Abstract
We evaluated anti-Campylobacter jejuni activity among >1,200 isolates of different lactic acid bacteria. Lactobacillus salivarius strain NRRL B-30514 was selected for further study. The cell-free, ammonium sulfate precipitate from the broth culture was termed the crude antimicrobial preparation. Ten microliters of the crude preparation created a zone of C. jejuni growth inhibition, and growth within the zone resumed when the crude preparation was preincubated with proteolytic enzymes. Bacteriocin OR-7, derived from this crude preparation, was further purified using ion-exchange and hydrophobic-interaction chromatography. The determined amino acid sequence was consistent with class IIa bacteriocins. Interestingly, OR-7 had sequence similarity, even in the C-terminal region, to acidocin A, which was previously identified from L. acidophilus and had activity only to gram-positive bacteria, whereas OR-7 had activity to a gram-negative bacterium. Bacteriocin activity was stable following exposure to 90°C for 15 min, also consistent with these types of antibacterial peptides. The purified protein was encapsulated in polyvinylpyrrolidone and added to chicken feed. Ten day-of-hatch chicks were placed in each of nine isolation units; two groups of birds were challenged with each of four C. jejuni isolates (one isolate per unit). At 7 days of age, one group of birds was treated with bacteriocin-emended feed for 3 days, and one group was left untreated. At 10 days of age, the birds were sacrificed and the challenge strain was enumerated from the bird cecal content. Bacteriocin treatment consistently reduced colonization at least one millionfold compared with levels found in the untreated groups. Nonchallenged birds were never colonized by C. jejuni. Bacteriocin from L. salivarius NRRL B-30514 appears potentially very useful to reduce C. jejuni in poultry prior to processing.
The consumption of improperly prepared poultry products continues to result in human intestinal disease (24). In particular, Campylobacter spp., especially Campylobacter jejuni, have been implicated etiologies. Poultry serves as an important reservoir for Campylobacter in the food supply and creates a potential health risk (15). This microorganism may colonize poultry gastrointestinal (GI) tracts without deleterious effects upon the birds (31), and asymptomatic carriers freely spread the microorganisms during production and processing, resulting in further contamination of both live birds and processed carcasses (30). Reduction of colonization by food-borne pathogens in live poultry during production should be a goal to reduce consumer exposure.
A number of factors contribute to the colonization and continued presence of target bacteria within the digestive tracts of animals. These factors have been extensively reviewed by Savage (28). Factors include gastric acidity (11), bile salts (11, 12, 19), peristalsis, digestive enzymes, immune response, and indigenous microorganisms and antibacterial compounds which the bacteria produce. The first four factors depend upon the phenotype of the host and are not easily modified variables. Altering the immune response in the GI tract is also difficult. Indigenous microorganisms and the metabolites they produce are dependent on the colonization of other competing bacteria and the composition of that normal GI tract flora.
One potential approach to control Campylobacter colonization is to manipulate the flora through competitive exclusion. Nurmi and Rantala (26) demonstrated effective control of Salmonella colonization by feeding healthy adult poultry intestinal materials to young chicks whose microflora had not yet been established. Administration of undefined competitive exclusion preparations to day-of-hatch chicks speeds the maturation of gut flora in birds and provides a substitute for the natural process of transmitting microflora from the adult hen to its offspring. Results from administering competitive-exclusion preparations to chickens provide evidence of benefit for Campylobacter species control (22), and reduced levels of C. jejuni in the feces of colonized birds have been documented (29). These researchers reported a significant reduction in broiler colonization by C. jejuni through the application of carbohydrate supplements together with three identified antagonists: Citrobacter diversus 22, Klebsiella pneumoniae 23, and Escherichia coli 25. There is further evidence of a significant decrease in C. jejuni organisms in intestinal samples from colonized broilers after treatment with poultry-isolated cultures of Lactobacillus acidophilus and Streptococcus faecium (21).
Intestinal bacteria produce a variety of compounds which confer antibacterial properties. One group of these compounds, the bacteriocins, consists of bactericidal polypeptides employing a mechanism of action similar to that of ionophore antibiotics (27). Bacteriocins are often active against species which are closely related to the producer organism. The widespread occurrence of bacteriocins among bacterial species isolated from complex microbial communities suggests that bacteriocins may have a regulatory role in terms of population dynamics within bacterial ecosystems. Tagg et al. (36) defined bacteriocins as compounds produced by bacteria having a biologically active protein moiety with bactericidal action. Bacteriocins may be effective against wider or narrower spectra of bacterial groupings.
Lactic acid bacteria are among the most frequently applied probiotic microorganisms. Lactic acid-producing bacteria include Lactobacillus spp. and are used widely throughout the fermented dairy, food, and meat processing industries (4). Most of the bacteriocins produced by this genus are active only against other lactic acid bacteria, but several display antibacterial activity against more phylogenetically divergent gram-positive bacteria and occasionally against gram-negative bacteria. The C-terminal region is an important determinant of target cell specificity (7).
Lactobacilli have been extensively studied for production of antagonistic compounds. These include several well-characterized bacteriocins (2, 6, 9, 13). Klaenhammer (17) classified the bacteriocins of lactic acid bacteria into four major groups. Class I includes lantibiotics, which are small peptides of <5 kDa containing the unusual amino acids lanthionine and β-methyl lanthionine. Nisin is categorized as a class I bacteriocin. Class II includes small non-lanthionine-containing peptides and is a heterogeneous group of small peptides of <10 kDa. Class III includes large heat-labile proteins of >30 kDa. Class IV bacteriocins are complex proteins containing additional moieties, such as lipids and carbohydrates.
