Abstract
Monamines subserve many critical roles in the brain, and monoaminergic drugs such as amphetamine have a long history in the treatment of neuropsychiatric disorders and also as a substance of abuse. The clinical effects of amphetamine are quite variable, from positive effects on mood and cognition in some individuals, to negative responses in others, perhaps related to individual variations in monaminergic function and monoamine system genes. We explored the effect of a functional polymorphism (val158-met) in the catechol O-methyltransferase gene, which has been shown to modulate prefrontal dopamine in animals and prefrontal cortical function in humans, on the modulatory actions of amphetamine on the prefrontal cortex. Amphetamine enhanced the efficiency of prefrontal cortex function assayed with functional MRI during a working memory task in subjects with the high enzyme activity val/val genotype, who presumably have relatively less prefrontal synaptic dopamine, at all levels of task difficulty. In contrast, in subjects with the low activity met/met genotype who tend to have superior baseline prefrontal function, the drug had no effect on cortical efficiency at low-to-moderate working memory load and caused deterioration at high working memory load. These data illustrate an application of functional neuroimaging in pharmacogenomics and extend basic evidence of an inverted-“U” functional-response curve to increasing dopamine signaling in the prefrontal cortex. Further, individuals with the met/met catechol O-methyltransferase genotype appear to be at increased risk for an adverse response to amphetamine.
Amphetamine (AMP) and other psychostimulants are among the most effective psychotropic medications in clinical use and the mainstay of treatment for patients with attention deficit hyperactivity disorder (ADHD), narcolepsy, chronic fatigue syndrome, and apathy and anhedonia of diverse etiologies. There is general consensus that these drugs increase CNS alertness, modulate attention, and enhance mood and cognitive performance by potentiating monaminergic neurotransmission. Because of these effects, and the reinforcing properties of monoaminergic stimulation, AMP and related compounds are popular substances of abuse. Although it has been well known that there are dose- and behavior-dependent differential effects of psychostimulants (1, 2), there is also considerable evidence that the response to these drugs varies across individuals, even to fixed doses (3–5). These variable effects have been difficult to predict a priori and to date no neurobiological explanation for them has been established. It is possible that some of the intersubject differences can be explained by functional polymorphisms in monoamine system genes (e.g., synaptic proteins, metabolic enzymes, etc.) that effect baseline monoaminergic tone.
While AMP blocks the action of transporters at dopaminergic, serotonergic, and noradrenergic neurons, its positive effects on attention and cognition appear to be mediated principally at the prefrontal cortical level and to involve dopamine (DA) neurotransmission (6, 7). DA signaling, particularly through D1 receptors, has been shown to be critical for cognitive functions subserved by the prefrontal cortex (PFC), such as executive cognition and working memory (WM) (8). Experimental evidence also indicates that DA impacts on PFC function in accordance with an inverted U-shaped dose–response curve, such that the response is optimized within a narrow range of DA activity, with too little or too much DA having a relatively deleterious effect (8, 9).
Consistent with data implicating an inverted-U response function, pharmacological studies in animals (10) and in healthy human volunteers (3–5) indicate that the effect of AMP and other dopamimetic agents on the PFC depends on the baseline level of PFC function, which is presumably a reflection, at least in part, of baseline dopaminergic tone (i.e., relative position on the putative inverted U). Indeed, in healthy subjects, relatively poor performers on prefrontal cognitive tasks tend to improve after stimulants, whereas high performers show no response or get worse (3–5). An individual's location on this hypothesized inverted U may depend in part on individual differences in genes that affect baseline prefrontal DA signaling. Although there are numerous proteins involved in regulating synaptic DA activity, catechol O-methyltransferase (COMT), which inactivates released DA through enzymatic conversion to 3-methoxytyramine, appears to play a unique role in regulating DA flux in the PFC because of the low abundance and minimal role of DA transporters in the PFC (11–13). Studies in COMT-knockout mice, while showing no effect on DA levels in the striatum where DA transporters are abundant, show increased DA levels in the PFC (14, 15). The levels of other monoamines such as norepinephrine and 5-hydroxytryptamine (serotonin), which are regulated in the PFC by abundantly expressed transporters, are unaffected in the PFC of COMT-knockout mice. Further, COMT inhibitors have been shown to improve WM in animals (16) and in humans (17).
