Abstract
Most neurons in the ventral intraparietal area (VIP) of the macaque brain respond to both visual and tactile stimuli. The tactile receptive field is usually on the face, and the visual receptive field usually corresponds spatially to the tactile receptive field. In this study, electrical microstimulation of VIP, but not of surrounding tissue, caused a constellation of movements including eye closure, facial grimacing, head withdrawal, elevation of the shoulder, and movements of the hand to the space beside the head or shoulder. A similar set of movements was evoked by an air puff to the monkey's cheek. One interpretation is that VIP contributes to defensive movements triggered by stimuli on or near the head.
The ventral intraparietal area (VIP) in the monkey brain receives convergent input from visual, somatosensory, and motor areas (1, 2). Neurons in VIP respond to visual and somatosensory stimuli, with a relative emphasis on stimuli that are near, approaching, or touching the head (3–5). Many neurons are also sensitive to vestibular signals during head rotation (6). There are currently three main hypotheses about the function of VIP.
(i) VIP contributes to cross-modal attention, in which a sensory stimulus in one modality draws attention to the corresponding region of space in another modality (7, 8). According to this hypothesis, VIP contributes to attention with a heavy bias toward the space near the face. Consistent with the hypothesis, neurons in VIP are influenced by visual attention (9).
(ii) VIP helps to determine the monkey's direction of heading during locomotion (6). According to this hypothesis, VIP is specialized for navigation with respect to nearby objects such as branches or leaves passing close to or touching the face, whereas navigation with respect to more distant visual features may be processed in other brain areas. A high proportion of neurons in VIP respond to visual flowfield patterns; of these, most prefer expanding patterns to contracting patterns (5, 6). Many neurons have the same directional selectivity in both the tactile and visual modality (4, 6); these cells might encode the motion of objects passing near or rubbing against the face as the monkey moves forward. Neurons in VIP are influenced by vestibular signals that also could contribute to navigation (6).
(iii) VIP contributes to the control of defensive and avoidance movements that protect the body from collisions (10). This hypothesis is consistent with the high proportion of neuronal responses to stimuli that are near and approaching the face. Some cells respond selectively to visual stimuli moving on a trajectory aimed at the corresponding tactile receptive field on the face (3, 4). The vestibular signals found in VIP (6) could be related to ducking and turning movements of the head.
The three hypotheses about VIP function overlap. Navigation with respect to objects near the face (hypothesis ii) is partly a matter of collision avoidance (hypothesis iii). Multimodal attention (hypothesis i) presumably is closely related to monitoring potentially dangerous objects near or approaching the face (hypothesis iii). That is, VIP could serve a set of related functions emphasizing the space near the body.
One way to approach the function of VIP is to consider the function of the areas to which it projects. VIP sends a projection out of the parietal lobe to a part of premotor cortex that we have termed the polysensory zone (PZ) (2, 11, 12). PZ has visual and tactile response properties nearly identical to those found in VIP, including an emphasis on objects near, approaching, or touching the face (13, 14). Furthermore, electrical stimulation of PZ evokes short-latency, complex movements that resemble defensive movements (15). For example, for some sites, stimulation evokes blinking, squinting, turning of the head away from the sensory receptive fields, and a rapid lifting of the hand as if to block an impending impact to the head.
Would electrical stimulation of VIP evoke the same types of movements that were evoked by stimulation of PZ? In several previous studies (15–17), electrical stimulation in the parietal lobe evoked blinking, squinting, and other facial movements. One purpose of the present experiment was to test whether such movements are evoked specifically from the multimodal VIP and not from surrounding tissue. A second purpose was to compare the stimulation-evoked movements with movements evoked by an externally applied threat such as a puff of air to the face.
Methods
Recording and Stimulating.
All procedures were approved by the Princeton University Institutional Animal Care and Use Committee and the attending veterinarian. The monkey sat in a primate chair with the head fixed by a head bolt and the limbs and torso free. The monkey was rewarded with small pieces of fruit for sitting quietly during the testing session. No trained task was necessary because stimulation evoked consistent, measurable results in the awake, quietly resting state. If the monkey became active, testing was stopped until the monkey was again still.
A hydraulic microdrive was used to lower a tungsten microelectrode (0.1–1.5 MΩ; Frederick Haer, Bowdoinham, ME) along a vertical approach into the superior parietal lobe. Once the electrode tip was within cortex as indicated by the presence of cellular activity, we tested every 0.5–1.0 mm along the penetration. We studied the effect of electrical stimulation of 114 sites in VIP and 45 sites in the surrounding tissue in two hemispheres of two adult male Macaca fascicularis.
