Abstract
To test the hypothesis that establishing gastrointestinal colonization with multiresistant Enterococcus faecium (VRE) C68 results from expansion of the enterococcal population in the upper small bowel, we compared VRE quantities recovered from the proximal, middle, and distal segments of the small bowel from mice treated with different antimicrobial agents. Antibiotics associated with high-level VRE fecal colonization (cefotetan, ceftriaxone, clindamycin, and ticarcillin-clavulanic acid) increased VRE quantities in all small-bowel segments, whereas cefepime and piperacillin-tazobactam did not. Enterococcal expansion did not correlate with reductions in numbers of native gram-negative or anaerobic flora. Green fluorescence protein-expressing E. faecium bacteria were found adjacent to the small bowel epithelial lining in colonized mice. These data indicate that enterococcal bowel colonization begins within the proximal small bowel and does not correlate with inhibition of other cultivable flora. Host or enterococcal factors induced by exposures to certain antibiotics may play a role in facilitating E. faecium colonization of the mammalian gastrointestinal tract.
Multiresistant Enterococcus faecium (VRE) continues to be an important cause of nosocomial infection, especially in severely immunocompromised patients (10). Data from clinical studies indicate that gastrointestinal colonization frequently precedes clinical infection and that exposure to antimicrobial agents increases the risk of becoming colonized or infected with multiresistant E. faecium (9, 18). Prominent implicated antibiotics include third-generation cephalosporins, agents with potent activity against anaerobic bacteria, and vancomycin (2, 13).
Using a mouse model of VRE colonization designed to examine the impact of antimicrobial administration on the establishment of VRE gastrointestinal colonization, we have shown that some broad-spectrum antimicrobial agents (cefotetan and ceftriaxone) promote heavy colonization while others (aztreonam, cefazolin, cefepime, and piperacillin-tazobactam) do not (15). The tendency to promote fecal colonization appears to be related to the intrinsic activity of the agent against E. faecium and the extent to which the agent is concentrated in bile. In separate experiments designed to examine the impact of antimicrobial agents on the persistence of high-level fecal colonization, activity against anaerobic flora was the most important characteristic (4, 6).
Although the associations of different antibiotic classes with colonization in our mouse model now seem relatively clear, the mechanisms by which colonization occurs remain unknown. In particular, the location within the bowel where the expansion of the enterococcal population occurs is not known. A study by Pultz and colleagues (12) suggested that one area of significant antimicrobial impact is in the proximal large bowel (cecum), where treatment with clindamycin promotes enterococcal overgrowth in the cecal contents as well as increased enterococcal numbers within the cecal mucus layer. Jin and colleagues (8) showed that enterococci could adhere to the mucus isolated from the small bowel of piglets and, when given in a large inoculum, inhibited adherence of pathogenic Escherichia coli to the same mucus. The glycoprotein receptors for the two strains appeared to be distinct (8).
The ability of E. faecium to adhere to small-bowel mucus suggests that the small bowel may be an important location for expansion of the enterococcal population in response to the administration of antimicrobial agents. In this scenario, high concentrations of broad-spectrum antibiotics secreted into bile serve to significantly reduce the quantities of native small-bowel flora, permitting unrestricted growth of E. faecium bacteria (when they are highly resistant to the antimicrobial agent being administered). This unfettered growth results in a large inoculum of resistant E. faecium arriving at the cecum, allowing these strains to compete more effectively for the limited space and resources available in the colon. If this model is correct, then large quantities of multiresistant E. faecium bacteria should be detectable in the proximal small bowel under conditions that predispose to high-level fecal colonization. Moreover, we should be able to demonstrate that this increase in enterococcal numbers occurs concomitantly with decreases in numbers of other, susceptible bowel flora.
In the present study, we used our mouse model of colonization establishment to determine where within the mouse gastrointestinal tract expansion of the enterococcal population begins. We also investigated whether inhibition of competing flora (enteric gram-negative bacilli and anaerobes) occurs concomitantly with the expansion.
(These results were presented in poster form at the 43rd Meeting of the Infectious Diseases Society of America, San Francisco, Calif., abstr. 318, 6 to 9 October 2005.)
MATERIALS AND METHODS
Bacterial strains and plasmids.
