Abstract
Drug addiction poses serious social, medical, and economic problems, but effective treatments for drug addiction are still limited. Cocaine and morphine elevate dopamine levels in the nucleus accumbens (NAc), and the overwhelming actions of dopamine are implicated in reinforcement and addiction of abusive drugs. In our previous studies, we reported the regulatory role of acetylcholine (ACh) in the NAc function by selectively ablating the NAc cholinergic neurons with use of immunotoxin-mediated cell targeting. These studies indicated that ACh and dopamine acted convergently but oppositely on the NAc circuit and that cholinergic cell ablation enhanced long-lasting behavioral changes of cocaine addiction. In this investigation, we showed that immunotoxin-mediated ablation of the NAc cholinergic neurons enhanced not only the sensitivity to morphine in conditioned place preference but also negative reinforcement of morphine withdrawal in conditioned place aversion. Remarkably, acetylcholinesterase (AChE) inhibitors that act on the brain AChE suppressed both cocaine- and morphine-induced conditioned place preference and blocked the induction and persistence of cocaine-evoked hyperlocomotion. Importantly, this inhibition was abolished by ablation of the NAc cholinergic neurons. These results demonstrate that centrally active AChE inhibitors prevent long-lasting behavioral abnormalities associated with cocaine and morphine addictions by potentiating the actions of ACh released from the NAc cholinergic neurons. Centrally active AChE inhibitors could thus be approached as novel and potential therapeutic agents for drug addiction.
Drug addiction poses serious social, medical, and economic problems, but effective treatments for drug addiction are still limited (1, 2). The mesolimbic dopaminergic system serves as a vital and fundamental role in pathological behavioral changes that occur with repeated exposure of abusive drugs (3–5). In the mesolimbic dopaminergic pathway, dopaminergic neurons originate in the ventral tegmental area and project to the nucleus accumbens (NAc), the ventral part of the striatum (6, 7). The NAc is a key neural substrate that is implicated in reinforcement and addiction of cocaine and morphine (3–5). These abusive drugs elevate dopamine levels in the NAc (8), and the overwhelming actions of dopamine in the NAc lead to neural adaptation that underlies reinforcement and addiction of cocaine and morphine (3, 4).
The activities of the principal γ-aminobutyric acid-containing, medium-sized spiny neurons in the NAc are modulated by not only dopaminergic input but also cholinergic input (9). The cholinergic input is derived from aspiny cholinergic interneurons within the NAc (7, 10). Because acetylcholine (ACh) agonists or antagonists generated global effects on many brain regions, the role of ACh in reinforcement and addiction of abusive drugs was not well understood (11–15). In our previous study, we investigated the role of ACh in the NAc circuit by selectively ablating the NAc cholinergic neurons with use of immunotoxin (IT)-mediated cell targeting techniques (16, 17). These investigations revealed that ACh regulates the NAc circuit concertedly but oppositely to dopamine and that cholinergic cell ablation enhances long-lasting behavioral changes of cocaine addiction (16, 17). ACh from cholinergic neurons in the NAc thus plays a pivotal role in neural responses and adaptation that underlie cocaine reinforcement and addiction.
This investigation concerns whether ACh in the NAc commonly regulates morphine-induced behavioral changes and whether enhancement of ACh in the NAc prevents behavioral abnormalities of cocaine and morphine. To address the latter question, we used acetylcholinesterase (AChE) inhibitors that act on the brain AChE and elevate ACh levels in the striatum and other brain regions (18–20). We report here that cholinergic cell ablation in the NAc increases the sensitivity to morphine in both its rewarding effects and negative reinforcements of morphine withdrawal. We further report that centrally active AChE inhibitors block the induction and persistence of addictive behaviors of both morphine and cocaine via enhanced actions of ACh in the NAc.
Materials and Methods
Animals and Drugs.
Male C57BL/6 mice (9–13 weeks) were purchased from Japan SLC (Hamamatsu, Japan) and were used as wild-type mice. The IG17 line of heterozygous transgenic mice expressing the fusion protein of human IL-2 receptor α/GFP (21) and their wild-type littermates (9–13 weeks) were used for the IT-mediated cell targeting experiments. Behavioral analysis was carried out 2 weeks after IT injection (17). All procedures were performed according to the guidelines of Kyoto University Faculty of Medicine. The following drugs were obtained from the following sources: morphine hydrochloride and cocaine hydrochloride (both from Takeda, Osaka), naloxone hydrochloride (Sankyo), donepezil hydrochloride (Eisai, Tokyo), and galanthamine hydrobromide (Sigma).
