Abstract
Single-chain variable-fragment (scFv) anti-idiotypic antibodies of an HM-1 killer toxin (HM-1) from the yeast Williopsis saturnus var. mrakii IFO 0895 have been produced by recombinant DNA technology from the splenic lymphocytes of mice immunized by idiotypic vaccination with a neutralizing monoclonal antibody (nMAb-KT). The fungicidal activity of scFv anti-idiotypic antibodies against the isolates of four Candida species was assessed by MIC analysis. scFv antibodies were fungicidal at concentrations of 1.56 to 12.5 μg/ml in vitro against four Candida species. The scFv antibodies exerted a strong candidacidal activity in vitro, with 50% inhibitory concentration (IC50) values ranging from 7.3 × 10−8 to 16.0 × 10−8 M, and were neutralized by adsorption with nMAb-KT. Furthermore, all scFv antibodies effectively inhibited fungal β-1,3-glucan synthase activity in vitro, with IC50 values ranging from 2.0 × 10−8 to 22.7 × 10−8 M, values which almost coincide with the values that are inhibitory to the growth of fungal cells. Binding assays showed that the scFv antibodies specifically bind to nMAb-KT, and this binding pattern was confirmed by surface plasmon resonance analysis. The binding ability was further demonstrated by the competition observed between scFv antibodies and HM-1 to bind nMAb-KT. To the best of our knowledge, this is the first study to show that an antifungal anti-idiotypic antibody, in the form of recombinant scFv, potentially inhibits β-1,3-glucan synthase activity.
Many yeast strains secrete proteins called “killer toxins” to inhibit the growth of other strains of yeast. HM-1 killer toxin (HM-1) is one such protein produced by Williopsis saturnus var. mrakii IFO 0895 (previously known as Hansenula mrakii) and is strongly cytocidal against Saccharomyces cerevisiae (55, 56). HM-1 is a small protein consisting of 88 amino acids and five disulfide bridges, and its three-dimensional structure has been determined by using nuclear magnetic resonance analysis (1, 56). It affects sensitive yeast cells primarily in the growth stage, but it is not toxic to yeast cells in the resting stage or to mammalian cells (21). The mechanism of cytocidal activity of HM-1 has been studied extensively, and the accumulated data indicate that HM-1 kills yeast cells by extracellularly inhibiting β-glucan synthase, a transmembrane enzyme participating in cell wall synthesis of yeasts and fungi (19, 52, 55). This inhibition by HM-1 results in the formation of a pore at the distal tip of the developing bud and the protruding conjugation tube where cell wall synthesis is active, and cells treated with HM-1 die by discharging cellular materials from pores because of osmotic pressure (21).
The incidence of fungal infections is increasing worldwide because of increasing numbers of immunocompromised patients who are of advanced age, have AIDS or cancer, or are undergoing organ transplantation (18, 33). Human fungal pathogens are a highly divergent group of fungal species. Candida albicans especially is a most dangerous pathogenic fungus, causing severe systemic infections in immunocompromised populations (14). C. albicans is still the species most frequently isolated from patients with bloodstream infections (58), while for other groups of patients non-C. albicans species have surpassed C. albicans as a cause of candidemia. Candida parapsilosis and Candida tropicalis are isolated more frequently than C. albicans in some European and Latin American centers (6). The proportion of C. albicans infections has decreased, whereas infections due to other species, such as C. parapsilosis, C. tropicalis, and Candida glabrata, have increased. Such an increase in non-C. albicans species has also been seen in retrospective reviews of the epidemiology of candidemia (6, 35). An urgent need to develop new strategies for novel antifungal agents exists. β-Glucan synthase has been the target of antimycotic drug development to control pathogenic fungi because it is common to all pathogenic and nonpathogenic fungi for cell wall biosynthesis (4, 46, 13). To inhibit fungal growth, various efficacious antibiotics have been developed to interfere with cell wall synthesis by targeting β-1,3-glucan synthase (9, 11, 12, 37, 53). However, no antifungal antibody that can inhibit β-1,3-glucan synthase activity has ever been reported. A monoclonal antibody (MAb) that neutralizes the yeast killing activity of HM-1 (nMAb-KT) was produced and classified as immunoglobulin G1(κ) [IgG1(κ)] (49, 56). To apply the excellent biochemical properties of HM-1 to the development of new antifungal drugs, we tested whether anti-idiotypic antibodies having the internal image of HM-1 can be raised from nMAb-KT and if such anti-idiotypic antibodies inhibit β-1,3-glucan synthase and the growth of yeasts and pathogenic fungi.