This paper describes a Lactobacillus salivarius isolate antagonistic to C. jejuni, the characterization of its associated bacteriocin, and the addition of that bacteriocin into feed, resulting in reduction of C. jejuni colonization in chicken GI tracts.
MATERIALS AND METHODS
Microbiology.
Cecal contents from healthy adult, commercial broiler chickens were streaked directly onto MRS agar and incubated under an anaerobic atmosphere overnight at 37°C. At least 10 Lactobacillus species colonies were selected from each sample. Lactobacilli were identified using the API 50 CHL microtest system (bioMerieux, France). Following colony isolation, tests of >1,200 Lactobacillus species isolates for activity against Campylobacter jejuni were assayed under a microaerobic atmosphere (5% O2, 10% CO2, and 85% N2) as follows. Approximately 0.2 ml, containing ∼107 CFU of each candidate isolate, was suspended in normal saline, distributed onto MRS agar, and incubated at 37°C for 24 h. Agar blocks containing the isolate growth (∼0.5 cm3) were aseptically cut and transferred to brucella agar supplemented with 5% lysed blood and seeded with ∼107 cells of C. jejuni NCTC 11168. Plates were incubated at 42°C for approximately 24 to 48 h under a microaerobic atmosphere. Activity was evaluated by measuring the resulting diameters of growth inhibition in mm.
Bacteriocin purification.
Lactobacillus salivarius NRRL B-30514 was evaluated for bacteriocin production following the procedures of Muriana and Luchansky (23). Broth culture of the strain was grown in 6.5 liters of minimal medium (per liter of broth) containing 6 g K2HPO4, 0.2 g KH2PO4, 0.2 g (NH4)2SO4, 0.1 g MgSO4, 9.0 g glucose, 0.08 g histidine, and 0.02 g arginine in distilled H2O at a pH of 7.2 at 37°C for 18 h under quiescent aerobic conditions. The spent culture was centrifuged at 12,000 × g for 10 min to remove the cells. The supernatant was adjusted to pH 6.2 by adding 1 N NaOH and 130 U/ml catalase to remove organic acids and hydrogen peroxide. The soluble peptides were isolated from the supernatant by a combination of ammonium sulfate precipitation and dialysis to produce a crude antimicrobial preparation used as described below. The crude preparation sample (480 ml) was filtered through 0.22-μm filters (Millipore, Bedford, MA).
The molecular mass of the peptide was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (18). Crude preparations and bacteriocins were separated using a 1.5% agarose gel and 1% SDS (9 by 12 cm) in Tris-glycine buffer. After electrophoresis at 100 mA for 4 h, the gel was fixed with a solution containing 15% ethanol and 1% acetic acid. The gel was then washed with distilled water for 4 h. To determine the molecular masses of the protein fractions, the gel was stained with a solution containing 0.15% Coomassie brilliant blue R-250 (Sigma) in 40% ethanol and 7% acetic acid. The gel was washed sequentially with phosphate-buffered saline (pH 7.2) for 1.5 h and then with deionized water for 3 h. To measure bacteriocin activity, the stained gel comprising the separated peptides was placed onto semisolid brucella agar (0.75% agar) seeded with cells of C. jejuni NCTC 11168 (3). The plate was incubated at 42°C for 48 h under a microaerobic atmosphere. Assessment of activity was based on the observation of zones of inhibited growth in the presence of the protein band.
The crude preparation and purified bacteriocin were also placed on isoelectric focusing gels (pH 3.1 to 10.0) (Novex, San Diego, CA). The gel was run at 100 V for 1 h and then at 200 V for 2 h and 500 V for 30 min in an XCM II Mini-Cell per company instructions (Novex). The gel was washed with distilled water for 30 s without fixation and subsequently stained with Coomassie brilliant blue R-250 (Sigma) to determine the isoelectric point (pI) of the bacteriocin and its ability to inhibit the growth of the C. jejuni test strain, as described above.
The amino acid sequence of the purified bacteriocin was determined by Edman degradation using a 491cLC automatic sequencer (10) (Applied Biosystems). The bacteriocin was hydrolyzed in 6 M HCl under a vacuum at 110°C for 72 h. The molecular mass was determined by mass spectrometry using a Voyager-DERP (Perkin-Elmer). The matrix-assisted laser desorption ionization-time of flight system was used along with a matrix, 2-cyano-hydroxycinnamic acid, per the manufacturer's instructions. Following biochemical determination of the primary amino acid sequence, the predicted physical characteristics were analyzed (25) utilizing Protean of the DNASTAR (Madison, WI) software. The primary amino acid sequence was entered into BLAST (1) to search for proteins with similar sequences.
Chromatography.