Enterococcus faecium C68 is an ampicillin- and vancomycin-resistant (VanB type vancomycin MIC, 256 μg/ml) clinical stain. It has been previously described (3) and used in our previous animal studies (15, 16). E. faecium D344SRF is an ampicillin-susceptible derivative of D344R, described previously (14). E. faecium D344SRF(pLRM23) is a colonization-proficient strain in which a plasmid that promotes colonization in the mouse model (pLRM23) was introduced by conjugation into D344SRF (15). E. faecium D344SRF(pLRM23) is resistant to high levels of clindamycin, so this antibiotic was used to promote colonization for fluorescence microscopy experiments after incorporation of green fluorescence protein (GFP) plasmid pMV158GFP (Tetr) (kindly provided by Manuel Espinosa) (11). pMV158GFP was introduced into D344SRF(pLRM23) in the following manner. pMV158GFP was initially introduced into Enterococcus faecalis OGIX (Strr) (7), into which broad-host-range plasmid pAMβ1 (17) had been introduced by conjugation. Selection of transformants occurred by use of brain heart infusion agar plates containing streptomycin (2,000 μg/ml) and tetracycline (30 μg/ml). pAMβ1 facilitates mobilization of pMV158GFP into recipient strains during conjugation. Filter matings between OGIX(pAMβ1, pMV158GFP) and D344SRF(pLRM23) were performed using filter-mating techniques with selection for transconjugants on fusidic acid (25 μg/ml), rifampin (100 μg/ml), and tetracycline (30 μg/ml). The transfer frequency of pMV158GFP in these matings was ca. 104/recipient CFU. In preliminary experiments, we tested the colonization capacity of D344SRF(pLRM23,pMV158GFP) in mice preexposed to clindamycin (2.4 mg/day) according to previously established protocols (16). Colonization was readily established under these conditions, yielding ca. 108 CFU/gram of feces. However, pMV158GFP was not completely stable in this environment. GFP expression was present in approximately 1% of the E. faecium D344SRF CFU recovered in the feces. Since the measurements requiring GFP expression were qualitative (location) rather than quantitative, this level of stability was deemed sufficient to proceed with the experiments.
Mouse model of enterococcal colonization.
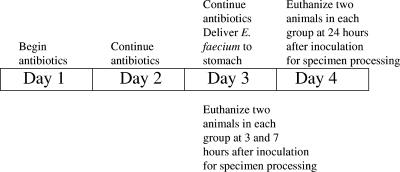
The details of mouse fecal colonization establishment have been previously described (5). Female CF1 mice (25 to 30 g; Harlan Sprague-Dawley) were used in these experiments. A total of eight mice per antibiotic were tested (the experiments were performed four times each). Experiments were performed in two different series. In the first series, four cephalosporin antibiotics were compared (cefepime [2.4 mg/day], cefotetan [3 mg/day], ceftazidime [3 mg/day], and ceftriaxone [2.4 mg/day]). In the second series, four antibiotics with potent activities against anaerobic bacteria were compared (clindamycin [2.4 mg/day], metronidazole [6 mg/day], piperacillin-tazobactam (8 mg/day), and ticarcillin-clavulanic acid [12 mg/day]). All antibiotics were given via subcutaneous injection two times a day in 12-h intervals, starting 2 days prior to gastric inoculation of E. faecium C68 [or D344SRF(pLRM23, pMV158GFP) for the fluorescence experiments]. Doses were chosen to approximate the total daily dose for each antibiotic (on a mg/kg basis) used in human infections. The time line for administration of antibiotics and harvesting of tissue is illustrated in Fig. 1. Forty-eight hours after the first dose of antibiotics, ca. 10,000 CFU from the test strain were administered via a gastric gavage with a 2-in. olive ball-shaped gavage needle (Popper and Sons, Inc, New Hyde Park, N.Y.). Mice were euthanized by carbon dioxide inhalation at 3, 7, and 24 h after gavage. The abdominal cavity of each mouse was opened, and the length of the small intestine was identified. Suture ligations were placed at the proximal duodenum and at the ileocecal junction. The duodenum was separated from the stomach and the terminal ileum from the cecum by using scissors, and the entire small bowel was then removed intact by blunt and sharp dissection to separate the bowel from abdominal connective tissue. After excision, the length of the small bowel was measured (in cm) and divided into three equal sections (proximal, medial, and distal). The intestinal contents were expressed from the individual segments, with avoidance of all contact between the contents or lumen of the intestine and anything but the inner surface of a sterile collection tube prefilled with 1 ml of 0.9% saline. After the tubes were weighed to determine the total mass of the intestinal contents that had been extracted, quantities of C68 were determined by performing serial tenfold dilutions on the sample and inoculating it onto Enterococcosel agar (BD, Franklin Lakes, N.J.) supplemented with 20 μg/ml of vancomycin (Sigma Chemical Company, St. Louis, Mo.) and incubated at 37°C for 48 h under aerobic conditions. After 48 h of incubation, enterococci were identified as colonies digesting esculin in the agar, leaving a black zone around the growing colonies. E. faecium C68 quantities were expressed as log10 numbers of CFU/gram of intestinal contents. MacConkey agar (BD) was used to quantify the number of gram-negative bacilli from the initial collection tube. MacConkey plates were inoculated with equivalent quantities of the sample and incubated at 37°C for 48 h under aerobic conditions. The lowest level of detection was established as 0.5 CFU (one colony growing from one of two duplicate spots) from 100 μl of nondiluted contents from the initial collection tube, equal approximately to a log10 of 2.
