Abstract
Bacterial lipopolysaccharides (LPS) are important triggers of the widespread inflammatory response, which contributes to the development of multiple organ failure during sepsis. The helical 37-amino-acid-long human antimicrobial peptide LL-37 not only possesses a broad-spectrum antimicrobial activity but also binds and neutralizes LPS. However, the use of LL-37 in sepsis treatment is hampered by the fact that it is also cytotoxic. To find a less toxic analog of LL-37, we used in silico analysis to identify amphipathic helical regions of LL-37. A 21-amino-acid fragment (GKE) was synthesized, the biological actions of which were compared to those of two equally long peptides derived from the N and C termini of LL-37 as well as native LL-37. GKE displayed antimicrobial activity against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Candida albicans, and Candida parapsilosis that was similar to or even stronger than LL-37. GKE, as well as the equally long control peptides, attracted granulocytes in a fashion similar to that of LL-37, while only GKE was as potent as LL-37 in inhibiting LPS-induced vascular nitric oxide production. GKE caused less hemolysis and apoptosis in human cultured smooth muscle cells than LL-37. In summary, we have identified an active domain of LL-37, GKE, which displays antimicrobial activity in vitro and LPS-binding activity similar to those of LL-37 but is less toxic. GKE therefore holds promise as a template for the development of peptide antibiotics for the treatment of sepsis.
Antimicrobial peptides naturally occur in the interface between an organism and the environment, such as the human dermis, epithelia of airways and gut, seminal and vaginal fluids, breast milk, and the vernix caseosa of the newborn (2). These peptides protect us from the invasion of microbes, and if invasion does occur, they constitute a first line of defense (32).
Many antimicrobial peptides share the similar features of hydrophobic and hydrophilic amino acid residues arranged in an amphipathic α-helix as well as having a positive net charge (2). Thus, antimicrobial peptides can bind to bacteria not only with hydrophobic interactions but also through electrostatic interactions (32). The way in which antimicrobial peptides inhibit the growth of microbes is not yet fully understood, but the disruption of the bacterial membrane integrity resulting in fatal depolarization of the bacterial cell and the activation of proteolytic enzymes have been proposed (32).
The 37-amino-acid-long human cathelicidin antimicrobial peptide LL-37 was discovered independently by three groups in 1995 (1, 8, 18). It is released from activated neutrophils and epithelial cells (8, 26). LL-37 has an amphipathic α-helical structure and carries a positive net charge of +6 at a physiological pH. LL-37 not only possesses extensive antibacterial properties against gram-positive and gram-negative bacteria as well as fungi but also binds and neutralizes lipopolysaccharides (LPS) from the cell wall of gram-negative bacteria (4, 18). Furthermore, LL-37 attracts neutrophils, monocytes, and T lymphocytes via activation of formyl peptide receptor-like 1 (FPRL1) (9).
Sepsis, a complex clinical syndrome caused by an infection with bacteria, viruses, or fungi, is triggered by microbial components such as LPS. The pathophysiology includes an overwhelming inflammatory host response, which can lead to the development of multiple organ failure, resulting in mortality rates of up to 45% (12). Numerous trials have been performed in order to evaluate anti-inflammatory agents directed against the action of inflammatory mediators released in sepsis (e.g., cytokines such as tumor necrosis factor alpha and interleukin-1) (7). Although results from in vivo studies with experimental animals have been promising, the results in the clinical setting have been disappointing.
The combination of antimicrobial and LPS-binding properties makes LL-37 an attractive candidate for adjuvant treatment of sepsis. Unfortunately, native LL-37 is toxic to human eukaryotic cells due to interactions with the eukaryotic-cell membrane (4, 14). The cytotoxicity is reduced in plasma due to plasma protein binding of LL-37, but the binding also reduces the antimicrobial activities of this peptide (14). We have previously shown that the removal of a few hydrophobic amino acid residues from the N terminus of native LL-37 not only reduces its cytotoxicity but also diminishes its plasma protein binding, sparing its antimicrobial and LPS-neutralizing actions (6).
The present investigation represents an extension of the previous work of identifying LL-37 variants with improved performance compared to the intact endogenous peptide. The goal of this study was to identify an optimal amphipathic fragment of LL-37 having a helical structure with high predicted internal stability and to investigate its antimicrobial, LPS-binding, and chemotactic abilities, as well as its toxicity. The overall objective of the work is to facilitate the development of novel peptide-based strategies for treating sepsis.
MATERIALS AND METHODS
Computational analysis and peptide synthesis.
In order to identify a region of LL-37 responsible for the antimicrobial activity of the peptide, a search for amphipathic, helical regions, with a high predicted internal stability was performed. An algorithm based on helix-coil transition theory, AGADIR, was used to predict helical propensity (17). Calculations were performed by submitting peptide sequences to the EMBL WWW Gateway to AGADIR Service (http://www.embl-heidelberg.de/Services/serrano/agadir/agadir-start.html). Input parameters were as follows: C terminus free, N terminus free, pH 7.4, temperature of 278 K, and ionic strength of 0.15 M. Amphipathicity of idealized helices was investigated by generating helical wheel diagrams. The peptides (Table 1) were synthesized by Innovagen AB, Lund, Sweden, by Fmoc chemistry. The purity of these peptides (>95%) was confirmed by mass spectrometry.
TABLE 1.