At each site, we first qualitatively tested the sensory responses of single neurons and multineuron activity to aid in localizing VIP. The details of this qualitative testing are described below (see Location of Stimulation Sites). Once the properties of neurons were qualitatively assessed, electrical stimulation was applied by an S88 stimulator and two SIU6 stimulus isolation units (Grass Instruments, Quincy, MA). Stimulation was triggered by a hand-held button and consisted of a train of pulses presented at 200 Hz. Each pulse had a negative followed by a positive phase; each phase was 0.4 ms in duration. The duration of each train was set to 500 ms. For 25 stimulation sites, trains of 100-ms duration also were tested. For 15 sites, trains of 50 ms also were tested.
For each site, we varied the current until an evoked movement was observed. The threshold, the current at which the movement was evoked 50% of the time, was determined by two observers. These threshold measurements thus were approximate but allowed us to set the current to an appropriate level for quantitative testing. The average threshold measured in this fashion for sites in VIP was 89.6 μA, with a SD of 75.3 μA and a range of 9–150 μA. These thresholds match previous reports for movements evoked from the parietal lobe (16, 17). In some cases, to confirm that stimulation of a site did not evoke any movement, the stimulating current was increased to 300 μA.
Three lines of evidence suggest that the electrical stimulation did not damage the brain. (i) The evoked movements remained undiminished after hundreds of stimulation trials. (ii) Immediately after stimulating, we still could record from single neurons through the same electrode at the same location, the neurons showed no abnormalities in their spike trains, and the neurons were still responsive. (iii) On histology, the tissue in monkey 1 showed no sign of electrolytic damage at the sites of stimulation. The monkey was perfused 5 days after the last of the daily stimulation experiments; thus, any electrolytic damage should have been marked by gliosis.
Data Acquisition.
Once qualitative testing and threshold testing were complete, we set the stimulation current to a level above threshold, usually 100 or 150 μA, and measured the evoked movement. The movements were measured in two ways: by videotape and by electromyogram (EMG).
Video Analysis.
The movements were recorded on videotape at 30 frames per s. A TTL (transistor–transistor logic) output from the Grass stimulator, indicating the time of onset of each stimulation pulse, was fed into the audio track of the video recorder to synchronize the images with the time of stimulation. Thus, the start of the stimulation train could be determined to the nearest video frame. Videotapes were analyzed off-line frame-by-frame to determine the type of movement evoked. Stimulation-evoked movements could be distinguished readily from spontaneous movements because the stimulation-evoked movements occurred in a consistent fashion on every trial with a consistent latency.
EMG Analysis.
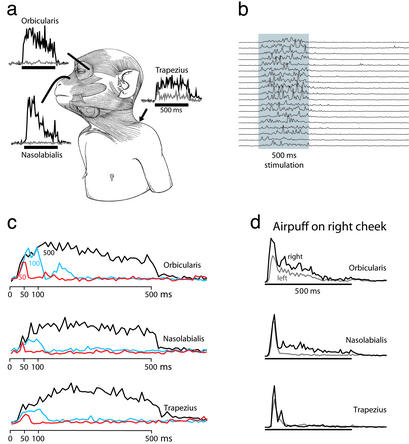
To study further the latency and reliability of stimulation-evoked movements, we measured EMG activity for 15 sites. The EMG activity was measured in the orbicularis muscle, the nasolabialis muscle, and the trapezius muscle (Fig. 4a).
Figure 4.
Stimulation-evoked EMG activity. (a) Activity evoked at one example site. Shown are EMG from orbicularis oculi muscle causing squint, EMG from nasolabialis muscle causing lifting of upper lip and facial skin, and EMG from trapezius muscle causing shoulder shrug. Dark lines, EMG from muscles on left side of body (contralateral to stimulation); gray lines, EMG from muscles on right side of body. Each EMG trace is the mean of 15 trials. Horizontal line, stimulation period. The y axis is in arbitrary units. Monkey drawing is adapted from ref. 18. (b) Consistency of EMG response across trials for one example site. Stimulation-evoked EMG activity from trapezius muscle on 19 consecutive trials is shown. Gray rectangle, stimulation period. (c) Mean EMG activity of 15 stimulation sites in VIP, tested with three stimulation durations; 500-, 100-, and 50-ms stimulation trains were tested in noninterleaved blocks for each site. (d) Mean EMG activity (15 trials) caused by air puff to the right side of the face. Dark line, EMG from muscle on right side; gray line, EMG from muscle on left side. Air puff, 500 ms in duration.
