Abstract
Lithium has long been a primary drug used to treat bipolar mood disorder, even though the drug's therapeutic mechanisms remain obscure. Recent studies demonstrate that lithium has neuroprotective effects against glutamate-induced excitotoxicity in cultured neurons and in vivo. The present study was undertaken to examine whether postinsult treatment with lithium reduces brain damage induced by cerebral ischemia. We found that s.c. injection of lithium dose dependently (0.5–3 mEq/kg) reduced infarct volume in the rat model of middle cerebral artery occlusion/reperfusion. Infarct volume was reduced at a therapeutic dose of 1 mEq/kg even when administered up to 3 h after the onset of ischemia. Neurological deficits induced by ischemia were also reduced by daily administration of lithium over 1 week. Moreover, lithium treatment decreased the number of neurons showing DNA damage in the ischemic brain. These neuroprotective effects were associated with an up-regulation of cytoprotective heat shock protein 70 (HSP70) in the ischemic brain hemisphere as determined by immunohistochemistry and Western blotting analysis. Lithium-induced HSP70 up-regulation in the ischemic hemisphere was preceded by an increase in the DNA binding activity of heat shock factor 1, which regulates the transcription of HSP70. Physical variables and cerebral blood flow were unchanged by lithium treatment. Our results suggest that postinsult lithium treatment reduces both ischemia-induced brain damage and associated neurological deficits. Moreover, the heat shock response is likely to be involved in lithium's neuroprotective actions. Additionally, our studies indicate that lithium may have clinical utility for the treatment of patients with acute stroke.
Keywords: cerebral ischemia‖heat shock factor 1‖ heat shock protein ‖neuroprotection‖DNA damage
Lithium has been extensively used in the treatment of bipolar mood disorder, although the mechanisms underlying the drug's therapeutic action remain unclear. There is growing evidence that lithium is neuroprotective against a variety of insults, such as glutamate-induced excitotoxicity, in cultured cells and animal models of diseases (1–3). The mechanisms underlying lithium-induced neuroprotection are complex and may include inactivation of N-methyl-d-aspartate receptors (4), activation of the phosphatidylinositol 3-kinase/Akt cell survival pathway (5), enhanced expression of cytoprotective Bcl-2 (6, 7), and inhibition of glycogen synthase kinase-3β (8).
The brain is extremely sensitive to ischemic insult, and the resulting brain damage is related to excitotoxicity. Development of neuroprotective agents against ischemia-induced brain damage has been a therapeutic strategy to reduce the mortality and morbidity associated with stroke. Animal models of brain focal ischemia primarily involve middle cerebral artery occlusion (MCAO). We previously found that lithium pretreatment decreased the infarct volume and neurological deficits in a permanent MCAO model of rats (9). The present study was undertaken to explore the possibility that lithium might have clinical efficacy for the treatment of acute ischemic stroke. Using a rat temporary focal ischemia model, we established the dose and time requirements for postinsult lithium treatment to elicit its neuroprotective effects against ischemia-induced cerebral infarction. Because glycogen synthase kinase-3β can be inhibited directly by lithium (10), and negatively regulates the activity of heat shock factor 1 (HSF1) (11), a transcription factor of heat shock protein 70 (HSP70), we also studied heat shock response in an attempt to elucidate possible mechanisms underlying lithium protection against ischemia-induced brain damage.
Materials and Methods
Rat MCAO/Reperfusion Model and Lithium Treatment.
Male Sprague–Dawley rats weighing 250–300 g were anesthetized with halothane (3% in a mixture of 70% N2O and 30% O2). A 4-0 nylon suture with silicon-coated tip was inserted from the left external carotid artery into the left internal carotid artery and then to the Circle of Willis to occlude the origin of the left middle cerebral artery. One hour after MCAO, the nylon suture was withdrawn and the ischemic brain tissue received blood reperfusion for various times before death. Blood gas and blood pressure were maintained within their normal ranges during surgery. Body temperature was also monitored during surgery with a rectal probe and maintained in the range of 36.5–37.5°C with a heating pad. Laser–Doppler flowmetry was used to evaluate the efficacy of MCAO and reperfusion. Sham-operated rats underwent the same procedures except for the MCAO. All animals were handled according to the guidelines of the National Institute of Mental Health Animal Care and Use Committee. For all experiments, LiCl at indicated times and doses was s.c.-administered to rats, with normal saline as the vehicle control.
Infarct Volume and Neurological Deficit Evaluation.
Rats were killed by CO2 inhalation, and the brains were removed and stained with 2% 2,3,5-triphenyltetrazolium chloride and then fixed with 10% formalin for evaluation of infarct volume. Infarct volume of six slices of 2-mm coronal sections of each brain was calculated in a blinded manner by capturing the images with a digital camera and then performing computerized analysis using National Institutes of Health IMAGE 1.61 software. Infarct volume determination was corrected for edema by subtracting the volume of normal tissue in the ischemic hemisphere from the volume of the contralateral hemisphere, essentially as described (12). Rats showing tremor and seizure were excluded from studies of brain infarction and neurological deficits.