Amino acid sequences, indices of helicity, net charges, and mean relative hydrophobic moments of the peptides used in this study
Based on the method described in reference 16.
CD.
The circular dichroism spectroscopy (CD) spectra of the peptides dissolved in a 10 mM Tris buffer (pH 7.4) were measured on a J-810 Spectropolarimeter (Jasco, Japan) at wavelengths in the range of 200 to 250 nm. The measurements were performed at 37°C in a 10-mm quartz cuvette with stirring at a peptide concentration of 10 μM in the presence or absence of 150 mM NaCl. Liposomes containing dioleoylphosphatidylcholine were prepared as for the liposome leakage assay described below. The effect of liposomes on the peptide secondary structure on peptide secondary structure was monitored at a lipid concentration of 100 μM. The only peptide conformations observed under the conditions investigated were α-helix and random coil. The calculation of the ratio between these conformations has been described elsewhere (25). In brief, the fraction of the peptide in α-helical conformation (Xα) can be calculated from the following equation: Xα = (A − Ac)/(Aα − Ac), where A is the recorded CD signal at 225 nm and Aα and Ac are the CD signals at 225 nm for reference peptides in 100% α-helix and 100% random coil conformations, respectively. The 100% α-helix and 100% random coil references were obtained from 0.133 mM (monomer concentration) poly-l-lysine (Mw = 79,000) in 0.1 M NaOH and 0.1 M HCl, respectively (11, 25). To account for the instrumental differences between measurements, the background value (detected at 250 nm, where no peptide signal is present) was subtracted. Signals from the bulk solution were also corrected for.
Antimicrobial testing: radial diffusion assay.
To assess the antimicrobial action of the peptides, Escherichia coli (strain 37,4), Pseudomonas aeruginosa (15159), Staphylococcus aureus (F18), Candida albicans (ATCC 90028), and Candida parapsilosis (ATCC 90018) isolates were grown for overnight at 28°C in 10 ml (3% [wt/vol]) tryptic soy broth (TSB; Becton Dickinson). To obtain mid-logarithmic-phase organisms, 200 μl of this culture was inoculated in 10 ml of fresh TSB and incubated for an additional 2 h (except P. aeruginosa and the Candida species, which were grown overnight) at 37°C. The bacteria were centrifuged at 900 × g for 10 min and washed once followed by resuspension in 10 ml cold 10 mM Tris buffer (pH 7.4). The optical density of the solution was measured at 620 nm. Radial diffusion assay was performed as follows and as described previously (19). One percent (wt/vol) of low-electroendosmosis-type agarose (Sigma-Aldrich, St. Louis, MO) with or without 150 mM NaCl and a final concentration of 0.02% (vol/vol) Tween 20 (Sigma-Aldrich) in 0.05% TSB was brought to ebullition, cooled to 50°C, and then mixed with bacterial suspension (4 × 106 CFU in all cases, except 3.3 × 106 CFU for C. albicans in 5 ml) and poured into a 10-cm petri dish. A series of wells (diameter, 4 mm) were punched in the plate after the agarose had solidified. Six microliters of peptide samples dissolved and diluted in sterile distilled water to concentrations of 0, 0.5, 1, 2.5, 5, 10, 20, 40, and 100 μM were applied in each well, and the plates were incubated for 3 h at 37°C. An overlay agar composed of 6% TSB and 0.5% (wt/vol) of low-electroendosmosis-type agarose was then pored over, and the plates were incubated upside down for 18 h in 37°C to allow visible growth of bacterial colonies. Antibacterial activity was indicated by a clear zone corresponding to the lack of bacterial growth around the well. The diameter of the clear zone surrounding the wells was measured with a metric scale to the nearest 0.1 mm. The gels were stained with a Coomassie brilliant blue solution containing 2 mg Coomassie blue R-250 (Merck, Darmstadt, Germany), 27 ml methanol, and 15 ml 37% formaldehyde (Sigma-Aldrich) in 63 ml water for 24 h. The staining solution was replaced with distilled water, and the gels were washed for 24 h and dried for permanent recording of the results.
Effects of serum on antimicrobial activity.
Blood was drawn from the antecubital vein of three healthy donors into glass tubes without additives and left to coagulate for one hour at room temperature. Serum was collected after centrifugation for 10 min at 2,000 × g. Peptides were diluted in sterile distilled water. Serum was added to achieve a peptide concentration of 20 μM in 0, 40, or 99% serum. The peptide-serum mixtures were applied to the wells of a radial diffusion assay using E. coli as described above.
Liposome preparation and leakage assay.
Dry lipid films were prepared by dissolving dioleoylphosphatidylcholine (60 mol%; Avanti Polar Lipids, Alabaster, Alabama) and cholesterol (40 mol%; Sigma-Aldrich) in chloroform and then removing the solvent by evaporation under vacuum overnight. Subsequently, buffer (10 mM Tris, pH 7.4) was added together with 0.1 M carboxyfluorescein (CF; Sigma-Aldrich). After hydration, the lipid mixture was subjected to eight freeze-thaw cycles consisting of freezing in liquid nitrogen and heating to 60°C. Unilamellar liposomes, of ≈100 nm, were generated by multiple extrusions through polycarbonate filters (pore size, 100 nm) mounted in a LipoFast miniextruder (Avestin, Ottawa, Canada) at 22°C. Untrapped CF was then removed by two gel filtrations (Sephadex G-50) at 22°C with the Tris buffer as the eluent. In the liposome-leakage assay, self-quenching of CF was used. Thus, at 100 mM, CF is self-quenched and the recorded fluorescence intensity from liposomes with entrapped CF is low. Upon leakage from the liposomes, released CF is dequenched and, hence, it fluoresces. The CF release was determined by monitoring the emitted fluorescence at 520 nm from a liposome dispersion (10 mM lipid in 10 mM Tris, pH 7.4). An absolute leakage scale is obtained by disrupting the liposomes at the end of the experiment by the addition of 0.8 mM Triton X-100 (Sigma-Aldrich), thereby causing 100% release and dequenching of CF. A Spex fluorolog 1650 0.22-m double spectrometer (Spex Industries, Edison, New Jersey) was used for the liposome-leakage assay.
