Abstract
In previous studies, we have shown that steroid hormone resistance in New World primates occurs in the absence of abnormal expression of cognate nuclear receptors. Rather, these animals have elevated levels of heterogeneous nuclear ribonucleoproteins (hnRNPs) that act as hormone response element-binding proteins and attenuate target gene transactivation. Here we present evidence for a similar mechanism in humans via a patient with resistance to the active form of vitamin D [1,25-dihydroxyvitamin D3 (1,25(OH)2D3)] who presented with normal vitamin D receptor (VDR) expression. Initial cotransfection studies showed that the cells of the patient suppressed basal and hormone-induced transactivation by wild-type VDR. Electrophoretic mobility-shift assays and Western/Southwestern blot analyses indicated that this suppressive effect was due to overexpression of a nuclear protein that specifically interacts with a DNA response element known to bind retinoid X receptor–VDR heterodimers. Ab blocking in electrophoretic mobility-shift assays indicated that this dominant-negative acting protein was in the hnRNPA family of nucleic acid-binding proteins. Further studies have shown that several members of this family, most notably hnRNPA1, were able to suppress basal and 1,25(OH)2D3-induced luciferase activity. We therefore propose that this case of vitamin D resistance in a human subject is similar to that previously described for New World primates in which abnormal expression of a hormone response element-binding protein can cause target cell resistance to 1,25(OH)2D3. That this protein is a member of the hnRNP family capable of interacting with double-stranded DNA highlights a potentially important new component of the complex machinery required for steroid hormone signal transduction.
Analysis of inherited forms of steroid hormone resistance has greatly helped to clarify the underlying mechanisms by which molecules such as cortisol, testosterone, estradiol, and vitamin D achieve their actions (1, 2). In almost all cases, end organ resistance has been attributed to inactivating mutations of genes for intracellular steroid hormone receptors, despite the fact that a range of accessory proteins is required for steroid hormone signal transduction (3–5). However, recent reports have described novel forms of hormone resistance that have presented in the absence of a specific receptor gene mutation. These include an androgen receptor (AR)-positive patient with androgen insensitivity syndrome whose hormone resistance seems to be due to abnormal expression of a coactivator protein that interacts with the AF-1 domain of the AR (6). Accessory protein abnormality has also been proposed as the molecular defect in female siblings who presented with partial resistance to multiple steroid hormones (7). In view of these observations, it is possible to speculate that other forms of hormone resistance may also be due to abnormal accessory protein expression and function (8, 9).
We have shown that another class of proteins possibly acts as dominant-negative modulators of steroid hormone-mediated transcription. Heterogeneous nuclear ribonucleoproteins (hnRNPs) are a family of >20 proteins that contributes to the complex around nascent pre-mRNA and are thus able to modulate RNA processing (10–12). The ability of hnRNPs to attenuate steroid hormone receptor gene transactivation has been demonstrated in studies of New World primates (NWPs) that are characterized by their relative target organ resistance to adrenal, gonadal, and vitamin D sterol/steroid hormones (13–20). Data indicate that NWPs exhibit forms of receptor-normal resistance to vitamin D and estrogen that are due to overexpression of hnRNP-like dominant-negative-acting hormone response element (RE)-binding proteins (REBiPs; refs. 21–23). The 42-kDa NWP estrogen REBiP is highly homologous to the hnRNPC subfamily (21, 22). In addition we have purified two vitamin D RE (VDRE)-binding proteins (BPs) that show strong sequence homology to members of the human hnRNPA family (23). In both cases, overexpression of the REBiP was able to squelch hormone-activated transcription via the appropriate RE. Therefore, we postulated that a similar molecular lesion could account for a receptor-normal vitamin D resistance in human as well as in nonhuman primates.
To address this hypothesis, we have characterized the molecular basis for end organ resistance in a patient who presented with skeletal abnormalities and biochemical features classically associated with vitamin D-resistant rickets. These included hypocalcemia [2.03 mmol/liter corrected for albumin (normal range, 2.25–2.55 mmol/liter)]; raised serum alkaline phosphatase [1101 units/liter (normal range, <300 units/liter)]; and raised circulating levels of 1,25(OH)2D3 [466–650 pmol/liter (normal range, 48–156 pmol/liter)] (24). Despite these abnormalities, the patient had normal vitamin D receptor (VDR) expression. Sequence analyses indicated that the coding regions (24) as well as the 5′ and 3′ UTRs (M.H. and L. Crofts, unpublished observations) of the VDR gene were normal. Furthermore, when extracted from cells, VDRs from the patient displayed normal binding capacity and affinity for the active form of vitamin D, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3). However, in intact cell assays the VDR of the subject was incapable of stable nuclear localization even after exposure to 1,25(OH)2D3 (24). The patient was without evidence of any other form of steroid-thyroid-retinoid hormone resistance, and correction of rachitic bone disease was accomplished with high-dose 1,25(OH)2D3 treatment (12 μg/day) and calcium supplements (1 g/day), although the alopecia persisted. Here we show that the underlying cause of insensitivity to 1,25(OH)2D3 in this patient is overexpression of a VDRE-interacting REBiP with similarity to the hnRNPA family. This study represents evidence for a human form of receptor-normal hormone resistance similar to that observed in NWPs.