Fine, insulated, stainless-steel wires were threaded into a 22-gauge syringe needle and inserted into the muscle. The wires had an exposed tip of 1–2 mm. Three wires were inserted and spaced ≈5 mm apart to provide input to a differential amplifier and its ground (single neuron amplifier, model 1800; A-M Systems, Everett, WA). The amplifier filters were set with a low cutoff at 300 Hz and a high cutoff at 1,000 Hz. Placement of the wires was confirmed by using electrical stimulation to cause a visible contraction of the target muscles. It also was confirmed by observing EMG during spontaneous movements such as blinking to an air puff on the face (orbicularis muscle), lifting of the lip during eating (nasolabialis muscle), and lifting of the shoulder during spontaneous arm movements (trapezius muscle). In all cases, EMG activity increased during the expected movement and not during other movements. To measure EMG activity during a stimulation train delivered to the brain, the time of each stimulation pulse was measured and the EMG signal was sampled once within each 5-ms interpulse interval. We confirmed that with this method the EMG signal could be measured without interference of artifact from the electrical stimulation. EMG thus was measured once every 5 ms, or at 200 Hz. It then was rectified. Traces shown have been integrated into 10-ms bins.
Latency was calculated by determining the first EMG measurement after the start of stimulation for which the signal was >2 SD above the baseline. In some cases, the monkey made a spontaneous movement that happened to occur just before the start of stimulation, thus complicating the assessment of latency. To reduce this source of noise, we divided the trials into two equal categories: those with high variance in the 200 ms before stimulation and those with low variance within the same prestimulation time period. Trials with low prestimulation variance corresponded to trials in which the muscle was not active before stimulation. These low-variance trials were used to calculate the latency of the EMG activity and to create the mean plots shown in Fig. 4c. Typically, each site was tested with 20 trials; thus, latency was determined by using the 10 trials that had the lowest prestimulation variance. When all trials were used to calculate latency, similar results were obtained but with greater variability.
Location of Stimulation Sites.
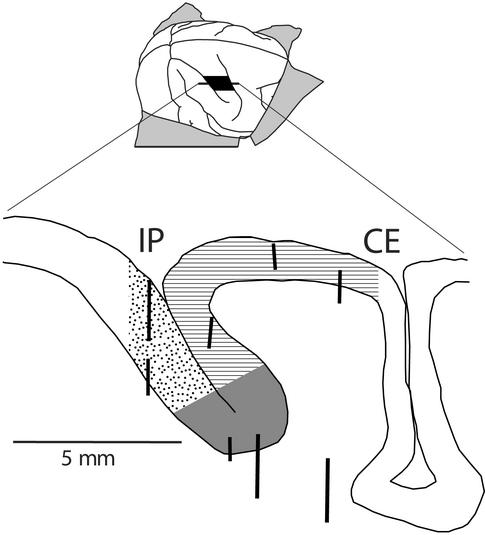
The brain of monkey 1 was sectioned parasagittally at 50 μm on a freezing Microtome and stained with cresyl violet. The location of the studied cortex is shown in Fig. 1. We believe that the studied area in monkey 2 was similar on the basis of reconstructed patterns of cellular activity and silence as the electrode passed through cortex and white matter and on the basis of patterns of response properties as described below. As shown in Fig. 1, we studied a strip of cortex ≈3 mm wide, extending from the intraparietal sulcus at its posterior end to the central sulcus at its anterior end. This strip was located ≈10 mm lateral to the midline of the brain, intersecting the intraparietal sulcus at a point at which VIP was estimated to be located on the basis of previous literature (1–3). Because only a strip of cortex was studied, the entire medial-to-lateral extent of VIP was not investigated; rather, we studied a cross section of VIP. It is therefore possible that the properties reported here within VIP are representative of only one part of this area.
Figure 1.
Location of stimulation sites in monkey 1. (Upper) Brain of monkey 1 traced from photo. Black area indicates approximate location of studied cortex extending from intraparietal sulcus (IP) to central sulcus (CE). (Lower) A parasagittal section through the studied cortex. Location of VIP, as determined by physiological properties, is gray (see Methods). Striped areas indicate studied cortex on the anterior bank of IP and on the cortical surface anterior to IP, and stippled areas indicate studied cortex on posterior bank of IP. Thick lines in the section indicate electrode tracks, distinguishable as streaks of gliotic cells in the cresyl violet stain.
To distinguish VIP from neighboring cortical areas, we tested each site qualitatively by monitoring single neurons and multineuron activity on an oscilloscope and over a loudspeaker during tactile and visual stimulation. Tactile stimuli included stroking of the skin with cotton swabs and passive manipulation of the joints. Visual stimuli consisted of a 3-cm-diameter ball mounted on the end of a wand, moved by hand in the space in front of the monkey. Different directions of movement, including movement in the frontoparallel plane and movement toward or away from the face, were tested. Also, different distances from the face, ranging from 5 cm to 1 m, were tested. Responses to visual stimuli and to tactile stimuli were distinguished from each other by presenting stimuli in the dark or with the monkey's eyes covered.