Conditioned Place Preference (CPP), Conditioned Place Aversion (CPA), and Morphine Withdrawal.
The CPP test was performed as described (17). Briefly, CPP was tested in a three-chamber apparatus (MED Associates, St. Albans, VT) in which the two large side chambers were separated by a small middle chamber. The two side chambers differed in floor and wall conditions. On day 0, mice were allowed to move freely in the three chambers for 30 min. On days 1–3, mice were confined to one large chamber for 20 min immediately after they had received saline. Four hours later, they received morphine or cocaine and were confined to the other side chamber for 20 min. On day 4, mice were placed in the middle chamber and allowed to move freely in the three chambers for 30 min. CPP was evaluated by calculating the time difference in which the time mice spent in the saline-paired chamber was subtracted from the time mice spent in the drug-paired chamber. Doses of morphine and cocaine administered at each day were 5 and 10 mg/kg, respectively, unless otherwise stated. For CPA analysis, morphine dependence was developed with twice daily i.p. morphine administration. Morphine administration was started with 10 mg/kg on day 1 and progressively increased with a 10-mg/kg increment from day 2 to day 4. Conditioning of naloxone-induced place aversion was conducted on day 5 in the three-chamber apparatus described previously. One hour after 50 mg/kg morphine treatment, saline was i.p. injected into the mice, and they were confined to one chamber for 20 min. Four hours later, they were again treated with 50 mg/kg morphine, and 1 h later, they were i.p. injected with 1 mg/kg naloxone and confined to the other chamber for 20 min. On day 6, the mice were allowed to move freely for 30 min, and CPA was evaluated by calculating the time difference in which the time mice spent in the saline-paired chamber was subtracted from the time mice spent in the naloxone-paired chamber. For examination of physical signs of morphine withdrawal, morphine dependence was developed over a period of 4 days as described previously. Saline or naloxone (1 mg/kg) was injected 1 h after 50 mg/kg morphine treatment on day 5. Jumping, rearing, and forepaw tremors were then counted during a 20-min period.
Other Behavioral Analyses.
The hot-plate test was conducted by placing mice on a hot plate at 55°C. The time at which the mice showed the first hind-paw licking or jumping was measured with a cut-off time of 30 sec. The tail-immersion test was carried out by immersing a distal half of the mouse tail in water at 53°C. The time at which the mice showed the first tail movement was measured with a cut-off time of 20 sec. Morphine was injected 20 min before the tests. Locomotor activity was measured with an infrared activity monitor (MED Associates) for a 10-min period immediately after cocaine injection with and without 10-min pretreatment of donepezil (1 mg/kg) or galanthamine (1 mg/kg).
Data Analysis.
Data are expressed as means ± SEM. Behavioral data were subjected to ANOVA, and post hoc comparisons were made with a Scheffé test.
Results
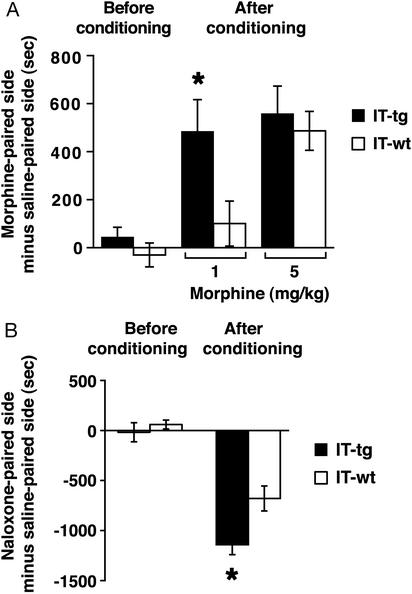
Cholinergic neurons in the NAc were selectively ablated by IT-mediated cell targeting techniques (IMCT; ref. 17). In the IMCT techniques, we generated transgenic mice in which the fusion protein of human IL-2 receptor α/GFP (hIL-2R/GFP) was driven by the promoter function of metabotropic glutamate receptor subtype 2 (16, 17, 21, 22). In these mice, hIL-2R/GFP was specifically expressed in cholinergic neurons within the cell population of the NAc (16, 17). The IT is composed of the Fv portion of a mAb reacting with hIL-2R fused to a 38-kDa fragment of Pseudomonas exotoxin (21). IT was injected at a single site on both sides of the NAc (17). The IT injection selectively eliminated >70% of the cholinergic neurons in the NAc cell population of transgenic mice (17). No such ablation was observed in the NAc of IT-treated wild-type mice (17). Two weeks after IT injection, we examined the effects of cholinergic cell elimination on CPP developed with repeated morphine administration (Fig. 1A). In the CPP paradigm, mice learn to associate the rewarding effect of morphine with a drug-paired environment (11). Before conditioning, both IT-treated wild-type and transgenic mice showed no preference in visiting drug- and saline-paired chambers that differed visually and textually. After conditioning with morphine for 3 days, cholinergic cell-eliminated transgenic mice exhibited a significant preference to a morphine-paired chamber with a low dose of morphine (1 mg/kg; Fig. 1A). The result indicates that ACh in the NAc controls long-lasting actions of morphine in a manner similar to that reported for cocaine (17).