Nitrate/nitrite accumulation.
The Institutional Review Board for the Care of Animal Subjects approved the study, and the care and handling of the animals were in accord with the National Institutes of Health guidelines. Seven male Sprague-Dawley rats (250-g body weight) were anesthetized to death with isoflurane (Abbott Scandinavia, Solna, Sweden). The thoracic aorta was removed, cleaned of adherent fat, and cut into 3-mm-long cylindrical segments. The segments were incubated for 24 h at 37°C with or without LPS (1 ng ml−1, from E. coli strain 0111:B4; Sigma-Aldrich, St. Louis, MO) together with LL-37, LLG, GKE, or FKR (0, 0.2, 2, and 20 μM) in 1 ml Dulbecco's modified Eagle's medium without phenol red (DMEM; Gibco, NY) saturated with a gas mixture containing 8% CO2 in oxygen. The DMEM contained l-arginine (1 mM), penicillin (2,000 U ml−1), and streptomycin (0.2 mg ml−1) (all from Sigma-Aldrich). After incubation, the accumulation of nitrate/nitrite in the incubation medium was measured as previously described (4); the results are expressed as nmol nitrate/nitrite per mg tissue and per 24 h.
Chemotactic activity.
Twenty milliliters of blood was drawn into plastic tubes containing EDTA (2 mg ml−1) from the antecubital vein of eight healthy donors. Polymorphonuclear cells were isolated by centrifugation over Polymorphprep (Axis-Shield PoC, Oslo, Norway) according to the manufacturer's instructions. The cells were washed and resuspended in RPMI 1640 containing l-glutamine (Gibco, NY) to achieve 2 × 106 cells ml−1. LL-37, LLG, GKE, or FKR (all diluted in RPMI 1640 to 0.01, 0.1, 1, or 10 μM) or N-formyl-Met-Leu-Phe (fMLP; diluted in RPMI 1640 to 0.1 μM; Sigma-Aldrich) were added to the lower wells of a 48-well microchemotaxis chamber (AP48; Neuro Probe Inc., Gaithersburg, MD). Fifty microliters of the cell suspension was added to the upper chamber, which was separated from the lower chamber by a polycarbonate membrane with 5-μm pores. The chamber was incubated for 30 min at 37°C in a humidified gas mixture containing 5% CO2 in air. After incubation, the number of transmigrated cells was counted as previously described (6), expressed as number of cells per mm2.
Hemolysis.
Blood was drawn into a plastic tube containing EDTA (2 mg ml−1) from the antecubital vein of five healthy donors. After centrifugation at 800 × g for 10 min, plasma and buffy coat were removed. The erythrocytes were rinsed three times by centrifugation for 10 min at 800 × g and resuspension at 5% (vol/vol) in phosphate-buffered saline (pH 7.4). Then, 400-μl erythrocyte suspension was incubated for 1 h at 37°C with gentle end-over-end rotation in the presence of LL-37, LLG, GKE, or FKR, at concentrations of 0 (negative control), 0.6, 2, 6, 20, or 60 μM. Triton X-100 at 2% (Sigma-Aldrich) served as a positive control. After incubation, the samples were centrifuged at 800 × g for 10 min. Release of hemoglobin was monitored by measuring the absorbance of the supernatant at 540 nm and is expressed as the percentage of Triton X-100-induced hemolysis.
DNA fragmentation assay.
Confluent human aortic vascular smooth muscle cells (CC-2571; BioWhittaker, Walkersville, Md.), passage 5 to 7, were cultured in 24-well plates and incubated in serum-free DMEM for 16 h in the absence (control) or presence of LL-37, LLG, GKE, or FKR at concentrations of 2, 6, 20, or 60 μM. Internucleosomal DNA fragmentation was measured using a cell death detection enzyme-linked immunosorbent assay kit (Roche Molecular Biochemicals, Mannheim, Germany) as previously described (5) and is expressed as the increase in absorbance (n-fold) over untreated controls. Cell morphology was examined with an inverted phase-contrast microscope immediately after the addition of the peptides and before the measurement of the DNA fragmentation.
Statistics.
Values are means ± standard errors of the means (see the figures). n is the number of rats, humans, or independent experiments as indicated. Mean values were compared by two-way repeated measurement analysis of variance followed by post hoc testing using the Holm-Sidak method. The factors were different peptides and peptide or serum concentration when appropriate. The significance level (P) was <0.05.
RESULTS
Molecular modeling.
