Abstract
Recent evidence suggests that intracellular Zn2+ accumulation contributes to the neuronal injury that occurs in epilepsy or ischemia in certain brain regions, including hippocampus, amygdala, and cortex. Although most attention has been given to the vesicular Zn2+ that is released into the synaptic space and may gain entry to postsynaptic neurons, recent studies have highlighted pools of intracellular Zn2+ that are mobilized in response to stimulation. One such Zn2+ pool is likely bound to cytosolic proteins, like metallothioneins. Applying imaging techniques to cultured cortical neurons, this study provides novel evidence for the presence of a mitochondrial pool distinct from the cytosolic protein or ligand-bound pool. These pools can be pharmacologically mobilized largely independently of each other, with Zn2+ release from one resulting in apparent net Zn2+ transfer to the other. Further studies found evidence for complex and potent effects of Zn2+ on isolated brain mitochondria. Submicromolar levels, comparable to those that might occur on strong mobilization of intracellular compartments, induced membrane depolarization (loss of Δψm), increases in currents across the mitochondrial inner membrane as detected by direct patch clamp recording of mitoplasts, increased O2 consumption and decreased reactive oxygen species (ROS) generation, whereas higher levels decreased O2 consumption and increased ROS generation. Finally, strong mobilization of protein-bound Zn2+ appeared to induce partial loss of Δψm, suggesting that movement of Zn2+ between cytosolic and mitochondrial pools might be of functional significance in intact neurons.
Zn2+ is present in all cells, but may have unique roles in the brain, where it is localized in presynaptic vesicles of many excitatory terminals, is released with synaptic activation, and appears to be a physiologically relevant modulator of excitatory neurotransmission (1). Under conditions of excessive presynaptic release, as occurs in epilepsy or ischemia, substantial synaptic Zn2+ levels may be achieved (1). Observations that Zn2+ accumulates in postsynaptic neurons and that extracellular Zn2+ chelators decrease both the Zn2+ accumulation and resultant neurodegeneration support the hypothesis that transsynaptic movement of Zn2+ (“Zn2+ translocation”) contributes to the neuronal injury observed in these conditions (1).
Recent observations suggest that translocation only explains a part of Zn2+ dynamics, with mobilization of cytosolic protein- or ligand-bound Zn2+ pools likely contributing critically to Zn2+ actions. Indeed, transgenic mice lacking the vesicular Zn2+ transporter, ZnT3, have no vesicular Zn2+ but still show cytosolic Zn2+ accumulation in pyramidal neurons after epilepsy (2), suggesting that strong synaptic activity can induce Zn2+ release from intracellular sites of sequestration in the absence of translocation. One likely source of such Zn2+ accumulation is Zn2+ binding proteins, like metallothioneins (MTs). These proteins are widely expressed, present at high levels in brain, and reversibly bind Zn2+ with high affinity and large capacity (seven or more Zn2+ ions per molecule) (3–6). Recent studies have indicated that Zn2+ is released from MTs in response to disulfide containing oxidants, such as oxidized glutathione (GSSG) or 2,2′-dithiodipyridine (DTDP), leading to the suggestion that these pools might be mobilized in response to changes in the redox potential of the cell (7, 8). Consistent with this idea, disulfide oxidants have been found to induce mobilization of intracellular Zn2+ in cultured neurons (9).
Mitochondria constitute another likely site of intracellular Zn2+ sequestration. In previous studies we have found that, on strong cytosolic loading, Zn2+ is taken up into these organelles, from which it can be subsequently released in a Ca2+ dependent fashion (10, 11). Zn2+ accumulation in mitochondria may not be benign, as Zn2+ has been found to interfere with the function of isolated mitochondria (12–19) and mitochondria are therefore likely to be sites at which this cation induces injurious effects on neurons (20, 21).
In this study we present novel evidence for the presence of Zn2+ pools in neuronal mitochondria under basal conditions. We further find that both mitochondrial and cytosolic Zn2+ pools can be mobilized largely independently of each other, with mobilization from either appearing to result in net Zn2+ transfer to the other. Additional studies demonstrate potent and complex effects of Zn2+ on isolated brain mitochondria, with submicromolar levels opening large conductance channels in the inner mitochondrial membrane, increasing O2 consumption and decreasing ROS generation. Finally, strong mobilization of presumptive cytosolic protein-bound Zn2+ in intact neurons appeared to induce partial loss of Δψm, suggesting possible functional significance of Zn2+ movement between cytosolic and mitochondrial pools.
Materials and Methods
Materials.
Dichlorodihydrofluorescein diacetate (H2DCFDA), FluoZin-3 AM, MitoTracker Green, and rhodamine 123 were purchased from Molecular Probes (Eugene, OR). RhodZin-3 AM was synthesized at Molecular Probes. MK-801 was purchased from Research Biochemicals (Natick, MA). d-(-)-2-amino-5-phosphonopentanoic acid (d-AP5) was purchased from Tocris (Ballwin, MO). 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline (NBQX) was kindly provided by Novo Nordisk (Malov, Denmark). 2,2′-dithiodipyridine (DTDP), cyclosporin A (CSA), carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone (FCCP), and N,N,N′,N′-tetrakis-(2-pyridylmethyl)ethylenediamine (TPEN) were obtained from Sigma. Tissue culture media and serum were from Life Technologies. All other chemicals and reagents were obtained from common commercial sources.