Figure 1.
Effects of cholinergic cell elimination on morphine-induced CPP and naloxone-induced CPA. (A) CPP was developed by repeated administration of indicated doses of morphine for 3 days (n = 7–12). Cholinergic cell-eliminated transgenic mice spent significantly more time at the morphine-paired chamber after conditioning with 1 mg/kg morphine (*, P < 0.05). (B) CPA was developed by naloxone injection (1 mg/kg) after establishment of morphine dependence (n = 8 each). Cholinergic cell-eliminated transgenic mice spent significantly less time at the naloxone-paired chamber (*, P < 0.05).
We next examined withdrawal responses of morphine addiction by using the CPA paradigm. Morphine dependence was developed by twice daily i.p. administration of morphine with a gradual increment from 10 to 40 mg/kg morphine during days 1–4. On day 5, the mice were place-conditioned by i.p. injection with saline in one chamber and then with the morphine antagonist naloxone in the other chamber, each injected 1 h after morphine administration (50 mg/kg). On day 6, CPA was tested by allowing the mice to visit freely the saline- and naloxone-paired chambers. Wild-type and transgenic mice showed CPA but cholinergic cell-eliminated transgenic mice were more aversive to naloxone than wild-type mice (Fig. 1B). Negative reinforcement with morphine withdrawal is also enhanced by elimination of cholinergic neurons in the NAc.
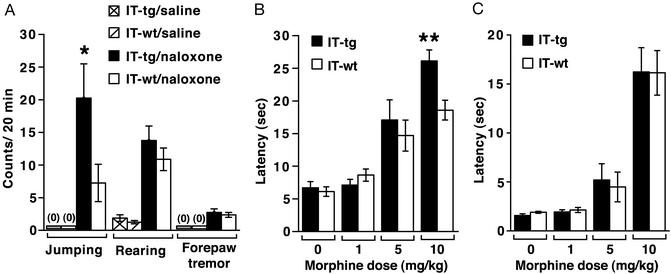
Mice also develop a physical morphine dependence after repeated morphine exposure. When the physical signs of naloxone-induced withdrawal symptoms were analyzed, both wild-type and cholinergic cell-eliminated mice showed jumping, forepaw tremor, and enhanced rearing (Fig. 2A). Jumping behavior is regarded as a dominant physical sign of morphine withdrawal (23). This behavior was significantly enhanced in cholinergic cell-eliminated mice as compared with wild-type mice (Fig. 2A). These findings indicate that not only a psychological but also a physical dependence of chronic morphine exposure is enhanced by cholinergic cell elimination.
Figure 2.
Effects of cholinergic cell elimination on naloxone-induced withdrawal behaviors and morphine antinoception. (A) Physical signs of morphine withdrawal were monitored during a 20-min period after naloxone injection in morphine-dependent mice (n = 8 each). After naloxone treatment, jumping, but not rearing or forepaw tremor, was most frequently induced in cholinergic cell-eliminated mice than IT-treated wild-type littermates (*, P < 0.05). No abnormal physical behaviors were observed in both genotypes by saline injection. (B and C) IT-treated wild-type and transgenic mice were treated with indicated doses of morphine, and latencies of antinociceptive responses in the hot-plate (B) and tail-immersion (C) tests were measured 20 min after morphine administration (n = 7–8). Cholinergic cell-eliminated mice showed a significantly prolonged latency of antinociceptive responses at 10 mg/kg morphine in the hot-plate test (**, P < 0.01).
