Abstract
The High Mobility Group protein HMGA2 is a nuclear architectural factor that plays a critical role in a wide range of biological processes including regulation of gene expression, embryogenesis and neoplastic transformation. Several studies are trying to identify the mechanisms by which HMGA2 protein is involved in each of these activities, and only recently some new significant insights are emerging from the study of transgenic and knock-out mice. Overexpression of HMGA2 gene leads to the onset of prolactin and GH-hormone induced pituitary adenomas in mice, suggesting a critical role of this protein in pituitary tumorigenesis. This was also confirmed in the human pathology by the finding that HMGA2 amplification and/or overexpression is present in human prolactinomas. This review focuses on recent data that explain the mechanism by which HMGA2 induces the development of pituitary adenomas in mice. This mechanism entails the activation of the E2F1 protein by the HMGA2-mediated displacement of HDAC1 from pRB protein.
Background
Pituitary tumors constitute 10% of intracranial neoplasms, and are mostly benign with slow growth [1]. Most pituitary neoplasms secrete hormone gene products, leading to disturbed endocrine functions. Prolactinomas account for the most common type of pituitary adenomas [1,2], while about one-third of pituitary adenomas are not associated with clinical hypersecretory syndromes, but with symptoms of an intracranial mass that leads to headaches, hypopituitarism or visual-field disturbances, which are classified as non-functioning pituitary adenomas (NFPAs). The genesis of pituitary tumors is still mainly unknown, but the actual model supposes that genetic alterations represent the initializing event that transforms pituitary cells, and that hypothalamic hormones and other local growth factors may play an important role in promoting the growth of already transformed cells. However, the classical gene alterations involved in cell transformation, such as ras, BRAF, Rb, do not appear to be responsible for the onset of pituitary adenomas [3]. Only up to 40% of sporadic human GH-secreting adenomas have missense mutations of the Gsα gene [4], and many functional adenomas present the overexpression of a recently discovered powerful transforming gene, PTTG, which is able to exert strong transforming effects both in vitro and in vivo [5].
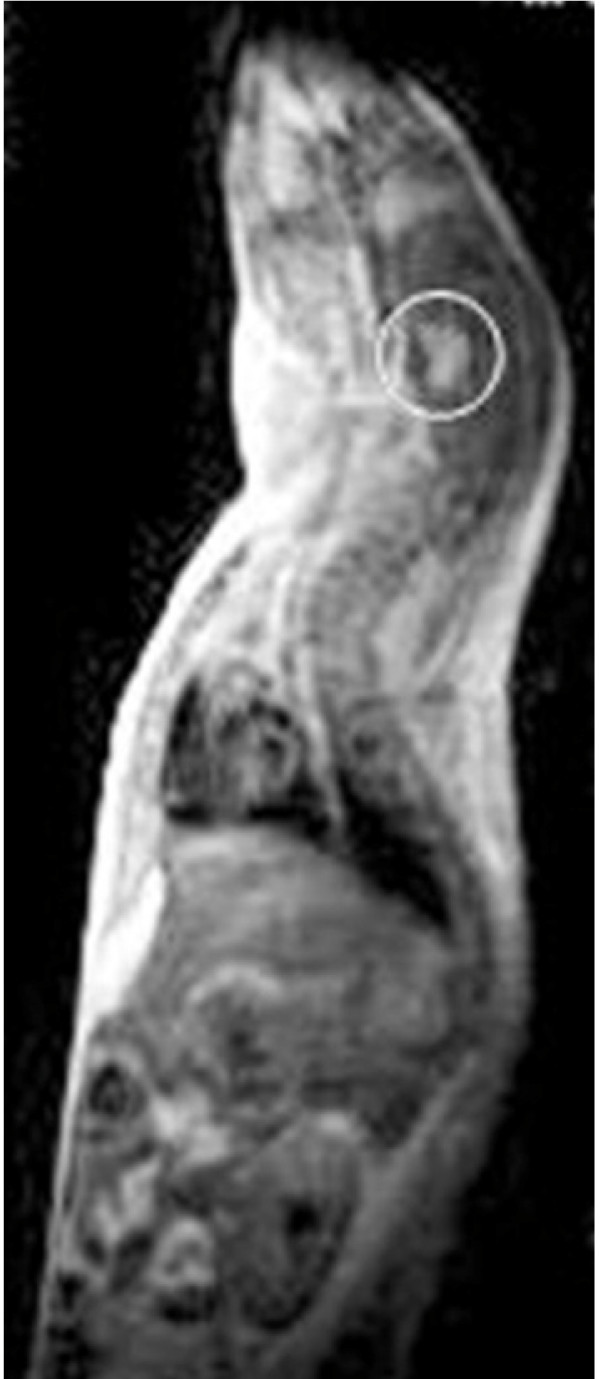
Recently, our group suggested a critical role for high-mobility group A2 (HMGA2) gene in pituitary oncogenesis. In fact, transgenic mice expressing high levels of the HMGA2 gene develop pituitary adenomas secreting prolactin and growth hormone [6], (Figure 1).