The neurological deficits in rats subjected to MCAO/reperfusion were evaluated, essentially as described (13) with some modification. Ten different tests for motor, sensation, and reflex abnormalities were performed by a blinded investigator. Six tests of motor performance (flexion of forelimb, flexion of hind limb, head movement >10° to the vertical axis within 30 s, inability to walk straight, circling toward the paralytic side, and falling down to the paralytic side) were included to evaluate hemiplegia in the extremities and trunk. In sensation tests, visual and tactile placement and a proprioceptive test were adapted to evaluate the sensory abnormalities of the body. In reflex tests, pinna and startle reflex were studied to evaluate the loss of responses in these animals. A score of 0 (normal) or 1 (unable to perform, abnormal in task performance, or deficit in reflex) was given to each test. Sham-operated rats did not show visible neurological deficits.
Terminal Deoxynucleotidyltransferase-Mediated dUTP-Digoxigenin Nick End Labeling (TUNEL) Staining.
The brains were removed and immersed in dry ice precooled isopentane. Coronal sections of 10-μm thickness were cut with a cryostat and mounted on slides. Cryosections were fixed in 1% paraformaldehyde in PBS and postfixed in a mixture of ethanol and acetic acid (2:1) at −20°C. TUNEL assay was performed with a fluorescein ApopTag kit (Intergen, Purchase, NY) according to the manufacturer's instructions by using affinity-purified fluorescein-conjugated antidigoxegenin sheep polyclonal antibody with the Fc portion removed. As a negative control, sections of ischemic brain were used after the standard procedures, but labeled dUTP was omitted. To counterstain with propidium iodide for detecting the total number of cells, the sections were mounted in Vectashield medium containing 1.5 μg/ml propidium iodide. For double immunofluorescence staining, TUNEL sections were washed in PBS and incubated overnight at 4°C with a monoclonal mouse antibody against neuronal marker protein NeuN (1:1,000, Chemicon) and this was followed by a 2-h incubation at room temperature with F(ab′) fragment of affinity-purified goat anti-mouse antibody conjugated to Alexa Fluor 546 (1:200; Molecular Probes). All sections were examined by light microscopy in three random middle cerebral artery areas in the inner border of the infarct in the ischemic fronto-parietal cortex of each rat, as described (14). After capturing images with a digital camera, quantification was performed by a blinded observer (see below).
Immunohistochemistry.
Coronal sections of 10-μm thickness were cut with a cryostat, fixed with 4% paraformaldehyde for 30 min, and rinsed with 1% Triton X-100 for 30 min. Endogenous peroxidase and peroxidase-like activity were blocked by incubating the sections in 1% H2O2 for 30 min. For immunostains, brain sections were incubated at 4°C overnight with a goat polyclonal IgG against HSP70 (1:50; Santa Cruz Biotechnology) and sequentially treated again at 4°C overnight with biotin-conjugated anti-goat IgG (1:400; Santa Cruz Biotechnology) and horseradish peroxidase-conjugated streptavidin (1:500; Upstate Biotechnology, Lake Placid, NY). Between each procedure, the brain sections were rinsed three times in PBS for 10 min and then incubated with 0.05% 3,3′-diaminobenzidine and 2.5% nickel ammonium sulfate. The omission of the first antibody was used as the negative control. Three random areas in the inner border of the infarct in the ischemic fronto-parietal cortex were examined under a microscope and counted for the number of HSP-positive cells and intensity of HSP labels (see below).
Western Blotting.
Brain tissues from ischemic cortex of left middle cerebral artery territory and the corresponding area of sham-operated rats and in the contralateral side of saline or lithium-treated rats were rigorously homogenized and sonicated for 30 s in a lysis buffer as described (7). Protein concentrations were determined and aliquots of 10 μg of the total proteins were separated by electrophoresis on SDS-polyacrylamide gels (10%). Proteins were subsequently transferred to a poly(vinylidene difluoride) membrane, which was then incubated with the mAb to HSP70 (1:1,000; Calbiochem) or β-actin (1:5,000; Sigma) at 4°C overnight. Membranes were washed three times with PBS and then incubated overnight at 4°C with a horseradish peroxidase-conjugated secondary antibody. Immunoreactivity was detected by enhanced chemiluminescent autoradiography.
Electrophoretic Mobility-Shift Assay (EMSA) and Supershift Assay.
Nuclear proteins from ischemic cortex in middle cerebral artery territory and the corresponding brain area of sham-operated rats and in the contralateral side of saline or lithium-treated rats were prepared as described (15). Briefly, brain tissues were gently homogenized and nuclear proteins were obtained with high salt extraction. Self-complementary consensus heat shock element (HSE) oligonucleotides (5′-CTA-GAA-GCT-TCT-AGA-AGC-TTC-TAG-3′), containing four perfect inverted 5′-NGAAN-3′ repeats after annealing (Integrated DNA Technologies, Coralville, IA) (16), were labeled with [γ-32P]ATP by a T4 polynucleotide kinase (Promega). Nuclear proteins (20 μg) were incubated with radiolabeled DNA probes (≈40,000 cpm) in the binding buffer (Promega) for 20 min at room temperature. Nonspecific binding was assessed by adding 60-fold unlabeled HSF1 probes to the reaction mixture. The sample was then electrophoresed on a 4.5% nondenaturing polyacrylamide gel in 0.5× Tris borate-EDTA buffer. Supershift assay was accomplished to identify the specificity of HSF1. EMSA was performed as described above except with the addition of 4 μl of rabbit anti-HSF1 antibody (200 μg/0.1 ml, Stressgen Biotechnologies, Victoria, Canada) to the EMSA mixtures during the binding reaction period. The autoradiogram was developed by exposing the vacuum-dried gel to x-ray film at −80°C for 24 h.