The human COMT gene contains a common and evolutionarily recent methionine (Met) for valine (Val) substitution at codon 158 (18), referred to here as val158-met polymorphism, which results in a thermolabile protein with three to four times lower activity. Thus, individuals with met alleles presumably have relatively more baseline dopaminergic signaling at synapses where COMT activity is critical than individuals with val alleles. Consistent with evidence that COMT is important in PFC DA flux, which is important in modulating prefrontal function (see above), Egan et al. (19) demonstrated that met allele carriers had superior performance on an executive cognition task and, by using functional MRI (fMRI) during a WM task, that val allele carriers consistently demonstrated a less efficient physiologic response in the PFC for a fixed level of task performance, (i.e., greater PFC activity) when compared with subjects with the met allele. They concluded that the COMT val allele, presumably by compromising the postsynaptic impact of the evoked DA response, reduces prefrontal neuronal signal-to-noise ratio and makes processing less efficient. Other groups have confirmed this effect of COMT val-met genotype on prefrontal cognition (20, 21).
Based on these various findings, we hypothesized that the val158-met functional polymorphism of the COMT gene would influence the effect of AMP on prefrontal cortical function. Specifically, we predicted that after AMP, which in the PFC increases DA levels by blocking extrasynaptic uptake at norepinephrine transporters (12, 13), normal individuals homozygous for the val allele would be shifted to more optimal DA levels, thereby improving their PFC function. We further predicted that individuals homozygous for the met allele, who tend to be superior performers on prefrontal cognitive tasks and presumably have baseline synaptic DA levels closer to the peak of the theoretical inverted-U curve, would be more likely to have their DA levels shifted by AMP beyond the optimal range with a resultant decrement in PFC function.
To test these hypotheses, we performed a double-blinded placebo (PBO)-controlled AMP trial in healthy normal volunteers who also underwent blood oxygen level-dependent (BOLD) fMRI while performing a PFC-dependent task, the N-back WM task with increasing levels of task difficulty (i.e., WM loads of 1-back, 2-back, and 3-back; ref. 22). The BOLD signal is sensitive to blood oxygenation and has been ascribed to changes in local field potentials in postsynaptic neurons (23), suggesting that activation of brain areas identified by BOLD fMRI reflects dynamic changes in neuronal information processing.
Earlier fMRI studies found that variations in DA signaling in the PFC affect the efficiency or signal-to-noise ratio of the physiologic response during the N-back task, i.e., the degree of cortical activation (reflected in the extent and/or amplitude of the BOLD response) for a fixed level of performance (4, 19, 24). Therefore, we predicted that the effect of genotype on the AMP response would be manifest as variations in cortical efficiency during this fMRI paradigm, even if performance did not change with AMP. That is, AMP and genotype would interact at the level of how WM information is processed in the PFC, which may or may not be manifest overtly as a change in behavior (cognitive performance). Specifically, individuals homozygous for the val allele would become more efficient after AMP, as DA signaling is pushed to more optimal levels (as modeled by the inverted-U–DA-response curve). In contrast, individuals homozygous for the met allele, who are hypothesized to be near the peak of the normal curve and who generally perform relatively better and more efficiently at baseline, would become less efficient on AMP because of a shift of DA levels onto the down slope of the curve. Because experimental data in animals indicate that increasing WM load also leads to increased synaptic DA release in the PFC (25, †) we anticipated an additive effect of genotype and task difficulty and further predicted that the greatest difference in the AMP effect between genotype groups would be at the highest levels of cognitive load. Before the fMRI sessions, subjects took an executive cognition test, the Wisconsin Card Sorting Task (WCST), as prior work has shown that the COMT genotype affects performance on this task (19–21, ‡).
Methods
Subjects.
One hundred twenty-three normal volunteers whose COMT genotype was known (val/val = 45 subjects, val/met = 52 subjects, and met/met = 26 subjects) and who had undergone extensive clinical evaluations were screened to participate in this study. Because our aim was to test a specific gene effect on the neuropharmacology and neurophysiology of the PFC, we deemed it necessary to control for other variables that also potentially contribute to the cortical response. Clearly, many factors other than genetic background will contribute to variance in the fMRI data, but these must be minimized to identify the genetic effect, which is likely to be relatively small. Thus, only subjects who were <45 years of age with similar educational background were contacted, because aging has an impact on the efficiency of the cortical response during our fMRI paradigm§ and education has an impact on task performance. Subjects were also excluded for any prior use of AMP or other psychostimulants (to control for potential sensitization effects), past and present history of neurological, psychiatric, and other medical problems, or medical treatment relevant to cerebral metabolism and blood flow. Smokers were also excluded. Because parameters such as IQ can affect performance and, thereby, the fMRI response, only subjects with an IQ of >90 were contacted. In addition to these criteria, some subjects declined to participate in studies that involved pharmacological challenges. A final sample of 27 healthy volunteers [11 males, 16 females; 10 val/val (mean ± SE, age = 31 ± 1.3 years; IQ = 111.2 ± 2.8), 11 val/met (mean ± SE, age = 32 ± 2 years; IQ = 106.5 ± 1.6), and 6 met/met (mean ± SE, age = 37 ± 1.7 years; IQ = 108 ± 4.5)] who gave written informed consent participated in this study, which had the approval of the National Institute of Mental Health Institutional Review Board. All of the data in this study are original and have not been published elsewhere.