Experimental Procedures
Cell Culture.
An Epstein–Barr virus-transformed B-lymphoblast cell line and primary dermal fibroblast cultures were established from a patient with classical symptoms of type II hereditary vitamin D-resistant rickets (HVDRRII) (24). Cultures of cells from an unrelated female of the same age with normal skeletal development were used for comparison. Both sets of cell lines were established when the subjects were 6 years old. B cells were routinely maintained in RPMI medium 1640 supplemented with 5% FCS (Omega, Tarzana, CA). Fibroblasts were maintained in DMEM (GIBCO/BRL) supplemented with 10% FCS. For transient transfection assays, fibroblast cultures were maintained in phenol red-free DMEM (GIBCO/BRL) supplemented with 10% charcoal-stripped FCS (Omega).
Analysis of Lymphocyte Proliferation.
B cell responses to 1,25(OH)2D3 were assessed by using aliquots of subconfluent cells (100 μl) plated in flat-bottomed 96-well plates. Cells were treated with 10 nM phorbol 12-myristate 13-acetate (PMA; Sigma) to stimulate clonal expansion and coincubated with 1,25(OH)2D3 (1–100 nM) for 72 h. Cell proliferation was then assessed by the addition of the thymidine analog [125I]iododeoxyuridine ([125I]dUR; Amersham Pharmacia, Little Chalfont, U.K.) for the last 6 h of each incubation. Nuclear incorporation of 125I-dUR was then measured by using a Titretek cell harvester followed by radioactive counting.
Transient Transfection of Promoter–Reporter Constructs.
Aliquots (5 × 105 cells) of patient or control fibroblasts were seeded into six-well plates and cultured as described in the previous section to 90% confluence. Transfections were performed in triplicate with aliquots of the following DNA preparations: 5.0 μg of VDRE-luciferase-reporter plasmid, with or without a VDR expression construct (0.5 μg of pRShVDR); and 5.0 μg of β-galactosidase plasmid with pGEM-3z vector DNA as carrier (Promega) to a final concentration of 20 μg of DNA per ml in LipoTAXI solution (Stratagene; ref. 23). Further cotransfection studies were carried out by using aliquots (5 μg) of expression constructs for various REBiPs: VDRE-BP1 and VDRE-BP2 (both NWPs) and hnRNPA1 and hnRNPA2 (both human). An equal volume of 20% FCS-supplemented, antibiotic-free medium was added to each well 5 h after transfection followed by the addition of 10 nM 1,25(OH)2D3. After an additional 48 h at 37°C, the cells were lysed, and luciferase and β-galactosidase activities were measured.
Electrophoretic Mobility-Shift Assays (EMSAs).
Preparation of nuclear extracts (NEs) was according to a modification (23) of the method of Zerivitz and Akusjarvi (25). In some experiments, extracts were digested with trypsin. Sequences of the various oligonucleotides used were as follows (consensus recognition sequences are underlined): direct repeat VDRE with three nucleotides between half-sites (VDRE-D3), 5′-CTAGTGCTCGGGTAG AGGTCACAGAGGTCACTCGACTCGT-3′; osteopontin VDRE (VDRE-OP), 5′-CTAGTGCTCGGGTAG-GGTTCACGAGGTTCACTCGACTCG-3; retinoid X RE (RXRE) with one nucleotide between half-sites, 5′-AGCTTCAGGTCAGAGGTCAGAGAGCT-3′; chicken ovalbumin upstream promoter transcription factor 1 (COUP-TF1-RE), 5′-TTCTATGGTGTCAAAGGTCAAAC-3′; estrogen RE (ERE), 5′-CTAGAAAGTCAGGTCACAGTGACCTGATCAAT-3′; and nuclear factor yin-yang-1 RE (YY1-RE), 5′-CGCTCCGCGGCCATCTTGGCGGGTGG T-3′. EMSA was performed as described (21–23).
Abs and Blotting Assays.
A panel of Abs was used in supershift EMSA and in Western blot analyses. The panel included anti-human VDR (N- and C-terminal), anti-human retinoid X receptor (RXR)α and RXRβ (all from Santa Cruz Biotechnology), and anti-human hnRNPA1/A2 (provided by G. Dreyfuss and W. Rigby, University of Pennsylvania, Philadelphia, and Dartmouth Medical School, Hanover, NH, respectively). Cell extracts from the patient with HVDRR and the control subject were subjected to electrophoresis on 10% SDS/PAGE and transferred to nitrocellulose membranes. Western and Southwestern analyses were performed as described (23).
Results
Suppression of RXR–VDR-Mediated Transactivation in HVDRR Cell Lines.
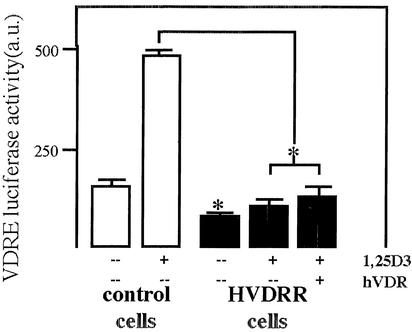
We have reported a patient with a classical HVDRRII phenotype including alopecia who presented with apparently normal VDR expression (24). Transfection of VDR cDNA from the patient into receptor-deficient (human) CV-1 cells resulted in normal transactivation in response to 1,25(OH)2D3 (24). These data suggested that an extra-VDR factor was interfering with the nuclear translocation and transactivation of endogenous VDR in cells from this patient. To investigate this further, promoter-reporter assays were carried out by using normal and HVDRR fibroblasts as transfection recipients (Fig. 1). Data confirmed the suppression of VDRE-mediated transactivation in HVDRR cells in the presence or absence of wild-type VDR. Furthermore, in contrast to control cells, HVDRR fibroblasts showed no induction of luciferase activity after treatment with 10 nM 1,25(OH)2D3.