Animal Cell Cultures.
All animal procedures were approved by the institutional animal care and use committee. Adult male Sprague–Dawley rats were used for isolated mitochondrial experiments. Murine cortical cultures were prepared from embryonic Swiss–Webster mice and neurons plated on astrocytic monolayers on polylysine + laminin coated coverslips as described (22).
Isolated Mitochondrial Experiments.
Isolated mitochondria were prepared largely as described (17, 23, 24). Mitoplast recording was carried out largely as originally described (25) with slight modifications. Mitochondria were placed in hypoosmotic media (30 mM KCl/20 mM Hepes, pH 7.2) for one half hour before recording. Patch clamp electrodes (40–80 MΩ) contained 150 mM KCl/20 mM Hepes, pH 7.2. Patch potential was maintained at voltages between −100 mV and +100 mV for periods of 10 s. Data were collected at 20 kHz and filtered at 500–1,000 Hz.
For other studies, mitochondria were suspended in respiration buffer (250 mM sucrose/20 mM Hepes/2 mM MgCl2/2.5 mM inorganic phosphates/0.1% BSA, pH 7.2). Mitochondria isolated under these conditions are well coupled with respiratory control ratios >5 (26). Δψm and oxygen consumption were measured by using electrodes sensitive to tetraphenylphosphonium cation (TPP+) and a miniature Clark-type electrode respectively, in a sealed, thermostated and continuously stirred chamber. Mitochondria were added to the chamber to yield a final protein concentration of 1 mg/ml. ROS production was measured by using the indicator H2DCFDA as described (24). Briefly, 200–250 μg of isolated mitochondrial protein was incubated in a total volume of 200 μl of respiration buffer at 37°C for 15 min in the presence of 10 μM H2DCFDA, which was made fresh before each use. The relative amounts of mitochondrial ROS and H2O2 produced were measured by using a fluorometric plate reader (excitation, 490 nm; emission, 526 nm).
Confocal Fluorescence Microscopy.
Mitochondrial studies used the newly synthesized mitochondrially sequestered dye, RhodZin-3, which had Kd for Zn2+ of ≈65 nM, but has no sensitivity to Ca2+ up to mM levels, has Fe3+ Kd of 20 μM, and is quenched by Fe2+. Cortical cultures were loaded with RhodZin-3 AM (10 μM + 0.1% pluronic acid) at 4°C for 30 min and then left at 37°C for 4 h for de-esterification. In some experiments, cultures were coloaded with MitoTracker Green FM (200 nM, 37°C, 30 min). Experiments were carried out by using a simplified Ca2+-free Hepes-buffered medium (HSS) whose composition was: 120 mM NaCl/5.4 mM KCl/0.8 mM MgCl2/20 mM Hepes/15 mM glucose/10 mM NaOH, pH 7.4. Series of confocal images (seven planes each 2 μm deep) were obtained by using either an Olympus fluoview (Olympus USA, Melville, NY), or a Bio-Rad MRC 600 (Bio-Rad) confocal system equipped with ×60 and ×40 objectives, respectively, and argon (Ex: 488 nm; Em: >510 nm, for MitoTracker Green) and krypton (Ex: 568 nm; Em: >648 nm, for RhodZin-3) lasers.
Non-Confocal Microfluorimetric Studies.
Fluorescence imaging was carried out by using an inverted microscope with a xenon lamp, filter wheel, a ×40, epifluorescence oil immersion objective, and a green fluorescence cube (Ex: 490 nm, dichroic: 510 nm, Em: 530 nm). Images were acquired with a 12-bit digital charge-coupled device camera, and analyzed (after background subtraction from a cell-free region of the dish) with METAFLUOR 4.0 software (Universal Imaging, West Chester, PA). The Zn2+ selective probe, FluoZin-3 AM, was loaded (5 μM + 0.2% pluronic acid) at room temp for 30 min, washed into HSS (as above but with 1.8 mM Ca2+) and kept in the dark for additional 30 min. The FCCP and/or DTDP exposures were carried out in the additional presence of glutamate antagonists to prevent possible effects caused by endogenous glutamate release. [Zn2+]i was determined as Kd [(F − Fmin)/(Fmax − F)] by using a Kd of 15 nM. Fmax was obtained at the end of each experiment by adding the Zn2+ selective ionophore Na+-pyrithione (50 μM) in the presence of 50 μM Zn2+ (the fluorescence rapidly approached a maximum), and Fmin was obtained by adding the cell permeable Zn2+ chelator TPEN (20 μM) (21). The ΔΨm-sensitive probe, rhodamine 123, was loaded (2 μg/ml) in bicarbonate containing buffer (25 min, 37°C/5% CO2). To compensate for cell-to-cell variability in dye loading, rhodamine 123 fluorescence measurements for each cell (Fx) were normalized to the fluorescence intensity for that cell at the beginning of the experiment (F0).
Results
Two Intraneuronal Pools from Which Zn2+ Can Be Mobilized.