FIG. 1.
Graphic representation of the time course of the experiments.
For the second series of experiments in which antibiotics with potent antianaerobic activity were compared, 400 μl from each tube was transferred into a separate tube that was taken immediately into an anaerobic chamber (Coy Laboratories; time, ∼5 min from exposure of lumen and contents of intestine to air/O2 until O2-free conditions were regained) for growth of anaerobic flora. Samples for determination of anaerobe contents were serially diluted, plated on Trypticase soy agar-5% sheep blood agar (Remel, Lenexa, Kans.), and incubated for 48 h at 37°C all under anaerobic conditions.
Microscopy and histopathology.
An Axiovert 200 microscope (Carl Zeiss, Germany) was used in fluorescence microscopy experiments to detect fluorescent E. faecium D344SRF(pLRM23, pMV158GFP). The structures of small-intestine wall on a section were identified by comparison with surrounding sections of small intestine stained with hematoxylin-eosin. Fluorescent E. faecium microorganisms were detected by their green fluorescence by using 630-fold magnification and reflector fluorescein isothiocyanate and were identified by their typical shape. Small-intestine segments were collected for frozen sectioning 24 h after introducing fluorescent E. faecium into the mouse gastrointestinal tract. Tissue was flushed with phosphate-buffered saline to eliminate intestinal contents, embedded in blocks filled with tissue glue (Tissue-Tech, Sakura Finetek, Torrance, Calif.), and frozen using ice-cold acetone. Blocks were kept frozen in a −80°C freezer until the sectioning. We used a Microtome Cryostat HM 505E to yield 8-μm-thick slices, which were fixed in mounting medium (Permount, Fisher Scientific, Pittsburgh, Pa.) and dried overnight. Hematoxylin-eosin staining of frozen sections was performed according to standard procedures. Control experiments were performed using mice colonized with non-GFP-producing E. faecium, to exclude the possibility that other components of the mouse gastrointestinal flora would fluoresce.
Statistics.
The differences between the effects of individual antibiotics were tested for statistical significance by one-way analysis of variance. The null hypothesis was chosen to be such that the effects of any two of the antibiotics that are tested are the same. When P is <0.05, it indicates that the effects of the two groups are significantly different. The results of the analysis of variance for each experiment are summarized below.
Three measurements (namely, proximal, medial, and distal) for each mouse (there were eight mice per treatment group) were first averaged before the statistical analysis of the data. The correlation coefficients for the measurements for the proximal and medial, proximal and distal, and medial and distal portions were calculated for each experiment in order to verify the use of the averages in place of the three measurements. The results of this correlation analysis were summarized, and the correlation coefficients as high as >0.7 for all the experiments allowed us to average the three measurements used in the analysis.
RESULTS
Comparison between cephalosporins.