The crude preparation containing the bacteriocin was first purified by cation exchange. SP Sepharose Fast Flow was equilibrated with 20 mM sodium phosphate, pH 4.8. Two-hundred fifty milliliters of crude preparation from the L. salivarius NRRL B-30514 fermentation was transferred to a centrifuge tube with 0.5 ml of the equilibrated SP Sepharose. The preparation was mildly agitated, held for 1 h at 20°C, and centrifuged at 12,000 × g for 15 min at 20°C. The supernatant was removed from the pellet containing the bacteriocin. The tube contents were then washed with a solution containing 20 mM Na2HPO4, pH 4.5, and centrifuged at 12,000 × g for 15 min at 20°C. This wash and centrifugation step was repeated. Saline (0.9 M NaCl) was added to fill each tube containing the pellet. The tubes were mildly agitated and subsequently incubated for 1 h at 20°C. The bacteriocin was now dissolved in the supernatant, which was again centrifuged at 12,000 × g for 15 min at 20°C. The supernatant was transferred to clean containers. Activities of the fractions were tested against Campylobacter jejuni NCTC 11168. The concentration of the proteins was measured as described by Lowry et al. (20).
The bacteriocin was further purified by hydrophobic interaction chromatography. Octyl Sepharose was equilibrated with 40 mM K2HPO4 at pH 4.5. The above-described supernatant was mixed at a ratio of 100:1 (vol/vol) with the Octyl Sepaharose by inverting the tubes, shaking them vigorously, and incubating them for 1 h at 20°C. The suspension was centrifuged at 12,000 × g for 15 min at 20°C. The supernatant was removed and the pellet washed in 20 mM K2HPO4 at pH 5.5. The suspension was centrifuged at 12,000 × g for 15 min at 20°C. These wash and centrifugation steps were repeated. After centrifugation, an elution buffer of 25 mM Tris-HCl, 15 mM K2HPO4 was added to the pellet, which was twice washed and centrifuged. Antimicrobial activity (“spot” test described below) and protein concentrations for each fraction were determined. The purified fractions were designated bacteriocin OR-7.
Bioassays.
One milliliter of the sterile crude preparation was diluted with 1 ml of sodium phosphate buffer (pH 7.0). Ten microliters was spot plated onto blood-supplemented brucella agar previously seeded with cells of C. jejuni NCTC 11168 using the methods of Zheng and Slavik (37). Plates were cultivated at 42°C under a microaerobic atmosphere. The activity of the crude preparation was expressed in arbitrary units per ml of the maximal dilution at which a visible zone of inhibition of C. jejuni growth appeared. All assays were conducted in duplicate.
The influence of enzymes, temperature, and pH on bacteriocin OR-7 activity was determined. Ten-microliter portions of each of the following enzymes were transferred into separate tubes containing 20 ml of bacteriocin: beta-chymotrypsin at 100 mg/ml, proteinase K at 200 mg/ml, papain at 60 mg/ml, lysozyme at 75 mg/ml, and lipase at 100 mg/ml (Sigma-Aldrich Corp., St. Louis MO). After 3 h of incubation at 37°C, the mixture of bacteriocin and enzyme was analyzed for antimicrobial activity using the spot test described above. The untreated bacteriocin served as the positive control.
To study thermostability, a solution of 2 mg OR-7 bacteriocin/ml was boiled in a water bath for 15 min, cooled in an ice bath, and assessed for its antimicrobial activity using the spot test described above. Two mg/ml of bacteriocin was used to evaluate the effect of pH. To 2 ml of the sterile bacteriocin solution, drops of 10 mM NaOH or 10 mM HCl were added to test stability at pH values of 3 to 10. Samples were incubated at 37°C for 2 h and 24 h and at 90°C for 20 min. After incubation, samples were adjusted to a pH of 7.2 by addition of 4 mM sterile phosphate buffer and analyzed for antimicrobial activity using the above-described spot test.
Feed emendation, poultry challenge, and assays.
Five hundred milligrams of purified bacteriocin OR-7 was mixed into a 25-ml (0.8 mol K2HPO4/liter) solution containing 1.25 g of completely dissolved polyvinylpyrrolidone (PVP) powder. The material was termed the bacteriocin-PVP solution. The bacteriocin-PVP solution was thoroughly mixed with 100 g of ground maize to produce highly concentrated treated feed. This feed was used to prepare the treated commercial feed with 100 g of highly concentrated treated feed mixed with 1,900 g of commercial feed to produce modified commercial feed having a bacteriocin concentration of 250 mg/kg of feed. Bacteriocin production, microencapsulation, and incorporation into the modified commercial feed was done at the State Research Center for Applied Microbiology facility and shipped to the Poultry Microbiological Safety Research Unit (PMSRU).
