Abstract
We studied the in vitro and in vivo antimicrobial activities of picolinic acid (PA) in combination with the antiprotozoal drug quinacrine against intramacrophage Mycobacterium avium complex (MAC). Quinacrine significantly potentiated the anti-MAC activity of PA, suggesting the usefulness of this combination in the clinical control of MAC infection.
Mycobacterium avium complex (MAC) pulmonary infections are challenging because of the absence of a reliably effective multidrug treatment regimens (16, 21). Particularly, the urgent problems are that therapeutic efficacies of the present multidrug regimens, in terms of sputum conversion, clinical improvement, and relapse rate after termination of chemotherapy, are still unsatisfactory, even when multidrug regimens are applied according to the guidelines of the Japanese Society for Tuberculosis, which correspond to those of the American Thoracic Society (1, 10). In many cases, MAC pulmonary disease gradually but steadily worsens over the long term despite chemotherapeutic efforts with multidrug regimens. Since the development of new classes of anti-MAC drugs has been unpromising to date, one promising and practical strategy has been to develop improved regimens for patients with refractory MAC diseases using ordinary antimycobacterial drugs in combination with adjunctive immunomodulators (9, 15, 18).
Picolinic acid (PA), a naturally occurring degradation product of tryptophan, is a cheap and safe drug facilitating zinc/chromium ion absorption from the intestine because of its metal ion-chelating activity (6). It was previously reported that PA reduced intramacrophage growth of MAC and that the effect of PA was dependent on host macrophage (Mφ) apoptosis (12, 13). Our recent study demonstrated that PA exhibited antimicrobial activity against extracellular MAC (MICs for the two MAC strains ranged from 25 to 50 mM) and intramacrophage MAC and potentiated the anti-MAC activities of clarithromycin/rifampin and fluoroquinolones (5), suggesting the usefulness of PA in immunoadjunctive therapy for MAC infections. It is of interest that the antiprotozoal drugs belonging to nitroheterocyclic compounds, including nitrothiazoles, nitroimidazoles, and nitrofurans, also possess antimycobacterial activities (2, 20). For instance, mefloquine, displays excellent anti-MAC activity (3, 4, 11). It is also noteworthy that another antiprotozoal drug, quinacrine, which is generally used for anti-Giardia therapy (7), possesses antimicrobial activity against extracellular and intramacrophage Cryptococcus neoformans (8). This encouraged us to examine the in vitro and in vivo anti-MAC activities of quinacrine, particularly in combination with PA.
The intramacrophage growth of MAC was measured as follows. Human THP-1 monocytic cells (5 × 104) were differentiated to mature Mφs by cultivating in 5% fetal bovine serum (FBS)-RPMI 1640 medium containing 50 ng of phorbol 12-myristate 13-acetate/ml in microculture wells at 37°C for 20 h. After a wash with 2% FBS-Hanks’ balanced salt solution, the resultant Mφ monolayer was infected with 106 CFU of test MAC organisms (M. avium N-444, N-339, and N-361 strains isolated from patients with pulmonary MAC infections) at 37°C for 1.5 h. After rinsing, infected Mφs were cultured in 5% FBS-RPMI 1640 medium (0.2 ml) with or without test drugs at 37°C for up to 7 days. At intervals, cultured Mφs were lysed with 0.07% sodium dodecyl sulfate for 10 min. After neutralization with 6% bovine serum albumin, the cell lysate was centrifuged at 2,000 × g for 20 min, and the number of CFU of recovered MAC organisms was counted on 7H11 agar plates. Next, in experimental infection studies, C3H/HeN female mice were infected intravenously with MAC (107 CFU). Infected mice were given no drug or subcutaneous injections of test agents, once daily, six times per week, from week 1 to week 9. Bacterial CFU in homogenates of infected lungs were then counted on 7H11 agar plates.
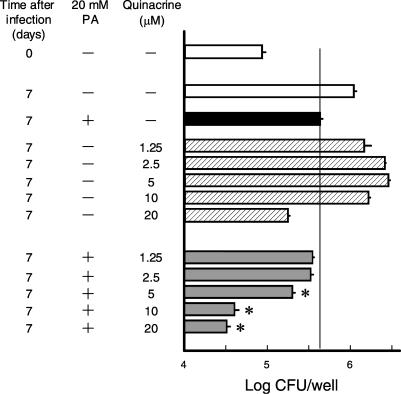
We first examined the antimicrobial activity of quinacrine against intramacrophage MAC when used in combination with PA. Quinacrine at 5 μM markedly increased the PA activity against intramacrophage MAC N-444 (Fig. 1A). Similar results were obtained for mouse peritoneal Mφs (unpublished observations). Significant combined effects were also observed when other MAC isolates, N-339 and N-361 strains (virulence to mice: N-339 > N-444 > N-361) (17), were used (Fig. 1B and C). These findings indicate that the combined effects of quinacrine with PA can be obtained regardless of the virulence of the target MAC organisms. Figures 2 and 3 show the dose dependency of the combined antimicrobial activity of quinacrine and PA against intramacrophage MAC. As shown in Fig. 2, 10 or 20 μM quinacrine strongly potentiated the activity of PA fixed at 20 mM, and the greatest combined effect was seen when quinacrine was added at 10 μM. In addition, Fig. 3 shows that PA at 2.5 to 20 mM PA markedly increased the activity of quinacrine fixed at 10 μM, and the combined effect was most evident with PA at 5 or 10 mM. In any case, these findings indicate that quinacrine in combination with PA significantly potentiates Mφ anti-MAC activity.