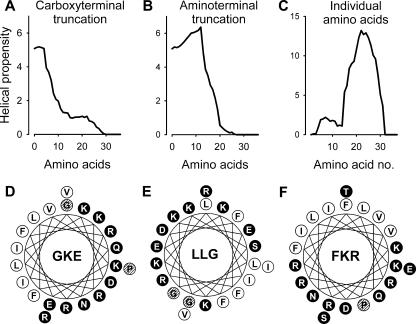
In order to identify an amphipathic, helical region of LL-37 with a high predicted internal stability, the amino acid sequence of native LL-37 was stepwise truncated in the C or N terminus and the predicted helix content calculated using AGADIR. After removal of the fifth C-terminal amino acid residue, P33, there was a marked decrease in the predicted helix content (Fig. 1A; Table 1). Similarly, after the removal of the 13th N-terminal amino acid residue, I13, there was also a decrease in the predicted helix content (Fig. 1B). Analysis of the helical propensity of the individual amino acids demonstrated a peak between residues K15 and V32 (Fig. 1C). It is well known that there are amino acid preferences for specific locations at the ends of α-helices. Although not established in free-standing peptides (15), helical regions are usually terminated with a G as the C-cap residue or with a P at the C-cap +1 position (22). Furthermore, it has been shown that antimicrobial peptides have a positional conservation for G in the N terminus (29).
FIG. 1.
Effect of C-terminal (A) or N-terminal (B) truncation as well as the contribution of individual amino acids (C) on the helical propensity of LL-37. Helical wheel diagrams of the peptides GKE (D), LLG (E), and FKR (F). Hydrophobic amino acids are represented by white circles, hydrophilic amino acids by black circles, and the indifferent amino acids, G and P, by hatched circles.
Combining the above information, we selected a 21-amino-acid-long internal region (G14-R34) that had a predicted helix content similar to that of intact LL-37 and contained the critical helix-capping residues (GKE). Equally long peptides (21 amino acid residues) from the N terminus (LLG) and C terminus (FKR) were synthesized for comparison. Assuming a helical conformation, the three peptides had amphipathic structures in which the hydrophobic and hydrophilic amino acids are preferentially located on opposing sides of the idealized helix (Fig. 1). However, GKE, compared to LLG and FKR, had the highest net charge, predicted helical content, and mean relative hydrophobic moment (see http://www.bbcm.univ.trieste.it/∼tossi/HydroCalc/HydroMCalc.html#K&D) (Table 1).
CD.
In order to provide an experimental comparison to the AGADIR prediction, CD measurements were performed. The results are shown in Table 2. The overall findings of the CD measurements correlate fairly well with predictions from AGADIR in the sense that the helix content is significantly lower for LLG than for FKR and GKE. However, experimentally, there is not much difference in helix content between the latter two peptides, in contrast to the AGADIR predictions. The CD measurements show that GKE and FKR both undergo coil-to-helix transitions on exposure to zwitterionic liposomes, whereas LLG remains largely unordered also in the presence of these liposomes. Interestingly, FKR is the one displaying the largest helix induction upon exposure to liposomes. The presence of NaCl (150 mM) did not affect (or marginally affected) the helical content of the peptides. Overall, within the comparisons made in the present study, the agreement between the AGADIR prediction and the experimental results is satisfactory.
TABLE 2.
Predicted indices of helicity and experimental helix contentsa
| Peptide | Index of helicity (AGADIR) | Helix contentb
|
|||
|---|---|---|---|---|---|
| Buffer
|
Liposomes
|
||||
| − Salt | + Salt | − Salt | + Salt | ||
| LLG | 0.97 | 13 | 11 | 12 | 10 |
| GKE | 5.16 | 26 | 26 | 32 | 28 |
| FKR | 2.93 | 26 | 27 | 37 | 38 |
Experiments performed in 10 mM Tris buffer (pH 7.4) in the presence (+ Salt) or absence (− Salt) of 150 mM NaCl.
Determined by circular dichroism spectroscopy of the peptides used in the study. Mean percent values of experiments performed in duplicate.
Antimicrobial activity.
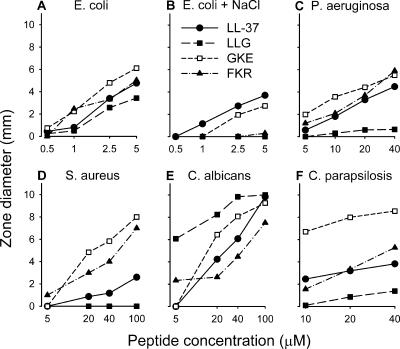
We compared the antimicrobial activities of the peptides in a radial diffusion assay. As shown in Fig. 2A, all peptides inhibited the growth of E. coli similarly at low salt concentrations (n = 8). It has previously been demonstrated that the antimicrobial activity of LL-37 can be reduced at physiological salt concentrations (6, 30). We found that the presence of NaCl at 150 mM (physiological salt concentration) did not affect the antimicrobial activities of LL-37 and GKE against E. coli to any major extent, while the activities of LLG and FKR were significantly lower (P < 0.001; n = 4) (Fig. 2B). LL-37 and FKR inhibited the growth of P. aeruginosa similarly, while GKE was statistically significantly more efficient than LL-37 (P < 0.001; n = 6) (Fig. 2C). The antimicrobial activity of LLG against P. aeruginosa was low at the concentrations studied. We have previously shown that an enhanced activity against S. aureus can be achieved by removal of as few as 2 or 6 N-terminal amino acids from LL-37 (6). This correlates with the present finding that the mainly N-terminally truncated peptides, GKE and FKR, displayed stronger antimicrobial activities against S. aureus than LL-37 and LLG (P < 0.001; n = 3) (Fig. 2D). All peptides inhibited the growth of C. albicans (Fig. 2E). The order of activity was as follows: LLG > GKE > LL-37 > FKR (P < 0.001; n = 3). GKE was found to be more active against C. parapsilosis than the other peptides (P < 0.001; n = 5) (Fig. 2F). No zones of inhibition of microbial growth were found around the wells containing the solvent of the peptides:distilled water.
