Abstract
Dietary restriction has been shown to have several health benefits including increased insulin sensitivity, stress resistance, reduced morbidity, and increased life span. The mechanism remains unknown, but the need for a long-term reduction in caloric intake to achieve these benefits has been assumed. We report that when C57BL/6 mice are maintained on an intermittent fasting (alternate-day fasting) dietary-restriction regimen their overall food intake is not decreased and their body weight is maintained. Nevertheless, intermittent fasting resulted in beneficial effects that met or exceeded those of caloric restriction including reduced serum glucose and insulin levels and increased resistance of neurons in the brain to excitotoxic stress. Intermittent fasting therefore has beneficial effects on glucose regulation and neuronal resistance to injury in these mice that are independent of caloric intake.
Keywords: caloric restriction‖hippocampus‖insulin‖β-hydroxybutyrate‖ ketosis
Aging refers to the biological changes that occur during a lifetime that result in reduced resistance to stress, increased vulnerability to disease, and an increased probability of death. The rate at which aging occurs is species-specific, suggesting a strong genetic influence. The only environmental variable that has been shown to markedly affect the rate of aging in a wide range of species is caloric intake: Restricting food intake to a level below that which would be consumed voluntarily results in a decrease in the rate of aging and an increase in average and maximum life span (1, 2). Dietary restriction (DR) reduces cancer formation (3, 4) and kidney disease (5) and increases the resistance of neurons to dysfunction and degeneration in experimental models of Alzheimer's and Parkinson's diseases as well as stroke (6–9). Two different DR paradigms have proven effective in increasing life span and disease resistance in rats and mice. In one paradigm animals are provided a daily food allotment that is typically 30–40% less than the ad libitum (AL) consumption of a control population; this limited daily feeding (LDF) paradigm involves a controlled caloric restriction and a corresponding reduction in body weight. In the second paradigm animals are subjected to intermittent (alternate-day) fasting (IF), which in rats results in reduced food intake over time and decreased body weight (10).
Restricting caloric intake causes the restricted population to weigh proportionally less than the AL-fed group. Indeed, weight reduction typically slightly exceeds the degree of food restriction, so that food intake per gram of body weight is reported consistently to be slightly higher in LDF animals than in their AL-fed counterparts (11). Rats and mice usually lose weight when maintained on an IF regimen, although some strains such as C57BL/6 mice may lose little or no weight (12). Both the IF and LDF paradigms are reported to result in dramatic increases in life span in comparison to AL-fed animals (13), but little else is known concerning the similarities and differences in their effects.
It seems reasonable to assume that both LDF and IF paradigms of DR extend life span through a common mechanism. To gain insight into the nature of the underlying mechanism, we compared the effects of LDF and IF diets on several parameters that have been postulated to play a role in the protective effects of DR including body weight, food intake, and fasting levels of serum insulin, glucose, and insulin-like growth factor 1 (IGF-1). In addition, recent studies have shown that rats and mice maintained on an IF regimen exhibit increased resistance of neurons in their brains to insults relevant to the pathogenesis of several different human neurological disorders including epileptic seizures and stroke (6, 8, 14). We therefore performed an experiment to determine whether LDF and IF diets exert similar beneficial effects on neurons in the brain.
Materials and Methods
Animals and Measurements of Body Weight and Food Intake.
Six-week-old C57BL/6 mice were obtained from Charles River Breeding Laboratories, housed in the National Institute on Aging Gerontology Research Center animal colony, and fed a standard NIH-07 diet (Harlan–Teklad, Indianapolis). Food was provided AL until 9 weeks of age. At that time, mice were assigned to one of three groups: AL, fed ad libitum; IF, provided access to food every other day; and LDF, provided with a limited daily food allotment of 60% of that eaten by the AL-fed animals. To control for caloric intake versus periodic food deprivation, a fourth group was added: These pair-fed (PF) mice were provided daily with a food allotment equal to the average daily intake of mice in the IF group. Water was available AL for all groups. The animals were kept in a 12-h light/12-h dark cycle (lights on at 6 a.m. and off at 6 p.m.); food was provided or removed at 10 a.m. Body weight was determined each week on the same day and time. Food intake was measured daily. To keep track of spillage, white bedding was used to allow easy separation of unconsumed food and powder from waste and bedding; spilled food was allowed to dry fully to avoid measurement of wet weight added by urine. Statistical comparisons were made by using ANOVA. All procedures were approved by the National Institute on Aging Gerontology Research Center Animal Care and Use Committee.
