Abstract
The widely spanning sensory cortex receives inputs from the disproportionately smaller nucleus of the thalamus, which results in a wide variety of travelling distance among thalamic afferents. Yet, latency from the thalamus to a cortical cell is remarkably constant across the cortex (typically, ≈2 ms). Here, we found a mechanism that produces invariability of latency among thalamocortical afferents, irrespective of the variability of travelling distances. The conduction velocity (CV) was calculated from excitatory postsynaptic currents recorded from layer IV cells in mouse thalamocortical slices by stimulating the ventrobasal nucleus of the thalamus (VB) and white matter (WM). In adults, the obtained CV for VB to WM (CVVB-WM; 3.28 ± 0.11 m/s) was ≈10 times faster than that of WM to layer IV cells (CVWM-IV; 0.33 ± 0.05 m/s). The CVVB-WM was confirmed by recording antidromic single-unit responses from VB cells by stimulating WM. Exclusion of synaptic delay from CVWM-IV did not account for the 10-fold difference of CV. By histochemical staining, it was revealed that VB to WM was heavily myelinated, whereas in the cortex staining became substantially weaker. We also found that such morphological and physiological characteristics developed in parallel and were accomplished around postnatal week 4. Considering that VB to WM is longer and more variable in length among afferents than is the intracortical region, such an enormous difference of CV makes conduction time heavily dependent on the length of intracortical region, which is relatively constant. Our finding may well provide a general strategy of connecting multiple sites irrespective of distances in the brain.
The timing of synaptic inputs onto postsynaptic neurons is of great importance, not only in the information processing (1–6) but also in the subsequent plasticity of the input (7–10). Yet, the expanse of cortex, in contrast with the disproportionately smaller nucleus of the thalamus (11, 12), makes the thalamocortical afferent trajectory crooked, resulting in a variety of travelling distances among the afferents. Nevertheless, a spike in thalamic cells arrives in cortical cells within a narrow window of time that peaks ≈2 ms (range: ≈1–4 ms), and this timing window seems well preserved, in some cases, even across the modality (visual, auditory, and somatosensory; refs. 2 and 13–16). This observation raises the following question: how is the timing of input (latency) kept within a narrow window of time, irrespective of travelling distances? Interestingly, white matter (WM) stimulation evokes monosynaptic responses in the cortex, whose latency falls in a window of time similar to that of thalamic stimulation, although the travelling distance is less than half. We therefore systematically examined the conduction velocity (CV) of thalamocortical fibers and found that the CV along this axon is not homogeneous. Instead, the CV of thalamus to WM was 10× faster than the rest of the afferents, because of a selective myelination. We also found that, in newborn animals, the difference of CVs was least remarkable and the adult pattern was attained during postnatal week 4.
Considering that the distance from the thalamus to WM is longer than that of the rest of the afferents and there is greater variability in length among afferents, such an enormous difference of CV by partial myelination serves as a mechanism that eliminates the timing disadvantage of long-distance connections, and helps to make the wide cortical area accessible from the thalamus within a narrow timing window.
Materials and Methods
Postnatal day (P)3-P60 C57/BL6 mice were used in this study. The day of birth was assigned as P0. Thalamocortical slices were prepared in a conventional way with a small modification by using a rotor slicer (Dosaka EM, Kyoto; refs. 17 and 18). Briefly, mice were deeply anesthetized with isoflurane and then were decapitated. The brain was rapidly removed and slices (400–500 μm) were bathed in ice-cold artificial cerebrospinal fluid (aCSF) consisting of the following (in mM): NaCl 124, KCl 3, KH2PO4 1.2, MgSO4 1.3, NaHCO3 26, CaCl2 2, and glucose 10, and were oxygenated in an atmosphere of 95% O2 and 5% CO2. The slices were allowed to recover in the aCSF at room temperature (27 ± 1°C) for at least 1 h before recording.
Electrophysiology.
Concentric bipolar stimulating electrodes were placed on the ventrobasal nucleus of the thalamus (VB) and WM (Fig. 1a). In some experiments another stimulating electrode was placed between the WM and the recording electrode (Fig. 3c). Extracellular field potentials or single-unit activity was recorded with a glass micropipette filled with 2% pontamine sky blue in 0.5% sodium acetate, with which a dye spot was made at the end of some experiments; otherwise, a pipette filled with 2 M NaCl was used as conventionally. To record synaptic current, a whole-cell patch pipette was used. The internal solution was as follows (in mM): 130 cesium methanesulfonate/10 Hepes/10 cesium chloride/0.5 EGTA with pH adjusted to 7.3 by cesium hydroxide. Results were recorded from the center of the primary somatosensory cortex (Fig. 1a), except for those experiments in Fig. 5. All recordings were amplified by Axopatch 1D or 2B (Axon Instruments, Foster City, CA). Extracellular potentials, both field and single-unit, and synaptic currents were low-pass filtered at 1 and 2 kHz, respectively, then were digitized (sampled at 10 kHz) and acquired by means of CLAMPEX 8 software (Axon Instruments). At the end of the experiments, photomicrographs of both the recording and stimulation sites were taken digitally (see Fig. 5) by a camera attached to the recording system. In other experiments, dye deposit of Pontamine sky blue was made iontophoretically, then slices were pictured. Then, distances between each site were measured on the printed-out pictures.