We next tested for antinociceptive responses to morphine. In the hot-plate test, antinociceptive effects became stronger at a higher dose of morphine in cholinergic cell-eliminated mice than wild-type mice (Fig. 2B). In contrast, no difference was observed between two types of mice in the tail-immersion test (Fig. 2C). This difference between the two tests is interesting, because it has been generally accepted that the hot-plate response involves supraspinal analgesia, whereas the tail-immersion response mainly occurs at the level of the spinal cord (24). In the hot-plate and tail-immersion tests, both wild-type and cholinergic cell-eliminated mice developed comparative antinociceptive tolerance by daily administration of 10 mg/kg morphine for 5 days (data not shown). The results indicate that cholinergic cell elimination influences supraspinal antinociceptive responses to acute challenge with a high dose of morphine but has no effect on morphine-induced desensitization.
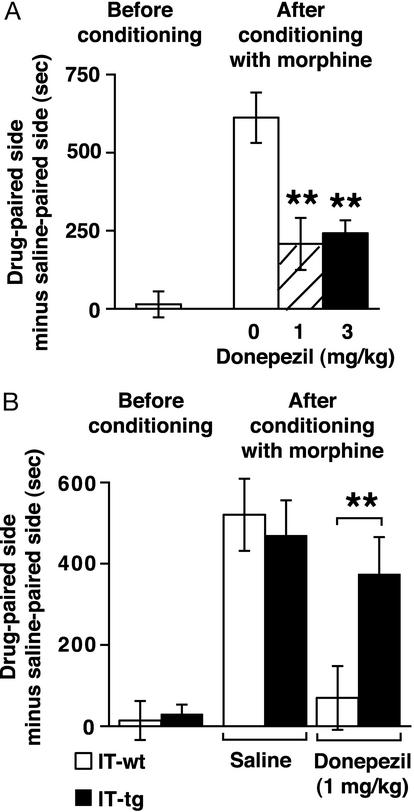
Ablation of intrastriatal cholinergic neurons significantly reduces ACh levels in the striatum (16). We addressed whether elevation of ACh in the NAc prevents behavioral changes associated with abusive drugs. We used donepezil, a selective, centrally active AChE inhibitor (18, 19) that elevates ACh levels in the striatum and other brain regions (20). Wild-type mice were i.p. injected with donepezil or saline 20 min before place-conditioning with daily administration of morphine (5 mg/kg) for 3 days. Pretreatment with donepezil strikingly reduced development of morphine-induced CPP (Fig. 3A).
Figure 3.
Effects of donepezil on morphine-induced CPP. (A) Wild-type mice were pretreated with donepezil (1 or 3 mg/kg) or saline 20 min before place-conditioning with 5 mg/kg morphine for 3 days (n = 7–11). Both doses of donepezil significantly reduced morphine-induced CPP (**, P < 0.01). (B) IT-treated wild-type and transgenic mice were place-conditioned by using the same protocol described in A (n = 7–9). Donepezil significantly reduced morphine-induced CPP in IT-treated wild-type mice but failed to suppress morphine-induced CPP in IT-treated transgenic mice (**, P < 0.01).
We then examined the effects of donepezil in cholinergic cell-eliminated transgenic mice to address whether ACh derived from the NAc cholinergic neurons was required for reduction of CPP by donepezil. Remarkably, cholinergic cell-eliminated transgenic mice failed to respond to donepezil and showed significant morphine-induced CPP comparable with saline-treated mice (Fig. 3B). The result indicates that ACh released from NAc cholinergic neurons is essential as a target of the AChE inhibitor for preventing morphine-induced CPP.
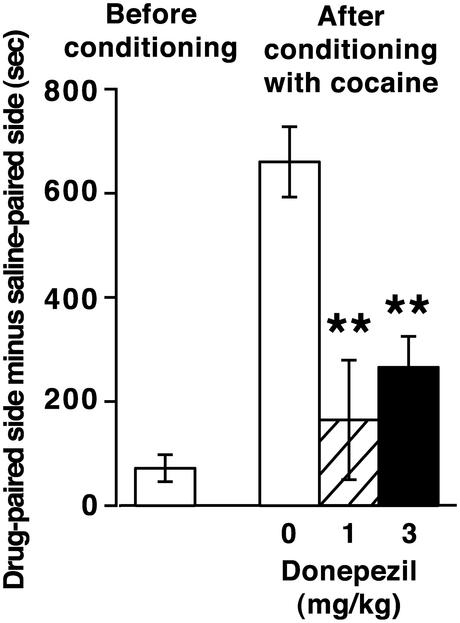
Our analysis of donepezil was extended to behavioral changes associated with cocaine addiction. We first examined the effects of donepezil on the development of cocaine-induced CPP. Wild-type mice were pretreated with donepezil or saline 20 min before place-conditioning with cocaine administration (10 mg/kg) for 3 days. This analysis revealed that donepezil significantly reduced cocaine-induced CPP (Fig. 4).