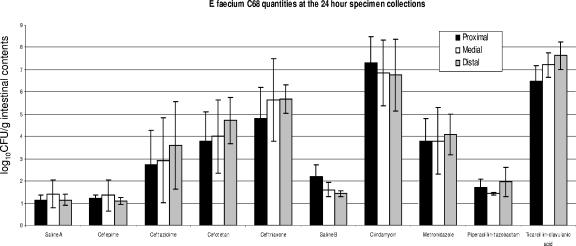
We first compared four cephalosporin antibiotics to determine their effects on the establishment of E. faecium C68 colonization in the small intestine. At 3 and 7 hours after gavage, we observed no statistically significant differences between colonization in animals administered any of the antibiotics and that in mice administered normal saline (data not shown). This lack of observed difference is likely explainable by the initial lag phase of growth lasting approximately 6 to 8 h in enterococci. It suggests that at 3 and 7 h after the introduction of E. faecium into the mouse gastrointestinal tract, only the initial inoculum of C68 was present and passing through the small intestine. At 24 hours after inoculation, however, significant differences were observed between the treatment groups (Fig. 2). Colonization of the small intestine by C68 in mice treated with ceftriaxone and cefotetan reached high levels compared to that in mice treated with normal saline (P ≤ 0.0001). Ceftazidime-treated mice yielded more colonization that those treated with cefepime (P = 0.0195) but less colonization than those treated with ceftriaxone (P = 0.0106). The difference between colonization associated with ceftazidime and that associated with cefotetan was not statistically significant (Fig. 2). No statistically significant difference in small-intestinal colonization was observed between the cefepime group and mice treated with 0.9% saline (Fig. 2). These results are consistent with previous experiments in which cefepime did not promote fecal colonization with E. faecium C68.
FIG. 2.
Graphic summary of the results from the colonization experiments, with separate data for the three portions of the small bowel (proximal, medial, and distal). Columns represent the quantities of E. faecium C68 per gram of intestinal contents in the designated segments collected at 24 h. Since the experiments were performed in two separate series, there were two separate saline control groups. The saline A group corresponds to the experiments testing cefepime, ceftazidime, cefotetan, and ceftriaxone. The saline B group corresponds to the experiments testing clindamycin, metronidazole, piperacillin-tazobactam, and ticarcillin-clavulanic acid. The error bars show the standard deviations for each set of experiments. As in previous experiments in which fecal colony counts were determined, cefotetan, ceftriaxone, clindamycin, and ticarcillin-clavulanic acid promote heavy colonization, whereas cefepime and piperacillin-tazobactam do not.
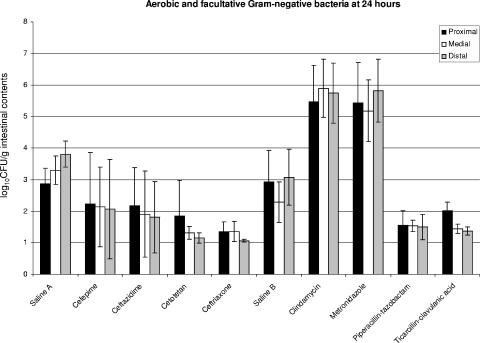
Growth on MacConkey plates revealed decreased numbers of gram-negative bacilli in mice treated with ceftriaxone, which is heavily concentrated in the gastrointestinal tract (Fig. 3). Overall numbers of gram-negative bacteria were relatively low (ca. 103 CFU/g), even in the saline-treated group. Reductions in numbers of gram-negative aerobes and facultative organisms did not achieve statistical significance for animals treated with cefepime, cefotetan, or ceftazidime.
FIG. 3.
Graphic summary of the 24-h results from the colonization experiments, with separate data for the three portions of the small bowel (proximal, medial, and distal). Columns represent the quantities of facultative and aerobic gram-negative bacteria per gram of intestinal contents in the designated segments collected at 24 h. Since the experiments were performed in two separate series, there were two separate saline control groups. The saline A group corresponds to the experiments testing cefepime, ceftazidime, cefotetan, and ceftriaxone. The saline B group corresponds to the experiments testing clindamycin, metronidazole, piperacillin-tazobactam, and ticarcillin-clavulanic acid. The error bars show the standard deviations for each set of experiments. The quantities of gram-negative bacteria in the saline group were relatively small. Differences between saline and ceftriaxone and between clindamycin and metronidazole are statistically significant (see text).