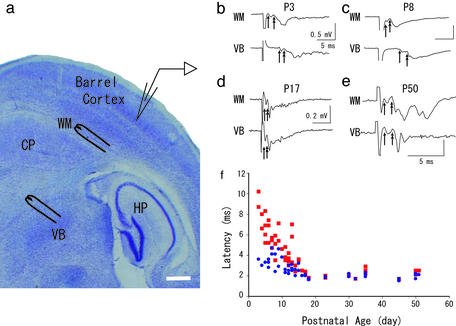
Figure 1.
(a) A photomicrograph of a thalamocortical slice, Nissl stained, representing our experimental procedure. Stimulation and recording sites are schematically illustrated. HP, hippocampus; CP, caudate putamen. (Bar, 500 μm.) (b–e) Four representative records of field potentials (an average of eight records) evoked by WM or VB obtained at indicated age are shown. Single- and double-headed arrows indicate the onset of the first and second components. (f) The latencies of the second components evoked by WM (blue circles) and VB (red squares) are plotted against postnatal age.
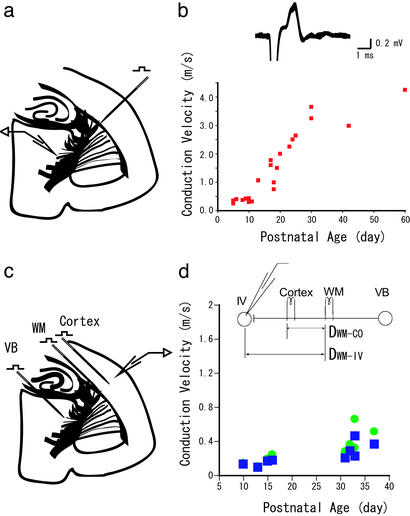
Figure 3.
(a) A schematic drawing of experiments for extracellular single-unit recording from VB evoked by antidromic stimulation of the WM. (b) (Upper) Sample recordings of an antidromic response. The superimposition of eight recordings is shown. The criteria for antidromic responses were the constancy of latency, surviving a double shock at a 7-ms interval, and resistance to Ca2+-free media. (Lower) CV calculated from antidromic responses are plotted against postnatal age. (c) A schematic drawing of experiments to obtain CVWM-IV, by placing an additional stimulating electrode between the WM and layer IV of the cortex (CO), to exclude the synaptic delay. (d) CV obtained by two methods plotted against postnatal age. ●, CV by differential distance (DWM-CO, Inset) divided by the differential latency, thus excluding the synaptic delay. ■, CV obtained by simple division of DWM-IV (Inset) over the latency of the WM, thus including the synaptic delay.
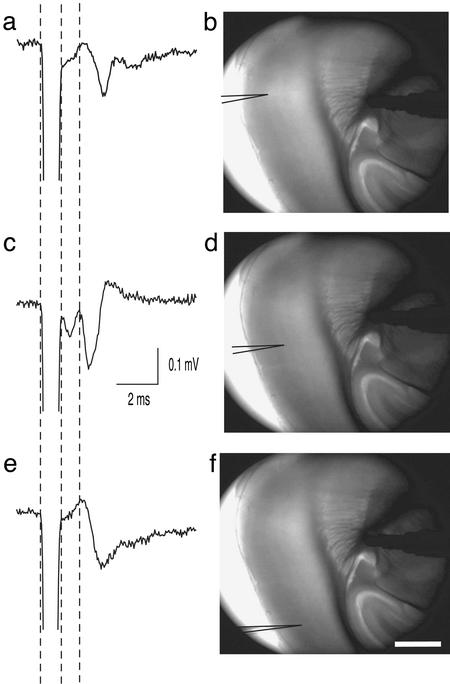
Figure 5.
