Abstract
To simplify the titration of infectious varicella-zoster virus (VZV), we generated a reporter cell line that produced luciferase in a dose-dependent manner upon infection with cell-free VZV. A few VZV-infected cells were detectable by coculturing with the cell line. We demonstrated the usefulness of the cell line for antiviral studies.
Varicella-zoster virus (VZV) causes varicella on primary infection and zoster upon reactivation. Both conditions may be associated with severe complications (1). For the treatment of VZV-associated diseases, acyclovir (ACV), valaciclovir, and famciclovir have been used widely (1, 25). The number of approved drugs for treating VZV diseases is limited, and all of them have the same mode of action. The development of resistant strains and the ineffectiveness of available antivirals at preventing complications have necessitated a search for alternative compounds (7, 8, 25, 33). Several new compounds have been under investigation (13, 20, 21, 31, 32). Plaque reduction assays have been routinely used to screen and evaluate candidate compounds, but this method is made laborious and time consuming due to the slow growth of VZV in tissue culture. Some methods aimed at reducing the inconvenience have been developed (26, 27, 34), as has a biochemical assay for characterization of ACV-resistant strains (29). However, the unavailability of a rapid, high-throughput assay for titrating VZV has hampered efforts both to screen new compounds and to detect drug-resistant strains in a timely fashion.
Previously, we established a reporter cell line for human herpesvirus 8 and demonstrated its practical uses (10, 11, 14). We report here the generation of a similar cell line for VZV by use of the following approach: (i) selection of the most suitable promoter from various promoters, (ii) identification of the minimum sequence length for efficient activation of the selected promoter to avoid nonspecific activation, (iii) establishment of a cell line that contains the reporter gene under the control of the minimum promoter, and (iv) characterization of the clone that performed best.
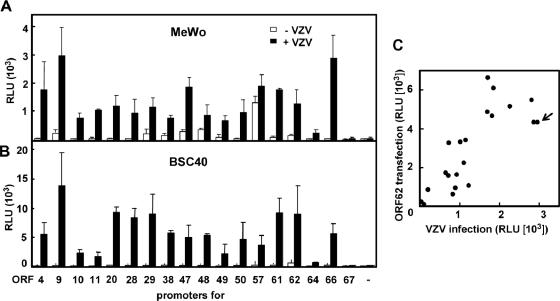
Characterization of individual VZV promoters and global VZV gene expression have been reported previously (4, 5, 12). On the basis of those studies, various DNA fragments containing known or putative VZV promoters (Table 1) were amplified from the nucleocapsid DNA of VZV pOka strain by PCR, cloned into a luciferase vector pGL3-Basic, and then analyzed for their responses to VZV infection in transient transfection experiments using BSC40 cells (3) and MeWo cells (9). Among the promoters examined, the ORF9 promoter was most strongly activated in both cell lines (Fig. 1). Activation of each promoter was also analyzed by cotransfection with a plasmid expressing VZV IE62. The correlation between infection- and IE62-mediated activation (Fig. 1C) was good, indicating that infection-mediated activation was VZV specific.
TABLE 1.
Comparison of VZV promoter activities
| ORF | Gene function(s)a | Promoter region analyzed
|
|
|---|---|---|---|
| Positionb | Length (bp) | ||
| 4 | Transactivator, tegument | 4,750-4,147 | 604 |
| 9 | Tegument, virion component | 9,886-10,993 | 1,108 |
| 10 | Tegument, transactivator | 11,140-12,137 | 998 |
| 11 | Tegument | 12,574-13,574 | 1,001 |
| 20 | Capsid | 31,520-30,490 | 1,031 |
| 28 | DNA polymerase | 50,647-51,646 | 1,000 |
| 29 | Single-stranded DNA binding protein | 49,848-50,847 | 1,000 |
| 38 | Virion protein | 71,309-70,309 | 1,001 |
| 47 | Protein kinase, tegument | 82,167-83,168 | 1,002 |
| 48 | DNase | 83,592-84,651 | 1,060 |
| 49 | Tegument | 85,216-86,215 | 1,000 |
| 50 | Glycoprotein | 88,927-87,898 | 1,030 |
| 57 | Cytoplasmic protein | 100,756-99,635 | 1,122 |
| 61 | Transcription regulator | 105,496-104,503 | 994 |
| 62 (71) | Transactivator, tegument | 110,149-109,149 | 1,001 |
| 64 (69) | Tegument | 110,550-111,549 | 1,000 |
| 66 | Protein kinase | 112,036-113,024 | 989 |
| 67 | Glycoprotein | 114,051-114,498 | 448 |
Based on known VZV function or function of HSV-1 homolog.