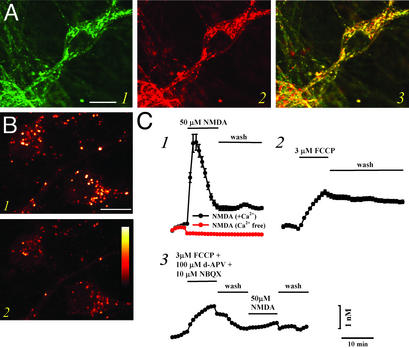
We have made use of newly available high-affinity Zn2+ selective fluorescent probes to characterize changes in cytosolic Zn2+ resulting from mobilization of either mitochondrial or cytosolic pools. Initial studies used the new Zn2+ selective probe, RhodZin-3 AM (an analog of the mitochondrial Ca2+ probe, rhod2; ref. 27), which is positively charged and follows the electrical gradient to accumulate in mitochondria, where it gets retained on de-esterification (Kd ≈ 65 nM). When coloaded with the mitochondrial marker, MitoTracker Green, a high degree of colocalization was evident, with both showing the distinct speckled pattern of fluorescence, most prominent in the perinuclear region, characteristic of mitochondria (Fig. 1A). These punctate regions of strong fluorescence were attenuated by addition of the membrane permeable Zn2+ selective chelator, TPEN (Fig. 1B; 23.3 ± 0.9% decrease; 180 neurons, 11 experiments), indicating that mitochondrial Zn2+ contributes to the RhodZin-3 signal. Conversely, consistent with our prior observations of mitochondrial uptake of cytosolic Zn2+ loads (10, 11), induction of Zn2+ entry into the neurons (by exposure to 50 μM Zn2+ in 60 mM K+ buffer, to cause neuronal depolarization and Zn2+ entry through voltage-sensitive Ca2+ channels), caused an increase in the mitochondrial signal (data not shown).
Figure 1.
Mobilization of Zn2+ from mitochondria. (A) Colocalization of RhodZin-3 and MitoTracker Green. Cultures were coloaded with the new Zn2+-sensitive mitochondrial probe RhodZin-3 (red fluorescence) and the mitochondrial marker, MitoTracker Green (green fluorescence), and imaged with confocal microscopy. Note the substantial overlap between these probes (yellow), indicating that they largely target the same intracellular organelles. Image is representative of 16 neurons from six experiments. (Bar = 10 μm.) (B) Mitochondria contain chelatable Zn2+. Cultures were loaded with RhodZin-3 and imaged under confocal microscopy before (1) and after (2) application of the cell-permeable Zn2+ chelator TPEN (20 μM). Note the decrement in speckled (mitochondrial) signal by TPEN, suggesting the presence of an intramitochondrial pool of chelatable Zn2+. Images are representative of 180 neurons from 11 experiments. (Bar = 10 μm; the pseudocolor bar shows the 12-bit fluorescence intensity range.) (C) Ca2+-dependent release of intramitochondrial Zn2+. FluoZin-3 loaded cultures were imaged before, during, and after 10-min exposures to NMDA (50 μM; 1), in a Ca2+-containing (black) or nominally Ca2+-free (red) buffer, to the mitochondrial protonophore, FCCP (3 μM; 2), or to FCCP followed by NMDA (3). Note that NMDA-induced Zn2+ rises depend on Ca2+ influx, and that prior exposure to FCCP occludes subsequent NMDA-triggered [Zn2+]i rises, indicating that these two manipulations target a common intracellular Zn2+ pool. Traces show time course of [Zn2+]i rises (±SEM) and are derived from 31–113 neurons from one experiment representative of 3–10.
In previous studies on neurons that had been subjected to cytosolic Zn2+ loading (as above, by depolarizing neurons in the presence of extracellular Zn2+), subsequent induction of mitochondrial depolarization (by addition of the mitochondrial protonophore, FCCP), or of rapid Ca2+ entry [by exposure to the glutamate receptor agonist, N-methyl-d-aspartate (NMDA)] induced release of mitochondrial Zn2+ to the cytosol (11). However, those studies used a relatively low-affinity Zn2+ probe, Newport Green, which was unable to detect endogenous Zn2+ in the absence of prior loading. In the present study, to examine the possibility that these stimuli can induce release of Zn2+ present in mitochondria under basal conditions, we have used a new high-affinity cytosolic Zn2+ probe, FluoZin-3 AM (Kd ≈15 nM; ref. 28). In cultures loaded with this dye, exposure to either FCCP (3 μM) or to NMDA (50 μM) caused a small (≈1–3 nM) increase in cytosolic free Zn2+ concentration ([Zn2+]i) (Fig. 1C). [Zn2+]i rises in response to FCCP occluded subsequent responses to NMDA (Fig. 1C), suggesting a common source of the Zn2+.
As discussed above, there is considerable evidence for the presence of substantial pools of protein-bound Zn2+ in neurons. To determine the size of these pools, FluoZin-3 loaded cultures were exposed to the disulfide oxidizing agent DTDP, which induces slow release of Zn2+ from MTs (29). DTDP exposure, when given alone caused a variable, but generally small [Zn2+]i rise (≈3 nM). However, combined exposure to DTDP and FCCP induced a [Zn2+]i rise that was considerably greater than that induced by either DTDP or FCCP alone, suggesting that these agents mobilize Zn2+ from distinct pools. Similarly, during a DTDP exposure, addition of NMDA resulted in a substantial further [Zn2+]i rise (Table 1).