Test Conditions and Drug Administration.
Subjects were studied in a double-blind, counterbalanced crossover design during two fMRI sessions separated by at least 72 h. All conditions were kept constant for the two visits and participants were closely monitored for 6 h after drug administration. Approximately 90 min after an oral dose of either PBO or dextroamphetamine (0.25 mg/kg of body weight), 26 of the 27 subjects completed the WCST, which has been a standard of neuropsychological testing of the prefrontal cortex in humans. For this study a computerized version of the WCST was used. Subjects viewed a computer screen that displayed five boxes. Subjects were asked to match the contents of the center box to one of the four outside boxes. Subjects were not informed on how to make the match, but had to determine from trial and error whether to match on the basis of color, shape, or numbers by using feedback displayed on the screen after each response. After the subject had made a series of correct responses, the “rule” changed and subjects had to determine a new rule for matching.
At ≈120 min after drug administration, fMRI was performed. Timing of testing was based on pharmacokinetic data indicating that plasma levels of AMP administered orally peak 1[1/2]-3 h after administration of the drug. Mood and anxiety scales were obtained after the fMRI scans on each test day. Blood pressure and heart rate were obtained at baseline and at 30- to 60-min intervals until discharge. Blood was drawn at the beginning and the end of each fMRI session and serum AMP levels were measured by using gas chromatography with a sensitivity of 50 ng/ml.
fMRI Data Acquisition.
Each subject was scanned by using a GE Signa (Milwaukee, WI) 3T scanner with a real-time functional imaging upgrade. BOLD fMRI (gradient echo planar imaging sequence, 24 axial 6-mm thick interleaved slices, relaxation time/echo time (TR/TE) = 2000/30 msec, flip angle = 90°, field of view = 24 cm, matrix = 64 × 64) was conducted while subjects performed three levels of the N-back WM task. N-back refers to the number of previous stimuli that the subject had to recall. The stimuli consisted of numbers (1–4) shown in random order and were displayed at the points of a diamond-shaped box at 1.5-sec intervals. Stimuli were presented by means of a back-projection system and the responses were recorded through a fiber optic response box with buttons arranged in the same configuration as the stimuli presented on the screen. During each treatment condition, four cycles of the WM task (1-back, 2-back, or 3-back) alternating with the 0-back (sensorimotor task) were administered. Each task combination was obtained in 4 min and 8 sec, 124 whole-brain scans with 4 cycles of 30 scans each (15 scans during WM (1-back, 2-back, or 3-back) and 15 scans during the 0-back task. The first four scans at the beginning of each time series were acquired to allow the signal to reach a steady state and were not included in the final analysis. The order of the task combinations was counter balanced across subjects but maintained within subjects across drug conditions.
Image Processing and Data Analysis.
Because of technical difficulties, imaging data from one subject (val/met) during one session could not be saved, and, because of time constraints, data acquisition in another subject (val/val) was limited to the 2-back task. The final image analysis, therefore, was limited to the data from the 25 subjects with complete data sets (9 val/val, 10 val/met, and 6 met/met). Whole-brain image analysis was completed using spm99 (www.fil.ion.ucl.ac.uk/spm). Images for each subject were realigned to the first volume in the time series to correct for head motion, were spatially normalized into a standard stereotactic space (Montreal Neurological Institute template) by using a 12-parameter affine model, and were smoothed to minimize noise and residual differences in gyral anatomy with a Gaussian filter, set at 10 mm full width at half maximum. Voxel-wise signal intensities were ratio normalized to the whole-brain global mean to control for systematic differences in global activity (for example, systematic drug effects, subject-to-subject differences in global activity, etc.). Data sets were also screened for high quality (scan stability) as demonstrated by small motion correction (<2 mm) and matched voxel variance across the drug and PBO sessions. Predetermined condition effects at each voxel were calculated by using a t statistic, producing a statistical image for the contrast of the WM task (1-back, 2-back, or 3-back) versus the sensorimotor control (0-back) for each subject for each drug condition. These individual contrast images were then used in a conservative second-level random effects model that account for both scan-to-scan and subject-to-subject variability.