Chicken challenge/treatment trials were conducted at PMSRU, ARS, USDA. Campylobacter jejuni isolates from a previous epidemiological study (32), encompassing three distinct geographical locations in the United States, were tested. Approval for the conduct of these experiments was provided by the Institutional Animal Care and Use Committee (PMS-03-03, “Control of Campylobacter in Poultry Production”). The trials were conducted over an ∼3-month period. Ninety healthy, day-of-hatch chicks (24 h old) obtained from local commercial hatcheries were placed in groups of 10 in separate isolation units. These units were equipped with feeders and water and were provided a filtered air supply along with ad libitum access to nonmedicated feed and water. Each group of 24-h-old chicks (positive control birds and treated) were challenged with the individual strains of C. jejuni. The challenge strains were administered individually by oral gavage. Each bird was provided a 0.2-ml suspension containing the specified C. jejuni isolate (∼108 CFU per chick). Within each strain trial, one colonized group of chicks served as the positive control and received free access to diet without bacteriocin. The groups of treated chickens were provided free access to the OR-7-emended commercial chicken feed at days 7 to 9 prior to sacrifice. Nonchallenged, nontreated groups of chickens served as negative controls. Groups of chickens were euthanized by cervical disarticulation 9 days after C. jejuni challenge. Ceca of the individual animals were aseptically dissected. Enumeration of C. jejuni organisms was accomplished by diluting 1 g of cecal content per 9 ml of phosphate-buffered saline (pH 7.2) solution (30). Tenfold serial dilutions were made in 0.1-ml portions, which were then surface plated onto Campy-Cefex (33). The minimum detection limit was 100 CFU per g cecal contents. Plates were incubated at 42°C for 48 h under a microaerobic atmosphere. Resulting characteristic Campylobacter colonies were counted after microscopic examination and latex agglutination assay confirmation. Arithmetic estimates of the numbers of the organism were transformed into a logarithmic format. Standard deviations for each experimental group were calculated and reported. Statistical analysis of Campylobacter numbers among the bacteriocin-treated versus nontreated chickens was made using an unpaired Student t test, with significance defined at the 95% level (P ≤ 0.05).
RESULTS
One lactic acid bacterial isolate created the largest zone of C. jejuni inhibition surrounding the transferred agar plug. This isolate, Lactobacillus salivarius NRRL B-30514, was a gram-positive, facultative-anaerobe, catalase-negative, nonmotile, pleomorphic rod. Isolated colonies were then grown on MRS agar. The isolate produced circular to regular-shaped, smooth, convex colonies with sharp margins that were 1 to 3 mm in diameter after microaerobic incubation for 18 h at 37°C. The strain produced lactic acid and H2O2. The isolate was deposited under the provisions of the Budapest Treaty with the USDA Agricultural Research Service Patent Culture Collection (National Center for Agricultural Utilization Research, 1815 N. University Street, Peoria, Illinois 61604).
Purification of bacteriocin OR-7 derived from fermenting L. salivarius NRRL B-30514 is presented in Table 1. With each succeeding step, the numbers of specific activity units per ml were substantially increased. The bacteriocin lost its antimicrobial activity after being treated with beta-chymotrypsin, proteinase K, and papain but retained activity when treated with lysozyme, lipase, or heat (to 90°C) (Table 2). The bacteriocin was stable at pH values ranging from 3.0 to 9.1 but became inactive at pH 10 (Table 3). Neither 37°C nor 90°C destroyed activity, nor did 24 h at 37°C influence activity.
TABLE 1.
Biochemical purification of bacteriocin OR-7 from the crude antimicrobial preparation of Lactobacillus salivarius NRRL B-30514
| Sample | Vol (ml) | Protein (mg/ml) | Sp act (AU/mg)a | Purity (%) |
|---|---|---|---|---|
| Culture supernatant | 6,500 | 1.2 | 14,000 | 0 |
| Crude (NH4)2SO4 precipitate | 480 | 3.2 | 29,000 | 9.1 |
| SP Sepharose cation exchange | 314 | 2.1 | 235,000 | 62.3 |
| Octyl-Sepharose hydrophobic interaction chromatography | 250 | 1.3 | 540,000 | 89.8 |
Specific activity against Campylobacter jejuni NCTC 11168 in 10 μl of preparation. AU, arbitrary units.
TABLE 2.
Effect of enzyme treatments and high temperature on anti-C. jejuni activity of bacteriocin OR-7
| Treatment | Activitya |
|---|---|
| β-Chymotrypsin | − |
| Proteinase K | − |
| Papain | − |
| Lysozyme | + |
| Lipase | + |
| 90°C, 15 min | + |
Activity against Campylobacter jejuni NCTC 11168 in 10 μl of preparation. +, presence of activity after treatment; −, loss of activity after treatment.
TABLE 3.
Effect of pH on the activity of bacteriocin OR-7
| pH | Activitya
|
||
|---|---|---|---|
| 2 h at 37°C | 24 h at 37°C | 20 min at 90°C | |
| 3.0 | + | + | + |
| 5.0 | + | + | + |
| 6.2 | + | + | + |
| 7.0 | + | + | + |
| 8.4 | + | + | + |
| 9.1 | + | + | + |
| 10.0 | − | − | − |
Activity against Campylobacter jejuni NCTC 11168 in 10 μl of preparation. +, presence of activity after treatment; −, loss of activity after treatment.