Table 1.
Compilation of peak [Zn2+]i rises observed in FluoZin-3 loaded cultures within 10 min after onset of indicated exposure
| Conditions | Peaks [Zn2+]i | SEM | n of expts | n of cells |
|---|---|---|---|---|
| 50 μM NMDA, 0 Ca2+ | −0.32 | 0.01 | 4 | 422 |
| 50 μM NMDA | 3.09 | 0.13 | 8 | 549 |
| 3 μM FCCP | 1.02 | 0.02 | 10 | 579 |
| 3 μM FCCP, pH 6.0 | 38.32 | 2.69 | 5 | 286 |
| 100 μM DTDP | 2.96 | 0.12 | 16 | 1122 |
| 100 μM DTDP, pH 6.0 | 12.09 | 0.75 | 14 | 985 |
| DTDP + FCCP | 20.68 | 1.44 | 7 | 500 |
| DTDP + NMDA | 10.39 | 0.41 | 12 | 812 |
| pH 6.0 | 0.62 | 0.02 | 16 | 1277 |
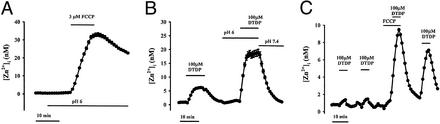
Prior studies had found that Zn2+ binding to MTs is decreased at acid pH (30). Thus, additional experiments used pH manipulations to examine the possible role of protein binding in maintenance of [Zn2+]i homeostasis. Lowering extracellular pH to 6, a maneuver that induces rapid equilibration between intracellular and extracellular pH (31) [which we have verified by using the pH-sensitive probe, 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF), to induce rapid intracellular acidification in our cultures; data not shown], caused a small and variable [Zn2+]i rise (0.6 ± 0.02 nM, n = 16). However, in marked contrast to observations at physiological pH, exposure to either FCCP or to DTDP at pH 6 resulted in far greater [Zn2+]i rises (>10 nM), suggesting that protein binding plays an important role in buffering [Zn2+]i rises (Fig. 2 A and B; Table 1). These findings are not because of direct pH effects on FluoZin-3, as the behavior of this probe is unaffected by changes in pH over a range from 6.0 to 9.0 (28).
Figure 2.
Mobilization of mitochondrial and protein-bound Zn2+ pools. (A and B) Acidosis greatly enhances intracellular Zn2+ mobilization. (A) FluoZin-3-loaded cultures were exposed to FCCP (3 μM) after a 10-min preincubation in acidic (pH 6.0) buffer. Note that the [Zn2+]i rise is nearly an order of magnitude greater than that induced by an identical exposure at physiological pH (Fig. 1C; Table 1). (B) FluoZin-3-loaded cultures were exposed to the oxidizing agent, DTDP (10 min, 100 μM) at physiological pH, and after a 10-min preincubation at pH 6.0. Note the increased [Zn2+]i response at pH 6 and the rapid recovery on restoration of physiological pH. (C) Relocation of Zn2+ from mitochondria to protein-bound sites. FluoZin-3-loaded cultures were exposed to consecutive 5-min pulses of DTDP (100 μM), FCCP (3 μM), and both before a final exposure to DTDP, as indicated. Note that combined exposure to DTDP and FCCP induced far greater [Zn2+]i rises than exposure to FCCP alone, suggesting that in the absence of oxidation, Zn2+ released from mitochondria gets rapidly bound by redox-sensitive proteins. Further note the markedly increased size of the final DTDP response, indicative of a relocation of Zn2+ from the mitochondrial compartment to redox-sensitive protein pools. Traces show mean [Zn2+]i (±SEM) of 63–83 neurons, from 1 experiment representative of 5–14.
We next used sequential exposures to FCCP and DTDP to examine the possibility that endogenous Zn2+ ions can move between these two sites of sequestration. After exposing neurons to FCCP, subsequent responses to DTDP were considerably increased, consistent with movement of Zn2+ from mitochondrial to protein-bound sites (Fig. 2C). However, the converse maneuver, DTDP followed by FCCP, did not result in an increased [Zn2+]i response to FCCP, possibly reflecting high-affinity protein binding (Kd of MTs for Zn2+ ≈1.4 × 10−13; ref. 5), resulting in rapid resequestration of Zn2+ on its release from mitochondria. Thus, in sum, the above data point to two distinct neuronal compartments, one mitochondrial, the other cytosolic and likely protein-bound, each of which can hold substantial quantities of Zn2+ and between which Zn2+ appears able to be exchanged.
Potent Zn2+ Effects on Isolated Brain Mitochondria.