A representative example showing that thalamic excitation reaches a wide range of the cortex with the least amount of variation in latencies. Field potentials (an average of five traces from P24 animals) shown in a, c, and e were recorded from three distantly separated sites in the barrel cortex in response to an almost identical stimulation site, as shown in b, d, and f, respectively. Latencies for the second component were 2.2, 1.9, and 2.1 ms for a, c, and e, respectively. The recording site in d was 361 μm away from that in b, and was 694 μm away from that of f. Stimulation was applied not exactly within, but slightly out of, the VB, where the thalamic afferents converge; thus, a large number of afferents could be activated. Stimulation in f was moved slightly (≈50 μm) to the hippocampus. (Bar, 500 μm.)
Myelin Staining.
To be able to see the myelin staining in the thalamocortical pathway, animals were first perfused intracardially with 1.25% glutaraldehyde and 1.0% paraformaldehyde in 0.1 M sodium phosphate buffer. Brains were stored in a postfixative that contained aldehydes at twice the concentrations given above. Thalamocortical slices (40 μm thick) were then sectioned on a Microtome (Yamato Kohki, Asaka, Japan). Sections were processed by a recently developed method (19).
Results
We first examined developmental changes in field potential latencies recorded from layer IV in response to thalamic and WM stimulation. Recorded field potentials in the barrel cortex consisted of multiple components throughout the development. The first component resisted high-frequency (10 Hz) stimulation and survived Ca2+-free media or the presence of an antagonist against excitatory amino acid receptor, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; data not shown), thus, it most likely represents presynaptic fiber activity, consistent with previous studies (17, 20–23). We noticed the latency of field potentials shortened during development, representative examples of which are shown in Fig. 1 b–e. At P3, latency for the synaptic component from VB was 5.9 ms, which was substantially longer than that from WM (2.7 ms). Both latencies did not shorten until P8 (7.7 ms and 2.7 ms from VB and WM, respectively). However, at P17, latency for VB shortened greatly (2.4 ms) and it became quite close to that from WM (2.0 ms), which was also the case through P50. Developmental changes of latencies is illustrated in Fig. 1f. This graph clearly demonstrates the following points: First, the latencies for VB were substantially longer than from WM in newborn animals, reflecting the distances. Second, both latencies, however, became smaller with age; then after around postnatal week 2, both latencies settled down to almost the same value, which remained constant throughout the rest of the lives of the animals. This finding raises a possibility that the CV of VB to WM (CVVB-WM) and WM to cortical cells (CVWM-IV) are differentially regulated during development, and, as a result, these two parts in a given thalamocortical fiber show quite distinct CV. We wanted to test this possibility, but because field response was not thought to be adequate for calculating precise CV, we performed whole-cell patch experiments.
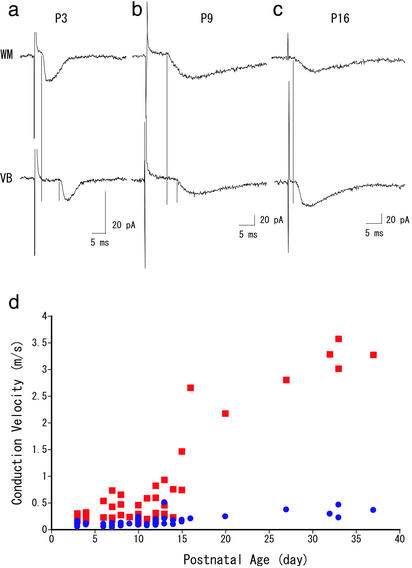
Latencies of excitatory postsynaptic currents evoked by VB or WM stimulation showed developmental changes similar to those of field potentials. As shown in Fig. 2 a–c, latencies from VB were substantially longer than those from WM at P3 and P9. However, the difference became very small at P16 (2.5 and 3.1 ms for WM and VB stimulation, respectively). On the basis of this latency, and the distances between the recording site and two stimulating sites, we calculated the CV (Fig. 2d). As seen in the graph, both CVWM-IV and CVVB-WM increased with age but to greatly different extents. At P3–P4, CVVB-WM was already 2 times faster than CVWM-IV, (0.10 ± 0.01 and 0.23 ± 0.03 m/s for CVWM-IV and CVVB-WM, respectively). During postnatal week 3, CVWM-IV increased ≈3-fold (0.33 ± 0.05 m/s, >P30, n = 4), whereas CVVB-WM increased >10-fold (3.28 ± 0.11 m/s, >P30, n = 4). The obtained value for CVWM-IV fell well within a range of CVs reported for vertical connections within the cortex (24–26). To obtain further support for exceptionally fast CVVB-WM, we recorded single-unit activities that were antidromically activated by a stimulation of WM (Fig. 3a). The resultant graph for CVVB-WM is illustrated in Fig. 3b, which turned out to be quite similar to that estimated from excitatory postsynaptic currents (Fig. 2d). Thus, we confirmed that CVVB-WM increased rapidly, and became almost 10 times faster than CVWM-IV during development.