Figure 4.
Effects of donepezil on cocaine-induced CPP. Wild-type mice were pretreated with donepezil (1 or 3 mg/kg) or saline 20 min before place-conditioning with 10 mg/kg cocaine for 3 days (n = 7–9). Both doses of donepezil significantly reduced cocaine-induced CPP (**, P < 0.01).
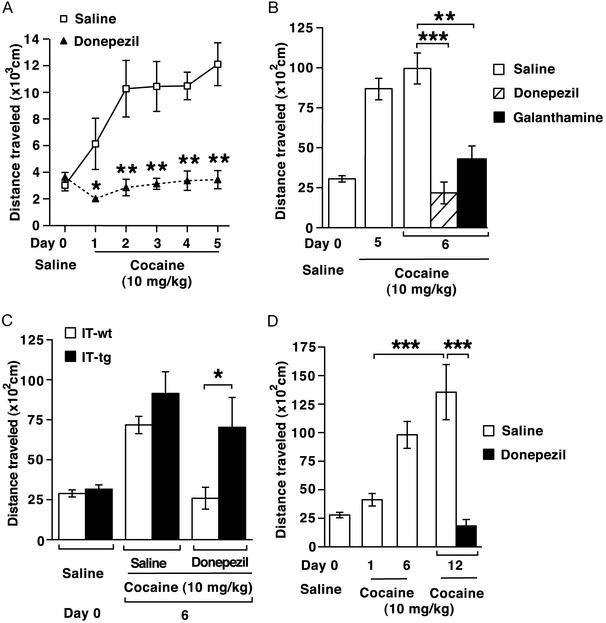
Repeated cocaine administration also induces a progressive increase in locomotor activity, called locomotor sensitization (refs. 17 and 25; Fig. 5A). Donepezil treatment before cocaine administration completely blocked cocaine-induced hyperlocomotion throughout the course of repeated cocaine exposure (Fig. 5A).
Figure 5.
Effects of donepezil on cocaine-induced locomotor sensitization. (A) Wild-type mice were pretreated with donepezil (1 mg/kg) or saline (n = 10 each) 10 min before daily cocaine administration (10 mg/kg). Locomotor activities were counted during a 10-min period immediately after cocaine administration. Repeated ANOVA showed that donepezil prevented cocaine-induced locomotor sensitization (F1,18 = 20.7, P < 0.001). The locomotor activity was significantly reduced when compared at each day (*, P < 0.05; **, P < 0.01). (B) Wild-type mice daily received cocaine (10 mg/kg) from day 1 to day 5. On day 6, the mice received saline (n = 12), donepezil (1 mg/kg, n = 7), or galanthamine (1 mg/kg, n = 5) 10 min before cocaine administration (10 mg/kg). The locomotor activities of both donepezil- and galanthamine-treated mice were significantly reduced as compared with that of saline-treated mice (***, P < 0.001; **, P < 0.01). (C) Wild-type and cholinergic cell-eliminated mice daily received cocaine (10 mg/kg) for 5 days. On day 6, the animals were pretreated with saline or donepezil (1 mg/kg) 10 min before cocaine administration (n = 7–11). Cholinergic cell-eliminated mice showed resistance to donepezil-mediated inhibition (*, P < 0.05). (D) Wild-type mice daily received cocaine (10 mg/kg) for 6 days. Five days after a cocaine-free interval (day 12), the animals were challenged with cocaine (10 mg/kg) 10 min after saline or donepezil (1 mg/kg) injection (n = 6). The locomotor activity on day 12 was significantly higher than that on day 1 (***, P < 0.001) and this high locomotor activity on day 12 was abolished by pretreatment with donepezil (***, P < 0.001).