FIG. 2.
Antimicrobial activity as determined using a radial diffusion assay for LL-37, LLG, GKE, and FKR (n = 3 to 8).
Effect of serum on antibacterial activity.
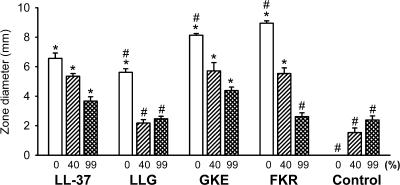
Binding to plasma proteins decreases the antimicrobial activity of native LL-37 (31). Using a radial diffusion assay, we investigated the influence of serum on the inhibitory effect the peptides on the growth of E. coli. In the absence of serum, all peptides inhibited bacterial growth, as indicated by clear zones around the wells containing the peptides (Fig. 3). The antibacterial activities of the peptides were reduced in the presence of serum in a concentration-dependent manner. The reduction was most pronounced for LLG and FKR. In the presence of serum at 99%, the antibacterial activities of these peptides did not differ from those elicited by serum alone. In contrast, LL-37 and GKE still retained antibacterial activity even at 99% serum.
FIG. 3.
Inhibition of antibacterial activity by serum assessed by an radial diffusion assay using E. coli. *, statistically significantly different from the value for serum alone at the same dilution. #, statistically significantly different from the value for LL-37 in the presence of serum at the same dilution (n = 3).
Effects on liposomes.
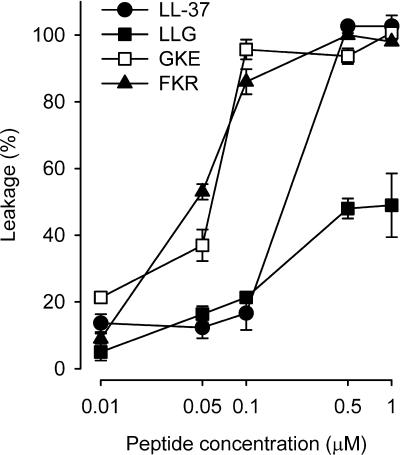
Liposome experiments were performed in order to investigate the correlation between bacterial mortality and defect formation in lipid membranes. There were clear differences between the different peptides concerning leakage induction, with GKE and FKR being the most potent ones, followed by LL-37 and LLG (Fig. 4). LLG was significantly less efficacious in inducing leakage. The ranking of the peptides regarding leakage induction in liposomes correlates well with the inhibition of growth of the bacterial strains tested, suggesting a pivotal role of the partial destruction of the lipid membrane as a key component in the antibacterial action of these peptides.
FIG. 4.
Permeabilizing effects of the peptides on liposomes. All peptides induced a concentration-dependent leakage from the liposomes, but GKE and FKR were more potent than LL-37. LLG was considerable less efficacious than the other peptides in inducing leakage (n = 3).
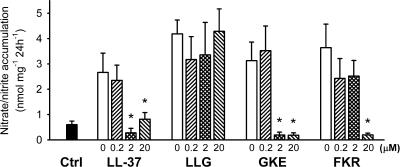
Nitrate/nitrite accumulation.
Next, we wanted to see if the peptides could inhibit the LPS-induced nitric oxide production in isolated rat aorta, leading to a subsequent decrease in the nitrate/nitrite accumulation in the incubation medium. LPS increased the nitrate/nitrite accumulation (Fig. 5). LL-37 and GKE inhibited the nitrate/nitrite accumulation efficiently and with similar potencies. The concentration of FKR had to be increased 10-fold in order to achieve a comparable inhibition. At the concentrations tested, LLG did not affect the LPS-induced nitrate/nitrite production.
FIG. 5.
Production of nitrate/nitrite from segments of rat aorta. LPS alone (open bars) increased the nitrate/nitrite production compared to control (Ctrl). *, statistically significantly different from the value for LPS alone (n = 7).
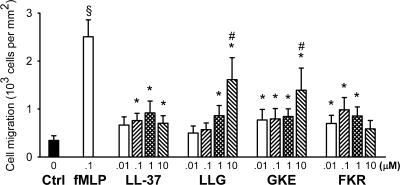
Chemotactic activity.