Blood Collection and Analyses.
At 29 weeks of age, all mice were fasted for 14 h before blood withdrawal. Blood was removed via intraorbital bleed under isoflurane anesthesia, and serum was isolated. In preliminary studies we found that the glucose concentrations in blood samples from fasted mice collected immediately after the mice succumb to the isoflurane inhalation anesthesia (within 1 min of exposure to the anesthetic) are essentially identical to the glucose concentrations in blood samples from fasted mice killed by decapitation within 10 seconds of removal from their cage. The concentrations of glucose and insulin were determined by using the glucose oxidase method (15) and RIA (16), respectively. IGF-1 concentrations were determined by using an enzyme immunosorbent assay kit (Diagnostic Systems Laboratories, Webster, TX), and β-hydroxybutyrate concentrations were determined by using an assay that measures the oxidation of β-hydroxybutyrate to acetoacetate by using a kit purchased from Sigma. Statistical comparisons were made by using single-factor ANOVA; if significant, pairwise comparisons were made by using a two-tailed t test assuming unequal variance.
Excitotoxin Administration and Quantification of Neuron Damage.
Mice were treated at 30–38 weeks of age. Kainate was injected stereotaxically into the dorsal aspect of the right hippocampus by using the methods described (14). Briefly, mice were anesthetized with Avertin and placed in a stereotaxic head holder, and the skull was exposed along the midline. A convulsant dose of kainate (0.2 μg dissolved in 0.5 μl of PBS, pH 7.2) was injected unilaterally into the dorsal hippocampus (stereotaxic coordinates were 2.0 mm posterior to bregma, 2.4 mm lateral to bregma, and 1.8 mm below the surface of the skull). The contralateral hippocampus of each mouse received an injection of 0.5 μl of PBS. Twenty-four hours after kainate administration, mice were anesthetized with isoflurane and perfused transcardially with saline followed by cold phosphate-buffered 4% paraformaldehyde. Coronal brain sections were cut on a freezing microtome and stained with cresyl violet. Nissl-positive undamaged neurons were counted in three ×40 fields in regions CA3 and CA1. Counts were made in five coronal brain sections per mouse (sections were chosen by unbiased sampling), and the mean number of cells per section was determined such that the value obtained for each animal represents an average total number of neurons counted per section (i.e., sum of six ×40 fields for each hippocampal region). Comparisons of numbers of undamaged neurons in hippocampal regions among treatment groups were made by using ANOVA followed by Scheffé's post hoc and Fisher's tests for pairwise comparisons.