Figure 1.
VDR-mediated transactivation is suppressed in cells from a patient with HVDRR. Primary fibroblast cultures from a patient with HVDRR and an age- and sex-matched control were transfected with a VDRE-DR3 luciferase promoter–reporter construct in the presence or absence of 1,25(OH)2D3 (D3) (10 nM) and/or cotransfected wild-type VDR cDNA. Data are shown as arbitrary luciferase units (a.u.) relative to internal standard, β-galactosidase. Values are the mean ± SD of three separate assays. *, statistically different from control, P < 0.01.
Vitamin D Resistance in HVDRR Cells Is Associated with Antagonism of VDR–RXRE–RE Complex Formation.
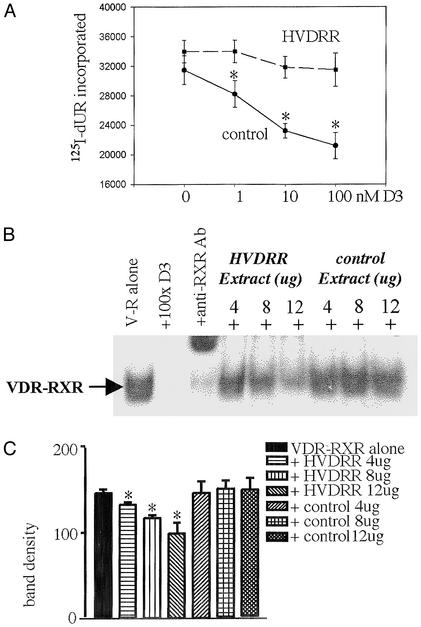
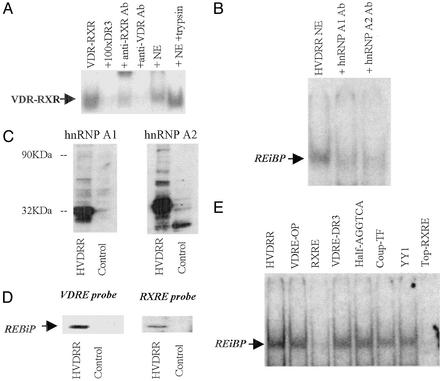
Based on data shown in Fig. 1, we hypothesized that cells from the patient with HVDRR constitutively overexpress a nuclear protein that competes with the VDR–RXR for binding to heterodimer cis recognition sequences. To test this hypothesis subsequent assays were carried out by using Epstein–Barr virus-transformed HVDRR and control B-lymphocytes, which could be cultured in large volumes to facilitate maximal protein recovery from NEs. We have reported that both fibroblasts and B-lymphocytes from the patient with HVDRR show abnormal nuclear localization of VDR when compared with an age- and sex-matched control (24). In further studies using only B-lymphocytes we were able to demonstrate the functional significance of these events by comparing the effects of 1,25(OH)2D3 on control and HVDRR cell proliferation. Results in Fig. 2A indicated that, in contrast to control lymphocytes, cells from the patient with HVDRR did not demonstrate any significant antiproliferative response to increasing doses of 1,25(OH)2D3 after mitogen (phorbol 12-myristate 13-acetate) activation. To investigate this observation further, EMSAs were carried out with NEs from control and HVDRR cells. Initial EMSA reactions were carried out by incubating recombinant human VDR and RXR with an idealized 3 nucleotide-spaced direct repeat VDRE (VDRE-DR3) probe in the presence or absence of increasing amounts of NE from control or HVDRR cells (Fig. 2B). Data showed that addition of the HVDRR NE competitively displaced RXR–VDR binding to the VDRE in a dose-dependent fashion. In contrast, NEs from control cells were without effect. This observation was confirmed by densitometric analysis of EMSA band density (Fig. 2C).
Figure 2.
Vitamin D resistance in HVDRR cells is associated with antagonism of normal VDR–RXR–VDRE nuclear complex formation. (A) Transformed B cells from the patient with HVDRR are resistant to 1,25(OH)2D3. Control and HVDRR lymphocytes were treated with the mitogen phorbol 12-myristate 13-acetate (PMA; 10 nM) in the presence or absence of increasing doses of 1,25(OH)2D3 (1–100 nM) for 72 h. Cell proliferation was then assessed by nuclear incorporation of [125I]iododeoxyuridine ([125I]dUR). *, statistically different from PMA-only controls cells in unpaired t tests, P < 0.01. (B) NEs from the HVDRR cells compete with VDR–RXR for association with a VDRE. Addition of increasing amounts of NEs from HVDRR cells (fourth through sixth lanes) but not the vitamin D-responsive control subject (seventh through ninth lanes) competed away recombinant human VDR–RXR binding to a labeled VDRE-DR3 double-stranded probe in EMSA (lane 1, V-R alone). Binding was also displaced by a 100-fold excess of unlabeled VDRE-DR3 (lane 2, +100 × DR3) and anti-RXR Ab (lane 3, +anti-RXR Ab). (C) Densitometric analysis of band density in replicate EMSAs (n = 3) representative assay shown in B (mean ± SD). Significant (P < 0.05) diminishment of probe-VDR–RXR complex formation with increasing doses of HVDRR NEs was achieved compared with equivalent control extracts.