We then examined the effects of donepezil on hyperlocomotor activity after establishment of cocaine-induced locomotor sensitization. Wild-type mice were repeatedly treated with cocaine in the absence of donepezil for 5 days. On day 6, the cocaine-exposed animals were pretreated with saline or donepezil, and their cocaine-induced locomotion was tested (Fig. 5B). Donepezil was effective in blocking hyperlocomotion after the establishment of locomotor sensitization. In this experiment, we also tested the ability of a different AChE inhibitor, galanthamine (18), to suppress cocaine-induced hyperlocomotion. Galanthamine was comparably effective in inhibiting cocaine-induced hyperlocomotion (Fig. 5B).
Effects of donepezil on locomotor sensitization were then tested between wild-type and cholinergic cell-eliminated mice (Fig. 5C). Chronic cocaine exposure induced much higher locomotion in cholinergic cell-eliminated mice than in wild-type mice (ref. 17; Fig. 5C). This sensitized hyperlocomotion was clearly resistant to donepezil in cholinergic cell-eliminated transgenic mice (Fig. 5C). These results indicate that ACh derived from cholinergic neurons in the NAc is targeted by the action of AChE inhibitors and the resulting enhancement of ACh in the NAc is critical for preventing cocaine-induced hyperlocomotion.
The adaptive response to cocaine persists even in the absence of cocaine administration after chronic cocaine exposure (3, 4, 26). To examine the effects of donepezil on long-lasting cocaine-induced adaptation, wild-type mice were sensitized with a daily injection of cocaine for 6 days and kept cocaine-free for the next 5 days. On day 12, these animals were challenged with cocaine after pretreatment with saline or donepezil. In saline-injected animals, the locomotion was significantly higher on day 12 than the initial cocaine-induced locomotion on day 1 (Fig. 5D). Importantly, this adaptive hyperlocomotion was completely suppressed by donepezil (Fig. 5D). The result indicates that the long-lasting cocaine-induced adaptation is also blocked by AChE inhibitors.
Discussion
Cholinergic neurons fill the NAc with highly radiating dendritic trees and a dense plexus of axonal branches (10). These cells thus provide rich connections with the principal medium-sized spiny neurons throughout the NAc. ACh released from these neurons acts concertedly but oppositely to dopamine on the principal medium-sized spiny neurons in the NAc (9, 16, 17). The convergent interactions between dopamine and ACh would thus contribute to regulation of neural responses and adaptation in the NAc circuit. However, earlier studies with ACh agonists and antagonists failed to indicate the regulatory role of ACh in abusive drugs because these agents exhibited global effects on many other brain regions (11–15). The behavioral studies combined with cholinergic cell ablation now reveal that ACh from cholinergic neurons plays a pivotal role in reinforcement and addiction of both cocaine and morphine. Importantly, centrally active AChE inhibitors blocked the development and persistence of behavioral changes associated with addiction of these drugs. This inhibition is derived from enhancement of ACh in the NAc, because depletion of ACh sources by cholinergic cell elimination markedly attenuated the blocking effects of the AChE inhibitor on drug-induced abnormal behaviors. This conclusion was supported further by contrasting effects of AChE inhibitors and the muscarinic receptor agonist pilocarpine on cocaine-induced CPP (data not shown). This receptor agonist effectively blocked cocaine-induced CPP in cholinergic cell-eliminated transgenic mice. Thus, ACh released presynaptically from cholinergic neurons serves as a target of AChE inhibitors and controls the induction and persistence of addictions of cocaine and morphine.
A variety of compounds acting on neurotransmitter receptors and their intracellular effectors was developed as therapeutic agents for drug addictions (1, 2). However, available medications for drug addictions are still limited or lacking (1, 2). Because dopamine is a key neurotransmitter for many categories of drugs that are abused (3, 4), the convergent interaction between dopamine and ACh in the NAc may also control behavioral changes of other addictive drugs and alcoholism. Importantly, centrally active AChE inhibitors such as donepezil and galanthamine have been shown to result in a long-lasting brain ACh elevation (18–20) and are widely used for preventing the progression of human Alzheimer's disease (18, 19). Thus, AChE inhibitors are not only useful for a better understanding of common mechanisms underlying drug addictions but also could be approached as potential therapeutic agents for patients who abuse drugs and alcohol.
Acknowledgments
We thank Akira Kakizuka for invaluable advice and Eisai Co., Ltd., for a gift of donepezil. This work was supported in part by research grants from the Ministry of Education, Science, and Culture of Japan and the International Resource Program of the National Cancer Institute.