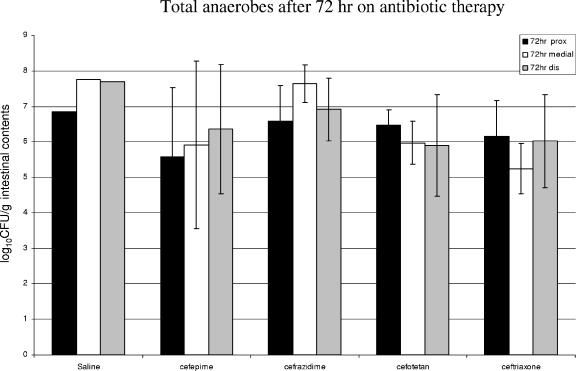
We did not obtain anaerobic cultures of material harvested from cephalosporin-treated mice in the first series of experiments. In order to obtain some information about the impact of cephalosporin antibiotics on the anaerobic flora, we administered the four tested cephalosporins (and saline) to a group of mice in a separate experiment. We then determined anaerobic bacterial counts in the intestinal segments at 72 h after cephalosporin initiation (approximating the time of the 24-h collection in the first series of experiments). The results are shown in Fig. 4. None of the cephalosporins (including the potent antianaerobic antibiotic cefotetan) significantly reduced the numbers of anaerobic bacteria in the bowel in these experiments.
FIG. 4.
Results of separate experiments in which total anaerobes were quantified after 72 h of treatment with cephalosporin antibiotics. These experiments are not strictly comparable to the others described in this paper since an E. faecium inoculum was not given at 48 h. Standard deviation bars are not included since there was only one animal given saline in this experiment. The other treatment groups contained three animals each. None of the cephalosporin antibiotics exhibited a significant effect on the total anaerobic flora of the small bowel.
Antianaerobic antibiotics.
Similar to what was found for cephalosporin-treated mice, we observed no statistically significant differences in E. faecium C68 colonization at 3 and 7 hours after gavage with C68 compared with that in the normal saline group (data not shown). Ticarcillin-clavulanic acid (P < 0.0001) and clindamycin (P < 0.0001) induced the highest VRE colonization levels in the small intestine compared to normal saline (Fig. 2). Metronidazole administration was also associated with increased C68 colonization compared with saline (P = 0.0018) but on average yielded about 3 log10 CFU/gram fewer bacteria than either clindamycin or ticarcillin-clavulanic acid. Piperacillin-tazobactam-treated mice showed no difference in C68 colonization compared to the normal saline group.
Piperacillin-tazobactam (P = 0.0063) and ticarcillin-clavulanic (P = 0.0068) acid both decreased the quantities of facultative and aerobic gram-negative bacilli recovered compared to those found in saline-treated mice (Fig. 3), but since the numbers of CFU in the saline group were modest, the average decrease was only about 1 log10 CFU/g. There were statistically significant increases in the growth of gram-negative organisms in the mice exposed to clindamycin (P = 0.00009) and metronidazole (P = 0.00037), reflecting their strong antianaerobic activities and lack of activity against aerobic and facultative gram-negative bacilli.
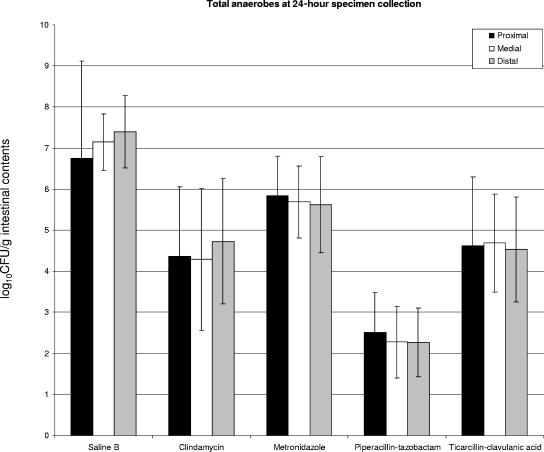
Total anaerobes were quantified after 48 h of incubation under anaerobic conditions at 37°C, and statistically significant decreases were observed at 3, 7, and 24 hours in all antibiotic groups tested in the second series of experiments (Fig. 5). Piperacillin-tazobactam reduced the number of total anaerobes to the greatest extent, showing a greater decrease in number of indigenous anaerobic flora than clindamycin (P = 0.0017) or ticarcillin-clavulanic acid (P = 0.0006) at 24 h. Metronidazole decreased the number of total anaerobes to a lesser extent than the other antibiotics (Fig. 5).
