Abstract
VRS1 is the first isolated strain of vancomycin-resistant Staphylococcus aureus (VRSA) found to carry the vanA gene complex previously described in Enterococcus. Under vancomycin pressure, VRS1 makes aberrant cell walls consisting of stem tetrapeptide and depsipeptide that lack the terminal d-Ala-d-Ala residues targeted by vancomycin. Previous data have suggested that this aberrant cell wall is not cross-linked by PBP2a, the enzyme responsible for cell wall transpeptidation in the presence of β-lactam antibiotics. We examined the efficacy of treating VRS1 with a combination of vancomycin and β-lactam antibiotics in vitro and in vivo. We found that the MIC of oxacillin for VRS1 decreased from >256 μg/ml to <1 μg/ml in the presence of vancomycin. Using the rabbit model of endocarditis, we treated VRS1-infected rabbits with nafcillin alone, vancomycin alone, or a combination of nafcillin and vancomycin. Treatment with nafcillin in combination with vancomycin cleared bloodstream infections within 24 h and sterilized 12/13 spleens (92%), as well as 8/13 kidneys (62%), following 3 days of treatment. Mean aortic valve vegetation counts were reduced 3.48 log10 CFU/g with the combination therapy (compared to untreated controls) and were significantly lower than with either vancomycin or nafcillin given alone. VRS1 was extremely virulent in this model, as no untreated rabbits survived the 3-day trial. Treatment of clinical infections due to VRSA with the combination of vancomycin and β-lactams may be an option, based on these results.
Staphylococcus aureus causes serious infections in both the hospital and the community. The growing prevalence of methicillin-resistant Staphylococcus aureus (MRSA) as a cause of these infections has increased the use of the glycopeptide antibiotic vancomycin over the past 3 decades (17). As a result, vancomycin-resistant S. aureus (VRSA) strains that exhibit two different resistance mechanisms have emerged. The first, initially noted in Japan in 1996 and subsequently in the United States in 1997, is believed to be due to a thickened cell wall that produces intermediate vancomycin MICs of 4 to 16 μg/ml (2, 14). The second, first noted in 2002 in S. aureus, is identical to the mechanism seen in vancomycin-resistant Enterococcus, involving the van genes that produce an altered stem pentapeptide with reduced affinity for vancomycin (1). The terminal peptide in the stem pentapeptide of the peptidoglycan layer of the cell wall is altered from d-Ala-d-Ala, the target of vancomycin, to the depsipeptide, d-Ala-d-Lac, which is not recognized by vancomycin. This mechanism of resistance results in high-level resistance to vancomycin, with MICs greater than 32 μg/ml.
Four cases of VRSA infection due to acquisition of the van gene complex have been described (1, 3, 4). Although the plasmid and van genes carrying the resistance determinant have varied, all have been of the vanA type, as originally described in Enterococcus (27). The first VRSA strain described was found in Michigan and is known as VRSA-MI, HIP11714, or VRS1. The genetics of this strain were examined by Weigel et al. (26). VRS1 was found to have acquired the vanA gene complex on Tn1546 from a strain of Enterococcus faecalis coisolated from the patient's infection site. Tn1546 was inserted on a conjugative plasmid between aac-aphD and blaZ carried on a plasmid, creating pLW1043. Tn1546 also contained the two-component regulatory genes, vanRS, indicating that, as in enterococci, the van resistance genes would not be fully expressed unless the bacterial cell were exposed to the inducer, vancomycin.
The interaction between vancomycin resistance due to the vanA gene complex and methicillin resistance due to mecA was initially studied by Severin et al., who transferred pLW1043 from VRS1 to the MRSA strain COL by conjugation, creating strain COLVA (20). The authors examined the muropeptide composition following growth in vancomycin or oxacillin and concluded that the two major resistance mechanisms carried in the same S. aureus strain functioned independently in cell wall assembly. In fact, growth of the vancomycin-resistant strain in the presence of low concentrations of oxacillin resulted in a prompt decrease in the vancomycin MIC, from 512 μg/ml to 12 μg/ml. In a subsequent study, Severin et al. determined that PBP2, and not PBP2a, encoded by the methicillin resistance determinant mecA, was essential for cell wall biosynthesis in COLVA grown in the presence of vancomycin (21). The authors noted that this was due to the inability of PBP2a to use the depsipeptide-containing precursor for transpeptidation.