Image and Data Analysis.
After capturing images with a digital camera, quantification of the results from histochemistry, Western blotting, and EMSA were performed with National Institutes of Health IMAGE 1.61 software. The total number of TUNEL-positive cells per image (area of 1.05 mm2 with resolution of 1,600 × 1,200 pix, objective ×10) was calculated. The mean pixel intensity values, within neuronal soma profiles traced under bright-field optics, were collected from 30 HSP70-positive neurons in each section (n = 3). In each section, three cortical areas outside labeled neurons were chosen randomly to obtain an average value for the subtraction of background. Specific binding of HSF1 was obtained by subtracting nonspecific binding from total binding. All results were expressed as mean ± SEM. Statistical analysis was performed by ANOVA and Fisher's protected least significant difference. A P value of ≤ 0.05 was considered to be statistically significant.
Results
Physiological Variables and Cerebral Blood Flow.
Lithium and saline-treated rats showed similar values in body temperature, mean arterial blood pressure, and blood gases (Table 1). Immediately after MCAO, the cerebral blood flow in the ischemic area dropped to ≈20% of the preischemia basal value in both lithium- and saline-treated rats (Table 1). After 20 min of reperfusion, cerebral blood flow was restored to ≈90% of the preischemia basal value in both groups.
Table 1.
Physiological parameters in rats subjected to MCAO
| Parameter | Preischemia | Ischemia | Reperfusion |
|---|---|---|---|
| Body temperature, °C | |||
| Control | 37.0 ± 0.14 | 36.0 ± 0.25 | 36.8 ± 0.41 |
| Lithium | 37.0 ± 0.25 | 36.8 ± 0.27 | 37.0 ± 0.34 |
| Mean arterial blood pressure, mmHg | |||
| Control | 100 ± 11 | 96 ± 5 | 101 ± 6 |
| Lithium | 98 ± 12 | 94 ± 7 | 97 ± 4 |
| PH | |||
| Control | 7.43 ± 0.04 | 7.43 ± 0.04 | 7.43 ± 0.02 |
| Lithium | 7.44 ± 0.03 | 7.39 ± 0.03 | 7.38 ± 0.02 |
| Pco2, mmHg | |||
| Control | 39.6 ± 2.6 | 40.8 ± 2.5 | 41.3 ± 1.3 |
| Lithium | 39.8 ± 2.6 | 39.7 ± 1.5 | 39.5 ± 0.9 |
| Po2, mmHg | |||
| Control | 143 ± 1.9 | 142 ± 1.5 | 137 ± 3 |
| Lithium | 138 ± 4.6 | 143 ± 2.4 | 140 ± 2.8 |
| Relative cerebral blood flow | |||
| Control | 100% | 22.3% ± 2.6* | 89.5% ± 4.3 |
| Lithium | 100% | 21.6% ± 3.4* | 91.7% ± 3.8 |
The preischemia, ischemia, and reperfusion values were determined 20 min before the onset of MCAO, 20 min after the onset of MCAO, and 20 min after the onset of reperfusion, respectively. Rats were s.c. injected with saline or LiCl (1 mEq/kg) immediately after the onset of MCAO. Data are mean ± SEM from five rats in both saline control and lithium-treated groups.
, P < 0.01, compared with preischemia values.
Lithium Reduces Infarct Volume.
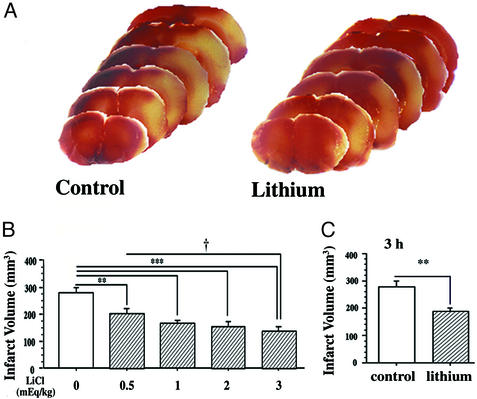
In rats subjected to MCAO for 1 h followed by reperfusion for 23 h, extensive infarction was detected in the cerebral cortical and subcortical areas over a series of brain sections, and a single s.c. injection with LiCl (3 mEq/kg) immediately after the onset of MCAO resulted in a reduction of infarct volume detected by 2,3,5-triphenyltetrazolium chloride staining (Fig. 1A). Lithium markedly reduced infarct volume in the dosage range of 0.5 to 3.0 mEq/kg (Fig. 1B). When LiCl (1 mEq/kg) was injected 3 h after the onset of MCAO and the rats were killed 24 h after the ischemic insult, the infarct volume was still significantly reduced (Fig. 1C). Plasma lithium levels of rats injected with 1, 2, or 3 mEq/kg LiCl were in the therapeutic range of 0.5–1.0 mmol/liter at 12 h and 0.2–0.8 mmol/liter at 24 h after injection.
Figure 1.
Postinsult lithium treatment reduces MCAO/reperfusion-induced brain infarction. Rats were s.c.-injected with normal saline or LiCl after MCAO and killed at different time points, and the brains were stained with 2,3,5-triphenyltetrazolium chloride. (A) The animals were subjected to ischemia for 1 h followed by reperfusion for 23 h and then killed for the determination of infarct volume. LiCl (3 mEq/kg) was injected immediately after cerebral ischemia induction. (B) A single injection of the indicated dose of LiCl was performed in rats immediately after the onset of MCAO and the animals were killed 24 h later. (C) LiCl (1 mEq/kg) was injected at 3 h after the onset of MCAO, and the animals were killed at 24 h after the onset of MCAO. Data are means ± SEM from eight rats in each group. **, P < 0.01; ***, P < 0.001 compared with the control (saline-injected group). †, P < 0.05, between the groups of 0.5 mEq/kg and 3.0 mEq/kg shown in B.