The luciferase gene locates the downstream of the numbers indicated at the right side. The base position numbers are based on Dumas strain (6).
FIG. 1.
Comparison of VZV promoter activities. MeWo (7 × 104 cells/well) (A) and BSC40 (2.4 × 104 cells/well) cells (B) in 96-well plates were transfected with 125 ng of a reporter plasmid containing the promoter for the indicated open reading frame (ORF) along with 4 ng of a Renilla luciferase-expressing plasmid (pRL-CMV; Promega) as an internal control, by using FuGENE 6 (Roche). pGL3-Basic, a vector plasmid used to clone the promoters, was used as a negative control (−). At 24 h after transfection, the cells were infected with VZV at an multiplicity of infection of ∼0.02, and 1 day later, both firefly and Renilla luciferase activities were determined by a chemiluminescent assay reaction (Dual-Glo luciferase assay system; Promega) followed by measurement of relative light units (RLU) with a luminometer (JNR AB2300; ATTO, Japan). Means and standard deviations (SDs) of RLU from triplicate wells are shown. (C) Correlation of IE62- and infection-dependent promoter activation. MeWo cells were transfected with each reporter plasmid along with an IE62-expressing plasmid, and 2 days later their luciferase activities were measured and compared with those represented in panel A. Each circle represents one promoter-reporter construct. An arrow indicates ORF9.
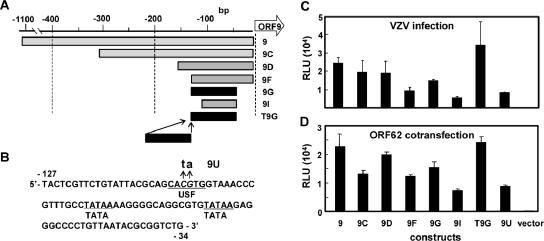
The minimal essential region for the ORF9 promoter was mapped to a 93-bp-long sequence by progressively truncating the promoter (Fig. 2). The patterns of relative promoter activity among the constructs were similar in infected and IE62-transfected cells. A 2-bp substitution introduced into the upstream stimulating factor (USF) motif reduced the promoter activity, supporting the importance of the USF-binding site reported for some VZV promoters (16, 17, 19). Since the presence of intact USF sites did not always correlate with promoter activity, further study will be needed to identify the sequence or context that makes the ORF9 promoter more responsive than the others.
FIG. 2.
Minimum ORF9 promoter region for responses to VZV infection and to IE62. (A) Reporter plasmids containing truncated ORF9 promoter sequences, 9C, 9D, 9F, 9G, and 9I were constructed. A duplicated form of the minimum essential 93-bp sequence (9G) was cloned into BamHI site of pGL3-Basic, resulting in pGL-T9G. (B) The sequence of the minimum essential region of the ORF9 promoter (9G) and the USF binding motif in the region are shown. A 2-bp alteration (CACGTG to CAtaTG) of the USF binding motif (9U) was introduced into the sequence. (C and D) Activities of the modified promoters represented in panels A and B were analyzed in transient transfection experiments. Means and SDs of luciferase activities from triplicate wells are shown. pGL3-Basic (vector) used for cloning of the promoters was used as a negative control.
Duplication of the minimal promoter region between 34 and 127 bp upstream of ORF9 (T9G) increased the promoter activity twofold. As such, our VZV reporter cell line was generated by transfecting MeWo cells with pGL-T9G, together with a plasmid encoding the G418-resistant gene. The cell clone MV9G was selected from 86 G418-resistant clones based on two criteria: high luciferase activities on VZV infection and low background activity in the absence of the infection.