We hypothesized that treatment of infections due to vancomycin-resistant S. aureus with combinations of vancomycin and β-lactams would be effective. When strains were induced with vancomycin, forcing the cell to make only cell walls containing d-Ala-d-Lac depsipeptide, the cell walls could not be cross-linked by PBP2a. Thus, the cells would be, in effect, methicillin susceptible, and infections would respond to a β-lactam antibiotic. In order to test this hypothesis, we assessed the efficacy of vancomycin-β-lactam combinations, both in vitro and in vivo, in the rabbit model of infective endocarditis.
MATERIALS AND METHODS
Antimicrobial susceptibility testing.
Vancomycin and oxacillin MICs were determined by Etest (AB Biodisk, Piscatway, NJ). A single colony of VRS1 was picked from a Mueller-Hinton agar (MHA) plate containing 32 μg/ml vancomycin, inoculated into 5 ml Mueller-Hinton broth (Becton Dickinson, Cockeysville, MD) with or without 32 μg/ml vancomycin (Sigma-Aldrich, St. Louis, MO), and shaken at 220 rpm at 37°C overnight. The culture was diluted to 0.5 McFarland standard and plated on brain heart infusion (Becton Dickinson) agar with or without 16 μg/ml vancomycin. The appropriate Etest strip was placed on the plate and incubated at 37°C. MICs were determined at 24 and 48 h.
Time-kill assays were performed in Mueller-Hinton broth inoculated with VRS1 grown in the absence of antibiotic at approximately 1 × 105 CFU/ml. The culture was grown at 37°C with shaking at 220 rpm for 2 h. The single culture was divided into four 25-ml cultures and treated with no antibiotic, 25 μg/ml nafcillin (Sigma-Aldrich), 30 μg/ml vancomycin, or nafcillin and vancomycin in combination. Bacterial counts were determined at −2, 0, 1, 4, 6, 8, and 24 h (where time zero represents the time when drug was added) by plating 0.1 ml of 10-fold serial dilutions onto Mueller-Hinton agar containing 32 μg/ml vancomycin.
Experimental infection.
The rabbit model of aortic-valve endocarditis, which has been described previously, was used to evaluate treatment regimens (9, 11, 19). Seventy-two hours after transcarotid placement of a polyethylene catheter across the aortic valve, rabbits were injected intravenously through the marginal ear vein with 1 ml of an overnight culture of VRS1 containing 5 × 106 to 1 × 107 CFU/ml. Blood samples were taken 24 h following infection, and the rabbits were randomly assigned to one of the following treatment groups and treated for 3 days with the following regimens: no treatment (control), nafcillin (Bristol-Meyer Squibb, Princeton, NJ) given at 200 mg/kg of body weight intramuscularly every 8 h, vancomycin (Abbott Laboratories, Chicago, IL) given at 30 mg/kg intravenously every 12 h, or nafcillin given at 200 mg/kg intramuscularly every 8 h and vancomycin given at 30 mg/kg intravenously every 12 h. Pharmacokinetic data for similar dosing regimens has been previously described (9, 16). Briefly, the mean peak serum nafcillin concentrations were 58 mg/liter when given intramuscularly at 180 mg/kg, while mean peak serum vancomycin concentrations were 69.16 ± 6.46 mg/liter when given intravenously at 30 mg/kg. The surviving animals were euthanized with intravenous pentobarbital after 3 days of antibiotic treatment. Additional rabbits were treated for 7 days with vancomycin (30 mg/kg intravenously every 12 h) or the combination of vancomycin (30 mg/kg intravenously every 12 h) and nafcillin (200 mg/kg intramuscularly three times a day). Rabbits with a negative blood culture 24 h after infection were excluded from subsequent analysis. To reduce the possibility of antibiotic carryover, the rabbits were not euthanized until at least 14 h after administration of the last dose. The heart, kidneys, and spleen were removed aseptically from each rabbit. Aortic-valve vegetations were removed from each rabbit's heart and weighed, and serial dilutions of vegetation homogenates were made. The kidneys were examined, and areas of abscess or infarct were removed, weighed, homogenized in Mueller-Hinton broth, and serially diluted. The spleens were weighed, and serial dilutions of the homogenate were made. Tissue homogenates were plated on Mueller-Hinton agar containing no antibiotic, agar containing 6 μg/ml oxacillin, and agar containing 32 μg/ml vancomycin. The cultures were read after 48 h at 37°C. Titers of bacteria were expressed as log10 CFU/gram of vegetation or tissue. Sterile vegetations of aortic valves contained ≤2 log10 CFU/g (limit of detection), and kidney or spleen tissue contained ≤1 log10 CFU/g (limit of detection).