Since the main goal of this study was to explore the impact of the COMT val-met polymorphism on the effect of AMP on prefrontal cortical function, we focused on the data from the two extreme genotype groups, i.e., individuals with the high enzyme activity val/val and low enzyme activity met/met genotypes. We first performed analysis of covariance (using performance as a nuisance variable) on data from all of the val/val and met/met subjects who had complete data sets (nine val/val and six met/met). We then performed a similar analysis after a finer pairwise matching of subjects across the two groups for age, sex, and IQ (six val/val and six met/met; three males and three females in each group; age mean ± SEM, val/val = 34 ± 3 years; met/met = 37 ± 1.2 years; IQ mean ± SEM, val/val = 111 ± 4.3; met/met = 108 ± 4.5). Because of our strong a priori hypothesis and our use of a rigorous statistical model, a statistical threshold of P < 0.05, with a small volume correction for multiple comparisons, was used to identify significant responses for all comparisons.
The effect of AMP on individuals with the intermediate activity val/met genotype was explored separately. Analyses were performed on all 10 subjects with this genotype and also on a smaller sample (five subjects, two males and three females) who were matched for age, sex, and IQ with the val/val and met/met groups (three females and two males; mean ± SEM, age = 34 ± 2.2 years; IQ = 109 ± 2.4).
Posthoc analysis was then performed by using a region-of- interest approach. A volume of interest was defined in the PFC encompassing voxels that showed a significant genotype by drug interaction (left PFC, Brodmann's area 9/46, Talaraich coordinates −38, 45, and 24, P < 0.01, small volume corrected). The mean signal intensity for each time point in each time series was then extracted to calculate the mean percentage change in BOLD signal for each WM task state and each drug condition in every subject. To allow for the hemodynamic response delay and to preclude signal contamination from the preceding task state (i.e., WM or control task), the first 10 sec of each task state were not included in these calculations.
Statistical Analysis of Clinical Variables.
Changes in physiological variables (blood pressure and heart rate), mood scales, and task performance (percent correct and reaction time (RT) on the N-back and percent preservative errors and number of categories on the WCST were assessed by using a repeated-measures ANOVA followed by post hoc analysis (Duncan test).
Genetic Analysis.
DNA was extracted by standard methods. COMT val108/158-met genotype was determined by 5′ exonuclease allelic discrimination TaqMan assay (26) that uses the 5′ nuclease activity of Taq DNA polymerase to detect a fluorescent reporter signal generated after PCRs.
Results
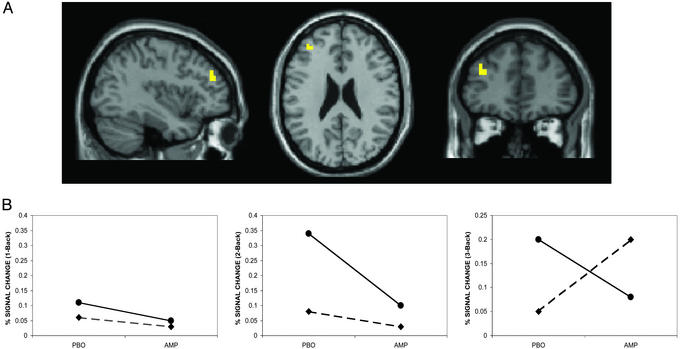
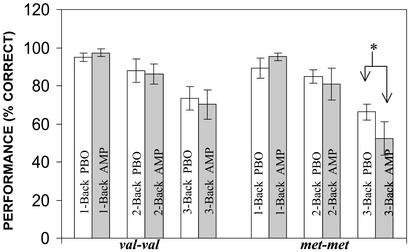
Consistent with earlier reports (4, 19), we found significant main effects of COMT genotype and of AMP on the distributed cortical activation patterns associated with this task, with prominent locales of activation in prefrontal and parietal cortices (data not shown), but a significant genotype by drug interaction was restricted to the PFC (Fig. 1A). This result was observed when data from all subjects were analyzed as well as when they were restricted to the subsample of subjects who were more precisely matched for IQ, age, and gender across genotypes (see Methods). At all levels of WM load, subjects with the val/val genotype had a more efficient prefrontal activation response i.e., a reduction in BOLD signal on AMP compared with PBO for the same level of performance (Fig. 1B). This response was associated with a significant improvement in reaction time (reaction time ANOVA F(1,15) = 4.4, P = 0.05) despite no change in accuracy (ANOVA F(1,15) = 0.2, P = 0.70). This pattern of behavioral and physiologic results suggests that AMP enhanced the efficiency (i.e., signal-to-noise ratio) of PFC information processing in these subjects. This finding is in accordance with the observations of Mehta et al. (5), who also reported enhanced PFC efficiency, i.e., a reduction in activation while on a stimulant, albeit with an improvement in accuracy. In contrast, in subjects with met/met genotype, there was no effect of AMP on prefrontal activation at the 1-back and 2-back conditions, but at 3-back there was an increase in PFC activity (i.e., decreased efficiency). This paradoxical decrease in efficiency at 3-back was associated with a significant decrement in performance (decreased accuracy; Fig. 2) and increased reaction time (see Table 1, which is published as supporting information on the PNAS web site, www.pnas.org). This pattern of behavioral and physiologic results suggests that AMP compromised the efficiency of information processing in these subjects.