Lithium Facilitates Functional Recovery.
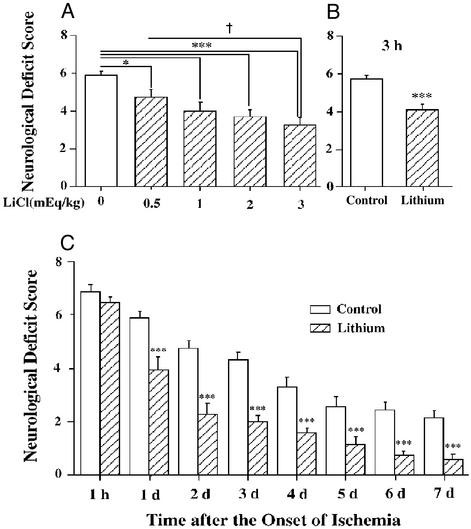
Postinsult lithium administration reduced neurological deficits in the dosage range of 0.5 to 3.0 mEq/kg (Fig. 2A). Rats injected with lithium at 3 h after the onset of MCAO also showed a significant decrease in neurological deficits when examined at 24 h (Fig. 2B). Lithium administered immediately after the onset of ischemia and once each day thereafter markedly reduced the neurological deficit scores compared with controls during days 1–7 (Fig. 2C). The lithium-induced reduction of neurological deficits persisted up to 14 days after ischemia (data not shown).
Figure 2.
Lithium treatment reduces MCAO/reperfusion-induced neurological deficits. (A) Rats were subjected to MCAO for 1 h followed by reperfusion for 23 h before determination of neurological deficits. When used, indicated doses of LiCl were injected immediately after MCAO. (B) Rats were injected with LiCl (1 mEq/kg) 3 h after the onset of MCAO and evaluated for neurological deficits 24 h postinsult. (C) The animals were injected with LiCl (1 mEq/kg) or normal saline immediately after MCAO and followed by daily injections for up to 6 days. Neurological deficits were evaluated once a day throughout a 7-day postischemic period. Data are means ± SEM from eight rats in each group. *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared with the corresponding saline-control group; †, P < 0.05 between the groups of 0.5 mEq/kg and 3.0 mEq/kg shown in A.
Lithium Protects Against Ischemia-Induced Cell Death.
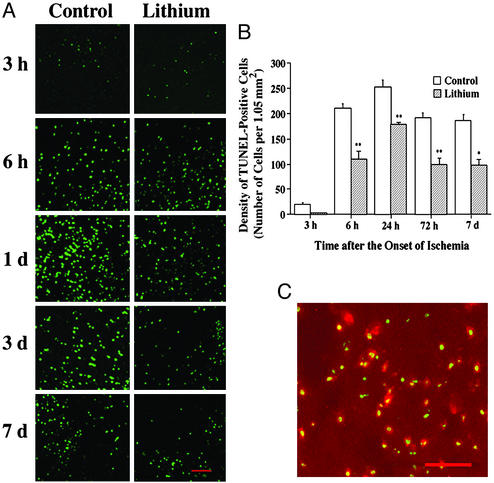
Ischemia-induced brain damage and the neuroprotective effects of lithium treatment were also assessed by TUNEL assay. Three hours after the onset of MCAO, TUNEL staining was found in the nuclei of cells within the ischemic cortical area (Fig. 3A, green). The number of TUNEL-positive cells time-dependently increased after the ischemia (from 6 h to 7 days) and this increase was suppressed by lithium treatment in the corresponding areas within the cortical penumbra (Fig. 3 A and B). The peak response of TUNEL staining occurred around 24 h after MCAO, which was followed by a gradual decline at 3 days and 7 days, an observation similar to that made previously (17). The gradual decline of TUNEL could reflect, at least in part, continuous apoptotic cell death, as 3 days after MCAO the number of activated caspase 3-positive cells was still markedly elevated and this was also suppressed by lithium treatment (Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org). The total number of cells (intact plus damaged cells) was determined by propidium iodide staining and not found to be significantly different after lithium treatment (data not shown). A large majority of these TUNEL-positive cells were colabeled with NeuN (Fig. 3C, red), yielding yellow labels, and indicating that neurons were affected.
Figure 3.
Lithium suppresses MCAO/reperfusion-induced DNA damage in rat brain. Rats were subjected to MCAO for 1 h followed by reperfusion for the indicated times. LiCl (1 mEq/kg) or normal saline was injected immediately after MCAO and daily thereafter as indicated. Brains were sliced with a cryostat into sections of 10-μm thickness and evaluated by TUNEL assay. The sections were also double-stained with NeuN. (A) Representative time-dependent changes in TUNEL staining in a defined locus within the cortical ischemic penumbra area of saline- and lithium-treated rats. (B) Quantification of the density of TUNEL-positive cells shown in A. (C) At 72 h after the ischemic insult, a great majority of TUNEL-positive cells (green) were colabeled with NeuN (red) in the ischemic penumbra area. Bar graphs shown are means ± SEM of TUNEL-positive cells from four rats in each group. *, P < 0.05; **, P < 0.01, compared with the corresponding saline-control group. (Bar: 100 μm.)
