulated with anti-IgM and IL-2. The shaded bars represent medians for each patient group. (B) Expression of CD25 and CD80 on activated B cells of representative HIV-infected viremic and aviremic patients.
Discussion
In the present study, we have demonstrated that active HIV replication, as manifested by plasma viremia, has a direct, detrimental effect on B cell responsiveness to CD4+ T cell help. B cell responsiveness to CD4+ T cell help depends on the expression of CD154 on CD4+ T cells, the availability of IL-2, and the expression of activation markers on responding B cells. In both cross-sectional and longitudinal analyses, poor responses of B cells to CD4+ T cell help were directly correlated with an inability of B cells to up-regulate CD25 despite the presence of normal expression of CD154 on the surface of CD4+ T cells. Similar defects in B cell proliferation in response to IL-2 were seen in viremic HIV-infected individuals even when cells were stimulated via the BCR, suggesting that a fundamental defect in the ability of B cells to up-regulate CD25 in response to normal immunologic stimulation is associated with HIV viremia.
We and others have shown that B cells of HIV-infected individuals respond poorly to surrogates of antigenic stimulation and that loss of B cell function is clearly related to high levels of plasma viremia (10, 15). However, this study establishes a more physiologic portrait of B cell dysfunction by investigating the response of B cells to activated CD4+ T cells in an autologous system. The importance of our findings is underscored by recent studies (25, 27) and our unpublished observations showing that soluble forms of CD154 or membrane forms expressed on CD154-transfected cells do not adequately substitute for CD154 expression on activated CD4+ T cells in the induction of IL-2-dependent B cell proliferation. These observations indicate that CD154 alone in the presence of IL-2 is not sufficient for the induction of B cell proliferation and that CD154 expression on the surface of activated CD4+ T cells is necessary for this IL-2–CD25 interaction.
The effect of HIV infection on the expression of CD154 on CD4+ T cells of HIV-infected patients has been the subject of conflicting reports, some showing decreased levels before or after in vitro stimulation (28–31), others showing either no effect or enhancing effects (20, 32, 33). Although many of these discrepancies may be related to differences in immunologic and virologic status of the patient (29), others may be related to the lability of CD154 expression after CD40 ligation (34) and differences between preformed or induced forms of the protein (21, 35). Most studies that showed reduced induction of CD154 on activated CD4+ T cells of HIV-infected patients used assay conditions that would be more consistent with preformed levels of CD154 (28, 30, 31), whereas our assay conditions would be more consistent with induced levels of CD154. Furthermore, cell membrane alterations induced by chronic activation in HIV (A.M. and S.M., unpublished observations) and other inflammatory diseases (21) potentially could have impacted CD154–CD40 interactions involved in B cell responses that we and others have reported. Hence, although our findings do not exclude defects in CD4+ T cell helper function, they suggest that the defect is not caused by inadequate induction of CD154.
Lack of B cell proliferation in response to CD4+ T cell help in HIV-infected patients was found to correlate with impaired induction of cell surface markers associated with B cell activation. Of particular relevance to the IL-2-dependent system described herein was the depressed induction of CD25, the component of the IL-2 receptor complex that is required for high-affinity IL-2 binding and signal transduction (36). The induction of CD25 on the surface of B cells of HIV-infected viremic patients was found to be defective in response to activated CD4+ T cells as well as in the setting of BCR stimulation (Fig. 4; A.M., unpublished observations), suggesting an intrinsic B cell defect in HIV-infected viremic patients. Reports on the effect of HIV infection on CD25 expression have been somewhat controversial, with one report showing enhanced levels of CD25 on B cells of HIV-infected patients (37) and other studies reporting either no or depressed effects (5, 38, 39). These latter findings, combined with our data, agree with several studies on unfractionated PBMCs and CD4+ and CD8+ T cells (40, 41), suggesting a generalized loss or inhibition of CD25 expression in HIV infection. Whether this possibility reflects particular pathways of HIV-induced immune activation that exclude CD25 or a particular defect in CD25 induction brought on by HIV viremia remains to be determined. In either case, defects in CD25 induction with consequential effects on IL-2-dependent responses may have implications in numerous facets of CD4+ T cell help to B cells. The importance of IL-2 in B cell responses is underscored by recent studies demonstrating that both Th1 and Th2 CD4+ T cells can be effective mediators of B cell help in vivo (24, 42), and that IL-2-producing uncommitted CD4+ T cells constitute a substantial fraction of antigen-primed cells with capacity to differentiate into either Th phenotype (43).
In summary, we demonstrate that defective B cell responsiveness to CD4+ T cell help in HIV-infected viremic patients is associated with impaired induction of B cell activation markers, most notably CD25. Defects in B cell responsiveness associated with IL-2–CD25 interactions may provide new insight into the inadequacy of antibody responses against HIV and associated opportunistic infections.