Figure 1.
(A) Representative sagittal, axial, and coronal slices from a group analysis showing locales of a drug × COMT genotype interaction in the left PFC (Brodmann's area 9/46, Talaraich coordinates −38, 45, and 24, Z score >2.3, P < 0.01, corrected) during the WM task (see Fig. 5, which is published as supporting information on the PNAS web site). (B) Percent change in BOLD signal in the left PFC during the N-back relative to the control task on AMP and PBO conditions. The percent change in BOLD signal was calculated post hoc by using the mean signal intensity values extracted from the cluster of voxels that showed a significant drug × COMT genotype interaction (see text for details). Of note, similar to the findings of Egan et al. (19), there is a main effect of genotype at baseline (PBO condition), as val/val (●) individuals are less efficient than met/met individuals (♦) at all levels of task difficulty. Other PFC areas that were significant on this interaction analysis include Brodmann's area 6 (8, −1, and 55, Z score >3, P < 0.001) and Brodmann's area 44 (−49, 11, and 10, Z score >2, P < 0.02). There were no areas outside the PFC that showed a significant drug × genotype interaction.
Figure 2.
N-back performance based on genotype and drug condition. *, AMP caused a significant decrease in performance on the 3-back task in met/met individuals (P < 0.05; Duncan post hoc test). There was no significant difference in serum AMP levels across the three genotypes (mean ± SEM; val/val = 54 ± 3.6 ng/ml, val/met = 53 ± 2.9 ng/ml, and met/met = 56 ± 2.7 ng/ml; ANOVA F(2,22) = 0.27, P = 0.8) and no effect of genotype on any of the clinical variables. Further details on clinical variables are available in Supporting Text.
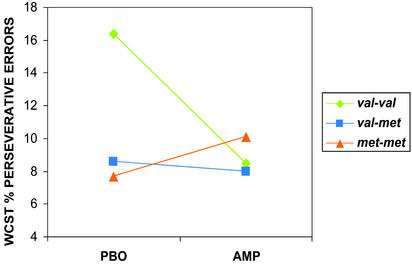
There was also a significant effect of the COMT genotype and AMP on the WCST. As in earlier reports (19–21, ‡), subjects with the val/val genotype made more perseverative errors on PBO then did met/met individuals. However, performance of the val/val group significantly improved on AMP. In contrast, the performance of the subjects with the met/met genotype deteriorated on AMP (Fig. 3).
Figure 3.
WCST percent perseverative errors on AMP and PBO showing a significant drug × genotype interaction (matched groups; ANOVA F(2,14) = 5.2, P < 0.02). Note that individuals with the val/val genotype perform better on AMP (fewer errors), whereas individuals the met/met genotype get worse (more errors) and individuals with the val/met genotype show no discernable effect on performance. Analysis of data from all subjects (val/val = 10, val/met = 10, and met/met = 6) who performed the task revealed a similar drug × genotype interaction (ANOVA F(2,23) = 3.7, P < 0.04). In addition, analysis using percent total errors (perseverative and nonperseverative errors; a measure of general performance) also revealed a significant drug × genotype interaction (ANOVA F(2,22) = 4.3, P < 0.02). Subjects with the val/val genotype showed better overall performance scores (i.e., percent fewer total errors) on AMP, whereas subjects with the met/met genotype showed the opposite response. In essence, this suggests that, although the val/val subjects on PBO made more total errors than they did on AMP, and met/met subjects on AMP made more total errors than on PBO, perseverative errors made up a major portion of the total errors. Thus, AMP did not induce perseverative errors independent of genotype.
Analysis of data from subjects with the intermediate activity val/met genotype showed that despite stable performance (see Supporting Text and Table 2, which are published as supporting information on the PNAS web site), prefrontal activation was reduced on AMP compared with PBO, particularly on the 2-back condition (Brodmann's area 10/46, Talaraich coordinates −34, 44, and 10, Z score >2.84, P < 0.002; Brodmann's area 46, Talaraich coordinates 45, 30, and 10, Z score >1.92, P < 0.03) suggesting that, similar to subjects with the val/val genotype, AMP enhanced cortical signal-to-ratio noise with the subjects as well. However, in contrast to, and perhaps intermediate between, the two homozygous groups, these subjects showed no effects from AMP on their WCST performance (Fig. 3).