Figure 2.
(a–c) Three representative excitatory postsynaptic currents (an average of five records) evoked by WM and VB stimulation recorded at P3 (a), P9 (b), and P16 (c) are presented. Vertical lines indicate the onset of excitatory postsynaptic currents. (d) The CV of two portions of thalamocortical axon, calculated by the latencies and distances between stimulated (VB and WM) and recorded sites. CVVB-WM (red squares) was calculated from the distance between VB and WM divided by the latency difference between VB and WM stimulation. CVWM (blue circles) was obtained by the simple calculation using distance between stimulated (WM) and recorded sites, divided by the latency evoked by WM stimulation.
Although the calculated value for CVWM-IV fit well with previously reported values, our value was not precise because it included a synaptic delay. To rule out the possibility that this delay might cause the difference between CVVB-WM and CVWM-IV, we placed an additional stimulating electrode between the WM and the recording site and calculated more precise CVWM-IV without a synaptic delay. We found that even when we excluded the synaptic delay, the CVWM-IV for >P30 (0.31 ± 0.06 m/s, n = 9) was still ≈10-fold smaller than the CVVB-WM (3.28 ± 0.11 m/s). The calculated synaptic delay for nine cells was 0.61 ± 0.11 ms, which is comparable with the previously reported values for thalamocortical synapses (27–29).
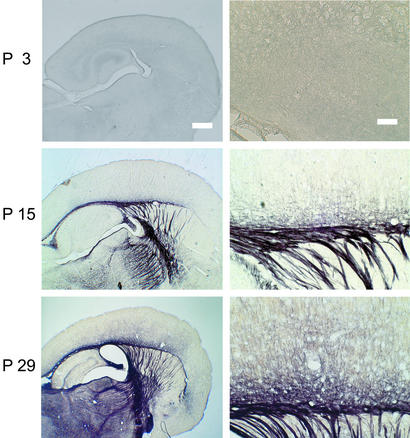
What could account for such 10-fold difference of CV? There are at least two possible ways to increase CV: myelination and the increment of an axon diameter. Theoretically, the CV is proportional to the diameter in a myelinated axon, and to the square root of the diameter in an unmyelinated axon (30, 31). Thus, if the thalamocortical axon was totally unmyelinated and the 10-fold difference was accounted for entirely by the diameter difference, a 100-fold difference of diameter would be necessary, which is most unlikely. The exclusion of this possibility would predict that the thalamocortical axon would be myelinated, at least to a certain extent. Thus, we attempted to investigate the extent of myelination along the thalamocortical fiber to see whether there was any detectable difference of myelination between the inside and outside of the cortex. For this experiment, we performed myelin staining in total of 18 mice from P1 to P36. Sections were made so that the thalamocortical pathway was preserved as we prepared for electrophysiological experiments. Fig. 4 shows the result of myelin staining at three representative ages. At P3, when both CVVB-WM and CVWM-IV were the slowest, there was no detectable staining of myelin. This result is consistent with the previous studies showing that myelination starts after P2 only for spinal cord, and the rest of the brain was totally unmyelinated (32, 33). At P15, when CVVB-WM was speeding (Figs. 2d and 3b) and latency for VB stimulation was shortening (Fig. 1f), there was a clear staining of myelin from the VB to the cortex, yet staining suddenly disappeared on entering the cortex (Fig. 4 Center Right). The lack of staining in the cortex is consistent with slow CVWM-IV (Figs. 2d and 3d), and only a little shortening of latency was observed in the field potentials evoked by WM stimulation (Fig. 1f). At P29, when CVVB-WM almost reached plateau (Figs. 2d and 3b), further staining of myelin was observed between the thalamus and cortex, whereas staining was again suddenly reduced on entering the cortex, although it was to some extent darker than at P15. This result clearly demonstrates that the observed difference of the CV between the inside and outside of the cortex was accounted for, although not exclusively, but at least mainly, by location-specific myelination in the thalamocortical axons.
Figure 4.
Photomicrographs of myelin staining at three representative ages, P3, P15, and P29. (Right) Higher magnifications of the transition between the WM and the cortex at each age. [Bars, 500 μm (Left) and 100 μm (Right).]