Identification of an REBiP in NEs from HVDRR Cells.
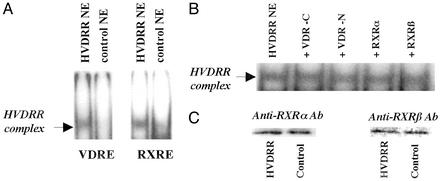
Further EMSAs were then carried out in the absence of exogenously added recombinant VDR and RXR. Data (Fig. 3) indicated that NEs from HVDRR cells contained a protein(s) that retarded the mobility of labeled VDRE-DR3 (cis sequence AGGTCAcag-AGGTCA; Fig. 3A Left). A similar observation was made when the highly homologous consensus RXRE was used as probe (cis sequence AGGTCAgAGGTCA; Fig. 3A Right). The presence of this RE complex was specific for the NE of the patient with HVDRR as equimolar concentrations of the control NE did not retard the VDRE or RXRE in this fashion. Likewise, the RXRE binding complex was not observed in other vitamin D-responsive cell lines such as Old World primate Rhesus monkey breast cells, Colobus lymphocytic cells, or MCF-7 human breast cancer cells (data not shown). Neither the mobility nor the intensity of the retarded HVDRR complex was altered by antihuman RXR or VDR Abs (Fig. 3B), and HVDRR cells showed apparently normal (i.e., wild-type) levels of RXRα and RXRβ expression (Fig. 3C). Furthermore, preincubation of NEs with 100 nM 1,25(OH)2D3, retinoic acid (RAR ligand), or 9-cis retinoic acid (RXR ligand) also had no effect on the mobility or intensity of the HVDRR complex (data not shown). These observations confirmed that neither RXR, VDR, nor their respective ligands participated in retardation of the RE probe by HVDRR NEs.
Figure 3.
NEs from HVDRR cells harbor a non-VDR, non-RXR-related REBiP. (A) EMSA analysis with consensus VDRE-DR3 and a consensus RXRE as probe incubated with NEs from HVDRR and control B cells. (B) Lack of EMSA supershift response by the RXRE-bound HVDRR NE complex in the presence of anti-VDR-C-terminal Ab (+VDR-C), anti-VDR-N-terminal Ab (+VDR-N), anti-RXRα Ab (+RXRα), and anti-RXRβ Ab (+RXRβ). (C) Western blot analyses showing normal expression of RXRα and RXRβ in NEs from control and HVDRR B cells.
Hormone RE Specificity of the HVDRR EMSA Complex.
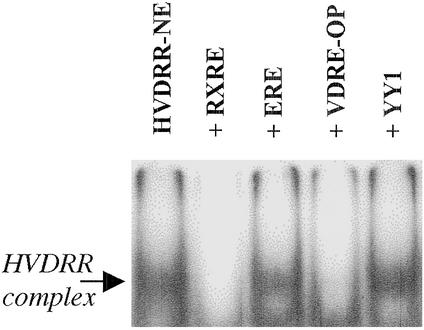
The cis element specificity of the HVDRR EMSA complex was assessed by competition analyses (Fig. 4). Data confirmed that RXRE and VDRE competed out the hormone RE-binding complex. An AGGTCA half-site corresponding to the chicken ovalbumin upstream promoter-transcription factor cis element also acted as a competitor (data not shown). However, consensus sequences for other types of cis elements such as the ERE showed no displacement of the RE binding complex (Fig. 4).
Figure 4.
EMSA analysis of RE sequence specificity for DNA complex formation by HVDRR extracts. NEs from HVDRR B cells (HVDRR-NE) were incubated with radiolabeled double-stranded RXRE probe in the absence (HVDRR-NE) or presence of an excess of unlabeled competitor probes. VDRE-OP, osteopontin VDRE; YY1, Ying Yang 1 transcription factor RE.
Characterization of the REBiP in HVDRR NEs.
Characterization of the RXRE/VDRE-binding protein was carried out by further EMSA analyses. The peptide nature of the complex component(s) was confirmed by using HVDRR NEs that had been digested by trypsin. Unlike untreated HVDRR extracts, these preparations were unable to compete out the EMSA complex formed by recombinant VDR–RXR (Fig. 5A). In studies of steroid hormone resistance in NWPs, we have demonstrated the presence of hnRNPs that are capable of squelching VDRE-mediated transactivation by acting as REBiPs (23). We therefore reasoned that a similar protein could also be associated with aberrant VDRE function in the HVDRR cells. Data (Fig. 5B) indicated that VDRE probe binding by HVDRR NEs was competed away by the addition of anti-hnRNPA1 and anti-hnRNPA2 Ab. These data suggested that, in a fashion similar to that in NWP cells, HVDRR cells expressed an REBiP capable of interacting with the VDRE.