Most sites on the floor of the intraparietal sulcus, in presumed VIP, responded to visual stimuli (74%, 81 of 109 tested). In addition to the visual responses, most sites in presumed VIP responded to tactile stimuli (96%, 109/114). The tactile receptive fields usually included the face (90%, 98/109) and also sometimes included the trunk (23%, 25/109) or the arms (23%, 25/109). These properties, namely, (i) a high proportion of visual responsiveness, (ii) a high proportion of tactile responsiveness on the face, and (iii) location on the floor of the intraparietal sulcus, match the known properties of VIP and of no other area. In addition, we tested a sample of 15 sites in presumed VIP for directional preference. At all 15 sites, both the tactile and visual responses were directionally selective and the two directional preferences matched. For example, neurons at one site responded best to a tactile probe moving across the facial skin from left to right. When the eyes were uncovered, the neurons at the same site responded best to visual stimuli near the face moving from left to right. This match in tactile and visual directional preference is a distinguishing property of VIP.
The response properties that we observed in the floor of the intraparietal sulcus were different from the properties that we found in nearby cortical regions. Of 30 sites studied in the medial bank of the intraparietal sulcus and the cortical surface anterior to the sulcus, only 3 responded to visual stimuli, whereas 27 responded to tactile stimuli. Of 10 sites studied in the posterior bank of the sulcus, none responded to tactile stimuli, whereas 8 responded to visual stimuli. The sites outside of VIP may have been in the medial intraparietal area, the parietal reach region, area 5, area 2, and the lateral intraparietal area. Rather than attempt to assign the sites to specific areas with insufficient information, we refer to them collectively as “non-VIP” sites. The non-VIP sites also included five sites in the white matter 2–3 mm anterior and ventral to VIP.
Results
Video Analysis.
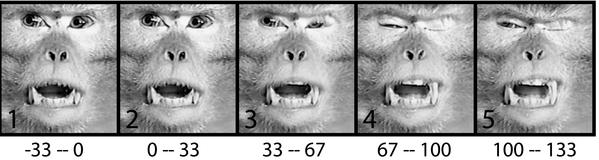
Four kinds of movements were commonly evoked by stimulation of sites within VIP. One was a squint or blink (evoked from 77% of VIP sites; 0% of non-VIP sites). Fig. 2 shows an example. When this site in the right hemisphere was stimulated, the left eye closed. The closure of the eye began within two video frames of stimulation onset. The latency of the observed movement thus was between 33 and 67 ms. A similar frame-by-frame analysis was performed on each of the 25 trials, and, in every case, the movement occurred between the first and second video frame after stimulation; thus, the latency was always 33–67 ms. This consistency indicates that the movement indeed was evoked by stimulation and was not a spontaneous movement that sometimes occurred around the time of stimulation. (The latency as measured by EMG is described below.) The evoked movement ended within a similar short latency (33–67 ms) after the stimulation train ended and the monkey returned to a quietly resting state with no sign of distress or agitation.
Figure 2.
An example of facial movement evoked by microstimulation of VIP. Shown are images of monkey 1 captured from video (30 frames per s). Numbers beneath each frame indicate time in milliseconds relative to stimulation onset. By frame 3, the monkey's left eye (contralateral to stimulation) began to close and the left upper lip began to lift, exposing the teeth. By frame 4, the lifting of the skin on the left snout was more pronounced, deforming the left nostril. The left eye was closed, the right eye was partially closed, and the left brow was lowered. Stimulation of this site also caused the ear to pull back and down and the left arm to move to the left.
A second commonly evoked movement was a contraction of the facial musculature causing the upper lip to lift and the skin on the snout to wrinkle upward toward the eye (81% of VIP sites; 2% of non-VIP sites). Fig. 2 shows an example. The movement occurred between the first and second video frame after stimulation onset on each of the 25 stimulation trials; thus, the latency was between 33 and 67 ms.
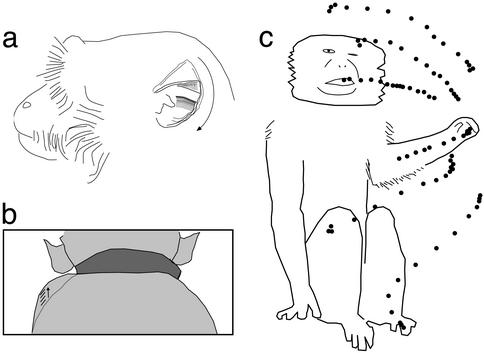
Third, stimulation evoked movements of the ear in which the pinna flattened back against the side of the head and rotated downward (77% of VIP sites; 9% of non-VIP sites). Fig. 3a shows an example. Stimulation at this site in the right hemisphere caused movement of the left pinna on every trial (n = 20) with a latency between 33 and 67 ms. During downward rotation, the pinna developed an accordion-like fold.