Anti-idiotypic antibodies can compete with external antigens for the binding sites of specific antibodies by mimicking the structures of the relative epitopes (38). Immunoglobulin variable domains of heavy chains (VH) and light chains (VL) that form the antigen-binding sites are expressed either as heterodimeric Fab fragments or as monomeric single-chain variable-fragment (scFv) regions in which the VH and VL domains are connected by a short peptide linker (54). This simplified structure makes them useful in the assessment and development of immunotherapeutic and immunodiagnostic applications (16, 30). In this context, anti-idiotypic antibodies with antifungal activity have previously been reported for a killer toxin (KT) from Pichia anomala ATCC 96603 (23, 44) which is apparently different from HM-1, based on its large molecular mass (115 kDa) and also on its specific interaction with cell wall receptors mainly consisting of β-glucans (15). P. anomala KT is strongly cytocidal against Candida albicans (39). On the other hand, HM-1 has a strong cytocidal effect on S. cerevisiae and is also capable of inhibiting the growth of various Candida species. C. albicans is less sensitive to HM-1 (MIC, 300 μg/ml), while non-C. albicans species such as C. glabrata (MIC, 0.4 μg/ml) and C. parapsilosis (MIC, 12.5 μg/ml) are more sensitive to HM-1 (57). Thus, killer toxins differ between species or strains and demonstrate diversity in biochemical structure, immunity, and mechanism of killing sensitive cells (24, 27).
In this study, we used the nMAb-KT and phage display technology to produce scFv anti-idiotypic antibodies specific to nMAb-KT. We generated four scFv anti-idiotypic antibodies and found that they inhibit β-1,3-glucan synthase activity, resulting in a strong cytocidal effect on the growth of S. cerevisiae and the pathogenic isolates of four Candida species, namely, C. albicans, C. tropicalis, C. parapsilosis, and C. glabrata. This study also shows that anti-idiotypic antibody medicines based on natural fungicidal proteins have a potential to be developed as new antimycotic drugs.
MATERIALS AND METHODS
Materials.
HM-1 was extracted from the culture broth of the yeast W. saturnus var. mrakii IFO 0895 and purified as described previously (52). C. albicans (ATCC 10231) and S. cerevisiae (A451) were obtained from Japan Roche Research Center. C. albicans (NBRC 0197, NBRC 0759, NBRC 1389, NBRC 1390, NBRC 1392, NBRC 1397, NBRC 1856, and NBRC 1974), C. tropicalis (NBRC 1400), C. parapsilosis (NBRC 1396), and C. glabrata (NBRC 0622) were purchased from NITE Biological Resource Center. C. albicans (IFM 40213) was a generous gift from Koji Yokoyama, Research Center for Pathogenic Fungi and Microbial Toxicoses, Chiba University, Japan. Saccharomyces bayanus (AKU 4103) was obtained from Kyoto University. The hybridoma clone that produces mouse nMAb-KT was a gift from Tadashi Mayumi, Jichi Medical School, whose group initially reported HM-1-specific nMAb-KT (56). The purified nMAb-KT was prepared at Technology Incubation & Transfer Ltd. (Saitama, Japan). Mouse monoclonal antibodies 1F1 and 4A2, which bind to the HM-1 molecule but have no neutralizing activity, were prepared and classified as IgG1(κ) (22). The purified anti-human c-myc MAb and the antihistidine mouse MAb were purchased from Chemicon International (Temecula, CA) and Calbiochem (San Diego, CA), respectively.
Preparation of anti-idiotypic antibodies.
The scFv anti-idiotypic antibodies used in this study were produced according to a procedure using the recombinant phage antibody system (Amersham Biosciences, Piscataway, NJ). Briefly, 50 μg of nMAb-KT was used to immunize female BALB/c mice (3 weeks old; 10 to 12 g) subcutaneously and intraperitoneally (booster injection). Three days after the final injection, the mice were killed and their spleens were removed. mRNA was isolated from the splenic lymphocytes and reverse transcribed with random hexamer primers. The genes encoding antibody variable regions of heavy and light chains were amplified and assembled into a single gene using a linker fragment and cloned into a specific phagemid vector, pCANTAB 5E. Recombinant phages produced in transformed Escherichia coli TG1 were repeatedly panned against nMAb-KT and screened using a conventional enzyme-linked immunosorbent assay (ELISA) with the nMAb-KT. The nonsuppressor E. coli HB2151 strain was infected with the selected recombinant phages to produce soluble recombinant scFv antibodies that were purified by using affinity chromatography with an anti-E tag Sepharose column (Amersham Biosciences). The protein concentration of the purified scFv antibodies was measured using the bicinchoninic acid method. The yield of the purified scFv antibodies ranged between 1.0 and 3.0 mg/liter. Four selected antibodies assayed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis gave a single band corresponding to a molecular mass of around 30 kDa, which agreed well with the calculated masses based on the amino acid sequences of the antibodies. Surface plasmon resonance (SPR) analysis confirmed that purified scFv antibodies bound to nMAb-KT but did not bind to the 1F1 or 4A2 MAb. Using the ELISA, it was confirmed that scFv antibodies specifically bound to nMAb-KT, whereas scFv antibodies did not bind to MAb 1F1, MAb 4A2, or the irrelevant isotype-matched anti-human c-myc and antihistidine mouse MAbs. A control scFv antibody clone which did not bind to any of the MAbs was selected before panning and produced as described above.