Figure 5.
The REBiP present in HVDRR cells is a member of the hnRNP family. (A) The ability of HVDRR NE to compete out VDR–RXR complex formation with a VDRE-DR3 probe is abrogated by enzyme digestion with trypsin. EMSA lanes are as follows: 1, VDR–RXR alone; 2, VDR–RXR with 100-fold excess unlabeled probe; 3, VDR–RXR with anti-RXR Ab; 4, VDR–RXR with anti-VDR Ab; 5, VDR–RXR with untreated HVDRR NE; and 6, VDR–RXR with HVDRR NE pretreated with 0.5 μg/μl trypsin for 5 min followed by inactivation of trypsin with BSA 14 mg/ml. (B) HVDRR NE complex formation with VDRE-DR3 is blocked by anti-hnRNPA1 and anti-hnRNPA2 Abs. (C) Western blot analysis of hnRNPA1 (Left) and hnRNPA2 (Right) in HVDRR and control lymphocytes. (D) Southwestern blot analysis of HVDRR lymphocytes identifies a protein that associates with RXRE and VDRE-DR3. NEs from HVDRR and control cells were separated by PAGE and probed with radiolabeled RXRE or VDRE-DR3. (E) REBiP can bind to single- and double-stranded DNA. In competition EMSAs, HVDRR NEs bound to a single-strand labeled probe corresponding to the upper strand of the RXRE and was competed out by an excess of unlabeled double-stranded DNA probes.
Characterization of the REBiP by Western blot analysis showed increased expression of hnRNPA1 and hnRNPA2 immunoreactive proteins in HVDRR cells (Fig. 5C). In subsequent Southwestern blots, NEs from HVDRR and control cells were subjected to SDS/PAGE, and the separated proteins were analyzed for their ability to bind radiolabeled VDRE probe (Fig. 5D Left) and RXRE probe (Fig. 5D Right). A VDRE-DR3 and RXRE-reactive protein was observed only in the NEs from the HVDRR cells. In view of the RNA-binding capacity of hnRNPs, further studies were carried out to assess REBiP binding to single-stranded nucleotide sequences. HVDRR NEs were incubated with a single-stranded radiolabeled DNA probe consisting of the upper strand of the RXRE. Data (Fig. 5E) indicated that the resulting REBiP complex could be competed out only by using an excess of unlabeled single- or double-stranded RXREs.
hnRNPs Suppress VDR-Mediated Transactivation.
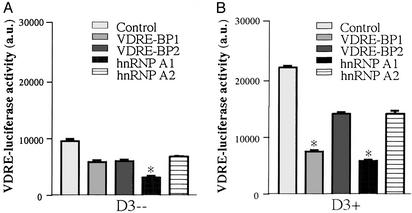
Data shown in Figs. 1–5 suggested that resistance to 1,25(OH)2D3 in the patient with HVDRR correlated with overexpression of an REBiP(s). To confirm the functional link between this and VDR-mediated transactivation, further reporter studies were carried out by using the VDR-positive human kidney cell-8 cell line cotransfected with expression constructs for human hnRNPA1 or hnRNPA2, and the NWP hnRNPs VDRE-BP1 and VDRE-BP2, which show strong homology to human hnRNPA1 and hnRNPA2, respectively (23). In the absence of exogenous 1,25(OH)2D3, all four REBiPs suppressed basal VDRE transcription, with hnRNPA1 showing the strongest response (Fig. 6A). The ability of REBiPs to squelch hormone-induced transcription was confirmed in cultures treated with 10 nM 1,25(OH)2D3. Both human and NWP REBiPs suppressed but did not completely block the stimulation of VDRE–luciferase activity, with VDRE-BP1 and hnRNPA1 having the greatest effect (Fig. 6B). This result contrasts studies using an ERE–reporter construct in which both human and NWP REBiP expression constructs were without effect (data not shown).
Figure 6.
hnRNPs antagonize VDRE-mediated transactivation. VDR-positive human kidney cells (HKC-8) were cotransfected with a VDRE-luciferase reporter plasmid together with expression constructs for hnRNPA1, hnRNPA2, VDRE-BP1 (NWP hnRNPA1), VDRE-BP2 (NWP hnRNPA2), or vector alone (control). Cells were cultured in the absence (A) or presence (B) of 10 nM 1,25(OH)2D3 for 24 h. Data are shown as arbitrary luciferase units (a.u.) relative to internal standard β-galactosidase measurements. Values are the mean ± SD of three separate assays. *, statistically different from equivalent control cells, P < 0.0001.
Discussion
Vitamin D-resistant rickets currently encompasses two distinct disorders, HVDRRI and HVDRRII. HVDRRI is a rare autosomal recessive disorder in which patients harbor an inactivating mutation of the gene encoding the enzyme 25-hydroxyvitamin-d-1α-hydroxylase (26, 27). Children with this disease present at birth with very low circulating levels of 1,25(OH)2D3, hypocalcemia, and an inability to normally calcify their developing skeleton. HVDRRII is another rare autosomal recessive disease but in this case the classical phenotype is elevated serum levels of 1,25(OH)2D3, chronic hypocalcemia, rickets, and sometimes alopecia (28–30). In common with other hormone-resistance disorders, HVDRRII is usually associated with abnormal expression and function of a cognate intracellular receptor, in this case the VDR (12–14).