Up-Regulation of HSP70 and HSF1 Binding Activity by Ischemia and Lithium.
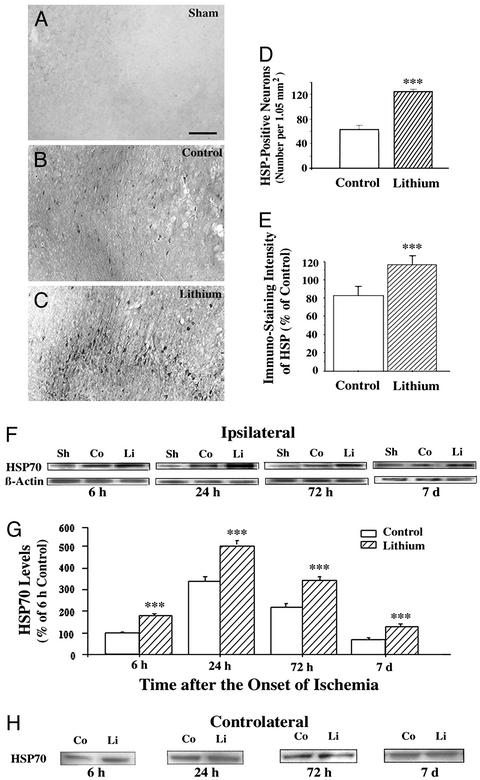
HSP70 is up-regulated shortly after ischemia and persists in the ischemic penumbra where recovery of neurons is most pronounced (for review, see ref. 18). To evaluate the role of HSP70 in mediating the neuroprotective effects of lithium, we found that at 24 h after MCAO HSP70-positive cells were detected within the ischemic penumbra of the cortical area in saline- and lithium-treated rats (Fig. 4 B and C). In contrast, there was no detectable HSP70 immunostaining in the corresponding brain sections of sham-operated rats (Fig. 4A). Morphological inspection and double-labeling with anti-NeuN antibody (data not shown) indicated that these HSP70-positive cells were mainly neuronal. Quantification revealed that the number of HSP70-positive cells was ≈2-fold greater in lithium-treated than saline-treated rats (Fig. 4D), and the intensity of HSP70-positive staining was also significantly higher in lithium-treated than saline-treated rats (Fig. 4E). Western blotting analysis revealed that HSP70 in the ischemic cortex was detectable 6 h after ischemic insult, reached a maximum at 24 h, and declined at 72 h and 7 days (Fig. 4 F and G). Lithium elicited an increase of the levels of HSP70 at every time point. In contrast, lithium affected neither the β-actin protein levels in the ipsilateral ischemic hemisphere (Fig. 4F) nor the HSP70 protein levels in the contralateral intact side (Fig. 4H).
Figure 4.
Lithium enhances MCAO/reperfusion-induced HSP70 levels. Rats were subjected to MCAO for 1 h followed by reperfusion until death at the indicated times. LiCl (1 mEq/kg) or saline was injected immediately after the onset of MCAO followed by daily injections. Representative immunohistochemistry for HSP70-positive cells within the penumbra of ischemic cerebral cortex or in the corresponding area of cortex was examined in sham-operated rats (A), saline-control rats (1 h of MCAO followed by 23 h of reperfusion) (B), and lithium-treated rats (1 h of MCAO followed by 23 h of reperfusion) (C). (D) The number of HSP70-positive cells from B and C were quantified in corresponding 1.05-mm2 areas. (E) The intensity of HSP70-positive cells from B and C were quantified by using National Institutes of Health IMAGE 1.61 software and the data are expressed as percentage of saline control. (F) Western blotting analysis of HSP70 protein levels in extracts from the entire ischemic cortex of the ipsilateral hemisphere of saline-treated control (Co) and lithium-treated (Li) rats and the corresponding area from sham-operated (Sh) rats. Levels of β-actin protein were used as the control. (G) Quantified results of Western blotting were expressed as means ± SEM of percentage of 6 h control from four rats in each group. ***, P < 0.001 compared with corresponding saline controls. (Bar: 200 μm.) (H) Western blotting analysis of HSP70 protein levels in extracts from the corresponding area in the cortex of the contralateral hemisphere of saline-treated control (Co) and lithium-treated (Li) rats.