Figure 3.
Components of stimulation-evoked movements illustrated for three typical sites from VIP in the right hemisphere of monkey 2. At all three sites, stimulation caused the left eye to squint, the left lip and facial skin to lift, and the left ear to flatten against the head and rotate downward. (a) Stimulation-evoked ear movement traced from two video frames: one frame (gray ear) before stimulation and one frame (black ear) during stimulation at time of maximum ear displacement (300–333 ms after stimulation onset). Movement included ear pulling back against the head, rotating down, and partially folding. (b) Stimulation-evoked shoulder shrug traced from seven consecutive video frames. Shown is the back view of monkey's head, shoulders, and collar (dark gray). Tracing of the left shoulder in frame 1 at stimulation onset is shown as a gray line. On subsequent frames, the monkey's left shoulder shrugged upward (arrow) to a maximum displacement at frame 7, shown as a black line. Line segments near the arrow show the position of the shoulder on successive frames between 1 and 7. At frame 2, the shoulder had not begun to move. (c) Stimulation at this site caused the left arm to move to the left. The six dotted lines show the frame-by-frame position of the hand for six different stimulation trials. The start point of each trajectory was near the midline of the monkey, and the endpoint was to the monkey's left.
Fourth, stimulation evoked a shoulder shrug (70% of VIP sites; 7% of non-VIP sites). Fig. 3b shows an example in which stimulation in the right hemisphere caused an elevation of the left shoulder. This shoulder movement occurred on every trial (n = 30) with a latency between 67 and 100 ms. The shoulder shrugs sometimes were accompanied by a lateral movement of the arm, illustrated in Fig. 3c. Stimulation at this site in the right hemisphere caused the left shoulder to shrug and the left arm to move to the left. The movement of the hand was approximately normal to the video angle; thus, the speed of hand movement could be estimated by measuring the distance between hand positions on successive video frames. For this site, the hand moved at a peak speed of 68.6 cm/s. For the 11 sites at which the peak hand speed was measured in this fashion, the mean was 82.5 cm/s (SD = 24).
We studied 114 sites in VIP. From 109 of them (96%), at least one of four movement types was evoked. From 63 sites (55%), all four movement types were evoked. For 91 of 109 sites at which a movement was evoked, the movement was observed exclusively on the left side of the body, contralateral to the stimulating electrode. For 18 sites, movement was observed on both sides of the body. At no site was movement evoked exclusively on the ipsilateral side. The average latency of the observed movement was between one and two video frames, or between 33 and 67 ms.
All sites were tested with 500-ms stimulation trains; 25 sites also were tested with 100-ms trains. For all 25 sites, the same movement components were evoked with 100- as with 500-ms trains. The evoked movement began with a latency between 33 and 67 ms after stimulation onset and stopped at a similar latency after stimulation offset. The only observed difference on videotape between long and short stimulation trains was the duration of the evoked behavior.
Movements that did not match the four types described above were evoked from 15 sites in VIP (13%). These other movements included twitches of the contralateral hand and fingers (six sites); movements of the contralateral forearm including supination, pronation, and rotation at the elbow (five sites); movements of the lips and chin (two sites); and movements of the torso (six sites). For 10 of these 15 sites, stimulation also evoked one of four main movement types described above.
In addition to the tests described above, eight sites in VIP were tested while the monkey's head was released from the head holder. For four of these sites, stimulation caused a head movement. In all four cases, the movement involved a retraction of the head from the contralateral side of space. For example, on stimulation of one site in the right hemisphere, the left eye squinted; the left upper lip lifted and the skin on the left side of the snout wrinkled; the left ear pulled down and back against the head; the left arm moved to the left side of the body; and, when the head holder was released, stimulation also caused the head to pull back and toward the right.
A difference was observed between sites within VIP and sites outside of VIP. As shown in Table 1, the four types of movement were almost never evoked from regions of cortex outside of VIP. Sites that had a tactile response on the face and limbs were common outside of VIP (60% of sites); stimulation of these sites sometimes evoked movements of the fingers, wrist, and forearm, but not the four movement types typically evoked from VIP. The sites outside of VIP included five within the white matter anterior and ventral to VIP. Movements were not evoked from these white matter sites. At sites where no movement was obtained, stimulation currents as high as 300 μA were used to confirm the absence of effect. That is, stimulation of VIP almost always evoked a specific cluster of movements, whereas even high current stimulation of surrounding tissue outside of VIP almost never evoked the same movements. These results indicate that the stimulation-evoked movements were the result of activation of VIP and its connected structures, not the result of passive current spread to surrounding white matter or neighboring cortical areas.