Results and Discussion
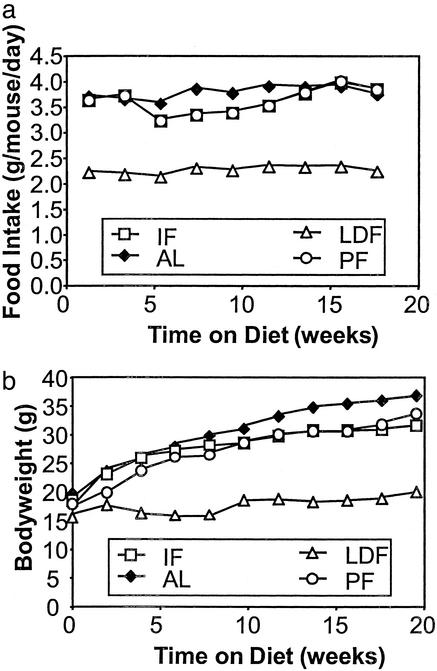
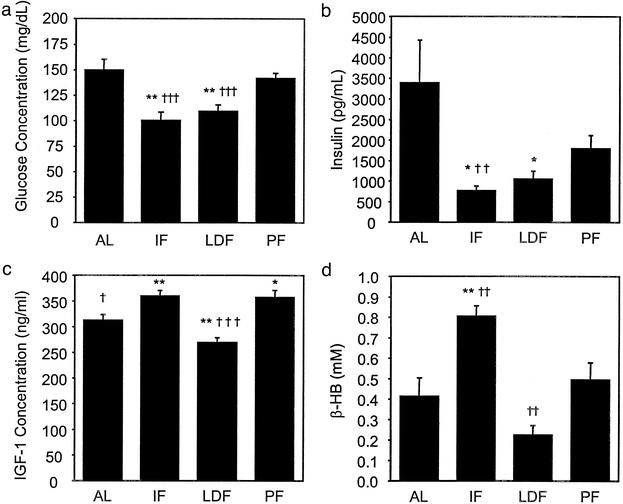
By the end of this study, male C57BL/6 mice subjected to IF were consuming essentially the same amount of food in a 48-h period as did those fed AL. On the days they had access to food, the IF mice ate roughly twice as much as did mice fed AL (Fig. 1a). Mice on the LDF regimen consumed 40% less food as provided: this was reflected in their body weights, which were 49% lower than those of the AL-fed group. In contrast, at the end of the study the body weights of mice maintained on the IF diet or PF on a daily basis were only slightly below those of the AL-fed group (Fig. 1b). A prominent physiological change that occurs in mammals maintained on reduced-calorie diets is increased insulin sensitivity, which often is reflected in decreased fasting plasma levels of glucose and insulin (17). Fasting serum concentrations of glucose and insulin in mice fed AL in the current study averaged 150 mg/dl and 3,400 pg/ml, respectively (Fig. 2 a and b). The concentrations of glucose and insulin were decreased significantly, to similar amounts, in mice maintained on either LDF or IF regimens with glucose and insulin concentrations dropping to 100 mg/dl and 700–1,100 pg/ml, respectively (Fig. 2). That similar changes are seen in IF and LDF groups in the current study suggests that despite an overall calorie intake similar to mice fed AL, IF has similar effects on circulating glucose and insulin levels.
Figure 1.
Male C57BL/6 mice compensate for periods of fasting by increasing their food intake and gaining weight at rates similar to mice fed AL. Shown are the average daily food intakes (calculated from 14-day intake) (a) and body weights (b) in mice maintained on one of four feeding regimens: AL, IF, PF, and LDF (40% reduction in calories relative to AL-fed). IF body weights are postfeeding values. Values are the mean of measurements made in eight mice per diet group. At the end of the study, body weights and food intakes of the LDF group were significantly lower than the body weights and food intakes of the AL-fed and IF groups (P < 0.001 in each case). Body weights and food intakes of the AL-fed and IF groups were not significantly different.
Figure 2.
Effects of IF and LDF on serum glucose, insulin, IGF-1, and β-hydroxybutyrate levels. Serum concentrations of glucose (a), insulin (b), IGF-1 (c), and β-hydroxybutyrate (d) were quantified in mice that had been maintained for 20 weeks on the indicated feeding regimens. Values are the mean and SEM of determinations made in eight mice per group. Statistical comparisons to the AL-fed group are indicated by * and to the PF group by †: * and †, P ≤ 0.05; ** and ††, P ≤ 0.01; *** and †††, P ≤ 0.001.