Figure 1.
Magnetic Resonance Image of a HMGA2 transgenic mouse showing a pituitary adenoma (indicated in circle).
The HMGA2 protein belongs to the HMGA family. The HMGA protein family members are non-histones, small, nuclear proteins, that bind the minor groove of AT-rich DNA sequences through their "AT-hook" domains localised in the N-terminal region of the proteins [7]. These proteins play key roles in chromatine architecture and gene control by serving as generalized chromatin effectors, either enhancing or suppressing the ability of more usual transcriptional factors to act in transcriptional regulation [8].
HMGA2 expression is restricted during embryogenesis, whereas it is absent or very low in normal adult tissues [9,10]. Induction of HMGA2 gene expression occurs in several human malignant neoplasias, including thyroid [11,12], pancreas [13], breast [14], and colorectum [15-17], and seems to play a critical role in cell transformation, since the block of its synthesis prevents rat thyroid transformation by murine transforming retroviruses [18]. Conversely, rearrangements of the HMGA2 gene are frequently detected in human benign tumors of mesenchymal origin [19]. Consistent with the onset of pituitary adenomas in HMGA2-transgenic mice, we have found the induction of HMGA2 expression in human prolactinomas in association with amplification and/or rearrangement of the gene [20], and, recently, we have shown that also the majority of NFPAs express HMGA2, but, in these cases, it is not associated to over-representation of the HMGA2 region [21].
HMGA2 binds to pRB and inhibits its function
The Retinoblastoma protein, pRB, has been suggested to be a key protein in the pituitary tumorigenesis because of the pituitary tumors developed by RB+/- mice [22], but no RB mutations, apart from few high aggressive pituitary carcinomas [23], have been so far reported in human pituitary pathology [24]. However, methylation of the RB gene-promoter region at a CpG island, resulting in loss of protein expression, has been described in human pituitary tumor cells [25], suggesting that pRB is indeed critical in human pituitary tumorigenesis.
pRB controls cell cycle progression through its interaction with the E2F family of transcription factors [26,27], whose activity is crucial for the expression of several genes required to enter the S phase of the cell cycle [28,29]. The transcriptional activity of E2F1 is repressed in non-proliferating cells by its interaction with pRB that masks the activation domain of E2F1, and prevents it to contact the general transcription machinery [30]. Conversely, in proliferating cells, pRB is phosphorylated at multiple sites by cyclin-dependent kinases [31,32], resulting in the release of E2F1 and, consequently, transcriptional activation of its target genes [33]. More recently, a new mechanism of pRB-mediated E2F1 repression has been suggested in addition to this one. It is an active repression that pRB exerts on E2F1-mediated transcription by recruiting class I histone deacetylase proteins (HDAC1) to the E2F1-sites. The HDACs repress transcription by removing acetyl groups from the histones, thereby facilitating the condensation of nucleosomes into chromatin and therefore blocking access to transcription factors [34].