Table 1.
Proportion of sites from which the different movement types were evoked
| Area | Movement type | No. of sites, %
|
||
|---|---|---|---|---|
| Monkey 1 | Monkey 2 | Combined | ||
| VIP | Total | 69 | 45 | 114 |
| Squint/blink | 53 (77%) | 35 (78%) | 88 (77%) | |
| Facial movement | 56 (81%) | 36 (80%) | 92 (81%) | |
| Ear movement | 54 (78%) | 34 (76%) | 88 (77%) | |
| Shrug/arm | 48 (70%) | 32 (71%) | 80 (70%) | |
| Non-VIP | Total | 16 | 29 | 45 |
| Squint/blink | 0 (0%) | 0 (0%) | 0 (0%) | |
| Facial movement | 0 (0%) | 1 (4%) | 1 (2%) | |
| Ear movement | 3 (19%) | 1 (4%) | 4 (9%) | |
| Shrug/arm | 2 (13%) | 1 (4%) | 3 (7%) | |
Squint/blink, movements related to musculature surrounding eye; facial movement, contraction of facial muscles that results in facial skin and upper lip lifting; ear movement, pinna pulling back against the head, rotating down, or folding; and shrug/arm, a fast shoulder shrug or a movement of the arm thrusting the hand to lateral space. These defensive-like movements were significantly more commonly evoked from area VIP than from surrounding areas (χ2 = 267.61, P < 0.0001).
EMG Analysis.
To study further the latency and repeatability of the stimulation-evoked movements, we measured EMG activity from the orbicularis, nasolabialis, and trapezius muscles for 15 sites. These muscles were selected because they participate in squinting and blinking (orbicularis muscle), lifting of the upper lip (nasolabialis muscle), and shoulder shrugging (trapezius muscle). Fig. 4a shows the results for one site. During stimulation of this site, the orbicularis muscle was active on the left side but not the right, corresponding to the closure of the left eye. The latency of this EMG activity was 18.7 ms (SD = 8.3 ms). The nasolabialis muscle was active on the left but not the right, corresponding to the lifting of the left upper lip. The latency of this EMG activity was 30.7 ms (SD = 15.8 ms). The trapezius muscle was active bilaterally, with a greater EMG activity on the left than on the right, matching the observed shoulder shrug that was bilateral and largest on the left. The latency of activity of the left trapezius was 24.7 ms (SD = 10.6 ms).
Fig. 4b shows trial-by-trial EMG traces from the trapezius muscle for another example site. Between trials, the monkey was sitting in a quiet state; thus, the muscle was relaxed before the stimulation began. The stimulation train evoked a consistent increase in muscle activity with a mean latency of 25.3 ms (SD = 9.1 ms). At the offset of the stimulation train, the muscle activity dropped back to baseline and the monkey returned to a quiet, resting state.
For the 15 sites studied in this fashion, the average latency for the orbicularis muscle was 35.3 ms (SD = 16.9 ms, range of 10–60 ms). The average latency for the nasolabialis muscle was 36.3 ms (SD = 13.0 ms, range of 20–60 ms). The average latency for the trapezius muscle was 35.5 ms (SD = 8.2 ms, range of 30–50 ms, with one outlier at 220 ms).
Previous studies of cortical microstimulation sometimes used stimulation trains shorter than 500 ms. To ensure that the effects we observed were not solely the result of using long stimulation trains, we studied the effect of the duration of the stimulation train by using three different durations (50, 100, and 500 ms) in noninterleaved blocks. Changing the duration of the stimulation train did not qualitatively change the evoked muscle activity; instead, the major effect was to change the time over which the movement occurred. Fig. 4c shows the mean result for 15 sites for the three muscles studied. A short latency increase in EMG activity was obtained for 50-, 100-, and 500-ms stimulation trains. The activity dropped back to baseline within a short latency after stimulation offset. For some sites, the eye blinked a second time after the initial stimulation-evoked movement. This second rise in activity can be seen in the traces for the orbicularis for 100- and 50-ms stimulation.
Movements Evoked by Air Puff to the Face.
We studied the movements evoked by a controlled, focused stream of air aimed at the side of the monkey's face. The air stream was delivered at 15 psi (1 psi = 6.89 kPa) from a nozzle 3 cm from the right cheek. The stimulus duration was 500 ms. The stimulus was delivered once every 10 s.