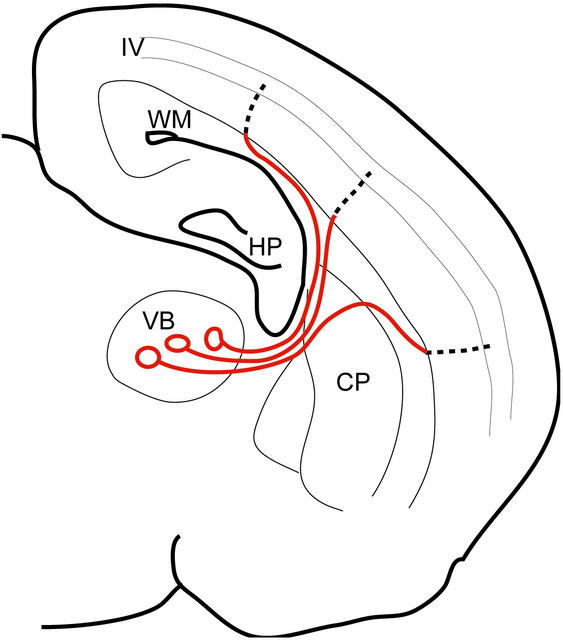
What would possibly be the advantage of such a drastic difference of CV based on a regional myelination? The cortical barrel area spans several millimeters in rodents. This causes the trajectory of thalamocortical axons to be crooked (see Fig. 6). Leaving the VB nucleus, the axons converge before entering the striatum, then they run in a straight path until they arrive at the WM, where they diverge widely. Within the WM, some axons ascend directly into the cortex, but others run in the subcortical WM for variable distances before ascending into the cortex (34). Thus, the total length of an axon differs from one axon to another, depending on each target region, and the difference amounts to almost two-fold (1,500–2,950 μm; ref. 34). In contrast, once the cortex is entered, the distance to layer IV is much shorter (less than ≈500 μm) and is comparatively constant. Based on these morphological characteristics, are cells that receive input from axons travelling a long distance temporally disadvantageous relative to those from axons travelling a short distance? If, as we have seen, the long and variable part, that is, from the thalamus before entering the cortex, had 10-fold the velocity by myelination relative to the rest of the axons, thalamic cells could send an excitation to the cortical cells at about the same time, irrespective of the travelling distance. We tested this hypothesis by stimulating the thalamocortical axon and recorded the field potentials from a wide range of the barrel cortex. We found that the results were consistent with this hypothesis. Fig. 5 presents a typical example, where field potentials were induced from a wide range of cortex (>1 mm) from a single stimulation site, but the difference of the latencies were in a submillisecond order (<0.3 ms; see Fig. 5 legend). Similar results were confirmed in five other animals, including the cases where constant latencies were observed across three successive slices. Also, such constant latency from the wide range of the cortex is quite a common observation during experiments using these thalamocortical slice preparations.
Figure 6.
A schematic presentation of the thalamocortical pathway, illustrating regional differences in CV. By having a 10-fold faster CV from the VB to the WM (red line), most of the conduction time is spent on the intracortical region (dotted line), whose length is generally constant; thus, the total latency from the VB to layer IV cells becomes comparable among the afferents. Abbreviations are as in Fig. 1.
Discussion
Our results demonstrated that a wide range of cortical regions are at temporally equivalent distances from the thalamus despite spatial inequalities, and this equivalence was achieved by a partial myelination of afferents, resulting in an almost 10-fold difference of CV between points before and after entering the cortex. We also showed that this property was developed during postnatal week 4. The strategy of making the CV of the longer and variable parts 10-fold faster than that of the shorter and constant parts seems quite exquisite for the purpose of eliminating the variability of travelling distances (Fig. 6). Most of the conduction time is spent while travelling the intracortical pathway, whose length is comparatively constant across the cortex. Thus, this phenomenon could theoretically be applied to other pathways as a common strategy of the brain, where multiple sources of input gather to postsynaptic cells and the input timing needs to be within a certain window (35, 36). However, even in the same thalamocortical pathway, conduction time is not necessarily the same when applied to motor projections. For example, the projections from thalamic ventrolateral and ventromedial nuclei to cortical area 4 in cats are significantly different, 1.8–2.3 and 2.8–3.0 ms, respectively (37). These findings, together with our results, would imply that the conduction time might be determined depending on the functions of that pathway. In addition, uniform conduction times in the rat olivocerebellar system (36) may be different from the same pathway in cats in which the conduction time varies linearly with the conduction distance (38). Thus, differences may also be observed depending on the pathways or species.