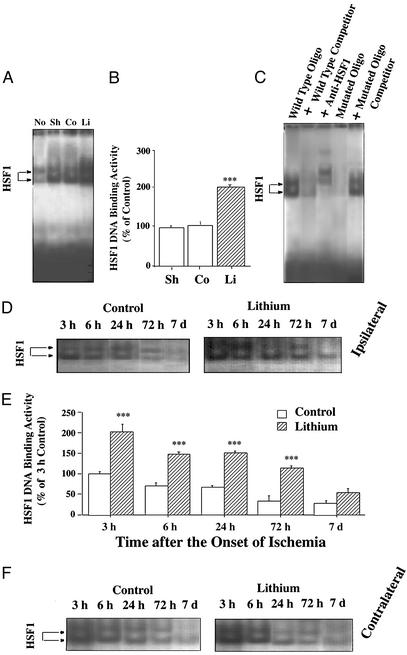
Because HSF1 is a transcription factor for HSP70 (for review, see ref. 19), we measured HSF1 DNA binding activity in extracts derived from the ischemic cortex. Three hours after the onset of MCAO, two bands of HSF1 binding activity were detected with EMSA, both of which were increased ≈2-fold by lithium treatment (Fig. 5 A and B). The specificity of HSF1 binding measured by EMSA was confirmed by the observations that both HSF1 binding bands were suppressed by the presence of excess unlabeled oligonucleotides and supershifted by HSF1-specific antibody (Fig. 5C). Moreover, mutated HSE oligonucleotides neither showed binding activity detected by EMSA nor competed with HSF1 binding to WT HSE oligonucleotides (Fig. 5C). At 3 h after the onset of ischemia HSF1 DNA binding activities were similarly elevated in extracts derived from sham-operated rats and MCAO/saline control rats (Fig. 5 A and B), compared with the activity in the normal brain. Time-course experiments showed that HSF1 DNA binding activity in the ipsilateral ischemic brain decreased from 3 h to 7 days after the ischemic insult (Fig. 5 D and E). At all time points, the HSF1 DNA binding activity was at least 2-fold greater in lithium-treated than saline-treated MCAO/reperfusion rats. Interestingly, HSF1 DNA binding activities in the contralateral side were also time- dependently increased by lithium after ischemia (Fig. 5F).
Figure 5.
Lithium increases HSF1 DNA binding activity in ischemic brain. (A) Rats were subjected to MCAO for 1 h followed by 2 h of reperfusion. LiCl (1 mEq/kg) or normal saline was injected immediately after MCAO. HSF1 DNA binding activities were determined by EMSA from extracts of the entire ischemic cortex of rats. The HSF–HSE complex migrated as a doublet in normal (No), sham-operated (Sh), saline control (Co), and lithium-treated (Li) groups as indicated by arrows. (B) Quantified EMSA results shown in A. (C) The specificity of HSF1 binding to HSE was assessed by the presence of 60-fold excess of unlabeled HSE, addition of 4 μl of rabbit anti-HSF1 antibody (200 μg/0.1 ml, Stressgen Bioreagents) to the EMSA mixture, the binding activity of 32P-labeled, mutated HSE (5′-CTA-TAA-TCT-TGT-ATA-AGT-TTG-TAG-3′), and WT HSE binding in the presence of 60-fold excess of unlabeled, mutated HSE. (D) Rats were subjected to MCAO for 1 h followed by reperfusion for the indicated times. LiCl (1 mEq/kg) or normal saline was injected immediately after MCAO followed by daily injections. The time course of lithium-induced enhancement of HSF1 DNA binding activity was determined by EMSA from extracts of entire ischemic cortex of saline-treated control and lithium-treated rats. (E) Quantified results of EMSA results shown in D. Data are means ± SEM of percentage of 3 h control from four rats in each group. ***, P < 0.001 compared with respective saline controls. (F) Time course of HSF1 DNA binding activity determined by EMSA in extracts of the corresponding areas in the contralateral hemisphere of saline-treated control and lithium-treated rats.
Discussion
The present study showed that lithium administered after the onset of ischemia was capable of reducing brain infarct volume in an MCAO/reperfusion model of rats. This neuroprotective effect occurred at therapeutic concentrations of this drug and the treatment time window was at least 3 h after the initiation of the insult. Lithium-induced neuroprotection was further demonstrated by a reduction in the number of neurons showing DNA damage. In the MCAO/reperfusion paradigm, neurological deficits declined progressively during the time period of 1 h to 7 days after ischemia. This spontaneous neurological recovery is likely caused by blood reperfusion after transient MCAO and is similar to the observations made in a clinical study (20). It should be noted that lithium treatment beginning immediately post-insult facilitated this neurological deficit recovery for at least 1 week after the insult. Such facilitation of functional recovery was also evident when the drug was administered 3 h after the onset of ischemia. These long-term benefits suggest that lithium might be useful as a therapeutic agent for acute stroke patients.
Lithium neuroprotection in the rat brain ischemia model might not be the result of increased cerebral blood flow, as the blood flow parameters determined by Laser–Doppler flowmetry shortly (20 min) after MCAO or reperfusion were similar in saline- and lithium-treated groups (Table 1). Body temperature was also not significantly affected by lithium (1 mEq/kg) determined either shortly after MCAO/reperfusion (Table 1) or throughout the 24-h survival period after the ischemic insult (data not shown). These results differed from those reported by Yoshida et al. (21) in which the body temperature was significantly decreased by i.p. injections of a relatively high dose (5 mEq/kg) of lithium. This discrepancy could arise from differences in the lithium dose, route, and duration of administration as well as animal species used in the studies.