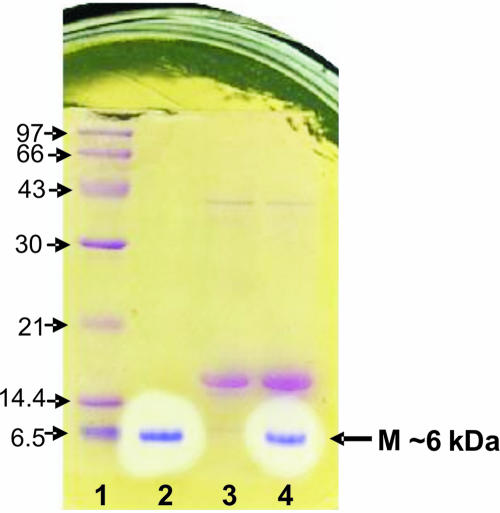
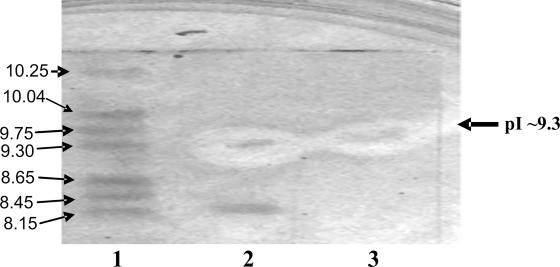
Figure 1 illustrates the direct detection of bacteriocin OR-7 after SDS-PAGE. As observed, the crude OR-7 preparation contained three protein fractions migrating at ∼6 kDa, 16 kDa, and 40 kDa. The 6-kDa fraction was found to be active against C. jejuni NCTC 11168. The band in lane 2 contained the pure bacteriocin, which corresponded to the antimicrobial activity manifesting a zone of growth inhibition and had the indicated mass. Similarly, the bands in lane 4 contained unabsorbed crude preparation from L. salivarius NRRL B-30514, with bactericidal activity detected only at 6 kDa. The band in lane 3 contained the crude preparation from L. salivarius NRRL B-30514 after absorption against C. jejuni NCTC 11168. None of those bands showed antimicrobial activity. Figure 2 demonstrates the isoelectric focusing of a crude preparation from L. salivarius NRRL B-30514. The active fraction observed at a pI value of 9.3 had growth antagonism against C. jejuni NCTC 11168.
FIG. 1.
Direct detection of bacteriocin OR-7 from the crude antimicrobial preparation of Lactobacillus salivarius NRRL B-30514 culture after SDS-PAGE. Following SDS-PAGE, the gel was laid onto a plate seeded with Campylobacter jejuni to determine which band(s) corresponded to the antimicrobial activity and its specific molecular mass. Lane 1 contains molecular mass markers (in kilodaltons). The bands and zones of inhibition in lanes 2 and 4 are pure bacteriocin and a crude preparation demonstrating antimicrobial activity. Lane 3 is the crude antimicrobial preparation after adsorption with C. jejuni. The zone of growth inhibition (arrow) had a mass (M) of ∼6 kDa.
FIG. 2.
Direct detection of OR-7 from the crude antimicrobial preparation of Lactobacillus salivarius NRRL B-30514 culture after isoelectric focusing. The focusing gel was laid over a plate seeded with Campylobacter jejuni to demonstrate antimicrobial activity at the specified isoelectric point. Lane 1 contains pI standards (pI values for Serva marker proteins were 10.25, 10.04, 9.75, 9.30, 8.65, 8.45, and 8.15). The zones of clearance surrounding the highlighted band (arrow) in lanes 2 and 3 are crude antimicrobial preparations and pure OR-7 bacteriocin demonstrating antimicrobial activity relating to the peptide migrating at pI ∼9.3.
The purified OR-7 bacteriocin contained a peptide comprised of 54 amino acid residues. The molecular mass of purified bacteriocin OR-7 was confirmed by matrix-assisted laser desorption ionization-time of flight mass spectrometry and averaged 5,123 Da. The result for amino acid sequencing of the bacteriocin was as follows: N terminus-KTYYGTNGVHCTKNSLWGKVRLKNMKYDQNTTYMGRLQDILLGWATGAFGKTFH-C terminus. The peptide was composed of 28.4% charged (RKHYCD), 3.3% acidic (DE), 13.3% basic (KR), 31.6% polar (NCQSTY), and 23.4% hydrophobic (AILFWV) amino acids with no glutamine (E) or proline (P). Computer analyses of the OR-7 amino acid sequence resulted in a peptide with a predicted molecular weight of 6,214 consisting primarily of amphipathic beta-turn regions in the interior portion of the peptide with a predicted isoelectric point of 9.5. This structure was as predicted for other membrane-disrupting cationic class IIa antibacterial peptides (8).
In total, eight experimental bird trials were conducted, with testing of four unique isolates of C. jejuni (Table 4). In each of the trials, the bacteriocin treatment significantly reduced the numbers of C. jejuni organisms compared to those found in the untreated control groups of birds (P ≤ 0.05), which were colonized at levels of 106.2 to 10>8.3 CFU per g of cecal material. Relatively younger birds were used in this phase of our study to conserve the amount of the bacteriocin needed and to verify the unproven efficacy of this treatment approach. Nonchallenged, nontreated birds were employed as a negative control for the trials, and none of these birds yielded detectable levels of Campylobacter.
TABLE 4.