MIC determination.
MICs were determined by the standardized protocol for yeasts developed by the National Committee for Clinical Laboratory Standards (34). Briefly, cells of the Candida species were suspended in sterile normal saline and diluted to a concentration of 5 × 105 cells/ml. The suspensions were diluted 1:1,000 in RPMI 1640 medium with l-glutamine, without bicarbonate, which had been buffered to pH 7.0 with 20 mM HEPES (Sigma, St. Louis, MO). Tubes containing 0.1-ml aliquots of scFv antibodies at 10 times the final drug concentration were inoculated with 0.9 ml of the diluted suspensions. The tubes were incubated at 30°C for 72 h with shaking. The MIC endpoints were read visually as the lowest concentration at which there was an absence of growth.
In vitro antifungal activity by scFv antibodies.
The antifungal activity of the purified scFv clones was tested in vitro by the CFU assay described previously (23). Approximately 5 × 102 cells of each of the four Candida species were suspended in 10 μl of phosphate-buffered saline, and 90 μl of various concentrations of purified scFv antibodies were added. An scFv antibody that did not bind to nMAb-KT was used as a control. In further control experiments, the same number of fungal cells was also added to scFv antibodies that were previously adsorbed with 20 μl nMAb-KT. After overnight incubation at 37°C with the respective reagents to allow cell replication, fungal cells were dispensed into Sabouraud dextrose agar petri dishes (three plates for each experiment). The plates were then incubated at 30°C and observed after 48 h for fungal CFU enumeration. The 50% inhibitory concentration (IC50) values were measured using semilogarithmic graphs.
Preparation of membrane fraction.
Membrane fractions of Candida species were prepared using a method described by Cabib and Kang (3) with some modifications. Cells in the mid-exponential phase were collected by centrifugation, washed with 1 mM EDTA, and then suspended in breaking buffer consisting of 50 mM Tris-HCl (pH 7.5), 0.5 M NaCl, 1 mM EDTA, and 1 mM phenylmethylsulfonyl fluoride. Cells were disrupted by vortexing them with glass beads and were centrifuged for 5 min at 1,000 × g at 4°C. The supernatant was centrifuged at 100,000 × g for 30 min at 4°C. The membrane fraction thus obtained was homogenized in a membrane buffer consisting of 50 mM Tris-HCl (pH 7.5), 10 mM EDTA, 1 mM β-mercaptoethanol, and 33% glycerol and was stored at −80°C.
Measurement of β-1,3-glucan synthase activity and IC50 values.
The β-1,3-glucan synthase assay was performed using the method described by Cabib and Kang (3). The reaction mixtures consisted of 5 mM UDP-d-[U-14C]glucose, 75 mM Tris-HCl (pH 7.5), 0.75% bovine serum albumin, 25 mM KF, 0.75 mM EDTA, 20 μM guanosine 5′-[γ-thio]triphosphate, and a 10-μl membrane fraction of either one of the four Candida species or S. cerevisiae in a total volume of 40 μl. The reaction was started by adding the membrane fraction, and the mixture was incubated at 30°C for 60 min. The scFv antibodies and the control scFv were added to the reaction mixture, and in a positive control experiment, 10 and 25 μg/ml of purified HM-1 were added to the reaction mixture of S. cerevisiae and C. albicans, respectively. The reaction was stopped by adding 250 μl cold 10% trichloroacetic acid, and the mixture was filtered through glass microfiber filters. The filters were washed with 10% trichloroacetic acid, followed by 95% ethanol. The radioactivities retained on the filters were determined using a liquid scintillation counter. To measure the IC50s of scFv antibodies against β-1,3-glucan synthase activity, various concentrations of scFv antibodies were added to the reaction mixture and the IC50 values were measured using semilogarithmic graphs. Each experiment was done three times.
Pore formation by scFv antibodies.
Pore formation was determined by observing the morphological changes of cells treated with scFv antibodies or HM-1, using phase-contrast microscopy as previously described (21). S. bayanus cells (4 × 106 cells/ml) were incubated with 4 μg/ml of HM-1 or 25 μg/ml of scFv anti-idiotypic antibodies in YPD (yeast extract-peptone-dextrose) medium containing 0.8 M sorbitol, and the mixture was shaken at 175 rpm at 30°C for 3 h. The cells were stained with 0.1% methylene blue in 0.8 M sorbitol and were photographed under a phase-contrast microscope using immersion oil.
SPR analysis.