The ability of the peptides to attract neutrophil granulocytes was studied in a microchemotaxis chamber. LL-37 and its C-terminal fragment, FKR, displayed similar concentration-dependent chemotactic activities on granulocytes (Fig. 6). The concentration-response curves for LL-37 and FKR were biphasic, with maximum effects at 1 and 0.1 μM, respectively. The maximum effects of these peptides amounted to nearly half of that achieved with the classical chemoattractant formyl peptide fMLP, which was used as a positive control at a single concentration of 0.1 μM. The concentration-response curve for GKE was also similar to that of LL-37 at concentrations of up to 1 μM. At 10 μM, there was a pronounced increase in chemotactic activity, which could be due to a loss of selectivity at higher concentrations, resulting in an agonistic activity on other chemoattractant receptors than the receptor activated by LL-37, FPRL1. We have previously demonstrated that the 18-amino-acid-long peptide 18-mer LLKKK (similar to the midportion of GKE) displays the same pattern of chemoattractant activity as found for GKE in the present study (6). The reason for the finding that LL-37, which contains the amino acid sequence of GKE, and FKR, having 15 of 21 amino acids in common with GKE, did not lose their selectivity at higher concentrations as GKE did cannot be determined on the basis of the present results. LLG was the least potent peptide in the chemotaxis assay, which could be explained by LLG being derived from the N terminus of LL-37 while the other peptides are derived from the C terminus. This suggests that the C terminus is important for the potent chemotactic activity of LL-37.
FIG. 6.
Chemotaxis of human neutrophils in response to LL-37, LLG, GKE, FKR, and the classical chemoattractant formyl peptide fMLP. fMLP (0.1 μM) displayed statistically significant chemoattractant activity (§) compared to the control (Ctrl; black bar). The peptides induced a statistically significant chemoattractant activity (*). At 10 μM, LLG and GKE induced a greater chemotactic response than LL-37 (#; n = 8).
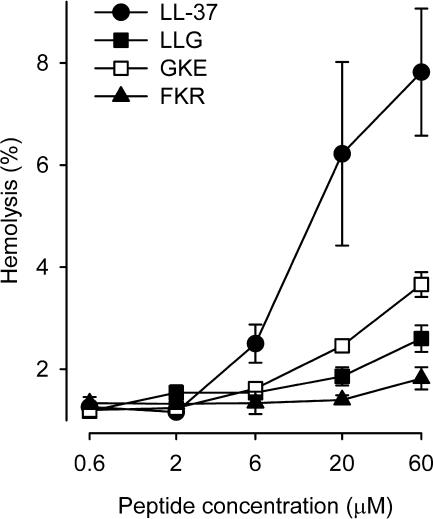
Hemolysis.
To study short-term cytotoxicity, we investigated to what extent the peptides could lyse isolated red blood cells. All peptides induced a concentration-dependent hemolysis (Fig. 7). Importantly, however, the LL-37 fragments LLG, GKE, and FKR were significantly less hemolytic than the native peptide.
FIG. 7.
Hemolytic activity of the peptides. The peptides displayed hemolytic activity in the following order: LL-37 > GKE > LLG = FKR (n = 5).
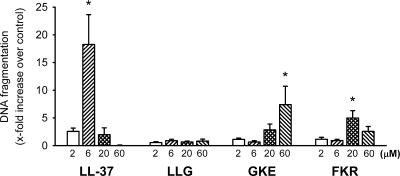
DNA fragmentation.
We also compared the toxicities of the peptides after a longer exposure time. As shown in Fig. 8, LL-37, GKE, and FKR induced statistically significant DNA fragmentations after 16 h in human cultured vascular smooth muscle cells compared to that of the control, which may at least partly be due to the development of apoptosis. Importantly, however, the difference in the threshold concentration required to induce DNA fragmentation between LL-37 and GKE was 10-fold. At higher concentrations, no significant LL-37- or FKR-induced DNA fragmentation was detected, probably due to severe membrane damage and cell death before the development of DNA fragmentation. This was confirmed by phase-contrast microscopy of the cells, which showed cytoplasmic shrinking within minutes after the addition of LL-37 at 20 or 60 μM or FKR at 60 μM. After the 16-hour incubation, similar cellular changes were observed also in the presence of LL-37 at 6 μM, GKE at 60 μM, and FKR at 20 μM. LLG did not induce any DNA fragmentation or cell morphology changes at the concentrations tested. Taken together, the results from the hemolysis and DNA fragmentation experiments demonstrate that the fragments of LL-37 studied are considerably less toxic towards human cells than the parent peptide.
FIG. 8.
DNA fragmentation in human cultured vascular smooth muscle cells after 16-h incubation with the peptides. *, statistically significant DNA fragmentation compared to the control (n = 5).
DISCUSSION
The present results clearly show that it is possible to find shorter and less-toxic variants of LL-37 with retained antimicrobial activity. The presently identified fragment, GKE, displays antimicrobial activity similar to or even stronger than LL-37 also at physiological salt concentrations and in serum.
As the results of the liposome experiments correlated with the antibacterial activity, it seems reasonable to believe that the induction of microbial lysis is an important mechanism for the inhibition of bacterial growth by all these peptides. No such correlation was observed for the inhibition of the growth of the two Candida species and the induction of hemolysis, suggesting that the mechanisms of the antifungal actions as well as the toxicity for human cells of these peptides are different from their antibacterial actions. It is also worth noting that LLG was more efficient in inhibiting the growth of C. albicans and that GKE was far more efficient in inhibiting the growth of C. parapsilosis than the other peptides (Fig. 2). The LL-37 precursor human CAP-18, as well as LL-37 itself, is subject to a diverse pattern of proteolytic processing in vivo, giving rise to a plethora of fragments (20, 27). Furthermore, LL-37 fragments are also produced by cleavage by bacterial proteinases (23, 24). The present results demonstrate that different LL-37-derived fragments differ widely in antimicrobial activity. Thus, the proteolytic processing of LL-37 could broaden the antimicrobial spectrum of the parent peptide.