We analyzed the video record for 67 trials. Five kinds of movement were evoked by the air puff: (i) a blink and a contraction of the musculature around the eye (67/67 trials); (ii) a lifting of the upper lip and wrinkling upward of the skin on the snout (67/67 trials); (iii) a rotation of the pinna downward and backward against the head (66/67 trials); (iv) a shoulder shrug [65/67 trials; the shrug was sometimes accompanied by a fast movement of the arm that brought the hand into upper lateral space on the side of the air puff (24/65 trials)]; and (v) a withdrawal of the head from the direction of the air puff when the monkey's head was released from the head holder (19/22 trials). Thus, all movement components typically evoked by electrical stimulation of VIP also were evoked by air puff to the cheek.
We measured the EMG activity from the orbicularis muscle (related to squinting), nasolabialis muscle (lifting of upper lip), and trapezius muscle (shrugging) during the air puff. The average activity is shown in Fig. 4d. Just as for stimulation of VIP, we found elevated activity in these muscles during the air puff. There were, however, two differences between the effect of VIP stimulation and the effect of air puff. First, the muscle activity evoked by VIP stimulation generally followed the time course of the stimulation train. As shown in the average EMG traces in Fig. 4c (black line), the activity rose at stimulation onset, remained high during stimulation, and fell within a short latency after stimulation offset. In contrast, as shown in Fig. 4d, the average muscle activity evoked by the air puff had a transient peak at stimulus onset and a reduction throughout the stimulus period. This pattern of muscle activity is not due to any drop in stimulus strength, because the stream of air maintained a steady pressure throughout the 500 ms.
A second difference between the effect of VIP stimulation and the effect of air puff was that VIP stimulation affected largely the contralateral musculature, as illustrated by the example in Fig. 4a. In contrast, a puff directed at one cheek caused an effect that was consistently bilateral (Fig. 4d). This bilaterality was most pronounced in the initial spike of EMG activity. In summary, puff-evoked movements were similar but not identical to those evoked by VIP stimulation.
Discussion
We found that electrical stimulation in VIP evoked a set of movements that were similar to those evoked by a puff of air to the side of the face. They also corresponded to the movements reported by others during startle and avoidance, including eye blink, squint, lifting of the upper lip, folding of the pinna against the head, elevation of the shoulder, rotation of the head away from the threat, and lifting of the forelimb into the space beside the head (19–21). Stimulation of surrounding cortex and white matter, even with high currents, did not evoke this set of movements.
Did the stimulation of VIP directly activate a motor pathway, or did it produce a sensory percept to which the monkey then reacted normally? VIP lies between traditional sensory and motor areas, receiving input from visual and somatosensory regions and sending projections to premotor cortex (1, 2, 11). It is difficult to know what mental sensation the monkey experienced when this sensorimotor pathway was activated.
Several observations, however, may be relevant. We found that an air puff on the cheek evoked an initial spike in muscle activity and an adaptation-like reduction throughout the stimulus period. The muscle activity dropped to baseline or near baseline levels before the end of the 500-ms stimulus. In contrast, VIP stimulation evoked, on average, a more sustained muscle activity that dropped abruptly to baseline within a short latency after the stimulation train stopped. In this respect, the muscle output seemed to be more temporally locked to VIP stimulation than to the tactile stimulus on the cheek. A second observation is that movements evoked by VIP stimulation were restricted mainly to the contralateral musculature, as if the outputs of VIP feed into a lateralized motor mechanism, whereas a puff of air to one cheek evoked movements that were more bilateral.
We would stress, however, that the question of whether the evoked movements were solely a motor output, a reaction to a sensory percept, or both, is not yet resolved. A purely mechanistic description is that we stimulated part of a pathway that links certain sensory events with certain motor events. This pathway may include the region of premotor cortex, PZ, to which VIP appears to project (2, 11).
The movements evoked by stimulation of VIP in the present study may be related to the known sensory properties of this brain area. Most VIP neurons (54%) respond best to visual stimuli within 20 cm of the face, and 34% respond best to “ultra-near” stimuli, within 5 cm (3). Most (78%) respond preferentially to visual stimuli approaching the face (5, 6). Most (70%) have a tactile receptive field spatially aligned with the visual receptive field (3). Some cells respond best to a visual stimulus that is approaching and on a collision course with the tactile receptive field on the face (3). Because of these neuron properties, and because of the present finding that activation of this tissue leads to repeatable, short-latency, defensive-like movements, we suggest that at least one function of VIP may be to protect the head from potentially damaging objects, with a relative emphasis on those objects that are near, approaching, or touching the face. It is important to note, however, that there may be other interpretations (e.g., ref. 16). One possibility is that VIP may serve a more general role encoding space on and around the face. It will be useful to deactivate VIP and study the resulting behavioral deficits to further specify its functions.