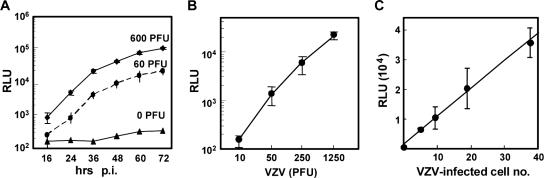
The luciferase activity in MV9G increased gradually after infection, and the activity increased in a dose-dependent manner with a detection limit of 50 PFU (Fig. 3). Coculturing of VZV-infected cells with MV9G cells induced luciferase activities with a detection limit of fewer than 10 infected cells (Fig. 3C). No luciferase activity was detected in MV9G cells cocultured with cells infected with human cytomegalovirus (Towne), human herpesvirus 6 (Z29) (15), or human herpesvirus 7 (SB) (2) or in MV9G cells directly infected with cell-free human cytomegalovirus. In contrast, MV9G cells infected with herpes simplex virus type 1 (HSV-1) resulted in luciferase activities similar to those seen with VZV-infected cells at 24 h after infection. However, at 48 h after infection, the infected cells showed strong cytopathic effect and their luciferase activities decreased, making it practical to distinguish HSV-1 infection from VZV infection.
FIG. 3.
Characterization of VZV reporter cell line MV9G. (A) Time-dependent activation of the reporter cell line after VZV infection. MV9G cells in 96-well plates were infected with 60 and 600 PFU of VZV and incubated for 2 h. After removal of the inoculums, cells were cultured for the indicated period (in hours), rinsed with phosphate-buffered saline, and stored at −80°C until luciferase activity was measured. Means and SDs of luciferase activities from triplicate wells are shown. (B) MV9G cells were infected with the indicated PFU of VZV and incubated for 2 h. After removal of the inoculums, the cells were cultured for an additional 46 h. Means and SDs of luciferase activities from triplicate wells are shown. (C) MV9G cells were infected with cell-associated virus, and their luciferase activities were measured 2 days later. MeWo cells were infected with the same cell-associated virus, and the numbers of the infected cells used for the inoculation were obtained by immunostaining using anti-VZV IE62 monoclonal antibody (MAb8616; Chemicon International). The luciferase activities were compared with the numbers of the infected cells.
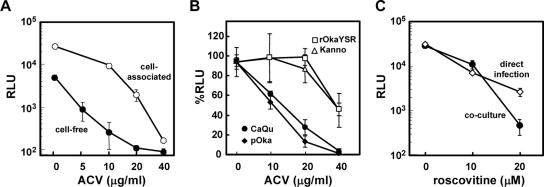
For proof of concept, we evaluated the usefulness of MV9G cells for antiviral studies. MeWo cells were infected with cell-associated VZV or with cell-free VZV, cultured in the presence of ACV, and then cocultured with MV9G cells (Fig. 4A). The 50% effective concentration (EC50) of ACV against infection with cell-free virus was <5 μg/ml, which is consistent with the reported EC50 (∼2 μg/ml) (13, 18, 28). The EC50 against infection with cell-associated virus was 10 to 20 μg/ml. Note that the EC50 of ACV is known to be higher when infections are induced using cell-associated virus (24). ACV treatment decreased luciferase activity only slightly in MV9G cells infected directly with cell-free virus; this was not unexpected, since the reporter gene expression in MV9G depends on a promoter that is active during the early phase of infection.
FIG. 4.