Discussion
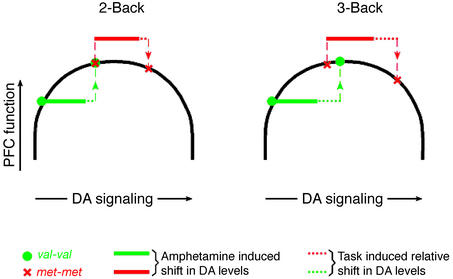
Our results suggest that the val158-met polymorphism in the COMT gene contributes to variability of the cortical response to AMP and implicate a heritable neurobiological mechanism for a variable clinical response to this drug. In line with data in experimental animals (10, 27), and in patients with Parkinson's disease (24, 28), that DA receptor activation (principally D1) can improve PFC functioning when baseline DA signaling is suboptimal, there was an improvement in the efficiency of PFC information processing in val/val individuals after AMP, presumably because of a shift of DA signaling from the lower end of the normal range to a higher level on the putative inverted-U curve. This effect was observed at all levels of task load. Also consistent with evidence that supranormal stimulation of DA D1 receptors can have detrimental effects on PFC function (2, 25, 27, †) there was a decrement in the efficiency of PFC information processing in met/met individuals on AMP at high load. A load-dependent effect of COMT in met/met individuals may indicate that despite their relative position near the peak of the DA-response curve, supraoptimal DA levels do not disrupt neuronal information processing until a critical threshold of DA signaling and associative processing load are exceeded. We suggest that the combined effects on DA levels of AMP and high WM load push individuals with the met/met genotype beyond the critical threshold at which compensation can be made. These points are illustrated in Fig. 4 in a theoretical model that accounts for variable effects on DA signaling and PFC function of COMT genotype, WM load, and AMP. Although our data cannot be interpreted to confirm this model, which is a theoretical construct based on empirical observations, we believe the data best fit this formulation. Our findings and this model are also in agreement with the classic clinical observations of Sprague and Sleator (1), who reported that at low psychostimulant doses, children with ADHD showed a remarkable improvement on a short-term memory test at all levels of task load, whereas at higher doses, there was a significant decrement in performance on the more difficult versions of the task.
Figure 4.
Theoretical inverted-U model describing the effects of COMT genotype, WM load, and AMP on PFC DA signaling and function. The model has three simplified assumptions: (i) fixed baseline positions for each genotype group based on differential COMT activity; (ii) greater DA release with increasing WM load; and (iii) a fixed pharmacological effect of AMP on increasing synaptic DA levels. Thus, at baseline, individuals homozygous for the val allele (who have relatively poorer prefrontal function, greater COMT activity, and presumably less DA) are located on the up slope of the normal range, whereas individuals homozygous for the met allele are located near the peak. In val/val individuals, AMP improves PFC function as DA signaling is shifted to more optimal levels at all load conditions. In contrast, in individuals homozygous for the met allele, AMP shifts DA levels onto the down slope of the inverted-U curve, which has no effect, or a deleterious effect, depending on the magnitude of additional shifts in DA levels associated with increasing processing demands. (See text for further discussion.) The model predicts that higher doses of AMP would also compromise prefrontal function in individuals with the val/val genotype.
Our clinical data also extend insights from basic experiments about the cellular mechanisms of how DA modulates PFC function. These studies suggest that DA strengthens the effects of strong depolarizing currents and enhances task-related neural activity (9, 29) through the activation of D1 receptors, which enhances persistent Na+, L-type Ca2+, and N-methyl-d-aspartate currents in PFC pyramidal neurons (30–33). The net result of D1 receptor stimulation is signal sharpening, or a gain-amplifying effect on a subset of inputs to PFC neurons (31). Evidence also indicates that too much DA activity in the PFC may disorganize networks of PFC neurons by activating inhibitory mechanisms, including inactivation of N-type Ca2+ channels (31), activation of GABAergic interneurons,¶ and pre- and postsynaptic reduction of glutamate-mediated synaptic responses (34). Thus, our observations are consistent with the hypothesized inverted-U cortical-response curve to increasing DA signaling in the PFC and suggest that the likelihood of a person being on the up or down slope of the inverted U after AMP administration depends not only on the environmental demands (e.g., task conditions) but also on an individual's COMT genotype. Indeed, val/val individuals on AMP appear in our paradigm similar to met/met individuals at baseline. met/met individuals on AMP, however, process the 3-back task more poorly than do val/val individuals at baseline.