Inclusion criteria.
For the final analysis, animals that met the following criteria were included: (i) positive blood culture at 24 h postinfection, (ii) survival for at least 24 h after the first treatment, (iii) proper placement of the catheter across the aortic valve at necropsy with macroscopic evidence of aortic-valve endocarditis (visible vegetations), and (iv) aortic-valve vegetations, kidney tissue, and spleen tissue yielding pure cultures of VRS1.
Statistical analysis.
The mean numbers of bacteria per gram vegetation, kidney tissue, and spleen tissue were compared by analysis of variance. The Student-Newman-Keuls test was used to adjust for multiple comparisons. For analysis of sterilization of tissue cultures, we used Fisher's exact test. A P value of <0.05 was considered statistically significant for all tests.
Cell wall preparation and HPLC.
Isolated cell walls were prepared by the method of Stranden et al. as described previously (22). Lyophilized peptidoglycan was digested with mutanolysin (Sigma), and the resulting muropeptides were reduced to their muramitol derivatives. Separation of muropeptides was achieved by reversed-phase high-pressure liquid chromatography (HPLC) using a Waters 2695 Separation Module system with the Waters 2487 Dual Lambda Absorbance Detecter. Samples were applied to a Beckman ODS column (4.5 mm by 250 mm) protected by an Altex Ultrapshere-ODS precolumn (4.6 mm by 4.5 mm). The column was eluted with a methanol-NaH2PO4 gradient as previously described (12). Muropeptides were detected at 206 nm.
RESULTS
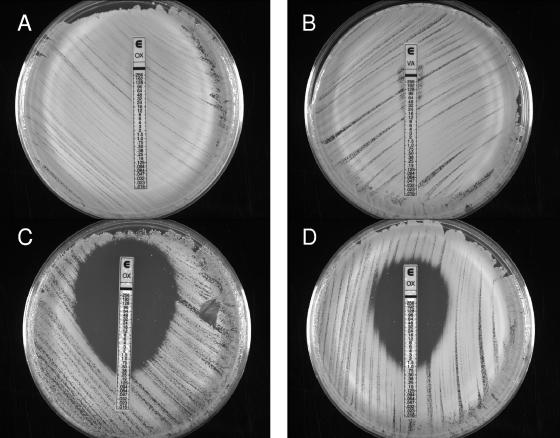
VRS1 susceptibility to each antibiotic was determined individually and in combination using the Etest (Fig. 1). The MIC of oxacillin or vancomycin alone for VRS1 was >256 μg/ml (Fig. 1A and B). In contrast, when the oxacillin Etest strip was placed on agar containing vancomycin, the oxacillin MIC was reduced to 0.38 μg/ml (Fig. 1C). When VRS1 was incubated overnight with vancomycin before being applied to vancomycin agar, the oxacillin MIC was still 1.0 μg/ml.
FIG. 1.
Susceptibility testing of VRS1. An overnight culture of VRS1 grown in the presence (D) or absence (A, B, and C) of 32 μg/ml vancomycin was diluted to 0.5 McFarland standard and swabbed onto brain heart infusion agar with (C and D) or without (A and B) 16 μg/ml vancomycin. Oxacillin (A, C, and D) or vancomycin (B) Etest strips were placed on the plates, and the plates were incubated at 37°C for 24 h. The marks within the zone of clearing on plates C and D are not colonies.
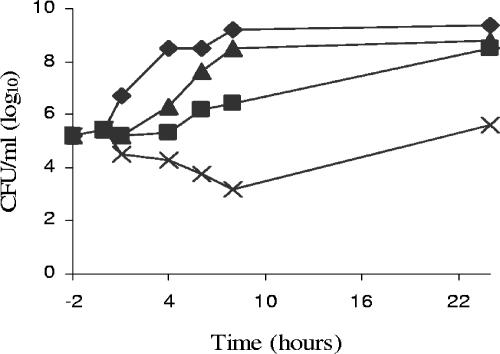
Figure 2 shows the results of a 24-h time-kill assay of VRS1. The antibiotic concentrations used were approximately half of the maximum serum antibiotic concentrations achievable in the rabbit (9). After 8 hours of incubation, the number of VRS1 CFU was increased over the starting number in the presence of either nafcillin or vancomycin alone. However, nafcillin and vancomycin in combination reduced the number of bacteria at 8 hours by 2 log units over the starting concentration and by 3 and 5 log units, respectively, over nafcillin and vancomycin alone.