Based on the striking mirror similarities between the phenotypes of pRB [22,35] and HMGA2 [36,37] animal models, our group has recently investigated a potential functional interaction between HMGA2 and the Retinoblastoma protein [38]. By co-immunoprecipitating HMGA2 and pRB in pituitary adenomas developed by HMGA2 mice, we demonstrated the interaction between the two proteins occurring in the tumor. This interaction was then repeated and confirmed in vitro with recombinant proteins, finding that one of the pRB domains involved in the interaction is the A/B pocket [30], the same domain that is also involved in the interaction with E2F1, HDAC1 and viral oncoproteins such as those produced by the E1A adenovirus [39,40]. This was very interesting because it suggested that HMGA2, similarly to the viral oncoproteins, could inhibit pRB function by displacing E2F1 and HDAC1 from pRB. By transfection, luciferase and colony assays, we could establish that the overexpression of HMGA2 antagonizes the activity of pRB. In fact it blocks the pRB-dependent inhibition of both E2F1 target gene transcription and cell proliferation. Interestingly, this positive role of HMGA2 on cell proliferation is due to the interaction with pRB, opening a new class of cell cycle related proteins: "the suppressors of the cell cycle inhibitors". As described above, HMGA2 is considered a bona fide oncogene because it induces both neoplastic transformation of cultured rat fibroblasts [41] and tumors in transgenic mice [6]. Interestingly, we found that the interaction between HMGA2 and pRB is crucial for the transforming activity of HMGA2 protein. In fact, in a focus assay on rat fibroblasts, HMGA2 mutants unable to bind pRB lost the capacity of the wild-type gene to transform cells. These results suggest that the binding between HMGA2 and pRB may be generally involved in HMGA2-mediated cell transformation.
HMGA2 displaces HDAC1 from E2F1 target promoters and causes acetylation of both histones and E2F1 protein
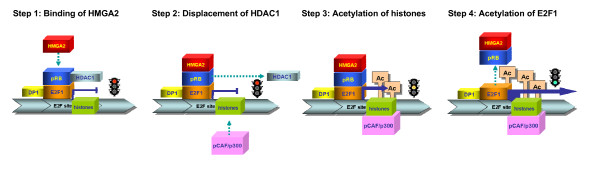
Using competitions with recombinant proteins and Chromatin Immonoprecipitation (ChIP) experiments, we demonstrated that following the binding of HMGA2 to pRB (Figure 2, step1), HDAC1 is displaced from the E2F1-target promoters (Figure 2, step 2) where it was recruited by pRB [34]. Consistently, HDAC1 activity associated to pRB is lower in cells and pituitary adenomas overexpressing HMGA2 than in mock-transfected cells and normal pituitary, respectively [38]. Histone acetyl transferases and histone deacetylases acetylate and deacetylate core histone tails that protrude from the nucleosome. Histone acetylation is thought to weaken the interaction between histone N-terminal tails and DNA, thus opening up the chromatin and increasing accessibility for activating transcription factors [42,43]. Therefore, the displacement of HDAC1 from pRB results in the recruitment of histone acetyl transferase to the E2F1-target promoters and acetylation of both histones and other proteins, including E2F1. This was convincingly demonstrated by ChIP experiments using antibodies against acetylated histone H3 and E2F1 [38]. The acetylation of both histones and E2F1 protein increase about two-fold the E2F1 transcriptional activity. In fact, as above described, the acetylation of histones opens up the chromatin and facilitates gene transcription (Figure 2, Step 3). Moreover, acetylation of E2F1 augments its DNA binding and stabilizes the protein in its "free" active form [44] (Figure 2, Step 4). Thus, as a consequence of the E2F1 acetylation, HMGA2 can indirectly also cause the displacement of E2F1 from pRB as it was observed by ChIP and re-ChIP experiments on the cyclin E1 promoter [38].
Figure 2.
Schematic model of E2F1 activation by HMGA2. Following HMGA2 overexpression, transcription through E2F1 sites switches from repression to activation through four steps: 1- HMGA2 binds to pRB, which is complexed with E2F1 and HDAC1 to form the active repression; 2- the interaction between HMGA2 and pRB displaces HDAC1; 3- in the absence of HDAC1, the histone acetylase enzymes are recruited and, by acetylating histones, relieve transcriptional repression; 4- histone acetylases also acetylate E2F1 causing the stabilization of its "free" active form.
Suppression of pituitary tumorigenesis in HMGA2 transgenic mice lacking E2F1
Does the afore-reported HMGA2-dependent molecular events result in enhanced E2F1-dependent gene transcription in pituitary adenomas? The affirmative answer comes once again from the study of the HMGA2 transgenic mice. In fact, pituitary adenomas excised from these mice were used in EMSA assays to analyze the E2F1-DNA binding in pituitary tumours compared to normal pituitary glands from wild-type mice [38]. The data obtained showed a drastic increase of the "free" active form of the E2F/DNA complex. Moreover, by RT-PCR and ChIPs on tissues, expression of E2F-target genes, such as CDC1 and TK1, was shown to be enhanced, and E2F1 to be more acetylated in adenomas compared to normal glands (unpublished data). This suggests that E2F1 activity is a critical event in pituitary tumorigenesis of HMGA2 mice.