Levels of circulating IGF-1 were decreased in mice on the LDF diet but increased in mice on the IF diet (Fig. 2c). These findings are of considerable interest in light of evidence that the insulin signaling pathway is linked to longevity in a variety of species (18), and that IGF-1 levels are reduced in rodents on calorie-restricted diets (19). Because mice on the IF regimen were not calorie-restricted, the difference in IGF-1 levels between the IF and LDF groups suggests a difference in the ways in which IF and caloric restriction influence the growth hormone (GH)–IGF-1 axis and insulin signaling pathways. Because insulin levels were decreased in IF mice to levels even lower than in LDF mice, these two DR regimens reveal a dissociation in the mechanisms regulating IGF-1 and insulin levels. Transgenic rats with reduced levels of GH exhibit a transgene dose-dependent reduction in levels of IGF-1; rats with a moderate reduction in IGF-1 levels live longer, whereas those with a greater decrease in IGF-1 levels have a reduced life span (20). The latter results suggest that there is an optimal level of the GH–IGF-1 axis to maximize survival in mammals. With regard to the neuroprotective effects of IF (see data below and refs. 6–9), studies have reported that IGF-1 signaling is neuroprotective in experimental models of neurodegenerative disorders (21, 22). It will be of considerable interest therefore to determine the mechanisms by which LDF and IF differentially affect IGF-1 levels and insulin signaling and how these changes influence energy metabolism, longevity, and disease resistance.
Fasting is known to result in an increased production of ketone bodies, which can be used as an energy source (23, 24) and are known to provide some protective effects including neuroprotection and resistance to epileptic seizures (25–27). We therefore measured serum levels of β-hydroxybutyrate after an overnight fast in mice that had been maintained on AL, LDF, and IF diets. Mice on the IF diet exhibited a 2-fold increase in the fasting serum concentration of β-hydroxybutyrate compared with mice fed AL (Fig. 2d). In contrast, the β-hydroxybutyrate concentration of mice on the LDF diet were decreased compared with mice fed AL.
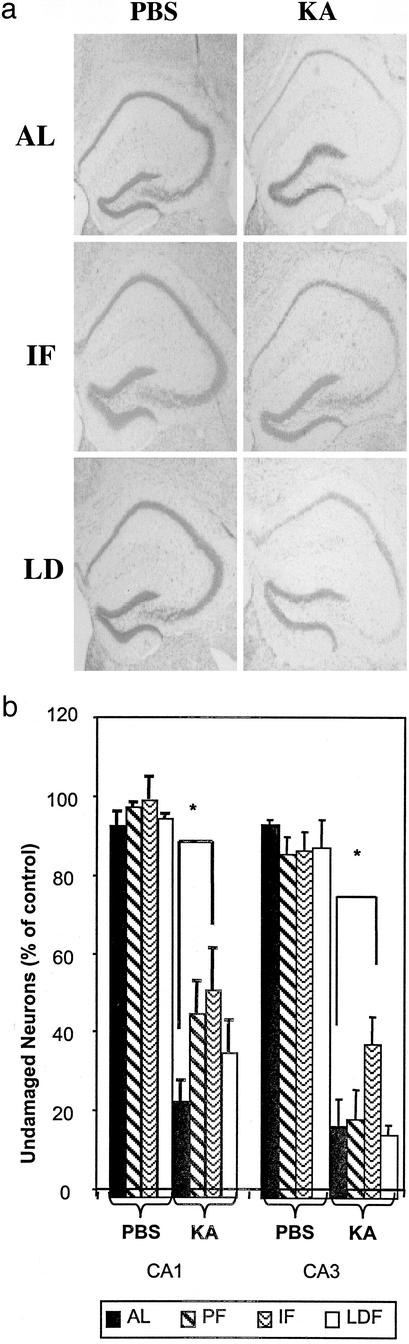
When the excitotoxin kainic acid (KA) is injected into the dorsal hippocampus of mice it induces seizures and damage to pyramidal neurons in regions CA3 and CA1 (14). KA was injected into the dorsal hippocampus of mice that had been maintained for 24 weeks on AL, LDF, and IF diets. All mice exhibited seizures of similar magnitude and duration (data not shown). Mice were killed 24 h after KA administration, tissue sections from their brains were stained with cresyl violet, and the numbers of neurons in regions CA3 and CA1 of each hippocampus were counted. KA caused a marked loss of CA3 and CA1 neurons in mice fed AL. As expected, many neurons in regions CA3 and CA1 of the hippocampus injected with KA degenerated (Fig. 3). There was a significant increase in the survival of CA3 and CA1 neurons in the IF mice compared with mice fed AL (Fig. 3). LDF also protected the CA1 neurons, albeit to a lesser amount than did IF, but did not protect CA3 neurons against KA-induced damage. Interestingly, the mice PF to the IF group also exhibited an increased resistance of CA1 neurons to KA-induced damage relative to either AL-fed or LDF mice. The major difference between the AL-fed and PF groups is that the latter were very slightly restricted early in the study (≈10%). This may imply that there is an optimal level of restriction for neuroprotective effects, a hypothesis that would require further study to verify. The 10% restriction in food intake of mice on the IF regimen may account for the differences between AL-fed and PF mice and also may be a factor in the effects of IF on some physiological parameters in this study.