FIG. 2.
Time-kill curves for VRS1. VRS1 was grown in the absence of antibiotic for 2 h. Antibiotics were added at 0 h. Control (⧫), nafcillin at 25 μg/ml (▴), vancomycin at 30 μg/ml (▪), and nafcillin at 25 μg/ml in combination with vancomycin at 30 μg/ml (×) are shown.
The muropeptide composition of VRS1 was examined by HPLC elution. VRS1 was grown in the absence of antibiotics, as well as in the presence of vancomycin (256 μg/ml) and oxacillin (32 μg/ml) alone. VRS1 grown in the absence of antibiotics demonstrated the presence of two major monomeric species corresponding to the native stem peptide and the altered muropeptide stem peptide terminating in d-Ala-d-Lac, as previously described (20). In the absence of antibiotics, there were nearly equal distributions of the native monomeric species and the altered muropeptide. Once exposed to high levels of vancomycin, the altered monomeric muropeptide replaced the native muropeptide species.
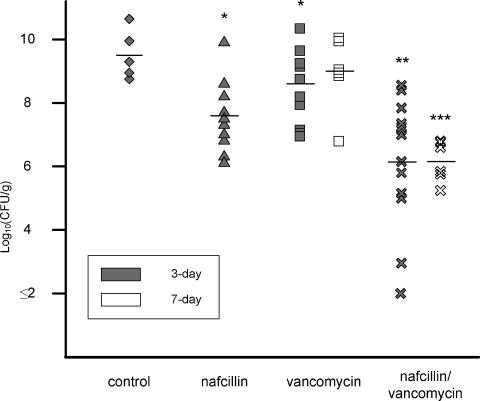
Based on the data generated in vitro demonstrating the bactericidal activities of vancomycin and β-lactam antibiotics against VRS1 when used in combination, we examined their effectiveness in the rabbit model of endocarditis. The results of both 3- and 7-day treatment regimens are summarized in Fig. 3 (valve vegetations) and Table 1 (kidneys and spleens). Infection with VRS1 among control rabbits was uniformly lethal, with all control rabbits dying within 48 h of infection. The mean aortic-valve vegetation counts were 9.64 ± 0.73 log10 CFU/g (± standard deviation), comparable to previously reported trials of MRSA experimental endocarditis (8, 13, 15). All treatment groups had significant reductions in mean aortic-valve vegetation counts compared to those of the controls. However, treatment with the combination of vancomycin and nafcillin was significantly better than treatment with either nafcillin or vancomycin monotherapy, with mean reductions in aortic-valve vegetation bacterial counts of 3.48 log10 CFU/g (P < 0.05).
FIG. 3.
Bacterial counts in valve vegetations after 3- or 7-day treatment. Control (⧫), nafcillin at 25 μg/ml (▴), vancomycin at 30 μg/ml (▪), and nafcillin at 25 μg/ml in combination with vancomycin at 30 μg/ml (×) are shown. The horizontal lines represent the mean log10 CFU/gram. Each symbol represents one rabbit. *, P < 0.05 compared to the control group. **, P < 0.05 compared to the control, nafcillin, or vancomycin group. ***, P < 0.05 compared to the vancomycin group (7-day).
TABLE 1.
Outcomes of 3- and 7-day treatments of experimental VRS1 aortic-valve endocarditis
| Treatment regimen | No. sterile at site/total no. of rabbits
|
Mean bacterial count (log10 CFU/g) ± SD
|
||||
|---|---|---|---|---|---|---|
| Valve vegetation | Kidney | Spleen | Valve vegetation | Kidney | Spleen | |
| 3-Day | ||||||
| Control | 0/5 | 0/5 | 0/5 | 9.64 ± 0.73 | 7.70 ± 1.56 | 5.89 ± 0.94 |
| Nafcillin | 0/10 | 1/10 | 1/10 | 7.61 ± 1.12a | 5.36 ± 3.16 | 3.21 ± 1.60a |
| Vancomycin | 0/10 | 2/10 | 1/10 | 8.54 ± 1.11a | 4.59 ± 2.31a | 4.14 ± 1.81a |
| Nafcillin-vancomycin | 1/13 | 8/13c | 12/13d | 6.16 ± 1.93b | 2.57 ± 2.13b | 1.06 ± 0.21b |
| 7-Day | ||||||
| Vancomycin | 0/6 | 0/6 | 0/6 | 8.93 ± 1.19 | 5.84 ± 2.79 | 4.88 ± 0.87 |
| Nafcillin-vancomycin | 0/6 | 5/6f | 4/6g | 6.18 ± 0.61e | 2.13 ± 2.76e | 1.30 ± 0.53e |
P < 0.05 compared to control group.