In order to be able to not only compare the effects of the different peptides on a certain microorganism but also assess the antimicrobial spectrum of each peptide, the conditions of the radial diffusion assay were standardized. However, the suspension of bacteria in a hypotonic Tris buffer and the addition of a detergent to the underlay medium are not always favorable for microbial growth and may enhance the antimicrobial actions of the peptides studied. The possibility that these conditions may have contributed to the relative lack of effect of salt and serum on the activity of LL-37 cannot be excluded.
Although conventional antibiotics may successfully kill pathogens involved in gram-negative sepsis, they cannot bind and neutralize LPS. In fact, bacteriolytic antibiotics, such as β-lactams, can even increase the amount of LPS (13). This means that despite the use of conventional antibiotics and support therapy, endotoxemia may still remain. LPS is one of the most powerful stimulants of the immune system (7). LPS activates Toll-like receptors on macrophages, monocytes, and neutrophils, which then release prototypic proinflammatory cytokines, e.g., interleukin-1, interleukin-6, and tumor necrosis factor alpha (7, 21). These cytokines trigger an inflammatory cascade. This can lead to disseminated intravascular coagulation (mortality, 25 to 30%) as well as vascular instability and a capillary leak, which contributes to the hypotension seen in septic shock (7, 28). By binding and neutralizing LPS, it would be possible to avoid these mechanisms, which seriously contribute to the pathophysiology of sepsis. Given this, it is important to note that of the LL-37 analogs tested, only GKE was as potent and effective in neutralizing LPS as intact LL-37 (Fig. 5).
LL-37 has been found to prevent sepsis in LPS-exposed neonatal rats (10). However, our previous results show that even low doses of LL-37 can induce apoptosis in human cultured smooth muscle cells (6). In the present study, all the truncated peptides were significantly less toxic than full-length LL-37, rendering them potential candidates for clinical use. Taken together with the antimicrobial activities of the peptides, these results demonstrate proof of the concept that in silico analyses may be useful in the design of shorter peptides with a lower toxicity than the naturally occurring LL-37.
Bowdish and colleagues have shown that LL-37 can also have immunomodulatory properties contributing to the host defense against infection (3). All the peptides tested possessed similar chemotactic activities against neutrophils. Whether this is important when peptides are to be used in the intravenous form for sepsis treatment remains unanswered.
In conclusion, we have identified a 21-amino-acid-long peptide constituting the midportion of LL-37, displaying antimicrobial and LPS-binding activities similar to those of LL-37 but which is less toxic. This peptide could serve as a template for the development of peptide antibiotics for the treatment of sepsis.
Acknowledgments
This work was supported by Swedish Research Council grants no. 2002-6270, 2004-3874, 13471, and 621-2003-4022, the Medical Faculty of Lund University, the Lund University Hospital Research funds, the Scandinavian Society for Antimicrobial Chemotherapy, the Region Skåne Research Council, the Royal Physiographical Society, and the LPS Foundation.
We also thank Lotta Wahlberg and Axel Nelson for expert technical assistance.
REFERENCES
- 1.Agerberth, B., H. Gunne, J. Odeberg, P. Kogner, H. G. Boman, and G. H. Gudmundsson. 1995. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc. Natl. Acad. Sci. USA 92:195-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boman, H. G. 2003. Antibacterial peptides: basic facts and emerging concepts. J. Intern. Med. 254:197-215. [DOI] [PubMed] [Google Scholar]
- 3.Bowdish, D. M., D. J. Davidson, Y. E. Lau, K. Lee, M. G. Scott, and R. E. Hancock. 2005. Impact of LL-37 on anti-infective immunity. J. Leukoc. Biol. 77:451-459. [DOI] [PubMed] [Google Scholar]
- 4.Ciornei, C. D., A. Egesten, and M. Bodelsson. 2003. Effects of human cathelicidin antimicrobial peptide LL-37 on lipopolysaccharide-induced nitric oxide release from rat aorta in vitro. Acta Anaesthesiol. Scand. 47:213-220. [DOI] [PubMed] [Google Scholar]
- 5.Ciornei, C. D., A. Egesten, M. Engström, K. Törnebrandt, and M. Bodelsson. 2002. Bactericidal/permeability-increasing protein inhibits endotoxin-induced vascular nitric oxide synthesis. Acta Anaesthesiol. Scand. 46:1111-1118. [DOI] [PubMed] [Google Scholar]
- 6.Ciornei, C. D., T. Sigurdardottir, A. Schmidtchen, and M. Bodelsson. 2005. Antimicrobial and chemoattractant activity, lipopolysaccharide neutralization, cytotoxicity, and inhibition by serum of analogs of human cathelicidin LL-37. Antimicrob. Agents Chemother. 49:2845-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen, J. 2002. The immunopathogenesis of sepsis. Nature 420:885-891. [DOI] [PubMed] [Google Scholar]