Axon diameters can be estimated from the CV in both myelinated and unmyelinated axons (30, 31). CV would be expected to increase linearly with increasing axonal diameter in a myelinated axon and to increase with the square root of the diameter in an unmyelinated axon. According to this hypothesis, the obtained CV indicates that extracortical myelinated fiber is 0.54 μm in diameter, including myelin [0.33 μm, myelin excluded, assuming that the axon vs. fiber diameter ratio is 0.6 (30)]. However, the diameter of the intracortical unmyelinated axon was calculated to be ≈0.02 μm. This prediction seems unlikely, because unmyelinated axons that are 0.3–0.4 μm in diameter are reported to be found at P13 (39), and in adults, they vary from 0.2 to 2 μm (E. L. White and D. L. Lev, personal communication). This discrepancy may presumably be due to an inappropriate ratio for the equation for this specific pathway. As discussed previously, the ratio for unmyelinated axon varies considerably because, in unmyelinated axons, unlike myelinated ones, specific membrane properties, probably such as density or kinds of channels, are different from one axon to the other (40). Thus, it seems we are not able to discuss the change of axon diameter between the extra- and intracortical portion, but we cannot rule out the possibility that the thinning of the axon might also contribute to the observed decrease of CV. In addition, although the axonal path is roughly perpendicular to cortical gray matter, although obviously not completely, and has significant branching (41). At the transition from myelinated to unmyelinated axon, there may also be a delay because of impedance mismatch (42). These factors could also contribute to the long conduction time in the cortex.
At both P15 and P29, dark staining was seen in the WM. Because the WM consists of heterogeneous fibers such as corticocortical, corticofugal, and corticothalamic, in addition to thalamocortical connections, observed staining may also represent other fibers besides thalamocortical ones. However, callosal and association fibers start to myelinate later than do thalamocortical fibers; callosal fibers are barely stained at P14, as are association fibers at P17 (32). Corticothalamic connections were also reported to myelinate later than they do in the thalamocortical path (43); in fact, staining at P58 was much darker (data not shown). Thus, the contribution of the thalamocortical afferent to the staining would be the greatest in these developing animals, compared with that in other pathways, although that of other fibers should not be ignored, especially in adults.
Changes of CV along an axon have been reported in other regions in the CNS (44), as well as between peripheral nerve roots and the spinal cord (45, 46). However, why CV needs to be different along a given fiber has not been discussed in these studies. Our results reasonably predict that similar functional significance may be underlying in the previously reported systems.
In the cortex, spiny stellate cells in layer IV receive input from neighboring cells, in addition to the thalamic cells, and interestingly, the latencies for those local connections are typically 1–3 ms, with a range of 1–6 ms (47, 48). This result indicates that, for a given layer IV stellate cell, thalamic input hardly has a temporal disadvantage, compared with neighboring cortical input, which might have significant implications in terms of possible subsequent change of the thalamic input. Recent experiments showed that the time at which the postsynaptic neuron receives inputs crucially affects whether the input is potentiated or suppressed subsequently (7–10). Thus, thalamic input is capable of competing with numerous types inputs of intracortical origin to obtain dominance in driving postsynaptic layer IV cells.
The timing of thalamic input to cortical cells is also important in generating synchronous activity in the cortex (4, 6, 49). Although we have not directly tested the relevance of our study to synchronous activity, the mechanism presented here theoretically enables the wide range of cortical regions to be at a temporally equivalent distance; thus it could underlie a thalamic-driven synchronous activity in the cortex. In addition, thalamocortical systems show characteristic low-frequency (7–14 Hz) synchronous oscillation, or spindles (50). The widespread synchrony and quasisimultaneous occurrence of these rhythms are generated in the thalamus by corticothalamic influences (51). This observation suggests, potentially, that the timing of corticothalamic projections may also be controlled by mechanisms similar to those described here.
The developmental time course of latency and CV did not match completely: there was a time lag of ≈1 week. A similar mismatch of latency and CV was also observed in other regions of the brain (52). In both cases, constant latency was maintained by a compensatory increment in the CV, while the brain was still growing in size during such a developmental period. These results again suggest that maintaining the timing of input is of primary importance.
In conclusion, our results demonstrated a mechanism for producing a timing of input that is consistent, regardless of varying travelling distance of signals, by implementing a regional myelination in the thalamocortical pathway.
Acknowledgments
We thank Drs. I. Sugihara, S. G. Waxman, E. White, and S. Nakamura for valuable discussions, and Drs. K. Samejima and Y. Sakumura for comments on the previous version of this article. M.S. was partially supported by the Ministry of Health and Medical Education of the Islamic Republic of Iran. This work was supported by a Grant-in-Aid from the Ministry of Education, Science, Sports, and Culture of Japan (to F.K.) and the Core Research for Evolutional Science and Technology–Japan Science and Technology Corporation (to T.T.).