To our knowledge, this is the first demonstration in humans of a genetic explanation for individual differences in the brain response to AMP. The observation that genetic differences can interact with other factors such as cognitive load to influence how dopaminergic agents modulate prefrontal function may prove useful in managing patients who receive these drugs, perhaps in preventing negative responses. Because symptoms of PFC dysfunction are characteristic of many neuropsychiatric disorders, including depression, schizophrenia, Parkinson's disease, ADHD, and traumatic brain injury, the COMT genotype may be a relevant factor to consider when implementing therapy with dopaminergic agents in these conditions. As noted above, it has been difficult to predict a priori who will show adverse responses to psychostimulants. Overall, subjects with negative responses represent a minority of patients treated with these agents. In populations of European ancestry, individuals with met/met genotypes constitute ≈15–20% of the population (35). Further studies are needed to determine to what degree this minority population of COMT genotypes and those individuals with a deleterious response to psychostimulants are one and the same.
Supplementary Material
Acknowledgments
We thank Saumitra Das, William Smith, and Sam Lee (Unit on Clinical Neuroimaging, Clinical Brain Disorders Branch, National Institute of Mental Health, National Institutes of Health) for research assistance; Dr. Andreas Meyer-Lindenberg (Unit on Integrative Neuroimaging, Clinical Brain Disorders Branch) for advice in fMRI data analysis; Dr. Karen F. Berman (Unit on Integrative Neuroimaging, Clinical Brain Disorders Branch) for helpful comments and discussion regarding the preparation of this manuscript; and Dr. Patricia Goldman-Rakic and Dr. Robert Desimone for helpful suggestions and criticisms. This work was supported by a Distinguished Investigator Grant from the Essel Foundation through the National Alliance for Research on Schizophrenia and Depression (to D.R.W.).
Abbreviations
- AMP
amphetamine
- fMRI
functional MRI
- WM
working memory
- DA
dopamine
- COMT
catechol O-methyltransferase
- BOLD
blood oxygen level-dependent
- ADHD
attention deficit hyperactivity disorder
- WSCT
Wisconsin Card Sorting Task
- PBO
placebo
- PFC
prefrontal cortex
Footnotes
Mattay, V. S., Fera, F., Tessitore, A., Callicott, J. H., Das, S., Meyer-Lindenberg, A., Berman, K. F., Goldberg, T. E. & Weinberger, D. R., 31st Annual Society for Neuroscience Meeting, San Diego, (Nov. 10–15).
Seamans, J., Gorelova, N., Durstewitz, D. & Yang, C., 30th Annual Society for Neuroscience Meeting, Nov. 4–9, 2000, New Orleans.
Ahn, S., Floresco, S. B. & Phillips, A. G., 30th Annual Society for Neuroscience Meeting, New Orleans, (Nov. 4–9).
This work was presented in part at Society for Neuroscience (Nov. 2–7, 2002, Orlando, FL) and American College of Neuropsychopharmacology (Dec. 7–12, 2002, San Juan, PR) conferences.
References
- 1.Sprague R L, Sleator E K. Science. 1977;198:1274–1276. doi: 10.1126/science.337493. [DOI] [PubMed] [Google Scholar]
- 2.Robbins T. Exp Brain Res. 2000;133:130–138. doi: 10.1007/s002210000407. [DOI] [PubMed] [Google Scholar]
- 3.Kimberg D Y, D'Esposito M, Farah M J. NeuroReport. 1997;8:3581–3585. doi: 10.1097/00001756-199711100-00032. [DOI] [PubMed] [Google Scholar]
- 4.Mattay V S, Callicott J H, Bertolino A, Heaton I, Frank J A, Coppola R, Berman K F, Goldberg T E, Weinberger D R. NeuroImage. 2000;12:268–275. doi: 10.1006/nimg.2000.0610. [DOI] [PubMed] [Google Scholar]
- 5.Mehta M A, Owen A M, Sahakian B J, Mavaddat N, Pickard J D, Robbins T W. J Neurosci. 2000;20:RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierce R C, Kalivas P W. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 7.Volkow N D, Chang L, Wang G J, Fowler J S, Ding Y S, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, et al. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 8.Goldman-Rakic P. Adv Pharmacol. 1998;42:707–711. doi: 10.1016/s1054-3589(08)60846-7. [DOI] [PubMed] [Google Scholar]