Applications of the reporter cell assay. (A) MeWo cells (7 × 104 cells/well) in 96-well plates were inoculated with cell-associated VZV (∼1 × 103 MeWo cells infected with VZV; cytopathic effect > 70%) (○, “cell-associated”) or with 250 PFU of cell-free VZV (•, “cell-free”) and cultured in the presence or absence of the indicated concentrations of ACV. Two days later, MV9G cells (7 × 104 cells/well) were added to each well and cocultured in the presence of ACV for 1 day. The cells were harvested, and their luciferase activities were measured. Means and SDs of luciferase activities from triplicate wells are shown. (B) MeWo cells (7 × 104 cells/well) in 96-well plates were inoculated with approximately 200 of MeWo cells infected with TK-positive (pOka [•] and CaQu [⧫]) or -negative (rOkaYSR [○] and Kanno [Δ]) strains (22, 23, 28), and cultured in the presence of ACV for 2 days. Then, MV9G cells were overlaid and cocultured for 1 day. The luciferase activities in the ACV-treated cultures were expressed as percent RLU, with the activities obtained in the culture without inhibitor treatment used as a standard. Means and SDs of luciferase activities from triplicate wells are shown. (C) MeWo cells (7 × 104 cells/well) were infected with cell-associated VZV, cultured for 2 days, and then cocultured with MV9G for 1 day (•, “coculture”). MV9G cells (7 × 104 cells/well) were directly infected with 400 PFU of cell-free VZV, and cultured for 2 days (○, “direct infection”). Roscovitine was added at the indicated concentrations throughout the processes. Means and SDs of luciferase activities from triplicate wells are shown.
We also examined the ability to detect ACV-resistant strains by coculturing MV9G cells with a small number of the cells that were infected and treated with ACV (Fig. 4B). The average EC50 of ACV for thymidine kinase (TK)-deficient strains was ∼40 μg/ml, which was much higher than that observed for TK-positive strains. The use of a reporter cell assay thus eliminates the need for laborious preparation of cell-free VZV stocks for the detection of ACV-resistant strains, providing a strong practical advantage.
Roscovitine, a kinase inhibitor, decreased luciferase activities, both in directly infected MV9G cells and in MV9G cells cocultured with MeWo cells that were infected in the presence of roscovitine (Fig. 4C). This suggests that at least part of its inhibitory effect occurs in the early phase of infection, which is consistent with previous reports (30). Cellular factors required for virus infection are attractive targets for the development of novel antiviral drugs. High-throughput screening of compounds in a cell-based assay is essential for that purpose, and MV9G appears to be ideally suited for that role.
In conclusion, the VZV reporter cell assays developed here are expected to foster the efficient evaluation of new antiviral compounds for the treatment of VZV-associated diseases.
Acknowledgments
We thank Jennifer Lynch for pGL-ORF47 and -ORF66 plasmids, Bill Ruyechan for pGL-ORF67 plasmid, and Koich Yamanishi for pOka.
This work was supported by a Grant-on-Aid for Science from the Ministry of Education, Science, Technology and Sports, Japan (N.I.), and by the Research on Health Sciences focusing on Drug Innovation program of Japanese Human Science Foundation (T.S., D.S.S., N.I.). G.-Q.W. was a fellow supported by the Japan Society for the Promotion of Science.
REFERENCES
- 1.Arvin, A. M. 2002. Antiviral therapy for varicella and herpes zoster. Semin. Pediatr. Infect. Dis. 13:12-21. [DOI] [PubMed] [Google Scholar]
- 2.Black, J. B., N. Inoue, K. Kite-Powell, S. Zaki, and P. E. Pellett. 1993. Frequent isolation of human herpesvirus 7 from saliva. Virus Res. 29:91-98. [DOI] [PubMed] [Google Scholar]