P < 0.05 compared to control, nafcillin, or vancomycin group.
P = 0.0288 compared to nafcillin group (Fisher's exact test).
P = 0.0001 compared to nafcillin or vancomycin group (Fisher's exact test).
P < 0.05 compared to vancomycin group.
P = 0.0076 compared to vancomycin group (Fisher's exact test).
P = 0.0303 compared to vancomycin group (Fisher's exact test).
After 3 days of treatment, the rates of sterilization of kidneys and spleens by treatment with nafcillin in combination with vancomycin showed the combination to be significantly (P = 0.0001) more effective than either nafcillin or vancomycin alone in spleen sterilization, while the combination was significantly (P = 0.0288) more effective than nafcillin alone in kidney sterilization.
The bacterial burdens in vegetations, kidneys, and spleens were also significantly decreased (P < 0.05) in the 7-day nafcillin-vancomycin combination group compared to the vancomycin monotherapy group. There was a mean reduction in aortic-valve vegetation bacterial counts of 2.75 log10 CFU/g in the nafcillin-vancomycin group compared to the vancomycin monotherapy group. Significantly more kidneys (P = 0.0076) and spleens (P = 0.0303) were sterile after 7-day combination treatment than after vancomycin monotherapy for 7 days. However, the 7-day nafcillin-vancomycin combination regimen did not significantly decrease bacterial burdens compared to the 3-day combination regimen.
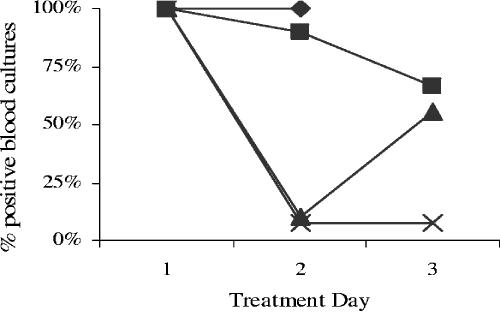
Treatment with nafcillin in combination with vancomycin cleared bloodstream infections in all rabbits within 24 h. In contrast, over half of the blood cultures returned to positive by 48 h after the first antibiotic treatment in the nafcillin-alone group. Only one-third of the rabbits treated with vancomycin alone had negative blood cultures 48 h after the initial dose of vancomycin, as shown in Fig. 4.
FIG. 4.
Percent positive blood cultures by treatment day for rabbits with endocarditis caused by VRS1. Control (⧫), nafcillin at 25 μg/ml (▴), vancomycin at 30 μg/ml (▪), and nafcillin at 25 μg/ml in combination with vancomycin at 30 μg/ml (×) are shown.
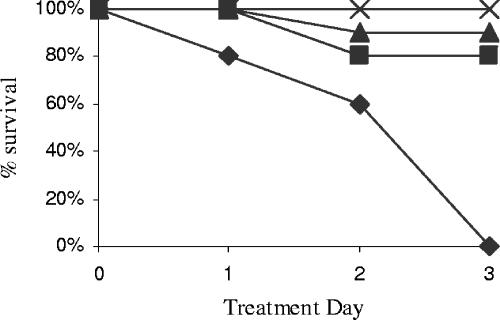
The only 3-day treatment regimen that achieved 100% survival of infected rabbits was the combination of nafcillin and vancomycin. In contrast, as can be seen in Fig. 5, 10 to 20% of the rabbits died during treatment with nafcillin or vancomycin alone. All rabbits that did not receive treatment died before the 3-day treatment period ended.
FIG. 5.
Survival curves for rabbits with endocarditis caused by VRS1. Control (⧫), nafcillin at 25 μg/ml (▴), vancomycin at 30 μg/ml (▪), and nafcillin at 25 μg/ml in combination with vancomycin at 30 μg/ml (×) are shown.