Mean log10 number of Campylobacter jejuni organisms per g of cecal contents in 10-day-old chickensa
| Trial no. | C. jejuni challenge strain | No. of chicks tested | Day of challenge | Log10 challenge | Treatment days | Day of sampling | Mean log CFU/gb |
|---|---|---|---|---|---|---|---|
| 1 | AL-22 | 10 | 1 | ∼8 | 0 | 10 | 7.2 ± 0.3 |
| 1 | AL-22 | 10 | 1 | ∼8 | 7-9 | 10 | ND |
| 2 | BH-6 | 10 | 1 | ∼8 | 0 | 10 | 7.1 ± 0.4 |
| 2 | BH-6 | 10 | 1 | ∼8 | 7-9 | 10 | 0.7 ± 1.2 |
| 3 | BL-1 | 10 | 1 | ∼8 | 0 | 10 | 7.8 ± 0.2 |
| 3 | BL-1 | 10 | 1 | ∼8 | 7-9 | 10 | 1.3 ± 1.8 |
| 4 | CL-11 | 10 | 1 | ∼8 | 0 | 10 | 6.6 ± 0.7 |
| 4 | CL-11 | 10 | 1 | ∼8 | 7-9 | 10 | ND |
| 1-4 | None (controls) | 10 | None | ND | |||
| 5 | AL-22 | 10 | 1 | ∼8 | 0 | 10 | 7.9 ± 0.7 |
| 5 | AL-22 | 10 | 1 | ∼8 | 7-9 | 10 | ND |
| 6 | BH-6 | 10 | 1 | ∼8 | 0 | 10 | 8.3 ± 0.3 |
| 6 | BH-6 | 10 | 1 | ∼8 | 7-9 | 10 | ND |
| 7 | BL-1 | 10 | 1 | ∼8 | 0 | 10 | 7.6 ± 0.3 |
| 7 | BL-1 | 10 | 1 | ∼8 | 7-9 | 10 | 0.4 ± 1.3 |
| 8 | CL-11 | 10 | 1 | ∼8 | 0 | 10 | 7.4 ± 0.6 |
| 8 | CL-11 | 10 | 1 | ∼8 | 7-9 | 10 | 1.2 ± 2.4 |
| 5-8 | None (controls) | 10 | None | ND |
Isolated individual groups of 10 chickens per trial served as colonized, positive controls (10 birds per challenge strain trial, eight trials), and two groups of noninoculated birds served as negative controls (one each for trials 1 to 4 and 5 to 8). All challenged chicks were provided 108 CFU C. jejuni per chick on the day of hatch. Treated birds were provided 250 mg bacteriocin OR-7/kg feed on days 7 to 9.
ND, not detected.
DISCUSSION
The relationship of poultry contamination and human infection is well recognized and documented. During broiler production and processing, fecal materials containing pathogens may be transferred onto meat, can cross-contaminate other foods, and may persist in food-processing kitchens (24). Thus, our goal was to intervene upstream in the colonization of C. jejuni during the production of broiler chickens.
After identifying in vitro cultural inhibition of C. jejuni, we wanted to learn more about the mechanism involved. Metabolites secreted by competing organisms may contribute to the control of C. jejuni. We realized this may have been due to a variety of compounds (fatty acids and peroxides, etc.) produced by the antagonistic isolate, causing antibacterial activity (11). Among these antibacterial compounds, bacteriocins consist of bactericidal polypeptides with a mechanism of action similar to that of ionophore antibiotics (36). As described in the current study, the occurrence of bacteriocin production among Lactobacillus spp. in competitive, complex microbial communities suggests that bacteriocins may have a regulatory role for population dynamics within bacterial ecosystems. Our bacterium-derived compound met the criteria for being considered a bacteriocin (36) and was sensitive to proteases, insensitive to 90°C for 10 min, and stable to acidic pH (>3.1) but unstable to alkaline conditions (pH, >10). The molecular weight of 5,123 and the provided amino acid sequence are unique. Results reported in this work point to the unique nature of selected bacteriocins. In a previous report (35), the bacteriocin produced by Paenibacillus polymyxa NRRL-B-30509 expressed a molecular mass (3,864 Da versus 5,123 Da), an isoelectric point (pI = 7.2 versus 9.3), and an amino acid sequence distinguishable from those of OR-7.
A BLAST search of the NCBI database resulted in a match with a previously reported antibacterial peptide, acidocin A, produced by L. acidophilus and active against Listeria monocytogenes (16). The OR-7 bacteriocin reported herein had a 65% similarity of exact identity with the acidocin A posttranslationally active peptide with another five conserved similar amino acid substitutions. Both peptides contained a “pediocin box”-like motif, YGNGVXCXnV, in the N-terminal region of the peptide typical of class IIa bacteriocins (5, 7, 27), except that a T was present as YGTNGV in the sequence. The C-terminal domains of class IIa bacteriocins are hypothesized to play roles in specificity for “listerial” activity (5, 14, 27). Acidocin A was determined to have activity against L. monocytogenes and other closely related gram-positive bacteria but not against gram-negative bacteria (16). Interestingly, OR-7 had antibacterial activity against the gram-negative C. jejuni, even though the C-terminal portion of acidocin A was highly conserved relative to that of OR-7.
Metabolites from competing organisms may dramatically contribute to the control of pathogens in poultry production. The novel antagonistic strain isolated from ceca of broilers produced a specific bacteriocin that substantially reduced the chicken colonization by four isolates of C. jejuni. It is likely that this bacteriocin also may inhibit the growth of other susceptible organisms and would alter the intestinal flora among treated broilers. Limited consequences for the intestinal bacteria would be anticipated if the treatment were provided immediately before chicken processing. Bacteriocins are produced in situ, killing proximal susceptible bacteria, and may exert the significant regulatory mechanism by which gut flora claims primacy among the community within the intestines of warm-blooded hosts. In the current study, we provided 250 mg/kg feed of the described bacteriocin to control C. jejuni. We anticipate that bacteriocin which did not adsorb to target bacteria was digested by the innate intestinal protease enzymes. We anticipate larger trials to determine the commercial feasibility for such an approach, serving to reduce pathogens in the chicken, thereby reducing the subsequent human exposure and disease.