In data presented here, we describe a form of human hormone insensitivity that is due to overexpression of a dominant-negative-acting REBiP. In this case the phenotype of vitamin D resistance could not be attributed to mutant forms or abnormal amounts of either the VDR or its principal binding partners, RXRα and RXRβ, and there was no clinical evidence for malfunction of other steroid hormone receptors. That REBiP is a DNA-binding protein and not a receptor-binding protein indicates that vitamin D resistance in this patient is not due to abnormal expression of a VDR accessory protein. Thus, the receptor-normal hormone resistance observed in this patient with HVDRRII is clearly different to that recently reported for a patient with androgen insensitivity syndrome (6). Nevertheless, the patient presented with classical symptoms of HVDRRII, including rickets and alopecia. We have therefore termed this HVDRRIII, with the patient representing the first reported human paradigm for the receptor-positive vitamin D resistance observed in NWPs. In the case of the patient with HVDRRIII and that of NWPs, the underlying cause of insensitivity to 1,25(OH)2D3 seems to be overexpression of an hnRNPA, with both the human (hnRNPA1 and hnRNPA2) and their NWP equivalents (VDRE-BP1 and VDRE-BP2) able to squelch VDRE-mediated transactivation.
The hnRNP family is a collection of more than 20 proteins that contribute to the complex around nascent pre-mRNA and are thus able to modulate RNA processing (10–12). Members of the group are characterized by their ability to bind to RNA with limited specificity and they are among the most abundant of all of the nuclear proteins (11). Despite its function in RNA handling, the precise physiological role of hnRNPs has yet to be fully defined and may include transregulatory effects. Recent studies have shown that the hnRNPs D0B (31), E2BP (32), and K (33) are able to bind to double-stranded DNA motifs within the complement receptor 2, hepatitis B virus, and c-myc promoters, respectively. In contrast to the data from the HVDRR cells, D0B, E2BP, and K all seem to activate gene transcription, suggesting a distinct function for members of the hnRNPA subgroup. It is therefore interesting to note that recent studies have identified a specific 36-bp target sequence for hnRNPA1 in double-stranded DNA (34). This sequence shows no overlap with established RNA target sequences for hnRNPA1 but notably includes two adjacent cryptic RXRE half-sites (ggctggtcttgaactcctgA/GCTCAAA/GGTGAtcctcc). It is also possible that the dominant-negative actions of the REBiP described here are not solely related to its ability to interact with double-stranded DNA. That the REBiP can also bind to a direct repeat of the AGGTCA half-site motif in single-stranded DNA suggests that its ability to squelch transcription may persist after DNA strand separation (35). It is therefore intriguing to postulate that eventual dislodgment of hnRNPs during transcription will make these single-strand nucleic acid-binding proteins available for interaction with other nearby cis elements such as the 3′ end of recently transcribed mRNAs.
That the hypocalcemia and rachitic bone disease in this patient with HVDRR were responsive to high-dose 1,25(OH)2D3 treatment (24) indicated that vitamin D resistance was not absolute but instead was likely to be determined by the relative abundance of the hnRNPA-related REBiP and competent [i.e., 1,25(OH)2D3-liganded] RXR–VDR heterodimer present in the target cell. If the balance in cis element binding favored REBiP, either because of a relative abundance of this protein and/or relative lack of the competitive RXR–VDR, then one would predict a 1,25(OH)2D3-reversible vitamin D-resistant phenotype in vivo. In fact, this prediction was compatible with the response of the patient to treatment with high-dose 1,25(OH)2D3; hypocalcemia and rachitic bone disease were corrected as long as the serum 1,25(OH)2D3 level remained high (24). These data also suggest that it is the VDRE-DR3 cis element which legislates the antirachitic action of the hormone in bone. In contrast, failure of the alopecia of the patient to be improved with an increase in serum 1,25(OH)2D3 levels suggests that (i) the VDRE-DR3 does not participate in control of genes rendering the hairless phenotype; (ii) maternal levels of 1,25(OH)2D3 are not high enough for fetal hair development; and/or (iii) as suggested by previous data from patients with HVDRR and VDR-ablated animals, the role of 1,25(OH)2D3-VDR in hair follicle development involves a prenatal mechanism. That the REBiP complex could not be competed-out with an ERE or YY1-RE suggests that the hnRNPA family has greater specificity for direct repeat half-sites. This observation is supported further by the elucidation of a double-stranded DNA target sequence for hnRNPA1 that includes two cryptic direct repeat half-sites (35). Furthermore, studies from our own group have shown that the ERE-BP in NWP belongs to the distinct hnRNPC family, which is likely to have an entirely different target sequence on double-stranded DNA. Collectively, these observations provide some explanation for the highly specific phenotype observed with the patient with HVDRR. However, in future studies it will be interesting to determine whether related REBiPs are associated with other syndromes of human hormone-resistant disease.