- 3.Brockman, W. W., and D. Nathans. 1974. The isolation of simian virus 40 variants with specifically altered genomes. Proc. Natl. Acad. Sci. USA 71:942-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen, J. I., and S. E. Straus. 2001. Varicella-zoster virus and its replication, p. 2707-2730. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 5.Cohrs, R. J., M. P. Hurley, and D. H. Gilden. 2003. Array analysis of viral gene transcription during lytic infection of cells in tissue culture with varicella-zoster virus. J. Virol. 77:11718-11732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davison, A. J., and J. E. Scott. 1986. The complete DNA sequence of varicella-zoster virus. J. Gen. Virol. 67:1759-1816. [DOI] [PubMed] [Google Scholar]
- 7.Field, A. K., and K. K. Biron. 1994. “The end of innocence” revisited: resistance of herpesviruses to antiviral drugs. Clin. Microbiol. Rev. 7:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbert, C., J. Bestman-Smith, and G. Boivin. 2002. Resistance of herpesviruses to antiviral drugs: clinical impacts and molecular mechanisms. Drug Resist. Updates 5:88-114. [DOI] [PubMed] [Google Scholar]
- 9.Grose, C., and P. A. Brunel. 1978. Varicella-zoster virus: isolation and propagation in human melanoma cells at 36 and 32 degrees C. Infect. Immun. 19:199-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue, N., T. Spira, L. Lam, J. L. Corchero, and W. Luo. 2004. Comparison of serologic responses between Kaposi's sarcoma-positive and -negative men who were seropositive for both human herpesvirus 8 and human immunodeficiency virus. J. Med. Virol. 74:202-206. [DOI] [PubMed] [Google Scholar]
- 11.Inoue, N., J. Winter, R. B. Lal, M. K. Offermann, and S. Koyano. 2003. Characterization of entry mechanisms of human herpesvirus 8 by using an Rta-dependent reporter cell line. J. Virol. 77:8147-8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy, P. G., E. Grinfeld, M. Craigon, K. Vierlinger, D. Roy, T. Forster, and P. Ghazal. 2005. Transcriptomal analysis of varicella-zoster virus infection using long oligonucleotide-based microarrays. J. Gen. Virol. 86:2673-2684. [DOI] [PubMed] [Google Scholar]
- 13.Kern, E. R., N. L. Kushner, C. B. Hartline, S. L. Williams-Aziz, E. A. Harden, S. Zhou, J. Zemlicka, and M. N. Prichard. 2005. In vitro activity and mechanism of action of methylenecyclopropane analogs of nucleosides against herpesvirus replication. Antimicrob. Agents Chemother. 49:1039-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krug, L. T., V. P. Pozharskaya, Y. Yu, N. Inoue, and M. K. Offermann. 2004. Inhibition of infection and replication of human herpesvirus 8 in microvascular endothelial cells by alpha interferon and phosphonoformic acid. J. Virol. 78:8359-8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez, C., P. Pellett, J. Stewart, C. Goldsmith, K. Sanderlin, J. Black, D. Warfield, and P. Feorino. 1988. Characteristics of human herpesvirus-6. J. Infect. Dis. 157:1271-1273. [DOI] [PubMed] [Google Scholar]
- 16.Meier, J. L., X. Luo, M. Sawadogo, and S. E. Straus. 1994. The cellular transcription factor USF cooperates with varicella-zoster virus immediate-early protein 62 to symmetrically activate a bidirectional viral promoter. Mol. Cell. Biol. 14:6896-6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michael, E. J., K. M. Kuck, and P. R. Kinchington. 1998. Anatomy of the varicella-zoster virus open-reading frame 4 promoter. J. Infect. Dis. 178(Suppl. 1):S27-S33. [DOI] [PubMed] [Google Scholar]
- 18.Morfin, F., D. Thouvenot, M. Turenne-Tessier, B. Lina, M. Aymard, and T. Ooka. 1999. Phenotypic and genetic characterization of thymidine kinase from clinical strains of varicella-zoster virus resistant to acyclovir. Antimicrob. Agents Chemother. 43:2412-2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahaus, M., N. Desloges, M. Yang, W. T. Ruyechan, and M. H. Wolff. 2003. Transcription factor USF, expressed during the entire phase of varicella-zoster virus infection, interacts physically with the major viral transactivator IE62 and plays a significant role in virus replication. J. Gen. Virol. 84:2957-2967. [DOI] [PubMed] [Google Scholar]