Schoeni and Wong (29) controlled broiler colonization by C. jejuni by administering carbohydrate supplements with three identified antagonists: Citrobacter diversus 22, Klebsiella pneumoniae 23, and Escherichia coli 25. Morishita et al. (21) reduced numbers of C. jejuni organisms among colonized chickens after treatment with cultures of Lactobacillus acidophilus and Streptococcus faecium isolated from birds. However, such approaches are not yet employed by the poultry industry.
In each of our reported chicken challenge/treatment trials, application of bacteriocin resulted in significant (P ≤ 0.05) reductions in Campylobacter colonization. These results are very similar to those we had already noted with another bacteriocin from Paenibacillus polymyxa NRRL-B-30509 (34). Although the bacteriocin treatment was not completely effective in each of the trials that have been conducted, the target reduction was always statistically significant. Among the control groups, as is often the case with commercial chickens, very high numbers (107 CFU/gm) of C. jejuni organisms were observed during poultry production. We have also gathered unpublished data to verify that birds of commercial processing age (42 days old) also manifest dramatic reductions in Campylobacter numbers when treated with bacteriocin.
Acknowledgments
This work was supported by the U.S. Department of State, the Russian Federation State Research Center for Applied Microbiology (SRCAM), the U.S. Department of Agriculture Agricultural Research Service (USDA-ARS) CRIS Interventions and Methodologies to Reduce Human Food-Borne Bacterial Pathogens in Chickens project no. 6612-32000-046-00, and the International Science and Technology Center (ISTC) project no. 1720.
Approval for the conduct of these experiments was provided by the Institutional Animal Care and Use Committee (PMS-03-03, “Control of Campylobacter in Poultry Production”). Appreciation is extended to Susan Brooks and Latoya Wiggins for technical assistance.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barefoot, S. F., and T. R. Klaenhammer. 1983. Detection and activity of lactacin B, a bacteriocin produced by Lactobacillus acidophilus. Appl. Environ. Microbiol. 45:1808-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhunia, A. K., M. C. Johnson, and B. Ray. 1987. Direct detection of an antimicrobial peptide of Pediococcus acidilactici in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J. Ind. Microbiol. 2:319-322. [Google Scholar]
- 4.Cleveland, J., T. J. Montville, and M. L. Chikindas. 2001. Bacteriocins: safe food preservatives of the future. Int. J. Food Microbiol. 71:1-20. [DOI] [PubMed] [Google Scholar]
- 5.Cotter, P. D., C. Hill, and R. P. Ross. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777-788. [DOI] [PubMed] [Google Scholar]
- 6.de Klerk, H. C., and J. A. Smit. 1967. Properties of a Lactobacillus fermenti bacteriocin. J. Gen. Microbiol. 48:309-316. [DOI] [PubMed] [Google Scholar]
- 7.Eijsink, V. G., M. Skeie, P. H. Middelhoven, M. B. Brurberg, and I. F. Nes. 1998. Comparative studies of class IIa bacteriocins of lactic acid bacteria. Appl. Environ. Microbiol. 64:3275-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fimland, G., O. Blingsmo, K. Sletten, G. Jung, I. F. Nes, and J. Nissen-Meyer. 1996. New biologically active hybrid bacteriocins constructed by combining regions from various pediocin-like bacteriocins: the C-terminal region is important for determining specificity. Appl. Environ. Microbiol. 62:3313-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fimland, G., L. Johnsen, B. Dalhus, and J. Nissen-Meyer. 2005. Pediocin-like antimicrobial peptides (class IIa bacteriocins) and their immunity proteins: biosynthesis, structure, and mode of action. J. Pept. Sci. 11:688-696. [DOI] [PubMed] [Google Scholar]
- 10.Garneau, S., N. Martin, and J. C. Vederas. 2002. Two-peptide bacteriocins produced by lactic acid bacteria. Biochimie 84:577-592. [DOI] [PubMed] [Google Scholar]
- 11.Gilliland, S. E., and M. L. Speck. 1977. Antagonistic action of Lactobacillus acidophilus toward intestinal and foodborne pathogens in associative cultures. J. Food Prot. 40:820-823. [DOI] [PubMed] [Google Scholar]
- 12.Hugdahl, M. B., J. T. Beery, and M. P. Doyle. 1988. Chemotactic behavior of Campylobacter jejuni. Infect. Immun. 56:1560-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joerger, M. C., and T. R. Klaenhammer. 1986. Characterization and purification of helveticin J and evidence for a chromosomally determined bacteriocin produced by Lactobacillus helveticus 481. J. Bacteriol. 167:439-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnsen, L., G. Fimland, and J. Nissen-Meyer. 2005. The C-terminal domain of pediocin-like antimicrobial peptides (class IIa bacteriocins) is involved in specific recognition of the C-terminal part of cognate immunity proteins and in determining the antimicrobial spectrum. J. Biol. Chem. 280:9243-9250. [DOI] [PubMed] [Google Scholar]
- 15.Jones, F. T., R. C. Axtell, D. V. Rives, S. E. Scheideler, F. R. Tarver, R. L. Walker, and M. J. Wineland. 1991. A survey of Salmonella contamination in modern broiler production. J. Food Prot. 54:502-507. [DOI] [PubMed] [Google Scholar]
- 16.Kanatani, K., M. Oshimura, and K. Sano. 1995. Isolation and characterization of acidocin A and cloning of the bacteriocin gene from Lactobacillus acidophilus. Appl. Environ. Microbiol. 61:1061-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klaenhammer, T. R. 1993. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 12:39-85. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 19.Lewis, R., and S. Gorbach. 1972. Modification of bile acids by intestinal bacteria. Arch. Intern. Med. 130:545-549. [PubMed] [Google Scholar]
- 20.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 21.Morishita, T. Y., P. P. Aye, B. S. Harr, C. W. Cobb, and J. R. Clifford. 1997. Evaluation of an avian-specific probiotic to reduce the colonization and shedding of Campylobacter jejuni in broilers. Avian Dis. 41:850-855. [PubMed] [Google Scholar]
- 22.Mulder, R., and N. Bolder. 1991. Reduction of Campylobacter infection of broilers by competitive exclusion treatment of day-old broiler chicks—a field study, p. 359-363. In L. C. Blankenship (ed.), Colonization control of human bacterial enteropathogens in poultry. Academic Press, San Diego, Calif.