The precise mechanism leading to overexpression of the REBiP in the patient with HVDRR remains unclear but presumably involves the inheritance of a mutant allele(s) leading to constitutive overexpression of an REBiP in fetal life. Relatively little is known about the regulation of hnRNPs in mammalian cells. However, hnRNPA1 levels are increased in myeloid progenitor cells expressing the p210 (BCR/ABL) oncoprotein, in mononuclear cells from patients with chronic myelogenous leukemia blast crisis (36), and in the early stages of lung cancer (37). In addition, hnRNPA2 is regulated by the tumor-suppressing von Hippel–Lindau protein, highlighting a potential link with both oncogenesis and oxygen sensing (38). These factors may not necessarily be implicated in pathophysiology of the patient with HVDRR described in this study, but aberrant expression of REBiPs may provide a potential mechanism for the hormone resistance that is frequently observed in tumor cells. This hypothesis is particularly relevant in the case of vitamin D where previous studies have highlighted resistance to the antiproliferative properties of 1,25(OH)2D3 and its synthetic analogs despite apparently normal expression of VDR (39, 40). Further analysis of the tissue and disease distribution of hnRNPs may help to provide a new insight to this problem.
Acknowledgments
We thank G. Dreyfuss and Dr. W. Rigby for provision of hnRNP Abs and L. Jameson and H. Kronenberg for helpful comments. This work was supported by National Institutes of Health Grant RO1AR37399 (to J.S.A.).
Abbreviations
- RE
response element
- VDRE
vitamin D RE
- VDRE-BP
VDRE-binding protein
- VDR
vitamin D receptor
- hnRNP
heterogeneous nuclear ribonucleoprotein
- EMSA
electrophoretic mobility-shift assay
- REBiP
RE-binding protein
- NWP
New World primate
- RXRE
retinoid X RE
- HVDRR
hereditary vitamin D-resistant rickets
- ERE
estrogen RE
- NE
nuclear extract
- RXR
retinoid X receptor
References
- 1.Chrousos G P, MacLusky N J, Brandon D D, Tomita M, Renquist D M, Loriaux D L, Lipsett M B. Adv Exp Med Biol. 1986;196:317–328. doi: 10.1007/978-1-4684-5101-6_21. [DOI] [PubMed] [Google Scholar]
- 2.Auchus R J, Fuqua S A. Semin Cell Biol. 1994;5:127–136. doi: 10.1006/scel.1994.1016. [DOI] [PubMed] [Google Scholar]
- 3.Ing N H, O'Malley B W. In: Molecular Endocrinology: Basic Concepts and Clinical Correlations. Weintraub B D, editor. New York: Raven; 1995. pp. 195–215. [Google Scholar]
- 4.Chen J D, Li H. Crit Rev Eukaryotic Gene Expression. 1998;8:169–190. doi: 10.1615/critreveukargeneexpr.v8.i2.40. [DOI] [PubMed] [Google Scholar]
- 5.Freedman L P. Cell. 1999;97:5–8. doi: 10.1016/s0092-8674(00)80708-4. [DOI] [PubMed] [Google Scholar]
- 6.Adachi M, Takayanagi R, Tomura A, Imasaki K, Kato S, Goto K, Ikuyama S, Nawata H. N Engl J Med. 2000;343:856–862. doi: 10.1056/NEJM200009213431205. [DOI] [PubMed] [Google Scholar]
- 7.New M I, Nimkarn S, Brandon D D, Cunningham-Rundles S, Wilson R C, Newfield R S, Vandermeulen J, Barron N, Russo C, Loriaux D L, O'Malley B W. J Clin Endocrinol Metab. 1999;84:4454–4466. doi: 10.1210/jcem.84.12.5786. [DOI] [PubMed] [Google Scholar]
- 8.Chrousos G P. J Clin Endocrinol Metab. 1999;84:4450–4453. doi: 10.1210/jcem.84.12.6073. [DOI] [PubMed] [Google Scholar]
- 9.Hughes I A. N Engl J Med. 2000;343:880–882. doi: 10.1056/NEJM200009213431210. [DOI] [PubMed] [Google Scholar]
- 10.Dreyfuss G, Henze M, Lamond A I. Cell. 1996;85:963–972. doi: 10.1016/s0092-8674(00)81298-2. [DOI] [PubMed] [Google Scholar]
- 11.Dreyfuss G, Matunis M J, Pinol-Roma S, Burd C G. Annu Rev Biochem. 1993;62:289–321. doi: 10.1146/annurev.bi.62.070193.001445. [DOI] [PubMed] [Google Scholar]
- 12.Pinol-Roma S, Dreyfuss G. Science. 1991;253:312–314. doi: 10.1126/science.1857966. [DOI] [PubMed] [Google Scholar]
- 13.Brown G M, Grota L J, Penney D P, Reichlin S. Endocrinology. 1970;86:519–529. doi: 10.1210/endo-86-3-519. [DOI] [PubMed] [Google Scholar]