In order to determine whether the treatment regimens altered antibiotic susceptibility, all rabbit valve vegetations and tissue homogenate dilutions were plated on MHA, MHA with 6 μg/ml oxacillin, and MHA with 32 μg/ml vancomycin to screen for the loss of either vancomycin or oxacillin resistance. For all animals treated for 3 days, bacterial counts on all plates with or without oxacillin or vancomycin were similar, indicating no loss of mecA or vanA in any of the treatment groups (data not shown). However, in one animal treated for 7 days with the combination of nafcillin and vancomycin, VRS1 appeared to lose vancomycin resistance. Further investigation of the vancomycin-sensitive strain recovered from the animal is under way.
DISCUSSION
The increasing incidence of infections caused by methicillin-resistant Staphylococcus aureus in conjunction with the emergence of S. aureus with reduced susceptibility or high-level resistance to vancomycin has heightened awareness of the limited number of treatment options for S. aureus infections. While VRS1 is reported to be susceptible to a number of other antibiotics available for treatment, most of the MICs reported for these agents are only one dilution below the susceptibility breakpoint (7). Additionally, two agents to which the Pennsylvania VRSA strain remains susceptible, linezolid and quinuprinstin-dalfopristin, show only bacteriostatic activity (23). Here, we have examined the potential to treat S. aureus resistant to vancomycin and β-lactam antibiotics with a combination of currently prescribed antibiotics, nafcillin and vancomycin. Previous work has shown different mechanisms of action for these antibiotics, as well as separate mechanisms of resistance (5, 18, 24, 25).
Combination therapy with nafcillin and vancomycin showed significant bacterial-load reduction and organ sterilization compared to monotherapy with either antibiotic or no treatment. Sixty-two percent of kidneys and 92% of spleens were sterile after 3-day combination therapy compared to 10 to 20% sterilization of end organs following 3-day monotherapy. The mean bacterial burden in valve vegetations was reduced by 1.5 log10 CFU/g or greater after 3 days of combination therapy compared to monotherapy.
Combination therapy with vancomycin plus a β-lactam antibiotic for treatment of a VRSA infection has been previously suggested but disregarded due to heterogeneous expression of vancomycin resistance during cotreatment with oxacillin in vitro. By population analysis profiling, Severin et al. showed that resistant subpopulations of COLVA were able to grow at 200 μg/ml oxacillin in the presence of 50 μg/ml vancomycin (20, 21). In contrast, our oxacillin Etest results for VRS1 grown in the presence of 16 μg/ml vancomycin gave an oxacillin MIC of ≤1 μg/ml and showed no resistant subpopulations within the zone of inhibition after 24 h of growth. Even after extending incubation to 48 h, very few colonies had formed within the zone of inhibition.
Both the oxacillin and vancomycin MICs of VRS1 are >256 μg/ml, which is well above the level achievable in vivo based on the previously established pharmacokinetics for the nafcillin and vancomycin treatment regimens used (9, 16). However, the VRS1 oxacillin MIC of ≤1 μg/ml achieved in the presence of vancomycin is below the trough level of nafcillin, 8 μg/ml, reached 7 h posttreatment in the study conducted by Miller et al. (16). This suggests that in the presence of vancomycin, the level of nafcillin in serum was above the MIC for the duration of treatment, accounting for the success of combination therapy.
It was previously suggested that VRSA strains may not be highly virulent because of their recovery from catheter exit sites and foot ulcers, where they were possibly only colonizing the site but not infecting the patients (6). The survival curve from this study contradicts this suggestion, demonstrating the virulence of VRS1, and likely other VRSA strains carrying the van genes, as well as the need to treat VRSA infections aggressively. All untreated rabbits died within 72 h of infection; their deaths were characterized by high bacterial counts in valve vegetations, kidneys, and spleens. Four other rabbits were excluded from the data due to death within the first 24 h of treatment.
Interestingly, treatment with either nafcillin or vancomycin alone, two antibiotics to which VRS1 is known to be homogeneously resistant, decreased the severity of the infection. In the case of vancomycin therapy, it is likely that the induction of van genes, leading to generation of an aberrant cell wall, reduces bacterial virulence. The limited success of nafcillin therapy is likely due to the basal level of tetrapeptide and depsipeptide achieved in the absence of vancomycin induction. The partial aberrant cell wall decreases bacterial virulence and cannot be cross-linked by PBP2a, making the bacterium susceptible to killing by β-lactam antibiotics.