Several studies have reported that levels of HSPs are increased in the ischemic penumbra of brain in animal models of focal ischemia, where many injured neurons recover (for reviews, see refs. 18 and 19). In our ischemia model, HSP70 levels were maximally increased at 24 h postinsult, and the increase persisted for at least 7 days in the ischemic cerebral cortex. HSP70 up-regulation was preceded by an increase in HSF1 DNA binding activity, suggesting the involvement of transcriptional regulation. More importantly, postischemic lithium treatment markedly enhanced the increase in HSP70 levels and HSF1 binding activity. Lithium-induced enhancements of heat shock responses could be the result of increased cell survival in the ischemic brain. However, the early detection of these lithium-induced increases in heat shock responses (Fig. 5), the lack of effects on β-actin protein levels (Fig. 4F), and the increase in not only the number of HSP-positive neurons but the intensity of the HSP immunostaining (Fig. 4 D and E) seem to speak against this possibility. The mechanism(s) underlying lithium-induced HSF1 activation here is most possibly through inhibition of glycogen synthase kinase-3β, which has been shown to negatively regulate both DNA binding activity of HSF1 and its subsequent transcriptional activation of HSP70 (8, 11). Thus, glycogen synthase kinase-3β inhibition by lithium, either by a direct action (10) or through activation of the phosphatidylinositol 3-kinase/Akt signaling pathway (5), may result in disinhibition of HSF1, leading to transcription and expression of HSP70. The observations that HSF1 binding activities are similar at 3 h in both sham-operated and MCAO/reperfusion rats (Fig. 5 A and B) and that lithium significantly increases levels of HSF1 binding, but not HSP70 protein, in the contralateral side (Figs. 4H and 5F) suggest that enhanced HSF1 DNA binding activity can be uncoupled from its transcriptional activation. The observations that HSF1 DNA binding and transactivating capabilities can be uncoupled are not unprecedented. This finding is exemplified in several studies using yeast where HSF binds DNA constitutively, but fails to activate transcription in the absence of further stimulus (for review, ref. 19). Moreover, in mammalian cells nonsteroidal antiinflammatory drugs such as salicylate and indomethalcin enhance HSF1 DNA binding, but not HSP70 transcriptional activation without heat shock stress (22, 23). Their results and ours suggest that drug-induced transcription and translation of HSP are multistep processes, highly regulated, and can be insult dependent.
HSPs such as HSP70 are molecular chaperones that bind to unfolded or malfolded protein to ensure proper folding and prevent intracellular protein aggregation (24). HSPs also exert their neuroprotective effects by antagonizing apoptosis-inducing factor (25). Recently, it was reported that gene transfer-induced HSP70 overexpression protects neurons from ischemic brain damage in an experimental rat model (26). Moreover, overexpression of HSP70 inhibits the activation of NF-κB (27), which is activated during ischemia and appears to promote apoptotic cell death (28). Lithium-induced activation of HSF1 and up-regulation of HSP70 have a relatively rapid time course and are temporally associated with the acute neuroprotective effects of lithium against MCAO/reperfusion-induced brain injury.
It is possible that other molecular and cellular actions of lithium also participate in lithium-induced neuroprotection in the transient focal ischemia model. These include lithium's ability to inhibit N-methyl-d-aspartate receptors (4, 29), up-regulate cytoprotective Bcl-2 (6, 7, 30), down-regulate proapoptotic p53 and Bax (7), inhibit glutamate-induced mitogen-activated protein kinase activation (31), facilitate glutamate uptake into presynaptic nerve endings (32), induce the expression of brain-derived neurotrophic factor in discrete brain areas (33, 34), and enhance neurogenesis in the hippocampus (35). These actions require long-term treatment and could be more likely involved in the “delayed” phase of the neuroprotective effects of this drug.
Agents potentially useful as new treatments for brain ischemia include glutamate antagonists, antiapoptotic agents, growth factors, activators of endogenous defensive responses, inhibitors of mitogen-activated protein kinases, and stimulators of neurogenesis (for review, ref. 36). Given that lithium has most, if not all, of their effects, clinical studies using lithium, alone or in conjunction with other therapy such as recombinant tissue-type plasminogen activator (37), to treat patients with acute stroke seem to be warranted.
Supplementary Material
Acknowledgments
We thank Drs. Yong Chen and John M. Hallenbeck of the National Institute of Neurological Disorders and Stroke, National Institutes of Health for assistance in the measurement of cerebral blood flow.
Abbreviations
- EMSA
electrophoretic mobility-shift assay
- HSE
heat shock element
- HSF1
heat shock factor 1
- HSP
heat shock protein
- MCAO
middle cerebral artery occlusion
- TUNEL
terminal deoxynucleotidyltransferase-mediated dUTP-digoxigenin nick end labeling
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Manji H K, Moore G J, Chen G. Biol Psychiatry. 1999;46:929–940. doi: 10.1016/s0006-3223(99)00165-1. [DOI] [PubMed] [Google Scholar]