The kinetics of scFvs binding to immobilized nMAb-KT were measured using SPR analysis with a Biacore X (Biacore AB, Uppsala, Sweden). The nMAb-KT or MAb 1F1 or 4A2 was diluted in 10 mM sodium acetate (pH 5.0) at a concentration of 35 μg/ml and immobilized on a CM5 sensor chip by use of an amine coupling kit, and the unreacted moieties of the surface were blocked with ethanolamine. One channel of each sensor chip, prepared in the same way but without monoclonal antibody, was used to monitor the nonspecific binding of scFvs. All measurements were done with HBS-EP buffer consisting of 10 mM HEPES (pH 7.4), 150 mM NaCl, 3.4 mM EDTA, and 0.005% surfactant P20 at a flow rate of 10 μl/min at 25°C. After each measurement, the chip surface was regenerated with 10 μl of 1 mM HCl. The binding of HM-1 and scFv antibodies was analyzed at concentrations of 15.8 to 250 nM. The equilibrium dissociation constant (KD) was evaluated from the kinetic sensorgram curves using BIAevaluation software.
Competitive binding of scFv antibodies with HM-1 to nMAb-KT.
ELISA plates were coated with 10 μg/ml of nMAb-KT and blocked with 3% dry milk in phosphate-buffered saline for 1 h at 37°C. The scFv anti-idiotypic antibodies were applied in ratios of 1:5 to 1:20, followed by the addition of HM-1 (50 ng/ml). Horseradish peroxidase-conjugated anti-E tag antibody was to detect scFv antibodies. Anti-HM-1 rabbit serum and horseradish peroxidase-conjugated anti-rabbit IgG goat antibody were used to detect HM-1. Using 0.022% of 2′,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)-diammonium salt in citric acid, the absorption was measured at 405 nm.
RESULTS
In vitro antifungal activity of scFv antibodies against Candida species.
The antifungal activity of recombinant scFv antibodies was examined for pathogenic strains of four Candida species, namely, C. albicans, C. tropicalis, C. parapsilosis, and C. glabrata. First, the in vitro susceptibilities of Candida species to scFv antibodies were examined; the results are summarized in Table 1. The MICs of scFv antibodies ranged from 1.56 to 12.5 μg/ml. Among the scFv antibodies, scFv-A2 was found to be the most active antifungal agent for the isolates of all the Candida species. Next, to measure the potency of scFv antibodies against Candida species, the numbers of CFUs were determined after incubation with increasing concentrations of scFv antibodies. Figure 1 shows the killing and neutralization assay of a representative Candida isolate after overnight incubation with scFv antibody and appropriate controls. Overall, the IC50 values of scFv antibodies ranged from 2.2 to 4.8 μg/ml (7.3 × 10−8 to 16.0 × 10−8 M) for all Candida species tested (Table 2), indicating the potent antifungal activity of scFv antibodies. Preincubation with nMAb-KT eliminated the inhibition of Candida growth by scFv antibodies, suggesting that the observed cytocidal effect of scFv antibodies was due to their structural resemblance to HM-1 (Fig. 1C).
TABLE 1.
MICs for in vitro susceptibilities of four Candida species to recombinant scFv anti-idiotypic antibodiesa
| Candida strain | MIC (μg/ml) of:
|
|||
|---|---|---|---|---|
| scFv-A1 | scFv-A2 | scFv-A3 | scFv-A4 | |
| C. albicans ATCC 10231 | 6.25 | 3.13 | 3.13 | 6.25 |
| C. albicans NBRC 0197 | 6.25 | 3.13 | 6.25 | 3.13 |
| C. albicans NBRC 0759 | 6.25 | 3.13 | 6.25 | 6.25 |
| C. albicans NBRC 1389 | 6.25 | 3.13 | 6.25 | 6.25 |
| C. albicans NBRC 1390 | 6.25 | 3.13 | 6.25 | 3.13 |
| C. albicans NBRC 1392 | 6.25 | 3.13 | 6.25 | 3.13 |
| C. albicans NBRC 1397 | 12.5 | 6.25 | 6.25 | 12.5 |
| C. albicans NBRC 1856 | 12.5 | 6.25 | 6.25 | 12.5 |
| C. albicans NBRC 1974 | 6.25 | 3.13 | 6.25 | 6.25 |
| C. albicans IFM 40213 | 6.25 | 3.13 | 6.25 | 3.13 |
| C. tropicalis NBRC 1400 | 6.25 | 3.13 | 6.25 | 3.13 |
| C. parapsilosis NBRC 1396 | 6.25 | 3.13 | 6.25 | 6.25 |
| C. glabrata NBRC 0622 | 3.13 | 1.56 | 3.13 | 1.56 |
The measurement of MICs for the cell growth of the Candida species is described in Materials and Methods.
FIG. 1.