Figure 3.
IF is superior to caloric restriction in protecting hippocampal neurons against excitotoxic injury. Mice that had been maintained for 20 weeks on the indicated diets were subjected to an intrahippocampal injection of the excitotoxin KA; PBS was injected into the contralateral hippocampus of each mouse. Mice were killed 24 h later, coronal brain sections were stained with cresyl violet, and the numbers of undamaged neurons in regions CA3 and CA1 of the hippocampus were quantified. Values are the mean and SD of determinations made in eight mice per group. (a) Representative images showing cresyl violet-stained neurons in KA- and PBS-injected hippocampi of mice from each diet group. (b) Quantification of damage in hippocampi injected with PBS or KA. *, P < 0.01.
A previous study compared the effects of LDF and IF on life span in male C57BL/6 mice, with the two DR regimens resulting in similar life-span extension despite a clear difference in body weight between the two groups (13). However, food intake was not determined, and it was assumed that the difference in body weight was due to some factor other than caloric intake. The present study establishes that it is the ability of this strain of mice to gorge on days when food is provided that allows them to maintain nearly AL body weight when fed every other day, and they thereby avoid a long-term calorie deficit. That IF feeding was more effective than either LDF or PF in protecting neurons from KA-induced damage demonstrates that the IF-feeding schedule itself is neuroprotective independent of overall caloric intake. In a study of F344 rats Masoro et al. (28) found that when calories are explicitly restricted to 60% of the AL intake, altering meal frequency within a 24-h period neither abrogates nor enhances the effect of the net caloric restriction on life span. Although providing evidence that meal frequency does not alter the ability of caloric restriction to extend life span, that study did not allow a conclusion as to whether IF can increase life span independent of overall calorie intake. The present study suggests that in the absence of explicit restriction, fasting may play a role in at least some of the effects of DR.
Because we did not determine life span in this study, we cannot conclude with certainty that the IF regimen used would extend life span. However, a previous study did establish a life-span-extending effect of the identical IF regimen in the identical strain of mice (12). Moreover, a recent study of mice with adipose tissue-selective disruption of the insulin receptor gene showed that the life span of mice can be increased without a reduction in calorie intake (29). Emerging data from this and other laboratories therefore support future studies to determine whether a reduction in caloric intake is the only dietary method to increase longevity or whether IF without caloric restriction might have beneficial effects as well. Thus, although caloric restriction is one important mechanism underlying the effects of different DR regimens on life span and disease susceptibility, at least some beneficial effects of DR regimens may result from a mechanism other than an overall reduction in calorie intake. One such possible mechanism is stimulation of cellular stress-resistance pathways, which are induced strongly by the IF regimen used in this study (7, 8, 14).