FIG. 5.
Graphic summary of the results from colonization experiments, with separate data for the three portions of the small bowel (proximal, medial, and distal). Columns represent the quantities of total anaerobic bacteria per gram of intestinal contents in the designated segments collected at 24 h. The bars above the columns represent the standard deviations for each set of experiments. All antibiotic groups demonstrated statistically significant reductions compared to the saline control group. Results for piperacillin-tazobactam in comparison to those for clindamycin and metronidazole were also statistically significant.
Location of E. faecium within the small bowel.
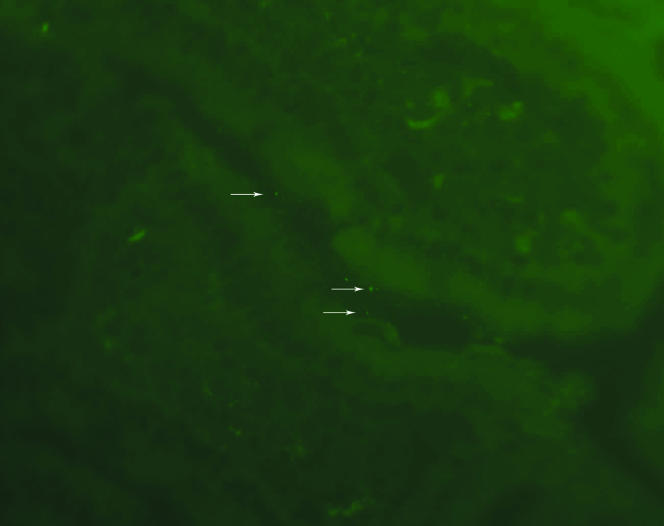
The results of experiments with colonization by E. faecium D344SRF(pLRM23,pMV158GFP) are shown in Fig. 6. Fluorescent E. faecium bacteria were found adjacent to the epithelium of villi, in the crypts of the small intestine, and in surrounding remnants of stool. We did not observe any fluorescent E. faecium bacteria penetrating to deeper parts of the intestinal wall. Similar fluorescent material was not observed in the small intestines of mice colonized with nonfluorescing bacteria (data not shown).
FIG. 6.
Histological section of small bowel from mouse colonized with E. faecium D344SRF(pLRM23, pMV158GFP). The white arrows indicate fluorescent E. faecium. E. faecium bacteria are located adjacent to the villi within the crypts of the small intestine.
DISCUSSION
The data accumulated in this work suggest that gastrointestinal colonization by multiresistant E. faecium includes expansion of the enterococcal population in the entire small intestine, rather than simply in the cecum. The mechanism(s) by which antibiotic administration promotes gastrointestinal colonization remains poorly defined at present. That the inhibition of competing flora would underlie the expansion of the enterococcal population seems logical. However, the data presented in this study are not consistent with this model. Ceftriaxone and cefotetan promote heavy enterococcal colonization, despite the fact that we were unable to detect significant reductions in the anaerobic population of the small bowel. Although ceftriaxone was associated with statistically significant decreases in the quantities of aerobic and facultative gram-negative organisms compared to saline, the relatively modest decrease in absolute numbers argues against the assumption that this decrease is of major importance. Moreover, clindamycin and metronidazole also promote heavy colonization despite the fact that the facultative and aerobic gram-negative populations dramatically increase after exposure to these agents, while the anaerobic flora is only modestly affected. Therefore, we do not have the data to support a model in which the overgrowth of E. faecium in the small intestine owes to antibiotic-induced reductions in numbers of normal flora.
It should be kept in mind that antibiotic exposure is critical to this level of colonization in mice. We are unable to establish significant colonization in the absence of antibiotics, and previous work has shown that high-level colonization induced by exposure to oral vancomycin wanes after roughly 3 weeks in mice (6). The duration of colonization in humans has often been observed to be longer than this and is frequently complicated by repeated exposures to antibiotics (4).