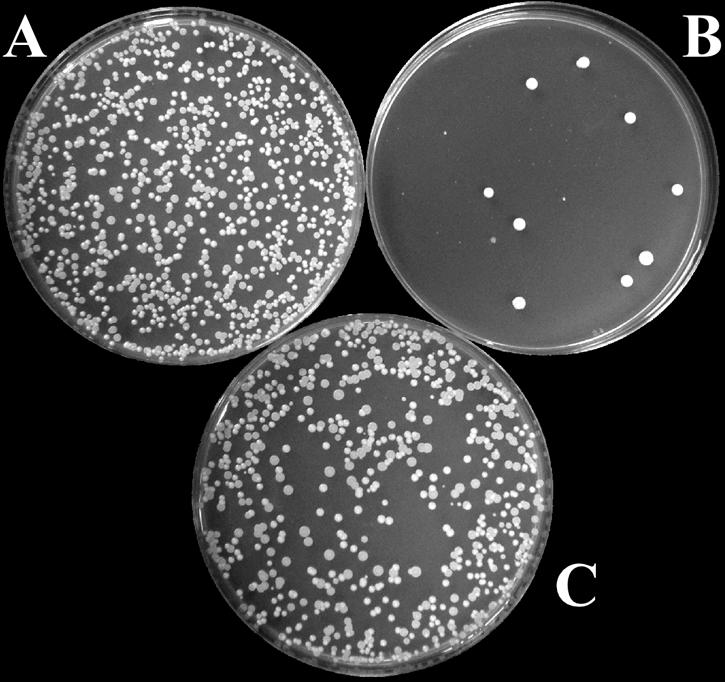
Candidacidal activity of an scFv antibody. The effect of scFv-A2 on the growth of C. albicans ATCC 10231 cells was measured by a CFU assay as described in Materials and Methods. (A) Fungal cells treated with the control scFv antibody; (B) fungal cells treated with 4 μg/ml of scFv-A2; (C) fungal cells treated with scFv-A2 neutralized with nMAb-KT. One of three plates used for each sample is shown.
TABLE 2.
IC50 values for recombinant scFv anti-idiotypic antibodies against the cell growth of four Candida speciesa
| scFv antibody | IC50 (10−8 M) for:
|
|||
|---|---|---|---|---|
| C. albicans ATCC 10231 | C. tropicalis NBRC 1400 | C. parapsilosis NBRC 1396 | C. glabrata NBRC 0622 | |
| ScFv-A1 | 12.7 | 16.0 | 14.0 | 11.3 |
| ScFv-A2 | 7.3 | 8.7 | 8.3 | 8.0 |
| ScFv-A3 | 8.7 | 15.7 | 15.0 | 9.7 |
| ScFv-A4 | 11.7 | 9.3 | 12.3 | 8.8 |
The measurement of IC50 values for recombinant scFv anti-idiotypic antibodies against the cell growth of isolates of the Candida species using a CFU assay is described in Materials and Methods.
Inhibition of yeasts and fungal β-1,3-glucan synthase by scFv antibodies.
HM-1 strongly inhibits β-1,3-glucan synthase activity in vitro and kills sensitive yeast cells (21, 19, 52, 55). To examine whether scFvs obtained from nMAb-KT affect β-1,3-glucan synthesis of yeasts and other fungal cells, we used an in vitro reaction system consisting of the β-1,3-glucan synthase of the membrane fractions of growing S. cerevisiae and C. albicans. All scFvs inhibited β-1,3-glucan synthase in S. cerevisiae and C. albicans by 75% at a concentration of 25 μg/ml, whereas the control scFv antibody did not (Fig. 2A and B). Under the same conditions, HM-1, included in this study as a positive control, showed almost the same levels of inhibition of β-1,3-glucan synthase activity.
FIG. 2.
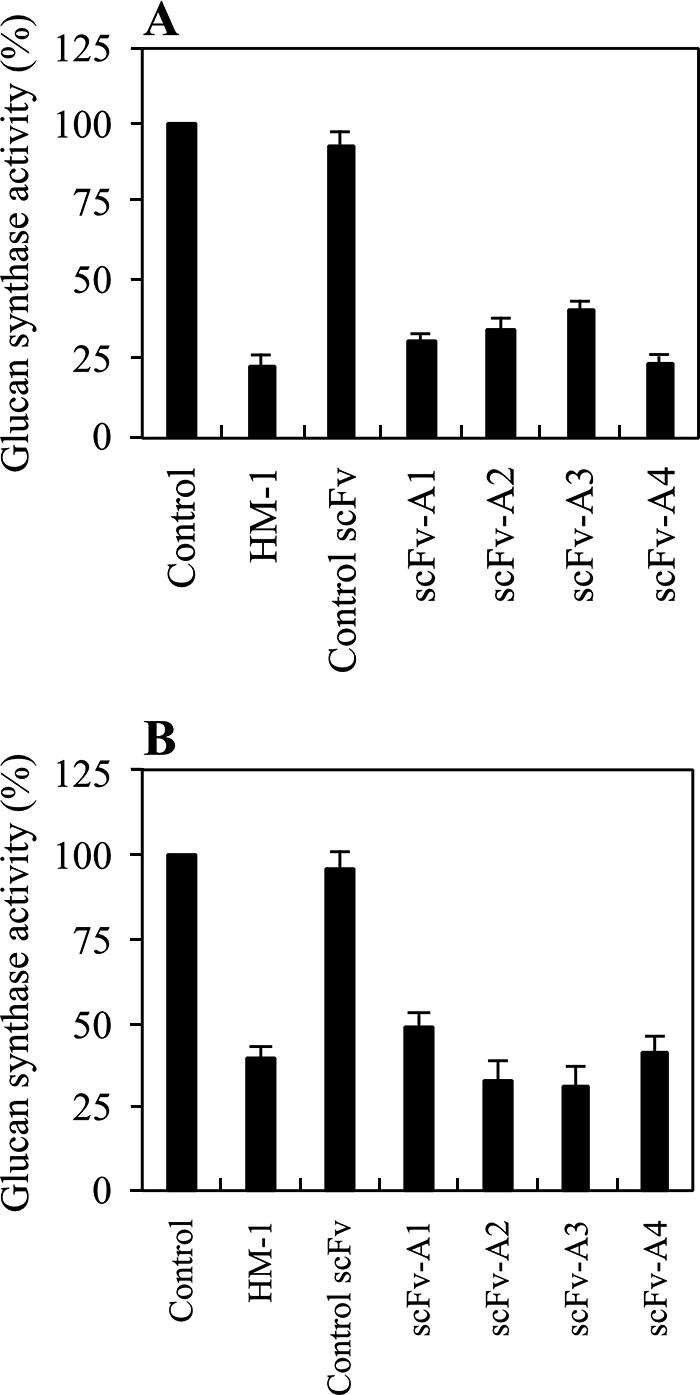
Inhibition of yeast and fungal β-1,3-glucan synthase activity by purified scFvs. The membrane fractions were obtained from S. cerevisiae A451 (A) and C. albicans ATCC 10231 (B). The scFvs were added to the reaction mixture at a concentration of 25 μg/ml, as described in Materials and Methods. The activity was expressed in comparison to the activity of the sample without scFv and HM-1 as 100%. Data represent the mean of results for triplicate experiments ± standard error of the mean.