- 20.Schang, L. M. 2005. Advances on cyclin-dependent kinases (CDKs) as novel targets for antiviral drugs. Curr. Drug Targets Infect. Disord. 5:29-37. [DOI] [PubMed] [Google Scholar]
- 21.Schnute, M. E., M. M. Cudahy, R. J. Brideau, F. L. Homa, T. A. Hopkins, M. L. Knechtel, N. L. Oien, T. W. Pitts, R. A. Poorman, M. W. Wathen, and J. L. Wieber. 2005. 4-Oxo-4,7-dihydrothieno[2,3-b]pyridines as non-nucleoside inhibitors of human cytomegalovirus and related herpesvirus polymerases. J. Med. Chem. 48:5794-5804. [DOI] [PubMed] [Google Scholar]
- 22.Shigeta, S., S. Mori, T. Yokota, K. Konno, and E. De Clercq. 1986. Characterization of a varicella-zoster virus variant with altered thymidine kinase activity. Antimicrob. Agents Chemother. 29:1053-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shigeta, S., T. Yokota, T. Iwabuchi, M. Baba, K. Konno, M. Ogata, and E. De Clercq. 1983. Comparative efficacy of antiherpes drugs against various strains of varicella-zoster virus. J. Infect. Dis. 147:576-584. [DOI] [PubMed] [Google Scholar]
- 24.Shiraki, K., H. Ochiai, J. Namazue, T. Okuno, S. Ogino, K. Hayashi, K. Yamanishi, and M. Takahashi. 1992. Comparison of antiviral assay methods using cell-free and cell-associated varicella-zoster virus. Antiviral Res. 18:209-214. [DOI] [PubMed] [Google Scholar]
- 25.Snoeck, R., G. Andrei, and E. De Clercq. 1999. Current pharmacological approaches to the therapy of varicella zoster virus infections: a guide to treatment. Drugs 57:187-206. [DOI] [PubMed] [Google Scholar]
- 26.Snoeck, R., D. Schols, C. Sadzot-Delvaux, J. M. Cloes, G. Andrei, E. De Clercq, J. Piette, and B. Rentier. 1992. Flow cytometric method for the detection of gpI antigens of varicella zoster virus and evaluation of anti-VZV agents. J. Virol. Methods 38:243-254. [DOI] [PubMed] [Google Scholar]
- 27.Standring-Cox, R., T. H. Bacon, and B. A. Howard. 1996. Comparison of a DNA probe assay with the plaque reduction assay for measuring the sensitivity of herpes simplex virus and varicella-zoster virus to penciclovir and acyclovir. J. Virol. Methods 56:3-11. [DOI] [PubMed] [Google Scholar]
- 28.Suzutani, T., M. Ogasawara, T. Shibaki, and M. Azuma. 2000. Susceptibility of protein kinase (ORF47)-deficient varicella-zoster virus strains to anti-herpesvirus nucleosides. Antiviral Res. 45:79-82. [DOI] [PubMed] [Google Scholar]
- 29.Suzutani, T., M. Saijo, M. Nagamine, M. Ogasawara, and M. Azuma. 2000. Rapid phenotypic characterization method for herpes simplex virus and varicella-zoster virus thymidine kinases to screen for acyclovir-resistant viral infection. J. Clin. Microbiol. 38:1839-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor, S. L., P. R. Kinchington, A. Brooks, and J. F. Moffat. 2004. Roscovitine, a cyclin-dependent kinase inhibitor, prevents replication of varicella-zoster virus. J. Virol. 78:2853-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villarreal, E. C. 2003. Current and potential therapies for the treatment of herpes-virus infections. Prog. Drug Res. 60:263-307. [DOI] [PubMed] [Google Scholar]
- 32.Visalli, R. J., and M. van Zeijl. 2003. DNA encapsidation as a target for anti-herpesvirus drug therapy. Antiviral Res. 59:73-87. [DOI] [PubMed] [Google Scholar]
- 33.Visse, B., B. Dumont, J. M. Huraux, and A. M. Fillet. 1998. Single amino acid change in DNA polymerase is associated with foscarnet resistance in a varicella-zoster virus strain recovered from a patient with AIDS. J. Infect. Dis. 178(Suppl. 1):S55-S57. [DOI] [PubMed] [Google Scholar]
- 34.Weinberg, A., J. C. Clark, S. A. Schneider, B. Forghani, and M. J. Levin. 1996. Improved detection of varicella zoster infection with a spin amplification shell vial technique and blind passage. Clin. Diagn. Virol. 5:61-65. [DOI] [PubMed] [Google Scholar]