FIG. 1.
Effects of quinacrine on the activity of PA in potentiating anti-M. avium function of THP-1 human Mφs. Mφs infected with M. avium strain N-444 (A), N-339 (B), or N-361 (C) were cultivated in the presence or absence of 20 mM PA and/or 5 μM (A) or 20 μM (B and C) quinacrine. Symbols: ○, none added; ▵, quinacrine; •, PA; ▴, PA plus quinacrine. Each plot indicates the mean ± the standard error of the mean (SEM; n = 3 or 4). *, Significantly different from the control value (none added) (P < 0.01); †, significantly different from the value of PA alone (P < 0.01). The experiments were repeated two or three times with similar results.
FIG. 2.
Effects of various concentrations of quinacrine on the activity of PA in potentiating anti-M. avium function of THP-1 human Mφs. M. avium N-444-infected Mφs were cultivated in the presence or absence of 20 mM PA and/or 1.25 to 20 μM quinacrine. Each bar indicates the mean ± the SEM (n = 4). *, Significant combined effect between PA and quinacrine (P < 0.01).
FIG. 3.
Effects of various concentrations of PA on the activity of quinacrine in potentiating anti-M. avium function of THP-1 human Mφs. M. avium N-444-infected Mφs were cultivated in the presence or absence of 10 μM quinacrine and/or 2.5 to 40 mM PA. Each bar indicates the mean ± the SEM (n = 4). *, Significant combined effect between PA and quinacrine (P < 0.01).
Next, we examined the therapeutic effects of PA and quinacrine against MAC infection. As shown in Fig. 4, bacterial growth in the lungs of infected mice was inhibited to some extent by either PA (100 mg/kg) or quinacrine (5 mg/kg) alone. Moreover, the combination of both drugs exerted a weak combined effect. However, none of the regimens tested here exhibited bactericidal effects against MAC organisms replicating in the lungs. The efficacy was much lower than the clarithromycin-ethambutol (20 and 15 mg/kg, respectively) regimen, which causes significant levels of bacterial elimination (0.66-log unit killing) in the lungs.
FIG. 4.
Therapeutic effects of PA and quinacrine individually or in combination against M. avium infection in mice. M. avium N-444-infected mice were given no drug or subcutaneous injections of PA (100 mg/kg) or quinacrine (5 mg/kg) individually or in combination, once daily, six times per week, from week 1 for 8 weeks. At week 9, mice were sacrificed and the bacterial loads in the lungs were examined. Drug-treated mice did not show any sign of significant adverse effects in terms of changes in body weights. Each bar indicates the mean ± the SEM (n = 5). *, Significantly smaller than the value in untreated control mice (P < 0.05). Bacterial load in the control mice (no drug) 1 week after infection was 4.13 ± 0.07 log units.
At present, quinacrine is widely used as a potent antigiardiasis drug, and some researchers consider the drug to be the most efficacious of any of the anti-Giardia therapeutics (7). Although antiprotozoal mechanisms of quinacrine has not been fully elucidated, it is known that the drug intercalates with protozoal DNA, causing inhibition of nucleic acid synthesis (19). In our separate experiments, quinacrine displayed combined antimicrobial effects with PA against extracellular MAC (unpublished observation). However, recent studies by Sotelo et al. indicated that quinacrine is a strong activator of host innate immunity and induces recruitment of activated immune cells, including Mφs (14). These findings suggest that quinacrine's antimicrobial activity against intramacrophage MAC is dependent on the Mφ-activating effects, as well as direct antimicrobial action against MAC organisms. In any case, certain multidrug anti-MAC regimens containing quinacrine and PA may be useful as new types of chemotherapeutic regimens against MAC infections. Further detailed studies are currently under way using in vitro and in vivo experimental systems.
Acknowledgments
We thank K. Sato for expert technical support and suggestions for the experimental infection studies carried out in the present investigation.