Two automated methods of vancomycin susceptibility testing, MicroScan (Dade Behring, Deerfield, Illinois) and Vitek (bioMérieux, Hazelwood, Missouri), failed to detect the second and third clinical cases of VRSA in Pennsylvania and New York, respectively (3, 23). This highlights the need for other, more sensitive tests for vancomycin resistance in S. aureus and, based on currently available screening methods, increases the time required to reliably detect vancomycin resistance levels to at least 24 h. The mortality data from this study suggest that this may be too long to wait before beginning effective antibiotic treatment, highlighting the potential use of combination therapy early in the course of a known vancomycin-resistant S. aureus infection.
Finally, it has been noted that vancomycin and β-lactam antibiotics also show synergy against S. aureus with reduced susceptibility to vancomycin mediated by a thickened wall (10). In each case of VRSA infection seen clinically thus far, the patient was infected with a MRSA strain prior to acquiring the van genes and developing a VRSA infection (1, 3, 4). Compared to vancomycin alone, the current drug of choice for many MRSA infections, vancomycin in combination with a β-lactam may improve the outcome for patients who acquire either S. aureus with reduced susceptibility to vancomycin or a VRSA infection.
Thus, when a patient is infected with S. aureus known to be resistant to vancomycin by any mechanism, combination vancomycin-β-lactam therapy may prove effective. The data from this study demonstrating the severity of the VRS1 infection, as well as previously described difficulties in rapidly detecting VRSA infections, indicate the need for implementation of an effective treatment protocol, such as the one examined here, early in the course of infection.
Acknowledgments
VRS1 was supplied by NARSA.
This work was supported by a VA Merit Grant (Climo) and NIH Grant 2 RO1 AI035705-13 (Archer).
REFERENCES
- 1.Anonymous. 2002. Staphylococcus aureus resistant to vancomycin—United States, 2002. Morb. Mortal. Wkly. Rep. 51:565-567. [PubMed] [Google Scholar]
- 2.Anonymous. 1997. Staphylococcus aureus with reduced susceptibility to vancomycin—United States, 1997. Morb. Mortal. Wkly. Rep. 46:765-766. [PubMed] [Google Scholar]
- 3.Anonymous. 2004. Vancomycin-resistant Staphylococcus aureus—New York, 2004. Morb. Mortal. Wkly. Rep. 53:322-323. [PubMed] [Google Scholar]
- 4.Anonymous. 2002. Vancomycin-resistant Staphylococcus aureus—Pennsylvania, 2002. Morb. Mortal. Wkly. Rep. 51:902. [PubMed] [Google Scholar]
- 5.Bugg, T. D., S. Dutka-Malen, M. Arthur, P. Courvalin, and C. T. Walsh. 1991. Identification of vancomycin resistance protein VanA as a d-alanine:d-alanine ligase of altered substrate specificity. Biochemistry 30:2017-2021. [DOI] [PubMed] [Google Scholar]
- 6.Bush, K. 2004. Vancomycin-resistant Staphylococcus aureus in the clinic: not quite Armageddon. Clin. Infect. Dis. 38:1056-1057. [DOI] [PubMed] [Google Scholar]
- 7.Chang, S., D. M. Sievert, J. C. Hageman, M. L. Boulton, F. C. Tenover, F. P. Downes, S. Shah, J. T. Rudrik, G. R. Pupp, W. J. Brown, D. Cardo, and S. K. Fridkin. 2003. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 348:1342-1347. [DOI] [PubMed] [Google Scholar]
- 8.Chiang, F. Y., and M. Climo. 2003. Efficacy of linezolid alone or in combination with vancomycin for treatment of experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 47:3002-3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Climo, M. W., S. M. Markowitz, D. S. Williams, C. G. Hale-Cooper, and G. L. Archer. 1997. Comparison of the in-vitro and in-vivo efficacy of FK037, vancomycin, imipenem and nafcillin against staphylococcal species. J. Antimicrob. Chemother. 40:59-66. [DOI] [PubMed] [Google Scholar]