Table 3 shows the IC50 values of scFv anti-idiotypic antibodies against β-1,3-glucan synthase in four Candida species. The IC50 values of the scFv anti-idiotypic antibodies ranged from 0.62 to 6.8 μg/ml (2.0 × 10−8 to 22.7 × 10−8 M) and corresponded well with the IC50 values obtained from the CFU assay.
TABLE 3.
IC50 values of recombinant scFv anti-idiotypic antibodies against the β-1,3-glucan synthase activity of four Candida speciesa
| scFv antibody | IC50 (10−8 M) for:
|
|||
|---|---|---|---|---|
| C. albicans ATCC 10231 | C. tropicalis NBRC 1400 | C. parapsilosis NBRC 1396 | C. glabrata NBRC 0622 | |
| ScFv-A1 | 19.7 | 16.5 | 11.7 | 4.2 |
| ScFv-A2 | 13.0 | 11.3 | 2.7 | 2.0 |
| ScFv-A3 | 12.7 | 20.7 | 22.7 | 11.4 |
| ScFv-A4 | 14.8 | 16.7 | 19.4 | 4.7 |
The measurement of IC50 values of β-1,3-glucan synthase activity of membrane fractions of Candida species is described in Materials and Methods.
Mechanism of antiyeast effect of scFv antibodies.
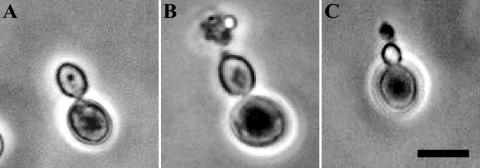
HM-1 inhibits the growth of yeast cells by forming a pore at the growing tip of the daughter cell, resulting in the formation of a protruding structure and eventual cell death (21). To examine whether scFv also forms protruding structures in growing cells, we added purified scFv antibodies to an S. bayanus cell culture and analyzed the change in morphology of the yeast cells. The microscopic study showed that most cultured cells treated with scFvs (3 h) had a pearlike structure with protruding materials, characteristic of pore formation and similar to the morphology change after treatment with HM-1 (Fig. 3B and C). This morphological change was caused by all four scFvs tested and was clearly distinguished from the smooth, round shape of untreated control cells (Fig. 3A). These data clearly indicate that the scFvs appear to have the same effect as HM-1 on sensitive yeast cells.
FIG. 3.
Pore formation in yeast cells by scFv antibodies shown by phase-contrast microscopy of S. bayanus AKU 4103 cells treated with scFv-A2 and HM-1. The sample preparation is described in Materials and Methods. (A) Control yeast cells; (B) HM-1-treated cells; (C) scFv-A2-treated cells. Bar, 5 μm.
Kinetic parameters of selected scFv antibodies.