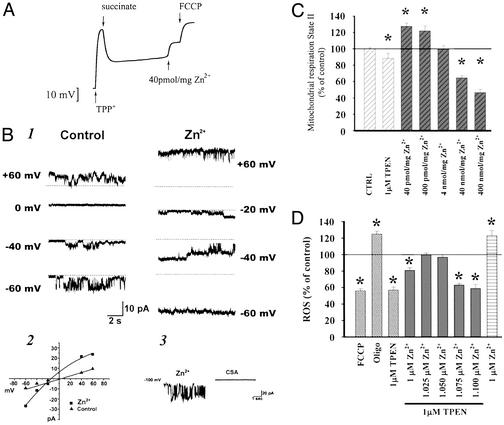
Might such Zn2+ movement be relevant to mitochondrial functioning either physiologically or under pathological conditions? In prior studies we found Zn2+ to be a remarkably potent inducer of swelling in isolated brain mitochondria (17). Extending these studies, we have begun to examine other effects of Zn2+ on isolated mitochondria. As Zn2+ dependent mitochondrial swelling is likely to reflect opening of the high conductance mitochondrial permeability transition pore (mPTP) (15, 17), we first assessed the potency with which Zn2+ induces loss of Δψm, by measuring changes in uptake of the charged compound TPP+ into the mitochondrial matrix (32). Paralleling prior studies on swelling, submicromolar levels (40 pmol/mg of mitochondrial protein) caused rapid loss of Δψm (Fig. 3A). Because either mPTP opening or inhibition of electron transport could account for loss of Δψm, further experiments examined Zn2+ effects on O2 consumption across a wide range of Zn2+ concentrations (40 pmol to 400 nmol per mg of protein). As would be expected on mPTP opening with consequent dissipation of the proton gradient and uncoupling of electron transport from ATP production, submicromolar levels of Zn2+ increased O2 consumption. Higher levels (40–400 nmol/mg) decreased respiration, consistent with known inhibitory effects on electron transport (12–14, 16, 19), (Fig. 3C).
Figure 3.
Potent effects of Zn2+ on isolated brain mitochondria. (A) Zn2+ depolarizes isolated brain mitochondria. Changes in Δψm are assessed by redistribution of TPP+ between the medium and the mitochondrial matrix. On addition of succinate as substrate, mitochondria generate Δψm (as indicated by the downward deflection of the trace). After a single pulse of Zn2+ (40 pmol/mg mitochondrial protein, likely corresponding to submicromolar free Zn2+) mitochondria undergo a sustained partial loss of Δψm, in comparison to the full loss of Δψm that occurs on addition of FCCP. Trace shows one experiment representative of six. (B) Zn2+ increases conductance and channel open probability of inner mitochondrial membranes. Traces show channel activity recorded in a patch of an isolated mitoplast from rat brain in the presence of 3 mM EGTA alone (Left) and in the presence of 300 nM Zn2+ (Right) (1). The patch was held at different voltages indicated (potentials refer to those of the patch electrode), and the dotted lines indicate the closed state. The membrane conductance and channel open probability are both increased. Current–voltage relationship for the two sets of recordings shown in 1 (2). CSA inhibits channel activity activated by Zn2+ (3). The traces show activity induced by 300 nM Zn2+ before (Left) and after (Right) addition of CSA (5 μM) to the bath. (C) Effects of Zn2+ on mitochondrial respiration. Isolated brain mitochondria are energized with 5 mM pyruvate/2.5 mM malate, and O2 consumption measured with an O2-sensitive electrode in the presence of TPEN or Zn2+ as indicated. Note the minimal decrease in respiratory rate induced by TPEN, as well as the biphasic effect of Zn2+, with low levels increasing and higher levels decreasing respiration. (D) Effects of Zn2+ on mitochondrial ROS generation. Isolated brain mitochondria are energized as above, and ROS generation is measured as changes in fluorescence of the oxidation-sensitive dye, H2DCFDA in the presence of FCCP (1 μM), oligomycin (1 μM), TPEN (1 μM), and/or Zn2+, as indicated. Note the decrease in ROS production induced by FCCP, consistent with uncoupling, and the expected increase in ROS generation caused by the mitochondrial ATPase inhibitor oligomycin (which hyperpolarizes mitochondria). Further note the decrease in ROS generation caused by TPEN, its reversal by addition of equimolar Zn2+, the decrease in ROS generation caused by a slight excess of the cation over TPEN (consistent with uncoupling), and finally the increase in ROS generation by addition of a larger amount of Zn2+ (consistent with inhibition of electron transport). In C and D, data were analyzed by using an ANOVA and subsequent Fisher's least significant difference test (n = 6). Bars represent group means ± SEM; asterisks indicate P < 0.01.
To more directly examine possible Zn2+ modulation of mitochondrial channel activity, we undertook direct electrophysiological recording of currents in isolated mitoplasts. The inner mitochondrial membrane contains a multiconductance, nonselective channel that is sensitive to CSA and is activated by high levels of matrix Ca2+ (33–36). We tested the possibility that opening of this channel might underlie the loss of Δψm observed in Zn2+-treated mitochondria. We recorded from mitoplasts, prepared by incubating mitochondria in hypotonic solution (23, 25, 33). Control recordings obtained in solutions lacking either Zn2+ or Ca2+ often contained multiconductance channel activity, but this activity could occasionally be inhibited by the addition of chelators (EGTA or TPEN) to the bath (data not shown). In over 50% of patches, addition of Zn2+ (300 nM) to the bath caused a marked increase in channel activity (n = 6/11; Fig. 3B). The channel activity resulted in a multiconductance state with conductances ranging from ≈50 pS to 1 nS. The current/voltage relationship of this activity was linear (Fig. 3B). To compare Zn2+-activated channel activity to the behavior of the mPTP, we recorded channels before and after the addition of CSA. When channel activity appeared in the presence of Zn2+, or was markedly increased by Zn2+, CSA (5 μM) inhibited this activity in three of seven patches (Fig. 3B). This finding suggests that the Zn2+-induced activity may be less sensitive to CSA than that described previously for the giant inner membrane channel (34, 37).