It is intriguing that in the experiments in which E. faecium D344SRF(pLRM23, pMV158GFP) was the colonizing strain, we observed fluorescent enterococci adjacent to the intestinal epithelium. Previous work suggests that the presence of fluorescent enterococci in this location represents its presence within the mucus layer of the small bowel (8, 12). The presence of fluorescent enterococci in this location suggests an interaction between the bacterium and the mucus or with the epithelium itself. It is conceivable that the presence of certain antimicrobial agents in sufficient concentrations in the upper gastrointestinal tract induces changes in the epithelial lining that promote enterococcal adherence. Such adherence would promote the presence of enterococci along the entire length of the small bowel, resulting in the relatively homogeneous expansion observed in our experiments. Without this adherence, the relatively rapid transit time through the mouse small bowel (heavy fecal colonization can be observed at 24 h) would sweep the inoculum through the small bowel in relatively short order, resulting in the arrival of relatively small, noncompetitive numbers at the cecum.
Alternatively, exposure to high concentrations of different antibiotics could induce a change in the enterococcus itself, resulting in the expression of factors that could promote adherence to the intestinal epithelial lining. A recently published study examining the transcriptional response of Enterococcus faecalis V583 to erythromycin exposure revealed that transport and binding proteins were the second-most-common group of genes identified as upregulated (1). At the present time, little is known about microbial factors that would promote enterococcal adherence and growth in the gastrointestinal tract. It does appear, however, that some enterococci express factors other than resistance determinants that promote colonization in the presence of antibiotics, as we demonstrated previously by transferring such a factor for C68 to D344SRF, resulting in D344SRF(pLRM23) (16).
In summary, we demonstrated that antimicrobial agents that promote fecal colonization with multiresistant enterococci do so by first creating a significant expansion of the enterococci throughout the small bowel, resulting in the delivery of large inocula of the colonizing strain to the colon, where further expansion likely occurs. Expansion in the small bowel is associated with the presence of E. faecium immediately adjacent to the bowel epithelium, likely within the mucus layer. Our data do not support the suppression of competing bacteria as the primary mechanism of this enterococcal expansion, suggesting that other factors favor the adherence and multiplication of E. faecium in the gastrointestinal tract of antibiotic-treated mammals. Defining the nature of these factors will be an important step toward the devising of intelligent strategies to minimize resistant enterococcal colonization in hospitalized patients.
Acknowledgments
Potential conflicts include the following: Louis B. Rice is a consultant for Elan, Wyeth, Merck, Pfizer, Cumbre, and Cubist; received grant support from Wyeth and Elan; and is a member of the speakers bureaus for Elan, Wyeth, Merck, and Cubist.
These studies were supported by grants from Elan Pharmaceuticals and Wyeth Pharmaceuticals, by a Merit Review from the Department of Veterans Affairs (L.B.R.), and by grant number AI 045626-05 from the National Institute of Allergy and Infectious Diseases (L.B.R.).
We are grateful for the assistance of Lenore Carias and Steve Marshall in processing microbiological specimens for this work.
REFERENCES
- 1.Aakra, A., H. Vebo, L. Snipen, H. Hirt, A. Aastveit, V. Kapur, G. Dunny, B. Murray, and I. F. Nes. 2005. Transcriptional response of Enterococcus faecalis V583 to erythromycin. Antimicrob. Agents Chemother. 49:2246-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonten, M. J. M., S. Slaughter, A. W. Ambergen, M. K. Hayden, J. van Voorhis, C. Nathan, and R. A. Weinstein. 1998. The role of “colonization pressure” in the spread of vancomycin-resistant enterococci. Arch. Intern. Med. 158:1127-1132. [DOI] [PubMed] [Google Scholar]