- 14.Chrousos G P, Renquist D, Brandon D, Eil C, Pugeat M, Vigersky R, Cutler G B, Jr, Loriaux D L, Lipsett M B. Proc Natl Acad Sci USA. 1982;79:2036–2040. doi: 10.1073/pnas.79.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chrousos G P, Loriaux D L, Brandon D, Schull J, Renquist D, Hogan W, Tomita M. Endocrinology. 1984;115:25–32. doi: 10.1210/endo-115-1-25. [DOI] [PubMed] [Google Scholar]
- 16.Chrousos G P, Brandon D, Renquist D M, Hogan W, Tomita M, Johnson E D L, Lipsett M B. J Clin Endocrinol Metab. 1984;58:516–520. doi: 10.1210/jcem-58-3-516. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi N, Suda S, Shinki T, Horiuchi N, Shiina Y, Tanioka Y, Koizumi H, Suda T. Biochem J. 1985;227:555–563. doi: 10.1042/bj2270555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams J S, Gacad M A, Baker A J, Gonzales B, Rude R K. Am J Primatol. 1985;9:219–224. doi: 10.1002/ajp.1350090307. [DOI] [PubMed] [Google Scholar]
- 19.Gacad M A, Deseran M W, Adams J S. Am J Primatol. 1992;28:263–270. doi: 10.1002/ajp.1350280404. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds P D, Ruan Y, Smith D F, Scammell J G. J Clin Endocrinol Metab. 1999;84:663–669. doi: 10.1210/jcem.84.2.5429. [DOI] [PubMed] [Google Scholar]
- 21.Chen H, Arbelle J E, Gacad M A, Allegretto E A, Adams J S. J Clin Invest. 1997;99:669–675. doi: 10.1172/JCI119210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H, Hu B, Gacad M A, Adams J S. J Biol Chem. 1998;273:31352–31357. doi: 10.1074/jbc.273.47.31352. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Hu B, Allegretto E A, Adams J S. J Biol Chem. 2000;275:35557–35564. doi: 10.1074/jbc.M007117200. [DOI] [PubMed] [Google Scholar]
- 24.Hewison M, Rut A R, Kristjansson K, Walker R E, Dillon M J, Hughes M R, O'Riordan J L H. Clin Endocrinol. 1993;39:663–670. doi: 10.1111/j.1365-2265.1993.tb02424.x. [DOI] [PubMed] [Google Scholar]
- 25.Zerivitz K, Akusjarvi G. Gene Anal Tech. 1989;6:101–109. doi: 10.1016/0735-0651(89)90016-2. [DOI] [PubMed] [Google Scholar]
- 26.Fu G K, Lin D, Zhang M Y, Bikle D D, Shackleton C H, Miller W L, Portale A A. Mol Endocrinol. 1997;11:1961–1970. doi: 10.1210/mend.11.13.0035. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Zhang M Y, Miller W L, Portale A A. J Clin Endocrinol Metab. 2002;87:2424–2430. doi: 10.1210/jcem.87.6.8534. [DOI] [PubMed] [Google Scholar]
- 28.Malloy P J, Pike J W, Feldman D. Endocr Rev. 1999;20:156–188. doi: 10.1210/edrv.20.2.0359. [DOI] [PubMed] [Google Scholar]
- 29.Malloy P J, Pike J K, Feldman D. In: Vitamin D. Feldman D, Glorieux F H, Pike J K, editors. San Diego: Academic; 1997. pp. 765–787. [Google Scholar]
- 30.Haussler M R, Haussler C A, Jurutka P W, Thompson P D, Hsieh J C, Remus L S, Selznick S H, Whitfield J K. J Endocrinol. 1997;154:S57–S73. [PubMed] [Google Scholar]
- 31.Tolnay M, Vereshchagina L A, Tsokos G C. Biochem J. 1999;338:417–425. [PMC free article] [PubMed] [Google Scholar]
- 32.Tay N, Chan S H, Ren E C. J Virol. 1992;66:6841–6848. doi: 10.1128/jvi.66.12.6841-6848.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomonaga T, Levens D. J Biol Chem. 1995;270:4875–4881. doi: 10.1074/jbc.270.9.4875. [DOI] [PubMed] [Google Scholar]
- 34.Gnatt A L, Cramer P, Fu J, Bushnell D A, Kornberg R D. Science. 2001;292:1876–1882. doi: 10.1126/science.1059495. [DOI] [PubMed] [Google Scholar]
- 35.Donev R M, Doneva T A, Bowen W R, Sheer D. Mol Cell Biochem. 2002;233:181–185. doi: 10.1023/a:1015504318726. [DOI] [PubMed] [Google Scholar]
- 36.Iervolino A, Santilli G, Trotta R, Guerzoni C, Cesi V, Bergamaschi A, Gambacorti-Passerini C, Calabretta B, Perrotti D. Mol Cell Biol. 2002;22:2255–2266. doi: 10.1128/MCB.22.7.2255-2266.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan-Sanders Y, Hammons G J, Lyn-Cook B D. Cancer Lett. 2002;183:215–220. doi: 10.1016/s0304-3835(02)00168-4. [DOI] [PubMed] [Google Scholar]
- 38.Pioli P A, Rigby W F. J Biol Chem. 2001;276:40346–40352. doi: 10.1074/jbc.M105391200. [DOI] [PubMed] [Google Scholar]
- 39.Zhao X Y, Peehl D M, Navone N M, Feldman D. Endocrinology. 2000;141:2548–2556. doi: 10.1210/endo.141.7.7549. [DOI] [PubMed] [Google Scholar]
- 40.Zhuang S H, Schwartz G G, Cameron D, Burnstein K L. Mol Cell Endocrinol. 1997;126:83–90. doi: 10.1016/s0303-7207(96)03974-3. [DOI] [PubMed] [Google Scholar]