A consistent hormonal response to a decrease in food intake in rodents, nonhuman primates, and humans (30, 31) is a reduction in insulin levels and an increase in insulin sensitivity. We found that mice subjected to IF exhibited decreases in serum levels of glucose and insulin to levels at or below those in mice fed daily but with a 40% reduction in caloric intake. The ability of IF to alter fasting levels of insulin and glucose was independent of overall caloric intake. It has been proposed that some beneficial effects of DR result from decreased blood glucose levels (integrated over time) (2). Glucose levels in the blood, integrated over time, have been postulated to lead to high levels of nonenzymatic glycation, a form of protein damage. Higher fuel availability may also lead to an increased frequency of mitochondrial state-four respiration, with consequent increases in reactive oxygen species production from the mitochondrial respiratory chain. DR has been shown to influence oxyradical production and damage (2) and nonenzymatic glycation (32). The present findings are consistent with a role for glucose in the beneficial effects of IF, because the animals spend a large proportion of their life span in a fasted state. However, it might be predicted that on feeding days, when IF mice gorge on food, their levels of oxyradical production and glycation are much higher than in mice on the LDF regimen. Apparently, confining bouts of high caloric intake to a limited time window with long intervening periods of fasting results in adaptive responses that do not occur when meals are more frequent. Previous studies have shown that there are large changes in respiratory quotient as restricted animals move from ingested to stored fuel (33). It may be that alternating periods of anabolism and catabolism play a mechanistic role in triggering increases in cellular stress resistance and the repair of damaged biomolecules or cells. Recent findings suggest that many of the beneficial effects of IF may result from a cellular stress response induced by the fasted state. For example, it was shown that levels of stress protein chaperones (8, 34) and neurotrophic factors (35) are increased in rats and mice maintained on an IF-feeding regimen. The superior neuroprotective efficacy of IF compared with LDF feeding documented in this study is consistent with enhancement of cellular stress resistance that results from the stress associated with fasting rather than an overall reduction in caloric intake.
Interestingly, although IF and LDF DR regimens had similar effects on insulin and glucose levels, they had different effects on serum IGF-1 levels (increased with IF but decreased with LDF) and serum β-hydroxybutyrate levels (increased with IF but decreased with LDF). Decreased IGF-1 levels have been proposed to contribute to the protective effect of DR against carcinogenesis (36). However, IF also protects against tumor growth (37, 38), suggesting that additional mechanisms must be operative in this beneficial effect of IF. Of particular interest with regard to the cytoprotective effect of IF was the large increase in the levels of β-hydroxybutyrate, a fat-derived fuel used when the carbohydrate supply is limiting. It is well known that utilization of these fuels increases in the fasting state in various tissues including the brain (39). Because mice on the IF regimen weigh a great deal more than mice on the LDF regimen they may have larger adipose reserves and a greater ketogenic response than that of LDF mice. This shift to ketogenesis may play a direct role in the cytoprotective effects of IF, because it has been reported that rats fed a ketogenic diet exhibit increased resistance to seizures (25), and that β-hydroxybutyrate protects neurons in rodent models of Alzheimer's and Parkinson's diseases (27). Ketogenic diets are prescribed for some patients with epilepsy (26) and are also implemented in several popular weight-loss programs (40, 41). The findings of this study suggest that IF can enhance health and cellular resistance to disease even if the fasting period is followed by a period of overeating such that overall caloric intake is not decreased.
The ability of both the IF and LDF paradigms to enhance stress resistance and extend life span provides a useful tool for discerning the mechanism by which DR exerts its effect. If a change such as an altered hormone level is proposed to play a fundamental mechanistic role, it should occur in response to both feeding regimes. Comparison of the two paradigms thus provides opportunities for discerning the mechanisms underlying the modulation of aging rate by DR.
Acknowledgments
We thank Dr. Josephine Egan and the Laboratory of Endocrinology at the National Institute on Aging for measurements of serum glucose and insulin and Dr. Julie Mattison for advice and assistance in blood collection.