Finally, like O2 consumption, mitochondrial ROS production depends on Δψm (38, 39); mPTP opening should cause mitochondrial uncoupling and decreased ROS generation. Consistent with such a mechanism, submicromolar Zn2+ (which reduced Δψm and increased respiration) also reduced ROS formation (detected with H2-DCFDA). Conversely, higher concentrations of Zn2+ (which inhibit respiration) increased ROS production. Surprisingly, removal of Zn2+ with TPEN (1 μM) caused an ≈50% decrease in ROS generation, an effect that was reversed by adding back an equimolar amount of Zn2+, hinting at a possible role of Zn2+ in normal mitochondrial function (Fig. 3D).
Does Endogenous Zn2+ Modulate Mitochondrial Function?
In light of the apparent ability of Zn2+ to move between cytosolic and mitochondrial pools, and indications that mitochondria are highly sensitive to low levels of Zn2+, it is pertinent to consider whether movement of Zn2+ in or out of neuronal mitochondria has physiological relevance in neurons. One level at which mitochondria can be regulated is their Δψm, which provides the proton motive force and is thus essential to ATP production. Changes in Δψm can be assessed by using charged mitochondrially sequestered dyes, like rhodamine 123, that accumulate in mitochondria in proportion to Δψm. With loss of Δψm, cellular rhodamine 123 fluorescence increases, because of dequenching of the dye on exiting mitochondria. When the protein-bound Zn2+ pool was mobilized by addition of DTDP at pH 6, a mild increase in fluorescence was seen, indicative of a partial loss of Δψm. Furthermore, the fluorescence increase was attenuated by TPEN (20 μM), indicating that Zn2+ contributes directly to the loss of Δψm (Fig. 4).
Figure 4.
Mobilization of protein-bound Zn2+ triggers partial loss of Δψm. Cultures were loaded with the Δψm-sensitive probe rhodamine 123 (an increase in fluorescence indicates loss of Δψm), and exposed to DTDP (100 μM) in an acidic environment (pH 6.0) to produce a strong mobilization of protein- bound Zn2+ pools. DTDP-induced rhodamine 123 fluorescence changes were compared with those observed on full loss of Δψm elicited by addition of FCCP (3 μM) at the end of the experiment. Note that inclusion of TPEN (20 μM) during the exposure substantially attenuated the rhodamine 123 fluorescence increase (Upper). Traces show mean (±SEM) of rhodamine 123 fluorescence changes from of 58–64 neurons from one experiment representative of 11. Bar graph (Lower) shows compiled rhodamine 123 fluorescence changes (means ± SEM), expressed as percent of maximal change induced by FCCP, at the end of 20-min DTDP exposures as above, at pH 7.4 and pH 6 (n = 567–964 in 8–11 experiments, asterisk indicates P < 0.01 by ANOVA and subsequent Bonferroni's test).
Discussion
Studies in recent years have provided compelling evidence that in addition to vesicular Zn2+, neurons contain substantial pools of intracellular Zn2+ that can be mobilized in response to stimulation in culture or slice (2, 9, 40, 41). A large component of these pools probably consists of Zn2+ binding proteins, like MTs. Present studies provide new evidence for the additional presence of endogenous Zn2+ in mitochondria. Furthermore, as observed on loading with exogenous Zn2+, these studies indicate that this Zn2+ can be released into the cytosol on loss of Δψm induced by the protonophore, FCCP, or by induction of rapid Ca2+ entry, by exposing neurons to NMDA.
At physiological pH, much of the Zn2+ released from mitochondria may be rapidly buffered by cytosolic proteins and ligands, thus preventing large increases in [Zn2+]i. Consistent with this idea, prior exposure to FCCP increased subsequent response to DTDP, suggesting the occurrence of a relocation of mitochondrial pools into cytosolic binding sites (Fig. 2C). However, whereas disulfides like DTDP cause a slow and reversible release of Zn2+ from MTs, acidification appears to rapidly destabilize the interaction between MTs and Zn2+, and thus shifts the equilibrium to favor free Zn2+ remaining in the unbound state (30). This model may be consistent with our observations that although acidification to pH 6 in itself had relatively little effect on [Zn2+]i, this maneuver dramatically increased the [Zn2+]i rises (often to tens of nM) seen on exposure to either FCCP or to DTDP.
Studies on isolated neuronal mitochondria document powerful and complex effects of Zn2+. In our prior studies we found submicromolar Zn2+ levels (40 pmol/mg) to induce swelling of brain mitochondria comparable to that elicited by far higher Ca2+ exposures (50 nmol/mg). Present studies provide further characterization of Zn2+ effects on mitochondria, demonstrating highly potent loss of Δψm. Furthermore, direct patch clamp recording of mitoplasts revealed that submicromolar levels of Zn2+ have powerful stimulatory effects on multiconductance cation channels in the inner mitochondrial membrane that were often blocked by CSA, consistent with Zn2+ activation of the mPTP. In addition, studies of effects on O2 consumption and ROS generation suggest biphasic effects of Zn2+, with low levels causing an increase in O2 consumption and decrease in ROS generation consistent with this increased membrane permeability, whereas higher levels decreased O2 consumption and increased ROS production, compatible with findings suggesting that Zn2+ inhibits components of both the TCA cycle and the electron transport chain in liver mitochondria (12–14, 16, 19). Surprisingly, addition of the high affinity Zn2+ chelator, TPEN, also decreased ROS generation, and this effect was reversible by adding back an equimolar amount of Zn2+, raising the possibility that Zn2+ present in mitochondria under basal conditions might have some effect on electron transport through the chain.