The binding specificities and kinetic parameters of purified scFv antibodies were determined by using SPR analysis. The Biacore sensorgram curve shows the interactions of scFv-A1, scFv-A2, scFv-A3, and scFv-A4 at different concentrations with immobilized nMAb-KT. Table 4 shows the calculated values of the association rate constant (kon), dissociation rate constant (koff), and equilibrium dissociation constant (KD), indicating that the interaction of scFv-A2 with nMAb-KT was the most effective of the four scFvs. Overall, the results of SPR analysis showed that all four scFvs have kinetic affinities similar to that of nMAb-KT, despite differences in their amino acid sequences. None of the four scFvs showed a signal curve with immobilized MAb 1F1 or 4A2 (data not shown). These results indicate that the scFv antibodies bind specifically to nMAb-KT but not to MAb 1F1 or 4A2, which binds to HM-1 but is unable to neutralize HM-1 antifungal activity. HM-1, included in this study as a positive control, showed the greatest affinity (KD = 5.48 × 10−9 M) for immobilized nMAb-KT.
TABLE 4.
Kinetic parameters for the binding of four scFv antibodies to nMAb-KT as measured by SPR analysisa
| Analyte | kon (105 M−1s−1) | koff (10−3 s−1) | KD (10−8 M) |
|---|---|---|---|
| scFv-A1 | 2.43 | 5.07 | 2.08 |
| scFv-A2 | 3.05 | 4.58 | 1.5 |
| scFv-A3 | 2.29 | 7.51 | 3.29 |
| scFv-A4 | 2.24 | 5.01 | 2.23 |
The interactions between HM-1 and the scFv antibodies with immobilized nMAb-KT were measured as described in Materials and Methods. kon, association rate constant; koff, dissociation rate constant; KD, dissociation constant. The constants were calculated by the equation KD = koff/kon.
Competitive binding of scFv antibodies with HM-1 to nMAb-KT.
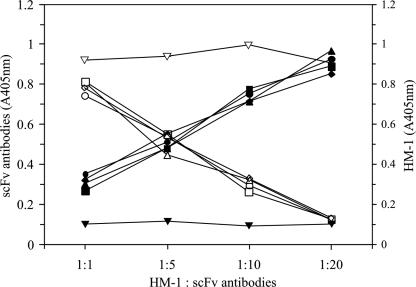
To verify whether scFv antibodies compete with HM-1 to bind nMAb-KT, a competitive binding ELISA was performed (Fig. 4). When the scFv antibody concentration was increased, the binding of scFv antibody to nMAb-KT was also increased, but the HM-1 binding to nMAb-KT was concomitantly decreased. In contrast, the control scFv antibody did not affect the binding of HM-1 to nMAb-KT. These results suggest that all four scFv antibody clones were homologous in competition with HM-1 for binding to nMAb-KT.
FIG. 4.
Competitive binding of scFv antibodies with HM-1 to nMAb-KT. ELISA plates coated with nMAb-KT were incubated with increasing concentrations of the selected scFv antibodies and the control scFv antibody as described in Materials and Methods. The symbols represent the respective binding of scFv antibodies (solid symbols) and HM-1 (open symbols) with nMAb-KT. •, scFv-A1; ▴, scFv-A2; ▪, scFv-A3; ♦, scFv-A4; ▾, control scFv antibody. The maximum binding activities (A405nm, absorption at 405 nm) of scFv-A1, -A2, -A3, and -A4 were 0.928, 0.968, 0.891 and 0.852, respectively.
DISCUSSION
Jerne proposed a biological significance for idiotypic determinants in the network theory of immune regulation (17). Idiotypes are epitopes that are unique to an antibody, associated with the heavy and light chains of the antibody in which the participation by variable regions that is usually needed for immune activity occurs (10, 38). Anti-idiotypic antibodies representing the internal image of some antigenic determinants have been proposed as surrogate vaccines (36), and antibodies conjugated with toxins have been proposed in the immunotherapy of cancer (30). In some cases, amino acid sequence homology between the protein antigen and the anti-idiotypic antibody variable region exists (2). Antigenic mimicry is usually more functional than biochemical, and the functional mimicry of ligands of biological receptors by anti-idiotypic antibodies has been extensively studied (10).
The ability of the phage display system to express antibody fragments offers several important advantages over hybridoma technology in identifying functional immunoglobulin domains (5, 54). Previously, anti-idiotypic antibodies with antimicrobial activity have been made with the neutralizing antibodies of KT from P. anomala ATCC 96603 (23, 44). Each killer toxin has unique properties, different targets, and an intrinsic strategy to attack the host organism, and these profiles also depend on the species and strains (20, 31). HM-1 inhibits β-1,3-glucan synthesis but does not inhibit the synthesis of protein, chitin, or mannan in S. cerevisiae protoplast cells (55). β-1,3-Glucan synthase from the S. cerevisiae membrane is inhibited in vitro by HM-1 at a concentration that coincides well with the cell growth inhibitory concentration, implying that inhibition of β-1,3-glucan synthase is responsible for the cytocidal activity of HM-1 (52). This inhibition is partly reduced by adding β-1,3-glucan fragments, suggesting that HM-1 binds both β-1,3-glucan and its synthase (19). HM-1 kills only growing cells and does not affect cells in the resting stage (19, 21). These excellent biochemical properties of HM-1 are important for antifungal drug development and encouraged us to generate recombinant immunoglobulin that has an internal image of the HM-1 active site responsible for β-1,3-glucan synthase inhibition and antifungal activity.