Acknowledgments
We thank L. McCandless, T. Mole, and J. Woronczuk. This work was supported by National Institutes of Health Grants EY-11347, MH-12336, and NS-41878 and Burroughs Wellcome Grant 992817.
Abbreviations
- VIP
ventral intraparietal area
- PZ
polysensory zone
- EMG
electromyogram
References
- 1.Maunsell J H R, Van Essen D C. J Neurosci. 1983;3:2563–2580. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis J W, Van Essen D C. J Comp Neurol. 2000;428:112–137. doi: 10.1002/1096-9861(20001204)428:1<112::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Colby C L, Duhamel J-R, Goldberg M E. J Neurophysiol. 1993;69:902–914. doi: 10.1152/jn.1993.69.3.902. [DOI] [PubMed] [Google Scholar]
- 4.Duhamel J R, Colby C L, Goldberg M E. J Neurophysiol. 1998;79:126–136. doi: 10.1152/jn.1998.79.1.126. [DOI] [PubMed] [Google Scholar]
- 5.Schaafsma S J, Duysens J. J Neurophysiol. 1996;76:4056–4068. doi: 10.1152/jn.1996.76.6.4056. [DOI] [PubMed] [Google Scholar]
- 6.Bremmer F, Klam F, Duhamel J R, Ben Hamed S, Graf W. Eur J Neurosci. 2002;16:1569–1586. doi: 10.1046/j.1460-9568.2002.02206.x. [DOI] [PubMed] [Google Scholar]
- 7.Driver J, Spence C. Curr Opin Neurobiol. 1998;8:245–253. doi: 10.1016/s0959-4388(98)80147-5. [DOI] [PubMed] [Google Scholar]
- 8.Ladavas E, di Pellegrino G, Farne A, Zeloni G. J Cognit Neurosci. 1998;10:581–589. doi: 10.1162/089892998562988. [DOI] [PubMed] [Google Scholar]
- 9.Cook E P, Maunsell J H. J Neurosci. 2002;22:1994–2004. doi: 10.1523/JNEUROSCI.22-05-01994.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graziano M S, Taylor C S, Moore T, Cooke D F. Neuron. 2002;36:349–362. doi: 10.1016/s0896-6273(02)01003-6. [DOI] [PubMed] [Google Scholar]
- 11.Luppino G, Murata A, Govoni P, Matelli M. Exp Brain Res. 1999;128:181–187. doi: 10.1007/s002210050833. [DOI] [PubMed] [Google Scholar]
- 12.Graziano M S A, Gandhi S. Exp Brain Res. 2000;135:259–266. doi: 10.1007/s002210000518. [DOI] [PubMed] [Google Scholar]
- 13.Rizzolatti G, Scandolara C, Matelli M, Gentilucci M. Behav Brain Res. 1981;2:147–163. doi: 10.1016/0166-4328(81)90053-x. [DOI] [PubMed] [Google Scholar]
- 14.Graziano M S A, Hu X, Gross C G. J Neurophysiol. 1997;77:2268–2292. doi: 10.1152/jn.1997.77.5.2268. [DOI] [PubMed] [Google Scholar]
- 15.Graziano M S A, Taylor C S R, Moore T. Neuron. 2002;34:841–851. doi: 10.1016/s0896-6273(02)00698-0. [DOI] [PubMed] [Google Scholar]
- 16.Thier P, Andersen R A. J Neurophysiol. 1998;80:1713–1735. doi: 10.1152/jn.1998.80.4.1713. [DOI] [PubMed] [Google Scholar]
- 17.Shibutani H, Sakata H, Hyvarinen J. Exp Brain Res. 1984;55:1–8. doi: 10.1007/BF00240493. [DOI] [PubMed] [Google Scholar]
- 18.Huber E. In: The Anatomy of the Rhesus Monkey. Hartman C F, Straus W L, editors. New York: Hafner; 1933. pp. 176–188. [Google Scholar]
- 19.Yeomans J S, Li L, Scott B W, Frankland P W. Neurosci Biobehav Rev. 2002;26:1–11. doi: 10.1016/s0149-7634(01)00057-4. [DOI] [PubMed] [Google Scholar]
- 20.Schiff W, Caviness J A, Gibson J J. Science. 1962;136:982–983. doi: 10.1126/science.136.3520.982. [DOI] [PubMed] [Google Scholar]
- 21.Landis C, Hunt W A, Strauss H. The Startle Pattern. New York: Farrer and Rinehart; 1939. [Google Scholar]