Interestingly, MTs were recently observed to be taken up into isolated liver mitochondria and inhibit electron transport in proportion to the amount of Zn2+ bound (18), providing precedent for the possibility that MT-bound Zn2+ might modulate mitochondrial function. Our studies of Zn2+ effects on isolated brain mitochondria demonstrate potent effects to occur at concentrations that may be comparable to those observed in intact neurons during strong mobilization of protein-bound pools. Indeed, present observations suggesting that strong mobilization of endogenous Zn2+ in intact neurons caused a partial loss of Δψm may be compatible with potent modulation of mitochondrial membrane conductances by Zn2+, and lend support to the possibility that intracellular Zn2+ pools play a role in physiological or disease associated alterations in mitochondrial function. For instance, increased mobilization of intracellular Zn2+ in an acidic environment could contribute to mitochondrial dysfunction in pathological conditions like cerebral ischemia, in which parenchymal acidosis is prominent (42, 43).
Acknowledgments
We thank Simin Amindari for expert assistance with the cell cultures. This work was supported by National Institutes of Health Grants NS30884 and AG00836 (to J.H.W.) and AG00919 (to S.L.S.), and a grant from the Alzheimer's Association (to J.H.W.).
Abbreviations
- CSA
cyclosporin A
- DTDP
2,2′-dithiodipyridine
- FCCP
carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone
- MTs
metallothioneins
- NMDA
N-methyl-d-aspartate
- ROS
reactive oxygen species
- TPEN
N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine
- [Zn2+]i
cytosolic free Zn2+
- Δψm
mitochondrial membrane potential
- DCFDA
dichlorodihydrofluorescein diacetate
References
- 1.Frederickson C J, Bush A I. Biometals. 2001;14:353–366. doi: 10.1023/a:1012934207456. [DOI] [PubMed] [Google Scholar]
- 2.Lee J Y, Cole T B, Palmiter R D, Koh J Y. J Neurosci. 2000;20:RC79. doi: 10.1523/JNEUROSCI.20-11-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aschner M, Cherian M G, Klaassen C D, Palmiter R D, Erickson J C, Bush A I. Toxicol Appl Pharmacol. 1997;142:229–242. doi: 10.1006/taap.1996.8054. [DOI] [PubMed] [Google Scholar]
- 4.Coyle P, Philcox J C, Carey L C, Rofe A M. Cell Mol Life Sci. 2002;59:627–647. doi: 10.1007/s00018-002-8454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kagi J H R. In: Metallothionein III: Biological Roles and Medical Implications. Kimura M, editor. Basel: Birkhauser; 1993. pp. 29–55. [Google Scholar]
- 6.Palumaa P, Eriste E, Njunkova O, Pokras L, Jornvall H, Sillard R. Biochemistry. 2002;41:6158–6163. doi: 10.1021/bi025664v. [DOI] [PubMed] [Google Scholar]
- 7.Maret W. Proc Natl Acad Sci USA. 1994;91:237–241. doi: 10.1073/pnas.91.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maret W. J Nutr. 2000;130:1455S–1458S. doi: 10.1093/jn/130.5.1455S. [DOI] [PubMed] [Google Scholar]
- 9.Aizenman E, Stout A K, Hartnett K A, Dineley K E, McLaughlin B, Reynolds I J. J Neurochem. 2000;75:1878–1888. doi: 10.1046/j.1471-4159.2000.0751878.x. [DOI] [PubMed] [Google Scholar]
- 10.Sensi S L, Yin H Z, Weiss J H. Eur J Neurosci. 2000;12:3813–3818. doi: 10.1046/j.1460-9568.2000.00277.x. [DOI] [PubMed] [Google Scholar]
- 11.Sensi S L, Ton-That D, Weiss J H. Neurobiol Dis. 2002;10:100–108. doi: 10.1006/nbdi.2002.0493. [DOI] [PubMed] [Google Scholar]
- 12.Skulachev V P, Chistyakov V V, Jasaitis A A, Smirnova E G. Biochem Biophys Res Commun. 1967;26:1–6. doi: 10.1016/0006-291x(67)90242-2. [DOI] [PubMed] [Google Scholar]
- 13.Kleiner D. Arch Biochem Biophys. 1974;165:121–125. doi: 10.1016/0003-9861(74)90148-9. [DOI] [PubMed] [Google Scholar]
- 14.Link T A, von Jagow G. J Biol Chem. 1995;270:25001–25006. doi: 10.1074/jbc.270.42.25001. [DOI] [PubMed] [Google Scholar]
- 15.Wudarczyk J, Debska G, Lenartowicz E. Arch Biochem Biophys. 1999;363:1–8. doi: 10.1006/abbi.1998.1058. [DOI] [PubMed] [Google Scholar]