- 2.Grimes C A, Jope R S. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 3.Chuang D-M, Chen R W, Chalecka-Franaszek E, Ren M, Hashimoto R, Senatorov V, Kanai H, Hough C, Hiroi T, Leeds P. Bipolar Disorders. 2002;4:1–9. doi: 10.1034/j.1399-5618.2002.01179.x. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto R, Hough C, Nakazawa T, Yamamoto T, Chuang D-M. J Neurochem. 2002;80:589–597. doi: 10.1046/j.0022-3042.2001.00728.x. [DOI] [PubMed] [Google Scholar]
- 5.Chalecka-Franaszek E, Chuang D-M. Proc Natl Acad Sci USA. 1999;96:8745–8750. doi: 10.1073/pnas.96.15.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen G, Zeng W Z, Yuan P X, Huang L D, Jiang Y M, Zhao Z H, Manji H K. J Neurochem. 1999;72:879–882. doi: 10.1046/j.1471-4159.1999.720879.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen R W, Chuang D-M. J Biol Chem. 1999;274:6039–6042. doi: 10.1074/jbc.274.10.6039. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Bijur G N, Jope R S. Bipolar Disorders. 2002;4:137–144. doi: 10.1034/j.1399-5618.2002.40201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nonaka S, Chuang D-M. NeuroReport. 1998;229:2081–2084. doi: 10.1097/00001756-199806220-00031. [DOI] [PubMed] [Google Scholar]
- 10.Klein P S, Melton D A. Proc Natl Acad Sci USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xavier I J, Mercier P A, McLoughlin C M, Ali A, Woodgett J R, Ovsenek N. J Biol Chem. 2000;275:29147–29152. doi: 10.1074/jbc.M002169200. [DOI] [PubMed] [Google Scholar]
- 12.Lin T N, He Y Y, Wu G, Khan M, Hsu C Y. Stroke. 1993;24:117–121. doi: 10.1161/01.str.24.1.117. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Chopp M, Chen J, Wang L, Gautam S C, Xu Y X, Zhang Z. J Cereb Blood Flow Metab. 2000;20:1311–1319. doi: 10.1097/00004647-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Savitz S I, Erhardt J A, Anthony J V, Gupta G, Li X, Barone F C, Rosenbaum D M. J Cereb Blood Flow Metab. 2000;20:1197–1204. doi: 10.1097/00004647-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Qin Z, H, Wang Y, Chen R W, Wang X, Ren M, Chuang D-M, Chase T N. J Pharmacol Exp Ther. 2001;297:78–87. [PubMed] [Google Scholar]
- 16.Higashi T, Nakai A, Uemura Y, Kikuchi H, Nagata K. Mol Brain Res. 1995;34:262–270. doi: 10.1016/0169-328x(95)00163-m. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki C, Hayashi T, Zhang W R, Warita H, Manabe Y, Sakai K, Abe K. Neurol Res. 2001;23:588–592. doi: 10.1179/016164101101199054. [DOI] [PubMed] [Google Scholar]
- 18.Lipton P. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 19.Pirkkala L, Nykanen P, Sistonen L. FASEB J. 2001;15:1118–1131. doi: 10.1096/fj00-0294rev. [DOI] [PubMed] [Google Scholar]
- 20.Barber P A, Davis S M, Infeld B, Baird A E, Donnan G A, Jolley D, Lichtenstein M. Stroke. 1998;29:2522–2528. doi: 10.1161/01.str.29.12.2522. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida S, Kirino T, Tamura A, Basugi N, Sano K. Stroke. 1991;22:84–89. doi: 10.1161/01.str.22.1.84. [DOI] [PubMed] [Google Scholar]
- 22.Jurivich D A, Sistonen L, Kroes R A, Morimoto R I. Science. 1992;255:1243–1245. doi: 10.1126/science.1546322. [DOI] [PubMed] [Google Scholar]
- 23.Lee B S, Chen J, Angelidis C, Jurivich D A, Morimoto R I. Proc Natl Acad Sci USA. 1995;92:7207–7211. doi: 10.1073/pnas.92.16.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hendrick J P, Hartl F U. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- 25.Ravagnan L, Gurbuxani S, Susin S A, Maisse C, Daugas E, Zamzami N, Mak T, Jaattela M, Penninger J M, Garrido C, Kroemer G. Nat Cell Biol. 2001;3:839–843. doi: 10.1038/ncb0901-839. [DOI] [PubMed] [Google Scholar]
- 26.Hoehn B, Ringer T M, Xu L, Giffard R G, Sapolsky R M, Steinberg G K, Yenari M A. J Cereb Blood Flow Metab. 2001;21:1303–1309. doi: 10.1097/00004647-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Feinstein D L, Galea E, Aquino D A, Li G C, Xu H, Reis D J. J Biol Chem. 1996;271:17724–17732. doi: 10.1074/jbc.271.30.17724. [DOI] [PubMed] [Google Scholar]
- 28.Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M. Nat Med. 1999;5:554–559. doi: 10.1038/8432. [DOI] [PubMed] [Google Scholar]
- 29.Nonaka S, Hough C J, Chuang D-M. Proc Natl Acad Sci USA. 1998;95:2642–2647. doi: 10.1073/pnas.95.5.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei H, Qin Z-H, Senatorov V V, Wei W, Wang Y, Qian Y, Chuang D-M. Neuroscience. 2001;106:603–612. doi: 10.1016/s0306-4522(01)00311-6. [DOI] [PubMed] [Google Scholar]
- 31.Chen R-W, Qin Z-H, Ren M, Kanai H, Chalecka-Franaszek E, Chuang D-M. J Neurochem. 2003;84:566–575. doi: 10.1046/j.1471-4159.2003.01548.x. [DOI] [PubMed] [Google Scholar]
- 32.Dixon J F, Hokin L E. Proc Natl Acad Sci USA. 1998;95:8363–8368. doi: 10.1073/pnas.95.14.8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukumoto T, Morinobu S, Okamoto Y, Kagaya A, Yamawaki S. Psychopharmacology. 2001;158:100–106. doi: 10.1007/s002130100871. [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto R, Takei N, Shimazu K, Christ L, Lu B, Chuang D-M. Neuropharmacology. 2002;43:1173–1179. doi: 10.1016/s0028-3908(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 35.Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji H K. J Neurochem. 2000;75:1729–1734. doi: 10.1046/j.1471-4159.2000.0751729.x. [DOI] [PubMed] [Google Scholar]
- 36.Lee J M, Grabb M C, Zipfel G J, Choi D W. J Clin Invest. 2000;106:723–731. doi: 10.1172/JCI11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.