- 9.Williams G, Goldman-Rakic P. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 10.Granon S, Passetti F, Thomas K, Dalley J, Everitt B, Robbins T. J Neurosci. 2000;20:1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis D A, Melchitzky D S, Sesack S R, Whitehead R E, Auh S, Sampson A. J Comp Neurol. 2001;432:119–136. doi: 10.1002/cne.1092. [DOI] [PubMed] [Google Scholar]
- 12.Mazei M S, Pluto C P, Kirkbride B, Pehek E A. Brain Res. 2002;936:58–67. doi: 10.1016/s0006-8993(02)02542-8. [DOI] [PubMed] [Google Scholar]
- 13.Moron J A, Brockington A, Wise R A, Rocha B A, Hope B T. J Neurosci. 2002;22:389–395. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gogos J, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M. Proc Natl Acad Sci USA. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huotari M, Gogos J, Karayiorgou M, Koponen O, Forsberg M, Raasmaja A, Hyttinen J, Mannisto P. Eur J Neurosci. 2002;15:246–256. doi: 10.1046/j.0953-816x.2001.01856.x. [DOI] [PubMed] [Google Scholar]
- 16.Liljequist R, Haapalinna A, Ahlander M, Li Y H, Mannisto P T. Behav Brain Res. 1997;82:195–202. doi: 10.1016/s0166-4328(97)80989-8. [DOI] [PubMed] [Google Scholar]
- 17.Gasparini M, Fabrizio E, Bonifati V, Meco G. J Neural Transm. 1997;104:887–894. doi: 10.1007/BF01285556. [DOI] [PubMed] [Google Scholar]
- 18.Mannisto P, Kaakkola S. Pharmacol Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- 19.Egan M, Goldberg T, Kolachana B, Callicott J, Mazzanti C, Straub R, Goldman D, Weinberger D. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malhotra A K, Kestler L J, Mazzanti C, Bates J A, Goldberg T, Goldman D. Am J Psychiatry. 2002;159:652–654. doi: 10.1176/appi.ajp.159.4.652. [DOI] [PubMed] [Google Scholar]
- 21.Joober R, Gauthier J, Lal S, Bloom D, Lalonde P, Rouleau G, Benkelfat C, Labelle A. Arch Gen Psychiatry. 2002;59:662–663. doi: 10.1001/archpsyc.59.7.662. [DOI] [PubMed] [Google Scholar]
- 22.Callicott J H, Mattay V S, Bertolino A, Finn K, Coppola R, Frank J A, Goldberg T E, Weinberger D R. Cereb Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- 23.Logothetis N, Pauls J, Augath M, Trinath T, Oeltermann A. Nature. 2001;412:150–156. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 24.Mattay V S, Tessitore A, Callicott J H, Bertolino A, Goldberg T E, Chase T N, Hyde T M, Weinberger D R. Ann Neurol. 2002;51:156–164. doi: 10.1002/ana.10078. [DOI] [PubMed] [Google Scholar]
- 25.Floresco S, Phillips A. Behav Neurosci. 2001;115:934–939. [PubMed] [Google Scholar]
- 26.Livak K J. Genet Anal. 1999;14:143–149. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 27.Arnsten A. Trends Cognit Sci. 1998;2:436–447. doi: 10.1016/s1364-6613(98)01240-6. [DOI] [PubMed] [Google Scholar]
- 28.Cools R, Stefanova E, Barker R A, Robbins T W, Owen A M. Brain. 2002;125:584–594. doi: 10.1093/brain/awf052. [DOI] [PubMed] [Google Scholar]
- 29.Sawaguchi T. Parkinsonism Relat Disord. 2001;7:9–19. doi: 10.1016/s1353-8020(00)00044-4. [DOI] [PubMed] [Google Scholar]
- 30.Yang C, Seamans J. J Neurosci. 1996;16:1922–1935. doi: 10.1523/JNEUROSCI.16-05-01922.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang C R, Seamans J K, Gorelova N. Neuropsychopharmacology. 1999;21:161–194. doi: 10.1016/S0893-133X(98)00112-2. [DOI] [PubMed] [Google Scholar]
- 32.Zheng P, Zhang X, Bunney B, Shi W. Neuroscience. 1999;91:527–535. doi: 10.1016/s0306-4522(98)00604-6. [DOI] [PubMed] [Google Scholar]
- 33.Seamans J K, Durstewitz D, Christie B R, Stevens C F, Sejnowski T J. Proc Natl Acad Sci USA. 2001;98:301–306. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Law-Tho D, Hirsch J C, Crepel F. Neurosci Res. 1994;21:151–160. doi: 10.1016/0168-0102(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 35.Palmatier M A, Kang A M, Kidd K K. Biol Psychiatry. 1999;46:557–567. doi: 10.1016/s0006-3223(99)00098-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.