Abbreviations
- VB
ventrobasal nucleus of the thalamus
- WM
white matter
- CV
conduction velocity
- CVVB-WM
CV from VB to WM
- CVWM-IV
CV from WM to layer IV
- Pn
postnatal day n
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Diesmann M, Gewaltig M O, Aertsen A. Nature. 1999;402:529–533. doi: 10.1038/990101. [DOI] [PubMed] [Google Scholar]
- 2.Usrey W M. Curr Opin Neurobiol. 2002;12:411–417. doi: 10.1016/s0959-4388(02)00339-2. [DOI] [PubMed] [Google Scholar]
- 3.Salinas E, Sejnowski T J. Nat Rev Neurosci. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Usrey W M, Reid R C. Annu Rev Physiol. 1999;61:435–456. doi: 10.1146/annurev.physiol.61.1.435. [DOI] [PubMed] [Google Scholar]
- 5.Stevens C F, Zador A M. Nat Neurosci. 1998;1:210–217. doi: 10.1038/659. [DOI] [PubMed] [Google Scholar]
- 6.Konig P, Engel A K, Singer W. Trends Neurosci. 1996;19:130–137. doi: 10.1016/s0166-2236(96)80019-1. [DOI] [PubMed] [Google Scholar]
- 7.Egger V, Feldmeyer D, Sakmann B. Nat Neurosci. 1999;2:1098–1105. doi: 10.1038/16026. [DOI] [PubMed] [Google Scholar]
- 8.Feldman D E. Neuron. 2000;27:45–56. doi: 10.1016/s0896-6273(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 9.Magee J C, Johnston D. Science. 1997;275:209–213. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L I, Tao H W, Holt C E, Harris W A, Poo M. Nature. 1998;395:37–44. doi: 10.1038/25665. [DOI] [PubMed] [Google Scholar]
- 11.Stevens C F. Nature. 2001;411:193–195. doi: 10.1038/35075572. [DOI] [PubMed] [Google Scholar]
- 12.Finlay B L, Darlington R B. Science. 1995;268:1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- 13.Bullier J, Henry G H. J Neurophysiol. 1979;42:1251–1263. doi: 10.1152/jn.1979.42.5.1251. [DOI] [PubMed] [Google Scholar]
- 14.Henry G H, Harvey A R, Lund J S. J Comp Neurol. 1979;187:725–744. doi: 10.1002/cne.901870406. [DOI] [PubMed] [Google Scholar]
- 15.Miller L M, Escabi M A, Read H L, Schreiner C E. Neuron. 2001;32:151–160. doi: 10.1016/s0896-6273(01)00445-7. [DOI] [PubMed] [Google Scholar]
- 16.Yen C T, Conley M, Jones E G. J Neurosci. 1985;5:1316–1338. doi: 10.1523/JNEUROSCI.05-05-01316.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agmon A, Connors B W. Neuroscience. 1991;41:365–379. doi: 10.1016/0306-4522(91)90333-j. [DOI] [PubMed] [Google Scholar]
- 18.Itami C, Samejima K, Nakamura S. Brain Res Protoc. 2001;7:103–114. doi: 10.1016/s1385-299x(01)00048-4. [DOI] [PubMed] [Google Scholar]
- 19.McNally K J, Peters A. J Histochem Cytochem. 1998;46:541–545. doi: 10.1177/002215549804600415. [DOI] [PubMed] [Google Scholar]
- 20.Morin D, Steriade M. Brain Res. 1981;205:49–66. doi: 10.1016/0006-8993(81)90719-8. [DOI] [PubMed] [Google Scholar]
- 21.Perl E R, Whitlock D G. J Neurophysiol. 1955;18:486–501. doi: 10.1152/jn.1955.18.5.486. [DOI] [PubMed] [Google Scholar]
- 22.Itami C, Mizuno K, Kohno T, Nakamura S. Brain Res. 2000;857:141–150. doi: 10.1016/s0006-8993(99)02352-5. [DOI] [PubMed] [Google Scholar]
- 23.Kimura F, Nishigori A, Shirokawa T, Tsumoto T. J Physiol (London) 1989;414:125–144. doi: 10.1113/jphysiol.1989.sp017680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura F, Fukuda M, Tsumoto T. Eur J Neurosci. 1999;11:3597–3609. doi: 10.1046/j.1460-9568.1999.00779.x. [DOI] [PubMed] [Google Scholar]
- 25.Fukuda M, Hata Y, Ohshima M, Tsumoto T. Neurosci Res. 1998;31:9–21. doi: 10.1016/s0168-0102(98)00018-2. [DOI] [PubMed] [Google Scholar]
- 26.Tanifuji M, Sugiyama T, Murase K. Science. 1994;266:1057–1059. doi: 10.1126/science.7973662. [DOI] [PubMed] [Google Scholar]