Abbreviations
- NAc
nucleus accumbens
- ACh
acetylcholine
- CPP
conditioned place preference
- CPA
conditioned place aversion
- AChE
acetylcholinesterase
- IT
immunotoxin
References
- 1.Pouletty P. Nat Rev Drug Discovery. 2002;1:731–736. doi: 10.1038/nrd896. [DOI] [PubMed] [Google Scholar]
- 2.Kreek M J, LaForge K S, Butelman E. Nat Rev Drug Discovery. 2002;1:710–726. doi: 10.1038/nrd897. [DOI] [PubMed] [Google Scholar]
- 3.Koob G F, Sanna P P, Bloom F E. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- 4.Nestler E J. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- 5.Wise R A. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- 6.Oades R D, Halliday G M. Brain Res Rev. 1987;12:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- 7.Pennartz C M A, Groenewegen H J, Lopes da Silva F H. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 8.Di Chiara G, Imperato A. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Chiara G, Morelli M, Consolo S. Trends Neurosci. 1994;17:228–233. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 10.Kawaguchi Y, Wilson C J, Augood S J, Emson P C. Trends Neurosci. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- 11.Tzschentke T M. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 12.Heidbreder C A, Shippenberg T S. Synapse. 1996;24:182–192. doi: 10.1002/(SICI)1098-2396(199610)24:2<182::AID-SYN10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Wang J Q, McGinty J F. Neuroscience. 1996;75:43–56. doi: 10.1016/0306-4522(96)00277-1. [DOI] [PubMed] [Google Scholar]
- 14.Rasmussen T, Sauerberg P, Nielsen E B, Swedberg M D B, Thomsen C, Sheardown M J, Jeppesen L, Calligaro D O, DeLapp N W, Whitesitt C, et al. Eur J Pharmacol. 2000;402:241–246. doi: 10.1016/s0014-2999(00)00442-8. [DOI] [PubMed] [Google Scholar]
- 15.Itzhak Y, Martin J L. Psychopharmacology. 2000;152:216–223. doi: 10.1007/s002130000537. [DOI] [PubMed] [Google Scholar]
- 16.Kaneko S, Hikida T, Watanabe D, Ichinose H, Nagatsu T, Kreitman R J, Pastan I, Nakanishi S. Science. 2000;289:633–637. doi: 10.1126/science.289.5479.633. [DOI] [PubMed] [Google Scholar]
- 17.Hikida T, Kaneko S, Isobe T, Kitabatake Y, Watanabe D, Pastan I, Nakanishi S. Proc Natl Acad Sci USA. 2001;98:13351–13354. doi: 10.1073/pnas.231488998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krall W J, Sramek J J, Cutler N R. Ann Pharmacother. 1999;33:441–450. doi: 10.1345/aph.18211. [DOI] [PubMed] [Google Scholar]
- 19.Sugimoto H, Ogura H, Arai Y, Iimura Y, Yamanishi Y. Jpn J Pharmacol. 2002;89:7–20. doi: 10.1254/jjp.89.7. [DOI] [PubMed] [Google Scholar]
- 20.Isomae K, Ishikawa M, Ohta M, Ogawa Y, Hasegawa H, Kohda T, Kamei J. Jpn J Pharmacol. 2002;88:206–212. doi: 10.1254/jjp.88.206. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe D, Inokawa H, Hashimoto K, Suzuki N, Kano M, Shigemoto R, Hirano T, Toyama K, Kaneko S, Yokoi M, et al. Cell. 1998;95:17–27. doi: 10.1016/s0092-8674(00)81779-1. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida K, Watanabe D, Ishikane H, Tachibana M, Pastan I, Nakanishi S. Neuron. 2001;30:771–780. doi: 10.1016/s0896-6273(01)00316-6. [DOI] [PubMed] [Google Scholar]
- 23.Bläsig J, Herz A, Reinhold K, Zieglgänsberger S. Psychopharmacologia. 1973;33:19–38. doi: 10.1007/BF00428791. [DOI] [PubMed] [Google Scholar]
- 24.Kieffer B L. Trends Pharmacol Sci. 1999;20:19–26. doi: 10.1016/s0165-6147(98)01279-6. [DOI] [PubMed] [Google Scholar]
- 25.Koob G F. Neuron. 1996;16:893–896. doi: 10.1016/s0896-6273(00)80109-9. [DOI] [PubMed] [Google Scholar]
- 26.Grimm J W, Hope B T, Wise R A, Shaham Y. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]