To address this hypothesis, we crossed HMGA2 transgenic mice with E2F1 knock-out mice to generate double mutants [38]. With our big satisfaction, the hypophysis of these mice was only rarely and however minimally interested to the adenomatous phenotype. In fact, the adenoma was diagnosed in only 25% of double mutant mice in respect to HMGA2 transgenic mice which all developed pituitary tumors. Moreover the tumours of the mice lacking E2F1 were smaller and slower growing than those developed by the HMGA2 mice. Interestingly, even in pituitary adenomas developed by HMGA2 mice lacking E2F1 the interaction between HMGA2 and pRB was present, however, the E2F "free" DNA binding activity did not show any significant increase compared to control wild-type glands. Conversely, an increase in E2F "free" DNA binding was always observed in pituitaries from single mutant HMGA2 mice even before the appearance of the pituitary tumour. Thus, even though HMGA2 is still able to bind pRB in the absence of E2F1, there are no other proteins belonging to the E2F family, whose DNA binding activity is enhanced following the HMGA2/pRB interaction. Therefore, it is likely that other E2F-independent mechanisms are responsible for the pituitary alterations observed in the minority of these mice.
Conclusion
Our data demonstrate that E2F1 activation is a crucial step required for the onset of pituitary adenomas in HMGA2 transgenic mice. Since HMGA2 amplification and overexpression has been detected also in human pituitary adenomas, we retain that E2F1 activation plays a critical role also in the human pituitary pathology.
These conclusions are not completely unexpected since several studies have previously demonstrated that alterations of the pRB/E2F pathway are critical for the development of pituitary adenomas in mice [45-47]. However, what appears to be really novel, is the mechanism that leads to E2F1 activation by HMGA2: the E2F1 protein is not displaced from the pRB complex, but an increased acetylation that is dependent on the removal of HDAC1 from pRB takes place. It would be very interesting to know whether the same mechanism may be induced by other proteins able to bind to the pRB complex and thereby are involved in pituitary tumorigenesis. To answer to this question, it would be interesting to evaluate the acetylation status of the E2F1 protein in pituitary adenomas when the HMGA2 is overexpressed or not. The presence of E2F1 hyperacetylation in the absence of HMGA2 overexpression would suggest the involvement of other proteins acting with the same or similar mechanism of HMGA2 protein, or alternatively other mechanisms that eventually lead to an increase in E2F1 acetylation and subsequent activation.
Acknowledgments
Acknowledgements
This work was supported by grants from the Associazione Italiana Ricerca sul Cancro (AIRC). We are very grateful to Dr. Carlo M. Croce for his continuous and friendly support to our research group.
Contributor Information
Monica Fedele, Email: mfedele@unina.it.
Giovanna Maria Pierantoni, Email: gmpieran@unina.it.
Rosa Visone, Email: rosavisone@hotmail.com.
Alfredo Fusco, Email: afusco@napoli.com.