REFERENCES
- 1.American Thoracic Society. 1997. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am. J. Respir. Crit. Care Med. 156:S1-S25. [DOI] [PubMed] [Google Scholar]
- 2.Barry, C. E., III, H. I. Boshoff, and C. S. Dowd. 2004. Prospects for clinical introduction of nitroimidazole antibiotics for the treatment of tuberculosis. Curr. Pharm. Des. 10:3239-3262. [DOI] [PubMed] [Google Scholar]
- 3.Bermudez, L. E., P. Kolonoski, M. Petrofsky, M. Wu, C. B. Inderlied, and L. S. Young. 2003. Mefloquine, moxifloxacin, and ethambutol are a triple-drug alternative to macrolide-containing regimens for treatment of Mycobacterium avium disease. J. Infect. Dis. 187:1977-1980. [DOI] [PubMed] [Google Scholar]
- 4.Bermudez, L. E., P. Kolonoski, M. Wu, P. A. Aralar, C. B. Inderlied, and L. S. Young. 1999. Mefloquine is active in vitro and in vivo against Mycobacterium avium complex. Antimicrob. Agents Chemother. 43:1870-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai, S., K. Sato, T. Shimizu, S. Yamabe, M. Hiraki, C. Sano, and H. Tomioka. 2006. Antimicrobial activity of picolinic acid against extracellular and intracellular Mycobacterium avium complex and its combined activity with clarithromycin, rifampicin, and fluoroquinolones. J. Antimicrob. Chemother. 57:85-93. [DOI] [PubMed] [Google Scholar]
- 6.Evans, G. W., and P. E. Johnson. 1980. Characterization and quantitation of a zinc-binding ligand in human milk. Pediatr. Res. 14:876-880. [DOI] [PubMed] [Google Scholar]
- 7.Gardner, T. B., and D. R. Hill. 2001. Treatment of giardiasis. Clin. Microbiol. Rev. 14:114-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison, T. S., G. E. Griffin, and S. M. Levitz. 2000. Conditional lethality of the diprotic weak bases chloroquine and quinacrine against Cryptococcus neoformans. J. Infect. Dis. 182:283-289. [DOI] [PubMed] [Google Scholar]
- 9.Holland, S. M. 2000. Cytokine therapy of mycobacterial infections. Adv. Intern. Med. 45:431-452. [PubMed] [Google Scholar]
- 10.Kobashi, Y., N. Okimoto, T. Matsushima, E. Shigetou, T. Kuraoka, H. Takeyama, R. Eda, S. Yano, K. Kobayashi, T. Ohnishi, K. Mori, Y. Ueda, T. Moritaka, K. Nishimura, and T. Abe. 2002. Effect of combined chemotherapy following the guidelines on treatment for Mycobacterium avium complex pulmonary disease. Kekkaku 77:435-441. [PubMed] [Google Scholar]
- 11.Nannini, E. C., M. Keating, P. Binstock, G. Samonis, and D. P. Kontoyiannis. 2002. Successful treatment of refractory disseminated Mycobacterium avium complex infection with the addition of linezolid and mefloquine. J. Infect. 44:201-203. [DOI] [PubMed] [Google Scholar]
- 12.Pais, T. F., and R. Appelberg. 2000. Macrophage control of mycobacterial growth induced by picolinic acid is dependent on host cell apoptosis. J. Immunol. 164:389-397. [DOI] [PubMed] [Google Scholar]
- 13.Pais, T. F., and R. Appelberg. 2004. Induction of Mycobacterium avium growth restriction and inhibition of phagosome-endosome interactions during macrophage activation and apoptosis induction by picolinic acid plus IFNγ. Microbiology 150:1507-1518. [DOI] [PubMed] [Google Scholar]
- 14.Sotelo, J., P. Guevara, B. Pineda, and C. Diaz. 2004. Interstitial quinacrine activates a distinctive immune response effective for tumor immunotherapy. Surgery 136:700-707. [DOI] [PubMed] [Google Scholar]
- 15.Tomioka, H. 2004. Adjunctive immunotherapy of mycobacterial infections. Curr. Pharm. Design. 10:3297-3310. [DOI] [PubMed] [Google Scholar]
- 16.Tomioka, H. 2004. Present status and future prospects of chemotherapeutics for intractable infections due to Mycobacterium avium complex. Curr. Drug Discovery Technol. 1:255-268. [DOI] [PubMed] [Google Scholar]
- 17.Tomioka, H., H. Saito, K. Sato, and D. J. Dawson. 1993. Comparison of the virulence for mice of Mycobacterium avium and Mycobacterium intracellulare identified by DNA probe test. Microbiol. Immunol. 37:259-264. [DOI] [PubMed] [Google Scholar]
- 18.Toossi, Z. 1998. Adjunctive immunotherapy of tuberculosis. Cytokine Cell Mol. Ther. 4:105-112. [PubMed] [Google Scholar]
- 19.Tracy, J. W., and L. T. Webster. 1996. Drugs used in the chemotherapy of protozoal infections, p. 987-1008. In J. G. Hardman and L. E. Limbird (ed.), The pharmacological basis of therapeutics, 9th ed. McGraw-Hill Book Co., New York, N.Y.
- 20.Upcroft, J. A., R. W. Campbell, K. Benakli, P. Upcroft, and P. Vanelle. 1999. Efficacy of new 5-nitroimidazoles against metronidazole-susceptible and -resistant Giardia, Trichomonas, and Entamoeba spp. Antimicrob. Agents Chemother. 43:73-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner, D., and L. S. Young. 2004. Nontuberculous mycobacterial infections: a clinical review. Infection 32:257-270. [DOI] [PubMed] [Google Scholar]