Abbreviations
- DR
dietary restriction
- AL
ad libitum
- IF
intermittent fasting
- LDF
limited daily feeding
- IGF-1
insulin-like growth factor 1
- PF
pair-fed
- KA
kainic acid
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Masoro E J. Exp Gerontol. 2000;35:299–305. doi: 10.1016/s0531-5565(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 2.Weindruch R, Sohal R S. N Engl J Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackwell B N, Bucci T J, Hart R W, Turturro A. Toxicol Pathol. 1995;23:570–582. doi: 10.1177/019262339502300503. [DOI] [PubMed] [Google Scholar]
- 4.Mukherjee P, El-Abbadi M M, Kasperzyk J L, Ranes M K, Seyfried T N. Br J Cancer. 2002;86:1615–1621. doi: 10.1038/sj.bjc.6600298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandes G, Yunis E J, Miranda M, Smith J, Good R A. Proc Natl Acad Sci USA. 1978;75:2888–2892. doi: 10.1073/pnas.75.6.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruce-Keller A J, Umberger G, McFall R, Mattson M P. Ann Neurol. 1999;45:8–15. [PubMed] [Google Scholar]
- 7.Duan W, Mattson M P. J Neurosci Res. 1999;57:195–206. doi: 10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 8.Yu Z F, Mattson M P. J Neurosci Res. 1999;57:830–839. [PubMed] [Google Scholar]
- 9.Zhu H, Guo Q, Mattson M P. Brain Res. 1999;842:224–229. doi: 10.1016/s0006-8993(99)01827-2. [DOI] [PubMed] [Google Scholar]
- 10.Goodrick C L, Ingram D K, Reynolds M A, Freeman J R, Cider N L. Exp Aging Res. 1983;9:203–209. doi: 10.1080/03610738308258453. [DOI] [PubMed] [Google Scholar]
- 11.Masoro E J, Yu B P, Bertrand H A. Proc Natl Acad Sci USA. 1982;79:4239–4241. doi: 10.1073/pnas.79.13.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodrick C L, Ingram D K, Reynolds M A, Freeman J R, Cider N. Mech Ageing Dev. 1990;55:69–87. doi: 10.1016/0047-6374(90)90107-q. [DOI] [PubMed] [Google Scholar]
- 13.Ingram D K, Reynolds M A. In: Evolution of Longevity in Animals. Woodhead A D, Thompson K H, editors. New York: Plenum; 1987. [Google Scholar]
- 14.Duan W, Guo Z, Mattson M P. J Neurochem. 2001;76:619–626. doi: 10.1046/j.1471-4159.2001.00071.x. [DOI] [PubMed] [Google Scholar]
- 15.Telfer Brunton W A, Percy-Robb I W. Clin Chim Acta. 1976;69:131–135. doi: 10.1016/0009-8981(76)90483-6. [DOI] [PubMed] [Google Scholar]
- 16.Dalpe-Scott M, Heick H M, Begin-Heick N. Can J Biochem. 1982;60:962–966. doi: 10.1139/o82-123. [DOI] [PubMed] [Google Scholar]
- 17.Kalant N, Stewart J, Kaplan R. Mech Ageing Dev. 1988;46:89–104. doi: 10.1016/0047-6374(88)90117-0. [DOI] [PubMed] [Google Scholar]
- 18.Wolkow C A. Trends Neurosci. 2002;25:212–216. doi: 10.1016/s0166-2236(02)02133-1. [DOI] [PubMed] [Google Scholar]
- 19.Dunn S E, Kari F W, French J, Leininger J R, Travlos G, Wilson R, Barrett J C. Cancer Res. 1997;57:4667–4672. [PubMed] [Google Scholar]