References
- Kovacs K, Horvath E. Atlas of Tumor Pathology Fascicle 21, II series. Washington, DC: AFPI; 1986. Tumors of the pituitary gland. [Google Scholar]
- Herman V, Fagin J, Gonsky R, Kovacs K, Melmed S. Clonal origin of pituitary adenomas. J Clin Endocrinol Metab. 1990;71:1427–33. doi: 10.1210/jcem-71-6-1427. [DOI] [PubMed] [Google Scholar]
- Asa SL, Ezzat S. The cytogenesis and pathogenesis of pituitary adenomas. Endocr Rev. 1998;19:798–827. doi: 10.1210/er.19.6.798. [DOI] [PubMed] [Google Scholar]
- Gilman AG. G-proteins: transducers of receptors-generated signals. Annu Rev Biochem. 1987;56:613–49. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Heaney AP, Horwitz GA, Wang Z, Singson R, Meemed S. Early involvement of estrogen-induced pituitary tumor transforming gene and fibroblast growth factor expression in prolactinoma pathogenesis. Nat Med. 1999;5:1317–20. doi: 10.1038/15275. [DOI] [PubMed] [Google Scholar]
- Fedele M, Battista S, Kenyon L, Baldassarre G, Fidanza V, Klein-Szanto AJ, Parlow AF, Visone R, Pierantoni GM, Outwater E, Santoro M, Croce CM, Fusco A. Overexpression of the HMGA2 gene in transgenic mice leads to the onset of pituitary adenomas. Oncogene. 2002;21:3190–8. doi: 10.1038/sj.onc.1205428. [DOI] [PubMed] [Google Scholar]
- Reeves R, Nissen MS. The A-T-DNA-binding domain of mammalian high mobility group I chromosomal proteins. A novel peptide motif for recognizing DNA structure. J Biol Chem. 1990;265:8573–82. [PubMed] [Google Scholar]
- Thanos D, Maniatis T. Virus induction of human IFN beta gene expression requires the assembly of an enhanceosome. Cell. 1995;83:1091–100. doi: 10.1016/0092-8674(95)90136-1. [DOI] [PubMed] [Google Scholar]
- Zhou X, Benson KF, Ashar HR, Chada K. Mutation responsible for the mouse pygmy phenotype in the developmentally regulated factor HMGI-C. Nature. 1995;376:771–4. doi: 10.1038/376771a0. [DOI] [PubMed] [Google Scholar]
- Chiappetta G, Avantaggiato V, Visconti R, Fedele M, Battista S, Trapasso F, Merciai BM, Fidanza V, Giancotti V, Santoro M, Simeone A, Fusco A. High level expression of the HMGA1 gene during embryonic development. Oncogene. 1996;13:2439–46. [PubMed] [Google Scholar]
- Chiappetta G, Bandiera A, Berlingieri MT, Visconti R, Manfioletti G, Battista S, Martinez-Tello FJ, Santoro M, Giancotti V, Fusco A. The expression of the high mobility group HMGA1 proteins correlates with the malignant phenotype of human thyroid neoplasms. Oncogene. 1995;10:1307–14. [PubMed] [Google Scholar]
- Chiappetta G, Tallini G, De Biasio MC, Manfioletti G, Martinez-Tello FJ, Pentimalli F, de Nigris F, Mastro A, Botti G, Fedele M, Berger N, Santoro M, Giancotti V, Fusco A. Detection of high mobility group I HMGI(Y) protein in the diagnosis of thyroid tumors: HMGI(Y) expression represents a potential diagnostic indicator of carcinoma. Cancer Res. 1998;58:4193–8. [PubMed] [Google Scholar]
- Abe N, Watanabe T, Izumisato Y, Masaki T, Mori T, Sugiyama M, Chiappetta G, Fusco A, Fujioka Y, Atomi Y. Diagnostic significance of high mobility group I(Y) protein expression in intraductal papillary mucinous tumors of the pancreas. Pancreas. 2002;25:198–204. doi: 10.1097/00006676-200208000-00015. [DOI] [PubMed] [Google Scholar]
- Chiappetta G, Botti G, Monaco M, Pasquinelli R, Pentimalli F, Di Bonito M, D'Aiuto G, Fedele M, Iuliano R, Palmieri EA, Pierantoni GM, Giancotti V, Fusco A. HMGA1 protein overexpression in human breast carcinomas: correlation with ErbB2 expression. Clin Cancer Res. 2004;10:7637–44. doi: 10.1158/1078-0432.CCR-04-0291. [DOI] [PubMed] [Google Scholar]
- Fedele M, Bandiera A, Chiappetta G, Battista S, Viglietto G, Manfioletti G, Casamassimi A, Santoro M, Giancotti V, Fusco A. Human colorectal carcinomas express high levels of high mobility group HMGI(Y) proteins. Cancer Res. 1996;56:1896–901. [PubMed] [Google Scholar]