- 16.Brown A M, Kristal B S, Effron M S, Shestopalov A I, Ullucci P A, Sheu K F, Blass J P, Cooper A J. J Biol Chem. 2000;275:13441–13447. doi: 10.1074/jbc.275.18.13441. [DOI] [PubMed] [Google Scholar]
- 17.Jiang D, Sullivan P G, Sensi S L, Steward O, Weiss J H. J Biol Chem. 2001;276:47524–47529. doi: 10.1074/jbc.M108834200. [DOI] [PubMed] [Google Scholar]
- 18.Ye B, Maret W, Vallee B L. Proc Natl Acad Sci USA. 2001;98:2317–2322. doi: 10.1073/pnas.041619198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gazaryan I G, Krasnikov B F, Ashby G A, Thorneley R N, Kristal B S, Brown A M. J Biol Chem. 2002;277:10064–10072. doi: 10.1074/jbc.M108264200. [DOI] [PubMed] [Google Scholar]
- 20.Manev H, Kharlamov E, Uz T, Mason R P, Cagnoli C M. Exp Neurol. 1997;146:171–178. doi: 10.1006/exnr.1997.6510. [DOI] [PubMed] [Google Scholar]
- 21.Sensi S L, Yin H Z, Carriedo S G, Rao S S, Weiss J H. Proc Natl Acad Sci USA. 1999;96:2414–2419. doi: 10.1073/pnas.96.5.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin H Z, Weiss J H. NeuroReport. 1995;6:2553–2556. doi: 10.1097/00001756-199512150-00025. [DOI] [PubMed] [Google Scholar]
- 23.Jonas E A, Buchanan J, Kaczmarek L K. Science. 1999;286:1347–1350. doi: 10.1126/science.286.5443.1347. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan P G, Geiger J D, Mattson M P, Scheff S W. Ann Neurol. 2000;48:723–729. [PubMed] [Google Scholar]
- 25.Sorgato M C, Keller B U, Stuhmer W. Nature. 1987;330:498–500. doi: 10.1038/330498a0. [DOI] [PubMed] [Google Scholar]
- 26. Sullivan, P. G., Dube, C., Dorenbos, K. D., Steward, O. & Baram, T. Z. (2003) Ann. Neurol., in press. [DOI] [PMC free article] [PubMed]
- 27.Hajnoczky G, Robb-Gaspers L D, Seitz M B, Thomas A P. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 28.Gee K R, Zhou Z L, Ton-That D, Sensi S L, Weiss J H. Cell Calcium. 2002;31:245–251. doi: 10.1016/S0143-4160(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 29.Maret W, Vallee B L. Proc Natl Acad Sci USA. 1998;95:3478–3482. doi: 10.1073/pnas.95.7.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang L J, Vasak M, Vallee B L, Maret W. Proc Natl Acad Sci USA. 2000;97:2503–2508. doi: 10.1073/pnas.97.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nedergaard M, Goldman S A, Desai S, Pulsinelli W A. J Neurosci. 1991;11:2489–2497. doi: 10.1523/JNEUROSCI.11-08-02489.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamo N, Muratsugu M, Hongoh R, Kobatake Y. J Membr Biol. 1979;49:105–121. doi: 10.1007/BF01868720. [DOI] [PubMed] [Google Scholar]
- 33.Petronilli V, Szabo I, Zoratti M. FEBS Lett. 1989;259:137–143. doi: 10.1016/0014-5793(89)81513-3. [DOI] [PubMed] [Google Scholar]
- 34.Szabo I, Zoratti M. J Biol Chem. 1991;266:3376–3379. [PubMed] [Google Scholar]
- 35.Szabo I, Bernardi P, Zoratti M. J Biol Chem. 1992;267:2940–2946. [PubMed] [Google Scholar]
- 36.Lohret T A, Kinnally K W. Biophys J. 1995;68:2299–2309. doi: 10.1016/S0006-3495(95)80412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zorov D B, Kinnally K W, Tedeschi H. J Bioenerg Biomembr. 1992;24:119–124. doi: 10.1007/BF00769538. [DOI] [PubMed] [Google Scholar]
- 38.Skulachev V P. Q Rev Biophys. 1996;29:169–202. doi: 10.1017/s0033583500005795. [DOI] [PubMed] [Google Scholar]
- 39.Votyakova T V, Reynolds I J. J Neurochem. 2001;79:266–277. doi: 10.1046/j.1471-4159.2001.00548.x. [DOI] [PubMed] [Google Scholar]
- 40.Cuajungco M P, Lees G J. Brain Res. 1998;799:118–129. doi: 10.1016/s0006-8993(98)00463-6. [DOI] [PubMed] [Google Scholar]
- 41.Frederickson C J, Cuajungco M P, LaBuda C J, Suh S W. Neuroscience. 2002;115:471–474. doi: 10.1016/s0306-4522(02)00399-8. [DOI] [PubMed] [Google Scholar]
- 42.Nemoto E M, Frinak S. Stroke. 1981;12:77–82. doi: 10.1161/01.str.12.1.77. [DOI] [PubMed] [Google Scholar]
- 43.Siesjo B K. Neurochem Pathol. 1988;9:31–88. doi: 10.1007/BF03160355. [DOI] [PubMed] [Google Scholar]