Acknowledgments
We thank Patricia Walsh for editorial assistance, the patients for their participation in this study, the National Institute of Allergy and Infectious Diseases study coordinators and case managers for recruiting and managing the patients, and Julie Metcalf for coordinating patient samples.
Abbreviations
- BCR
B cell receptor
- PBMC
peripheral blood mononuclear cell
- Th
T helper
References
- 1.Lane H C, Masur H, Edgar L C, Whalen G, Rook A H, Fauci A S. N Engl J Med. 1983;309:453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- 2.Morris L, Binley J M, Clas B A, Bonhoeffer S, Astill T P, Kost R, Hurley A, Cao Y, Markowitz M, Ho D D, Moore J P. J Exp Med. 1998;188:233–245. doi: 10.1084/jem.188.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shirai A, Cosentino M, Leitman-Klinman S F, Klinman D M. J Clin Invest. 1992;89:561–566. doi: 10.1172/JCI115621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pahwa R, Good R A, Pahwa S. Proc Natl Acad Sci USA. 1987;84:3826–3830. doi: 10.1073/pnas.84.11.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forster R, Schweigard G, Johann S, Emrich T, Kremmer E, Nerl C, Lipp M. Blood. 1997;90:520–525. [PubMed] [Google Scholar]
- 6.Horvath A, Banhegyi D, Biro A, Ujhelyi E, Veres A, Horvath L, Prohaszka Z, Bacsi A, Tarjan V, Romics L, et al. Immunobiology. 2001;203:756–768. doi: 10.1016/S0171-2985(01)80004-8. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Horowitz H W, Orlikowsky T, Hahn B I, Trejo V, Bapat A S, Mittler R S, Rayanade R J, Yang S Y, Hoffmann M K. J Infect Dis. 1999;180:1072–1079. doi: 10.1086/314974. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Maza O, Breen E C. Curr Opin Oncol. 2002;14:528–532. doi: 10.1097/00001622-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Steinhoff M C, Auerbach B S, Nelson K E, Vlahov D, Becker R L, Graham N M, Schwartz D H, Lucas A H, Chaisson R E. N Engl J Med. 1991;325:1837–1842. doi: 10.1056/NEJM199112263252603. [DOI] [PubMed] [Google Scholar]
- 10.Conge A M, Tarte K, Reynes J, Segondy M, Gerfaux J, Zembala M, Vendrell J P. AIDS. 1998;12:1437–1449. doi: 10.1097/00002030-199812000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Ballet J J, Sulcebe G, Couderc L J, Danon F, Rabian C, Lathrop M, Clauvel J P, Seligmann M. Clin Exp Immunol. 1987;68:479–487. [PMC free article] [PubMed] [Google Scholar]
- 12.Miedema F, Petit A J, Terpstra F G, Schattenkerk J K, de Wolf F, Al B J, Roos M, Lange J M, Danner S A, Goudsmit J, et al. J Clin Invest. 1988;82:1908–1914. doi: 10.1172/JCI113809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taoufik Y, Peguillet I, de Goer M G, Lambert M, Gubler B, Trylesinski A, Delfraissy J F, Lantz O. J Acquired Immune Defic Syndr. 2001;26:303–304. doi: 10.1097/00126334-200104010-00001. [DOI] [PubMed] [Google Scholar]
- 14.Notermans D W, de Jong J J, Goudsmit J, Bakker M, Roos M T, Nijholt L, Cremers J, Hellings J A, Danner S A, de Ronde A. AIDS Res Hum Retroviruses. 2001;17:1003–1008. doi: 10.1089/088922201300343681. [DOI] [PubMed] [Google Scholar]
- 15.Moir S, Malaspina A, Ogwaro K M, Donoghue E T, Hallahan C W, Ehler L A, Liu S, Adelsberger J, Lapointe R, Hwu P, et al. Proc Natl Acad Sci USA. 2001;98:10362–10367. doi: 10.1073/pnas.181347898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pantaleo G, Fauci A S. Annu Rev Immunol. 1995;13:487–512. doi: 10.1146/annurev.iy.13.040195.002415. [DOI] [PubMed] [Google Scholar]
- 17.Grossman Z, Feinberg M B, Paul W E. Proc Natl Acad Sci USA. 1998;95:6314–6319. doi: 10.1073/pnas.95.11.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hazenberg M D, Hamann D, Schuitemaker H, Miedema F. Nat Immunol. 2000;1:285–289. doi: 10.1038/79724. [DOI] [PubMed] [Google Scholar]