- 20.Shimokawa I, Higami Y, Utsuyama M, Tuchiya T, Komatsu T, Chiba T, Yamaza H. Am J Pathol. 2002;160:2259–2265. doi: 10.1016/S0002-9440(10)61173-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng B, Mattson M P. J Neurosci. 1992;12:1558–1566. doi: 10.1523/JNEUROSCI.12-04-01558.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsieh C C, DeFord J H, Flurkey K, Harrison D E, Papaconstantinou J. Mech Ageing Dev. 2002;123:1229–1244. doi: 10.1016/s0047-6374(02)00036-2. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell G A, Kassovska-Bratinova S, Boukaftane Y, Robert M F, Wang S P, Ashmarina L, Lambert M, Lapierre P, Potier E. Clin Invest Med. 1995;18:193–216. [PubMed] [Google Scholar]
- 24.Vazquez J A, Morse E L, Adibi S A. J Clin Invest. 1985;76:737–743. doi: 10.1172/JCI112029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bough K J, Valiyil R, Han F T, Eagles D A. Epilepsy Res. 1999;35:21–28. doi: 10.1016/s0920-1211(98)00125-9. [DOI] [PubMed] [Google Scholar]
- 26.Gilbert D L, Pyzik P L, Freeman J M. J Child Neurol. 2000;15:787–790. doi: 10.1177/088307380001501203. [DOI] [PubMed] [Google Scholar]
- 27.Kashiwaya Y, Takeshima T, Mori N, Nakashima K, Clarke K, Veech R L. Proc Natl Acad Sci USA. 2000;97:5440–5444. doi: 10.1073/pnas.97.10.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masoro E J, Shimokawa I, Higami Y, McMahan C A, Yu B P. J Gerontol A Biol Sci Med Sci. 1995;50:B48–B53. doi: 10.1093/gerona/50a.1.b48. [DOI] [PubMed] [Google Scholar]
- 29.Bluher M, Kahn B B, Kahn C R. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 30.Grassi G, Seravalle G, Colombo M, Bolla G, Cattaneo B M, Cavagnini F, Mancia G. Circulation. 1998;97:2037–2042. doi: 10.1161/01.cir.97.20.2037. [DOI] [PubMed] [Google Scholar]
- 31.Lane M A, Ball S S, Ingram D K, Cutler R G, Engel J, Read V, Roth G S. Am J Physiol. 1995;268:E941–E948. doi: 10.1152/ajpendo.1995.268.5.E941. [DOI] [PubMed] [Google Scholar]
- 32.Cefalu W T, Bell-Farrow A D, Wang Z Q, Sonntag W E, Fu M X, Baynes J W, Thorpe S R. J Gerontol A Biol Sci Med Sci. 1995;50:B337–B341. doi: 10.1093/gerona/50a.6.b337. [DOI] [PubMed] [Google Scholar]
- 33.Duffy P H, Leakey J E A, Pipkin J L, Turturro A, Hart R W. Environ Res. 1997;73:242–248. doi: 10.1006/enrs.1997.3714. [DOI] [PubMed] [Google Scholar]
- 34.Guo Z, Ersoz A, Butterfield D A, Mattson M P. J Neurochem. 2000;75:314–320. doi: 10.1046/j.1471-4159.2000.0750314.x. [DOI] [PubMed] [Google Scholar]
- 35.Lee J, Seroogy K B, Mattson M P. J Neurochem. 2002;80:539–547. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- 36.Kari F W, Dunn S E, French J E, Barrett J C. J Nutr Health Aging. 1999;3:92–101. [PubMed] [Google Scholar]
- 37.Kritchevsky D. Proc Soc Exp Biol Med. 1990;193:35–38. doi: 10.3181/00379727-193-42986. [DOI] [PubMed] [Google Scholar]
- 38.Siegal I, Liu T L, Neopmuceno N, Gleicher N. Cancer Invest. 1988;6:677–680. doi: 10.3109/07357908809078034. [DOI] [PubMed] [Google Scholar]
- 39.Pan J W, Rothman T L, Behar K L, Stein D T, Hetherington H P. J Cereb Blood Flow Metab. 2000;20:1502–1507. doi: 10.1097/00004647-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Anderson J W, Konz E C, Jenkins D J. J Am Coll Nutr. 2000;19:578–590. doi: 10.1080/07315724.2000.10718955. [DOI] [PubMed] [Google Scholar]
- 41.Willi S M, Oexmann M J, Wright N M, Collop N A, Key L L., Jr Pediatrics. 1998;101:61–67. doi: 10.1542/peds.101.1.61. [DOI] [PubMed] [Google Scholar]