- 27.Toyama K, Matsunami K, Ono T, Tokashiki S. Exp Brain Res. 1974;21:45–66. doi: 10.1007/BF00234257. [DOI] [PubMed] [Google Scholar]
- 28.Shirokawa T, Nishigori A, Kimura F, Tsumoto T. Exp Brain Res. 1989;78:489–500. doi: 10.1007/BF00230237. [DOI] [PubMed] [Google Scholar]
- 29.Komatsu Y, Fujii K, Maeda J, Sakaguchi H, Toyama K. J Neurophysiol. 1988;59:124–141. doi: 10.1152/jn.1988.59.1.124. [DOI] [PubMed] [Google Scholar]
- 30.Waxman S G, Bennett M V. Nat New Biol. 1972;238:217–219. doi: 10.1038/newbio238217a0. [DOI] [PubMed] [Google Scholar]
- 31.Waxman S G. Muscle Nerve. 1980;3:141–150. doi: 10.1002/mus.880030207. [DOI] [PubMed] [Google Scholar]
- 32.Jacobson S. J Comp Neurol. 1963;121:5–29. doi: 10.1002/cne.901210103. [DOI] [PubMed] [Google Scholar]
- 33.Jacobson M. Developmental Neurobiology. 3rd. Ed. New York: Plenum; 1991. [Google Scholar]
- 34.Bernardo K L, Woolsey T A. J Comp Neurol. 1987;258:542–564. doi: 10.1002/cne.902580406. [DOI] [PubMed] [Google Scholar]
- 35.Pelletier J G, Pare D. J Neurophysiol. 2002;87:1213–1221. doi: 10.1152/jn.00623.2001. [DOI] [PubMed] [Google Scholar]
- 36.Sugihara I, Lang E J, Llinas R. J Physiol. 1993;470:243–271. doi: 10.1113/jphysiol.1993.sp019857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steriade M. J Comp Neurol. 1995;354:57–70. doi: 10.1002/cne.903540106. [DOI] [PubMed] [Google Scholar]
- 38.Aggelopoulos N C, Duke C, Edgley S A. J Physiol (London) 1995;486:763–768. doi: 10.1113/jphysiol.1995.sp020851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White E L, Weinfeld L, Lev D L. Somatosen Mot Res. 1997;14:34–55. doi: 10.1080/08990229771204. [DOI] [PubMed] [Google Scholar]
- 40.Ritchie J M. In: The Axon. Waxman S G, Kocsis J D, Stys P K, editors. New York: Oxford Univ. Press; 1995. pp. 68–96. [Google Scholar]
- 41.Arnold P B, Li C X, Waters R S. Exp Brain Res. 2001;136:152–168. doi: 10.1007/s002210000570. [DOI] [PubMed] [Google Scholar]
- 42.Waxman S G, Brill M H. J Neurol Neurosurg Psychiatry. 1978;41:408–416. doi: 10.1136/jnnp.41.5.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsumoto T, Suda K. Dev Brain Res. 1982;256:323–332. doi: 10.1016/0165-3806(82)90144-4. [DOI] [PubMed] [Google Scholar]
- 44.Baker G E, Stryker M P. Nature. 1990;344:342–345. doi: 10.1038/344342a0. [DOI] [PubMed] [Google Scholar]
- 45.Traub R J, Mendell L M. J Neurophysiol. 1988;59:41–55. doi: 10.1152/jn.1988.59.1.41. [DOI] [PubMed] [Google Scholar]
- 46.Fraher J P. J Anat. 1978;126:509–533. [PMC free article] [PubMed] [Google Scholar]
- 47.Feldmeyer D, Egger V, Lubke J, Sakmann B. J Physiol (London) 1999;521:169–190. doi: 10.1111/j.1469-7793.1999.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stern P, Edwards F A, Sakmann B. J Physiol (London) 1992;449:247–278. doi: 10.1113/jphysiol.1992.sp019085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gray C M. Neuron. 1999;24:31–47. doi: 10.1016/s0896-6273(00)80820-x. [DOI] [PubMed] [Google Scholar]
- 50.Steriade M, Deschenes M, Domich L, Mulle C. J Neurophysiol. 1985;54:1473–1497. doi: 10.1152/jn.1985.54.6.1473. [DOI] [PubMed] [Google Scholar]
- 51.Contreras D, Destexhe A, Sejnowski T J, Steriade M. Science. 1996;274:771–774. doi: 10.1126/science.274.5288.771. [DOI] [PubMed] [Google Scholar]
- 52.Song W J, Okawa K, Kanda M, Murakami F. J Physiol (London) 1995;488:419–426. doi: 10.1113/jphysiol.1995.sp020976. [DOI] [PMC free article] [PubMed] [Google Scholar]