- Abe N, Watanabe T, Sugiyama M, Uchimura H, Chiappetta G, Fusco A, Atomi Y. Determination of high mobility group I(Y) expression level in colorectal neoplasias: a potential diagnostic marker. Cancer Res. 1999;59:1169–74. [PubMed] [Google Scholar]
- Chiappetta G, Manfioletti G, Pentimalli F, Abe N, Di Bonito M, Vento MT, Giuliano A, Fedele M, Viglietto G, Santoro M, Watanabe T, Giancotti V, Fusco A. High mobility group HMGI(Y) protein expression in human colorectal hyperplastic and neoplastic diseases. Int J Cancer. 2001;91:147–51. doi: 10.1002/1097-0215(200002)9999:9999<::AID-IJC1033>3.3.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Berlingieri MT, Manfioletti G, Santoro M, Bandiera A, Visconti R, Giancotti V, Fusco A. Inhibition of HMGI-C protein synthesis suppresses retrovirally induced neoplastic transformation of rat thyroid cells. Mol Cell Biol. 1995;15:1545–53. doi: 10.1128/mcb.15.3.1545. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Fedele M, Battista S, Manfioletti G, Croce CM, Giancotti V, Fusco A. Role of the high mobility group A proteins in human lipomas. Carcinogenesis. 2001;22:1583–91. doi: 10.1093/carcin/22.10.1583. Review. [DOI] [PubMed] [Google Scholar]
- Finelli P, Pierantoni GM, Giardino D, Losa M, Rodeschini O, Fedele M, Valtorta E, Mortini P, Croce CM, Larizza L, Fusco A. The high mobility group A2 gene is amplified and overexpressed in human prolactinomas. Cancer Res. 2002;62:2398–405. [PubMed] [Google Scholar]
- Pierantoni GM, Finelli P, Valtorta E, Giardino D, Rodeschini O, Esposito F, Losa M, Fusco A, Larizza L. High-mobility group A2 gene expression is frequently induced in non-functioning pituitary adenomas (NFPAs), even in the absence of chromosome 12 polysomy. Endocr Relat Cancer. 2005;12:867–74. doi: 10.1677/erc.1.01049. [DOI] [PubMed] [Google Scholar]
- Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA. Effects of an Rb mutation in the mouse. Nature. 1992;359:295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- Pei L, Melmed S, Scheithauer B, Kovacs K, Benedict WF, Prager D. Frequent loss of heterozygosity at the retinoblastoma susceptibility gene (RB) locus in aggressive pituitary tumors: evidence for a chromosome 13 tumor suppressor gene other than RB. Cancer Res. 1995;55:1613–6. [PubMed] [Google Scholar]
- Cryns VL, Alexander JM, Kilbanski A, Arnold A. The retinoblastoma gene in human pituitary tumors. J Clin Endocrinol Metab. 1993;77:644–6. doi: 10.1210/jc.77.3.644. [DOI] [PubMed] [Google Scholar]
- Simpson DJ, Hibberts NA, Mc Nicol AM, Clayton RN, Farrell WE. Loss of pRB expression in pituitary adenomas is associated with metylation of the RB1 CpG island. Cancer Res. 2000;60:1211–6. [PubMed] [Google Scholar]
- Chellappan SP, Hiebert S, Mudryj M, Horowitz JM, Nevins JR. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991;65:1053–61. doi: 10.1016/0092-8674(91)90557-F. [DOI] [PubMed] [Google Scholar]
- Helin K, Harlow E, Fattaey A. Inhibition of E2F-1 transactivation by direct binding of the retinoblastoma protein. Mol Cell Biol. 1993;13:6501–8. doi: 10.1128/mcb.13.10.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigo E, Müller H, Prosperini E, Hateboer G, Cartwright P, Moroni MC, Helin K. CDC25A phosphatase is a target of E2F and is required for efficient E2F-1 induced S phase. Mol Cell Biol. 1999;19:6379–95. doi: 10.1128/mcb.19.9.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller H, Bracken AP, Vernell R, Moroni MC, Christians F, Grassilli E, Prosperini E, Vigo E, Oliner JD, Helin K. E2Fs regulate the expression of genes involved in differentiation, development, proliferation, and apoptosis. Genes Dev. 2001;15:267–85. doi: 10.1101/gad.864201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Spencer J, Clements A, Ali-Khan N, Mittnacht S, Broceno C, Burghammer M, Perrakis A, Marmorstein R, Gamblin SJ. Crystal structure of the retinoblastoma tumor suppressor protein bound to E2F and the molecular basis of its regulation. Proc Natl Acad Sci USA. 2003;100:2363–8. doi: 10.1073/pnas.0436813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J-Y, Matsushime H, Hiebert SW, Ewen ME, Sherr CJ. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993;7:331–42. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- Brown VD, Gallie BL. The B-Domain Lysine Patch of pRB Is Required for Binding to Large T Antigen and Release of E2F by Phosphorylation. Mol Cell Biol. 2002;22:1390–401. doi: 10.1128/MCB.22.5.1390-1401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–30. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Magnaghi-Jaulin L, Groisman R, Naguibneva I, Robin P, Lorain S, Le Villain JP, Troalen F, Trouche D, Harel-Bellan A. Retinoblastoma protein represses transcription by recruiting a histone deacetylase. Nature. 1998;391:601–5. doi: 10.1038/35410. [DOI] [PubMed] [Google Scholar]
- Bignon YJ, Chen Y, Chang CY, Riley DJ, Windle JJ, Mellon PL, Lee WH. Expression of a retinoblastoma transgene results in dwarf mice. Genes Dev. 1993;7:1654–62. doi: 10.1101/gad.7.9.1654. [DOI] [PubMed] [Google Scholar]
- Battista S, Fidanza V, Fedele M, Klein-Szanto AJ, Outwater E, Brunner H, Santoro M, Croce CM, Fusco A. The expression of a truncated HMGI-C gene induces gigantism associated with lipomatosis. Cancer Res. 1999;59:4793–7. [PubMed] [Google Scholar]
- Fedele M, Battista S, Kenyon L, Baldassarre G, Fidanza V, Klein-Szanto AJ, Parlow AF, Visone R, Pierantoni GM, Outwater E, Santoro M, Croce CM, Fusco A. Overexpression of the HMGA2 gene in transgenic mice leads to the onset of pituitary adenomas. Oncogene. 2002;21:3190–8. doi: 10.1038/sj.onc.1205428. [DOI] [PubMed] [Google Scholar]
- Fedele M, Visone R, De Martino I, Troncone G, Palmieri D, Battista S, Ciarmiello A, Pallante P, Arra C, Melillo RM, Helin K, Croce CM, Fusco A. HMGA2 induces pituitary tumorigenesis by enhancing E2F1 activity. Cancer Cell. 2006;9:459–71. doi: 10.1016/j.ccr.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Hu QJ, Dyson N, Harlow E. The regions of the retinoblastoma protein needed for binding to adenovirus E1A or SV40 large T antigen are common sites for mutations. EMBO J. 1990;9:1147–55. doi: 10.1002/j.1460-2075.1990.tb08221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris EJ, Dyson NJ. Retinoblastoma protein partners. Adv Cancer Res. 2001;82:1–54. doi: 10.1016/s0065-230x(01)82001-7. [DOI] [PubMed] [Google Scholar]
- Wood LJ, Maher JF, Bunton TE, Resar LMS. The oncogenic properties of the HMG-I gene family. Cancer Res. 2000;60:4256–61. [PubMed] [Google Scholar]
- Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–52. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–80. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Martinez-Balbas MA, Bauer UM, Nielsen SJ, Brehm A, Kouzarides T. Regulation of E2F1 activity by acetylation. EMBO J. 2000;19:662–71. doi: 10.1093/emboj/19.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki L, Bronson R, Williams BO, Dyson NJ, Harlow E, Jacks T. Loss of E2F-1 reduces tumorigenesis and extends the lifespan of Rb1(+/-)mice. Nat Genet. 1998;18:360–4. doi: 10.1038/ng0498-360. [DOI] [PubMed] [Google Scholar]
- Lee EY, Cam H, Ziebold U, Rayman JB, Lees JA, Dynlacht BD. E2F4 loss suppresses tumorigenesis in Rb mutant mice. Cancer Cell. 2002;2:463–72. doi: 10.1016/S1535-6108(02)00207-6. [DOI] [PubMed] [Google Scholar]
- Ziebold U, Lee EY, Bronson RT, Lees JA. E2F3 loss has opposing effects on different pRB-deficient tumors, resulting in suppression of pituitary tumors but metastasis of medullary thyroid carcinomas. Mol Cell Biol. 2003;23:6542–52. doi: 10.1128/MCB.23.18.6542-6552.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]