- 8.Cowland, J. B., A. H. Johnsen, and N. Borregaard. 1995. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett. 368:173-176. [DOI] [PubMed] [Google Scholar]
- 9.De, Y., Q. Chen, A. P. Schmidt, G. M. Anderson, J. M. Wang, J. Wooters, J. J. Oppenheim, and O. Chertov. 2000. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 192:1069-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukumoto, K., I. Nagaoka, A. Yamataka, H. Kobayashi, T. Yanai, Y. Kato, and T. Miyano. 2005. Effect of antibacterial cathelicidin peptide CAP18/LL-37 on sepsis in neonatal rats. Pediatr. Surg. Int. 21:20-24. [DOI] [PubMed] [Google Scholar]
- 11.Greenfield, N., and G. D. Fasman. 1969. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry 8:4108-4116. [DOI] [PubMed] [Google Scholar]
- 12.Hotchkiss, R. S., and I. E. Karl. 2003. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 348:138-150. [DOI] [PubMed] [Google Scholar]
- 13.Jackson, J. J., and H. Kropp. 1992. β-Lactam antibiotic-induced release of free endotoxin: in vitro comparison of penicillin-binding protein (PBP) 2-specific imipenem and PBP 3-specific ceftazidime. J. Infect. Dis. 165:1033-1041. [DOI] [PubMed] [Google Scholar]
- 14.Johansson, J., G. H. Gudmundsson, M. E. Rottenberg, K. D. Berndt, and B. Agerberth. 1998. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J. Biol. Chem. 273:3718-3724. [DOI] [PubMed] [Google Scholar]
- 15.Kapp, G. T., J. S. Richardson, and T. G. Oas. 2004. Kinetic role of helix caps in protein folding is context-dependent. Biochemistry 43:3814-3823. [DOI] [PubMed] [Google Scholar]
- 16.Kyte, J., and R. F. Doolittle. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105-132. [DOI] [PubMed] [Google Scholar]
- 17.Lacroix, E., A. R. Viguera, and L. Serrano. 1998. Elucidating the folding problem of alpha-helices: local motifs, long-range electrostatics, ionic-strength dependence and prediction of NMR parameters. J. Mol. Biol. 284:173-191. [DOI] [PubMed] [Google Scholar]
- 18.Larrick, J. W., M. Hirata, R. F. Balint, J. Lee, J. Zhong, and S. C. Wright. 1995. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect. Immun. 63:1291-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehrer, R. I., M. Rosenman, S. S. Harwig, R. Jackson, and P. Eisenhauer. 1991. Ultrasensitive assays for endogenous antimicrobial polypeptides. J. Immunol. Methods 137:167-173. [DOI] [PubMed] [Google Scholar]
- 20.Murakami, M., B. Lopez-Garcia, M. Braff, R. A. Dorschner, and R. L. Gallo. 2004. Postsecretory processing generates multiple cathelicidins for enhanced topical antimicrobial defense. J. Immunol. 172:3070-3077. [DOI] [PubMed] [Google Scholar]
- 21.Opal, S. M., and C. E. Huber. 2002. Bench-to-bedside review: Toll-like receptors and their role in septic shock. Crit. Care 6:125-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richardson, J. S., and D. C. Richardson. 1988. Amino acid preferences for specific locations at the ends of alpha helices. Science 240:1648-1652. [DOI] [PubMed] [Google Scholar]
- 23.Schmidtchen, A., I. M. Frick, E. Andersson, H. Tapper, and L. Björck. 2002. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol. Microbiol. 46:157-168. [DOI] [PubMed] [Google Scholar]
- 24.Sieprawska-Lupa, M., P. Mydel, K. Krawczyk, K. Wojcik, M. Puklo, B. Lupa, P. Suder, J. Silberring, M. Reed, J. Pohl, W. Shafer, F. McAleese, T. Foster, J. Travis, and J. Potempa. 2004. Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus-derived proteinases. Antimicrob. Agents Chemother. 48:4673-4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sjögren, H., and S. Ulvenlund. 2005. Comparison of the helix-coil transition of a titrating polypeptide in aqueous solutions and at the air-water interface. Biophys. Chem. 116:11-21. [DOI] [PubMed] [Google Scholar]
- 26.Sørensen, O. E., P. Follin, A. H. Johnsen, J. Calafat, G. S. Tjabringa, P. S. Hiemstra, and N. Borregaard. 2001. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood 97:3951-3959. [DOI] [PubMed] [Google Scholar]
- 27.Sørensen, O. E., L. Gram, A. H. Johnsen, E. Andersson, S. Bangsbøll, G. S. Tjabringa, P. S. Hiemstra, J. Malm, A. Egesten, and N. Borregaard. 2003. Processing of seminal plasma hCAP-18 to ALL-38 by gastricsin: a novel mechanism of generating antimicrobial peptides in vagina. J. Biol. Chem. 278:28540-28546. [DOI] [PubMed] [Google Scholar]
- 28.Toh, C. H., and M. Dennis. 2003. Disseminated intravascular coagulation: old disease, new hope. Br. Med. J. 327:974-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tossi, A., L. Sandri, and A. Giangaspero. 2000. Amphipathic, alpha-helical antimicrobial peptides. Biopolymers 55:4-30. [DOI] [PubMed] [Google Scholar]
- 30.Turner, J., Y. Cho, N. N. Dinh, A. J. Waring, and R. I. Lehrer. 1998. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob. Agents Chemother. 42:2206-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, Y., B. Agerberth, A. Löthgren, A. Almstedt, and J. Johansson. 1998. Apolipoprotein A-I binds and inhibits the human antibacterial/cytotoxic peptide LL-37. J. Biol. Chem. 273:33115-33118. [DOI] [PubMed] [Google Scholar]
- 32.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]