- 19.De Milito A, Morch C, Sonnerborg A, Chiodi F. AIDS. 2001;15:957–964. doi: 10.1097/00002030-200105250-00003. [DOI] [PubMed] [Google Scholar]
- 20.Muller F, Aukrust P, Nordoy I, Froland S S. Blood. 1998;92:3721–3729. [PubMed] [Google Scholar]
- 21.van Kooten C, Banchereau J. J Leukocyte Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 22.Grewal I S, Flavell R A. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 23.Paul W E, Seder R A. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 24.Smith K M, Pottage L, Thomas E R, Leishman A J, Doig T N, Xu D, Liew F Y, Garside P. J Immunol. 2000;165:3136–3144. doi: 10.4049/jimmunol.165.6.3136. [DOI] [PubMed] [Google Scholar]
- 25.Johnson-Leger C, Christenson J R, Holman M, Klaus G G. J Immunol. 1998;161:4618–4626. [PubMed] [Google Scholar]
- 26.Moir S, Malaspina A, Li Y, Chun T W, Lowe T, Adelsberger J, Baseler M, Ehler L A, Liu S, Davey R T, et al. J Exp Med. 2000;192:637–646. doi: 10.1084/jem.192.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blanchard D, Gaillard C, Hermann P, Banchereau J. Eur J Immunol. 1994;24:330–335. doi: 10.1002/eji.1830240209. [DOI] [PubMed] [Google Scholar]
- 28.Poudrier J, Weng X, Kay D G, Pare G, Calvo E L, Hanna Z, Kosco-Vilbois M H, Jolicoeur P. Immunity. 2001;15:173–185. doi: 10.1016/s1074-7613(01)00177-7. [DOI] [PubMed] [Google Scholar]
- 29.Vanham G, Penne L, Devalck J, Kestens L, Colebunders R, Bosmans E, Thielemans K, Ceuppens J L. Clin Exp Immunol. 1999;117:335–342. doi: 10.1046/j.1365-2249.1999.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Gorman M R, DuChateau B, Paniagua M, Hunt J, Bensen N, Yogev R. Clin Diagn Lab Immunol. 2001;8:1104–1109. doi: 10.1128/CDLI.8.6.1104-1109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolthers K C, Otto S A, Lens S M, Van Lier R A, Miedema F, Meyaard L. AIDS Res Hum Retroviruses. 1997;13:1023–1029. doi: 10.1089/aid.1997.13.1023. [DOI] [PubMed] [Google Scholar]
- 32.Brugnoni D, Prati E, Airo P, Castelli F, Cattaneo R. Clin Immunol Immunopathol. 1995;74:112–114. doi: 10.1006/clin.1995.1016. [DOI] [PubMed] [Google Scholar]
- 33.Sousa A E, Chaves A F, Doroana M, Antunes F, Victorino R M. Clin Exp Immunol. 1999;116:307–315. doi: 10.1046/j.1365-2249.1999.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyashita T, McIlraith M J, Grammer A C, Miura Y, Attrep J F, Shimaoka Y, Lipsky P E. J Immunol. 1997;158:4620–4633. [PubMed] [Google Scholar]
- 35.McDyer J F, Li Z, John S, Yu X, Wu C Y, Ragheb J A. J Immunol. 2002;169:2736–2746. doi: 10.4049/jimmunol.169.5.2736. [DOI] [PubMed] [Google Scholar]
- 36.Gaffen S L. Cytokine. 2001;14:63–77. doi: 10.1006/cyto.2001.0862. [DOI] [PubMed] [Google Scholar]
- 37.David D, Bani L, Moreau J L, Treilhou M P, Nakarai T, Joussemet M, Ritz J, Dupont B, Pialoux G, Theze J. Proc Natl Acad Sci USA. 1998;95:11348–11353. doi: 10.1073/pnas.95.19.11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plaeger S, Bass H Z, Nishanian P, Thomas J, Aziz N, Detels R, King J, Cumberland W, Kemeny M, Fahey J L. Clin Immunol. 1999;90:238–246. doi: 10.1006/clim.1998.4646. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez C, Thomas J K, O'Rourke S, Stiehm E R, Plaeger S. Clin Immunol Immunopathol. 1996;81:191–199. doi: 10.1006/clin.1996.0176. [DOI] [PubMed] [Google Scholar]
- 40.Johnson N, Parkin J M. Cytometry. 1997;30:289–295. [PubMed] [Google Scholar]
- 41.Zola H, Koh L Y, Mantzioris B X, Rhodes D. Clin Immunol Immunopathol. 1991;59:16–25. doi: 10.1016/0090-1229(91)90078-o. [DOI] [PubMed] [Google Scholar]
- 42.Toellner K M, Luther S A, Sze D M, Choy R K, Taylor D R, MacLennan I C, Acha-Orbea H. J Exp Med. 1998;187:1193–1204. doi: 10.1084/jem.187.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Mosmann T. J Exp Med. 2001;194:1069–1080. doi: 10.1084/jem.194.8.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]