- 3.Carias, L. L., S. D. Rudin, C. J. Donskey, and L. B. Rice. 1998. Genetic linkage and cotransfer of a novel, vanB-containing transposon (Tn5382) and a low-affinity penicillin-binding protein 5 gene in a clinical vancomycin-resistant Enterococcus faecium isolate. J. Bacteriol. 180:4426-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donskey, C. J., T. K. Chowdhry, M. T. Hecker, C. K. Hoyen, J. A. Hanrahan, A. M. Hujer, R. A. Hutton-Thomas, C. C. Whalen, R. A. Bonomo, and L. B. Rice. 2000. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N. Engl. J. Med. 343:1925-1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donskey, C. J., J. A. Hanrahan, R. A. Hutton, and L. B. Rice. 2000. Effect of parenteral antibiotic administration on establishment of colonization with vancomycin-resistant Enterococcus faecium in the mouse gastrointestinal tract. J. Infect. Dis. 181:1830-1833. [DOI] [PubMed] [Google Scholar]
- 6.Donskey, C. J., J. A. Hanrahan, R. A. Hutton, and L. B. Rice. 1999. Effect of parenteral antibiotic administration on persistence of vancomycin-resistant Enterococcus faecium in the mouse gastrointestinal tract. J. Infect. Dis. 180:384-390. [DOI] [PubMed] [Google Scholar]
- 7.Ike, Y., R. A. Craig, B. A. White, Y. Yagi, and D. B. Clewell. 1983. Modification of Streptococcus faecalis sex pheromones after acquisition of plasmid DNA. Proc. Natl. Acad. Sci. USA 80:5369-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin, L. Z., R. R. Marquardt, and X. Zhao. 2000. A strain of Enterococcus faecium (18C23) inhibits adhesion of enterotoxigenic Escherichia coli K88 to porcine small intestine mucus. Appl. Environ. Microbiol. 66:4200-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreno, F., P. Grota, C. Crisp, K. Magnon, G. P. Melcher, J. H. Jorgensen, and J. E. Patterson. 1995. Clinical and molecular epidemiology of vancomycin-resistant Enterococcus faecium during its emergence in a city in southern Texas. Clin. Infect. Dis. 21:1234-1237. [DOI] [PubMed] [Google Scholar]
- 10.National Nosocomial Infections Surveillance System. 2004. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am. J. Infect. Control 32:470-485. [DOI] [PubMed] [Google Scholar]
- 11.Nieto, C., and M. Espinosa. 2003. Construction of the mobilizable plasmid pMV158GFP, a derivative of pMV158 that carries the gene encoding the green fluorescent protein. Plasmid 49:281-285. [DOI] [PubMed] [Google Scholar]
- 12.Pultz, N. J., U. Stiefel, S. Subramanyan, M. S. Helfand, and C. J. Donskey. 2005. Mechanisms by which anaerobic microbiota inhibit the establishment in mice of intestinal colonization by vancomycin-resistant Enterococcus. J. Infect. Dis. 191:949-956. [DOI] [PubMed] [Google Scholar]
- 13.Puzniak, L. A., J. Mayfield, T. Leet, M. Kollef, and L. M. Mundy. 2001. Acquisition of vancomycin-resistant enterococci during scheduled antimicrobial rotation in an intensive care unit. Clin. Infect. Dis. 33:151-157. [DOI] [PubMed] [Google Scholar]
- 14.Rice, L. B., L. L. Carias, R. Hutton-Thomas, F. Sifaoui, L. Gutmann, and S. D. Rudin. 2001. Penicillin-binding protein 5 and expression of ampicillin resistance in Enterococcus faecium. Antimicrob. Agents Chemother. 45:1480-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rice, L. B., R. Hutton-Thomas, V. Lakticova, M. S. Helfand, and C. J. Donskey. 2004. Beta-lactam antibiotics and gastrointestinal colonization with vancomycin-resistant enterococci. J. Infect. Dis. 189:1113-1118. [DOI] [PubMed] [Google Scholar]
- 16.Rice, L. B., V. Lakticova, M. S. Helfand, and R. Hutton-Thomas. 2004. In vitro antienterococcal activity explains associations between exposures to antimicrobial agents and risk of colonization by multiresistant enterococci. J. Infect. Dis. 190:2162-2166. [DOI] [PubMed] [Google Scholar]
- 17.Swinfield, T.-J., L. Janniere, S. D. Ehrlich, and N. P. Minton. 1991. Characterization of a region of Enterococcus faecalis plasmid pAMβ1 which enhances the segregational stability of pAMβ1-derived cloning vectors in Bacillus subtilis. Plasmid 26:209-221. [DOI] [PubMed] [Google Scholar]
- 18.Wells, C. L., B. A. Juni, S. B. Cameron, K. R. Mason, D. L. Dunn, P. Ferrieri, and F. S. Rhame. 1995. Stool carriage, clinical isolation, and mortality during an outbreak of vancomycin-resistant enterococci in hospitalized medical and/or surgical patients. Clin. Infect. Dis. 21:45-50. [DOI] [PubMed] [Google Scholar]