- 10.Climo, M. W., R. L. Patron, and G. L. Archer. 1999. Combinations of vancomycin and beta-lactams are synergistic against staphylococci with reduced susceptibilities to vancomycin. Antimicrob. Agents Chemother. 43:1747-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Climo, M. W., R. L. Patron, B. P. Goldstein, and G. L. Archer. 1998. Lysostaphin treatment of experimental methicillin-resistant Staphylococcus aureus aortic valve endocarditis. Antimicrob. Agents Chemother. 42:1355-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finan, J. E., G. L. Archer, M. J. Pucci, and M. W. Climo. 2001. Role of penicillin-binding protein 4 in expression of vancomycin resistance among clinical isolates of oxacillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:3070-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert, M., J. A. Boscia, W. D. Kobasa, and D. Kaye. 1986. Enoxacin compared with vancomycin for the treatment of experimental methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob. Agents Chemother. 29:461-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiramatsu, K., H. Hanaki, T. Ino, K. Yabuta, T. Oguri, and F. C. Tenover. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135-136. [DOI] [PubMed] [Google Scholar]
- 15.Jacqueline, C., D. Navas, E. Batard, A. F. Miegeville, V. Le Mabecque, M. F. Kergueris, D. Bugnon, G. Potel, and J. Caillon. 2005. In vitro and in vivo synergistic activities of linezolid combined with subinhibitory concentrations of imipenem against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 49:45-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller, M. H., M. A. Wexler, and N. H. Steigbigel. 1978. Single and combination antibiotic therapy of Staphylococcus aureus experimental endocarditis: emergence of gentamicin-resistant mutants. Antimicrob. Agents Chemother. 14:336-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Nosocomial Infections Surveillance System. 2002. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1992 to June 2002, issued August 2002. Am. J. Infect. Control 30:458-475. [DOI] [PubMed] [Google Scholar]
- 18.Nicas, T. I., C. Y. Wu, J. N. Hobbs, Jr., D. A. Preston, and N. E. Allen. 1989. Characterization of vancomycin resistance in Enterococcus faecium and Enterococcus faecalis. Antimicrob. Agents Chemother. 33:1121-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perlman, B. B., and L. R. Freedman. 1971. Experimental endocarditis. II. Staphylococcal infection of the aortic valve following placement of a polyethylene catheter in the left side of the heart. Yale J. Biol. Med. 44:206-213. [PMC free article] [PubMed] [Google Scholar]
- 20.Severin, A., K. Tabei, F. Tenover, M. Chung, N. Clarke, and A. Tomasz. 2004. High level oxacillin and vancomycin resistance and altered cell wall composition in Staphylococcus aureus carrying the staphylococcal mecA and the enterococcal vanA gene complex. J. Biol. Chem. 279:3398-3407. [DOI] [PubMed] [Google Scholar]
- 21.Severin, A., S. W. Wu, K. Tabei, and A. Tomasz. 2004. Penicillin-binding protein 2 is essential for expression of high-level vancomycin resistance and cell wall synthesis in vancomycin-resistant Staphylococcus aureus carrying the enterococcal vanA gene complex. Antimicrob. Agents Chemother. 48:4566-4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stranden, A. M., K. Ehlert, H. Labischinski, and B. Berger-Bachi. 1997. Cell wall monoglycine cross-bridges and methicillin hypersusceptibility in a femAB null mutant of methicillin-resistant Staphylococcus aureus. J. Bacteriol. 179:9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tenover, F. C., L. M. Weigel, P. C. Appelbaum, L. K. McDougal, J. Chaitram, S. McAllister, N. Clark, G. Killgore, C. M. O'Hara, L. Jevitt, J. B. Patel, and B. Bozdogan. 2004. Vancomycin-resistant Staphylococcus aureus isolate from a patient in Pennsylvania. Antimicrob. Agents Chemother. 48:275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ubukata, K., R. Nonoguchi, M. Matsuhashi, and M. Konno. 1989. Expression and inducibility in Staphylococcus aureus of the mecA gene, which encodes a methicillin-resistant S. aureus-specific penicillin-binding protein. J. Bacteriol. 171:2882-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ubukata, K., N. Yamashita, and M. Konno. 1985. Occurrence of a beta-lactam-inducible penicillin-binding protein in methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 27:851-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weigel, L. M., D. B. Clewell, S. R. Gill, N. C. Clark, L. K. McDougal, S. E. Flannagan, J. F. Kolonay, J. Shetty, G. E. Killgore, and F. C. Tenover. 2003. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science 302:1569-1571. [DOI] [PubMed] [Google Scholar]
- 27.Woodford, N. 2001. Epidemiology of the genetic elements responsible for acquired glycopeptide resistance in enterococci. Microb. Drug Resist. 7:229-236. [DOI] [PubMed] [Google Scholar]