- 23.Muriana, P. M., and J. B. Luchansky. 1993. Biochemical methods for purification of bacteriocins, p. 41-61. In D. G. Hoover and L. R. Steenson (ed.), Bacteriocins of lactic acid bacteria. Academic Press, New York, N.Y.
- 24.Neimann, J., J. Engberg, K. Moelbak, and H. C. Wegener. 1998. Foodborne risk factors associated with sporadic campylobacteriosis in Denmark. Dan. Veterinaertidsskn. 81:702-705. [Google Scholar]
- 25.Nishikawa, K., H. Nakashima, M. Kanehisa, and T. Ooi. 1987. Detection of weak sequence homology of proteins for tertiary structure prediction. Protein Seq. Data Anal. 1:107-116. [PubMed] [Google Scholar]
- 26.Nurmi, E., and M. Rantala. 1973. New aspects of Salmonella infection in broiler production. Nature 241:210-211. [DOI] [PubMed] [Google Scholar]
- 27.Papagianni, M. 2003. Ribosomally synthesized peptides and antimicrobial properties: biosynthesis, structure, function, and applications. Biotechnol. Adv. 21:465-499. [DOI] [PubMed] [Google Scholar]
- 28.Savage, D. C. 1983. Mechanisms by which indigenous microorganisms colonize gastrointestinal epithelial surfaces. Prog. Food Nutr. Sci. 7:65-74. [PubMed] [Google Scholar]
- 29.Schoeni, J. L., and A. C. Wong. 1994. Inhibition of Campylobacter jejuni colonization in chicks by defined competitive exclusion bacteria. Appl. Environ. Microbiol. 60:1191-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stern, N. J. 1994. Mucosal competitive exclusion to diminish colonization of chickens by Campylobacter jejuni. Poult. Sci. 73:402-407. [DOI] [PubMed] [Google Scholar]
- 31.Stern, N. J., J. S. Bailey, L. C. Blankenship, N. A. Cox, and F. McHan. 1988. Colonization characteristics of Campylobacter jejuni in chick ceca. Avian Dis. 32:330-334. [PubMed] [Google Scholar]
- 32.Stern, N. J., P. Fedorka-Cray, J. S. Bailey, N. A. Cox, S. E. Craven, K. L. Hiett, M. T. Musgrove, S. Ladely, D. Cosby, and G. C. Mead. 2001. Distribution of Campylobacter spp. in selected United States poultry production and processing operations. J. Food Prot. 64:1705-1710. [DOI] [PubMed] [Google Scholar]
- 33.Stern, N. J., B. Wojton, and K. Kwiatek. 1992. A differential-selective medium and dry ice-generated atmosphere for recovery of Campylobacter jejuni. J. Food Prot. 55:514-517. [DOI] [PubMed] [Google Scholar]
- 34.Stern, N. J., E. A. Svetoch, B. V. Eruslanov, Y. N. Kovalev, L. I. Volodina, V. V Perelygin, E. V. Mitsevich, I. P. Mitsevich, and V. P. Levchuk. 2005. Paenibacillus polymyxa purified bacteriocin to control Campylobacter jejuni in chickens. J. Food Prot. 68:1450-1453. [DOI] [PubMed] [Google Scholar]
- 35.Svetoch, E. A., N. J. Stern, B. V. Eruslanov, Y. N. Kovalev, L. I. Volodina, V. V. Perelygin, E. V. Mitsevich, I. P. Mitsevich, V. D. Pokhilenko, V. N. Borzenkov, V. P. Levchuk, O. E. Svetoch, and T. Y. Kudriavtseva. 2005. Isolation of Bacillus circulans and Paenibacillus polymyxa strains inhibitory to Campylobacter jejuni and characterization of associated bacteriocins. J. Food Prot. 68:11-17. [DOI] [PubMed] [Google Scholar]
- 36.Tagg, J. R., A. S. Dajani, and L. W. Wannamaker. 1976. Bacteriocins of gram-positive bacteria. Bacteriol Rev. 40:722-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng, G., and M. F. Slavik. 1999. Isolation, partial purification and characterization of a bacteriocin produced by a newly isolated Bacillus subtilis strain. Lett. Appl. Microbiol. 28:363-368. [DOI] [PubMed] [Google Scholar]