To generate recombinant single-chain antibodies, we used mRNA from the splenocytes of mice immunized by parenteral idiotypic vaccination with an nMAb-KT and a phagemid that enables expression of scFv recombinant antibodies as fusion proteins with phage gene III protein. This allowed the selection of phages that display anti-idiotypic antibodies and the production of soluble scFv anti-idiotypic antibodies. We selected four clones, scFv-A1, scFv-A2, scFv-A3, and scFv-A4, based on the strength of their binding activity to nMAb-KT and on their nucleotide sequences. The binding properties of scFv antibodies demonstrated by SPR analysis indicated that scFv antibodies react to nMAb-KT but not to MAb 1F1 or 4A2.
The wide spectrum of the antifungal activity of scFv antibodies against the Candida species was investigated because Candida species are pathogenic to humans. C. albicans especially has been the species most often associated with neonatal infections. Recent reports, however, have suggested an increasing number of infections attributable to C. parapsilosis associated with a common source (47). In terms of Candida species, there has recently been a shift towards more reports of non-C. albicans infections by some authors, especially with patients with hematological diseases and transplant patients (35, 45). In this study, we have determined the in vitro antifungal activity of scFv antibodies against pathogenic isolates of four Candida species. When the antifungal activity was evaluated by the CFU assay, the scFv antibodies showed strong antifungal activity against C. albicans, C. tropicalis, C. parapsilosis, and C. glabrata in vitro, with IC50 values ranging from 7.3 × 10−8 to 16.0 × 10−8 M. The fungal susceptibilities were also determined by MICs, and the scFv antibodies were found to be fungicidal at concentrations of 1.56 to 12.5 μg/ml in vitro against the Candida isolates.
All four scFv antibodies inhibited in vitro glucan synthesis catalyzed by β-1,3-glucan synthase in the membrane of S. cerevisiae as well as that of C. albicans (Fig. 2A and B). They also inhibited β-1,3-glucan synthase in four Candida species in vitro, with IC50 values ranging from 2.0 × 10−8 to 22.7 × 10−8 M. The good correlation of IC50 values in the inhibition of glucan synthesis and of cell growth support the speculation that scFv antibodies and HM-1 share a common target molecule(s) that is most likely to be the β-1,3-glucan synthase on the cell surface. Indeed, the binding of scFv antibodies to nMAb-KT was competitive with that of HM-1 (Fig. 4), and nMAb-KT that neutralized the killing activity of HM-1 eliminated the cytocidal activity of scFv (Fig. 1). In our established colorimetric β-1,3-glucan synthase assay system, HM-1 and scFv antibodies inhibit the enzyme reaction in a noncompetitive manner (unpublished results). These results suggest that the killing action of scFvs is the same as that of HM-1, which distorts the growing end of the cell wall (Fig. 3). The echinocandin analogues such as caspofungin, micafungin, and anidulafungin antifungals are the clinically useful antibiotics that inhibit fungal growth through the inhibition of β-1,3-glucan synthase (9, 11, 12, 37, 53). Although echinocandins are reported to be active against several medically important fungi, they are relatively inactive against Cryptococcus species (7, 8, 51). In this context, it should be noted that in addition to inhibiting Candida species, HM-1-derived scFv antibodies are also able to inhibit the cell growth and β-1,3-glucan synthase of Cryptococcus species (50).
The cloning system used in this study was based on a heterogeneous population of lymphocytes that does not necessarily ensure that the original pairing of the heavy and light chains will be conserved. Most pairings with binding activity are likely to be fortuitous. Nevertheless, the scFv clones were selected solely because of their high binding affinity to the idiotype nMAb-KT. We expected that the functional mimicking of HM-1 (i.e., β-1,3-glucan synthase inhibition, pore formation in the growing yeast, and the consequent antifungal activity) might reflect amino acid sequence similarity to HM-1. To our surprise, however, the selected scFv antibodies share no apparent homology with HM-1 in their amino acid sequences. Perhaps the three-dimensional structure, particularly that formed by the domain of VH shared by all four scFvs, is most responsible for the functional mimicry of HM-1 by the scFv antibodies.
Molecules able to selectively interact with microbial cell wall-related components that are not present in mammalian cells should rationally be considered as antimicrobial agents (48). From this point of view, some other promising candidacidal antibodies have been described. Magliani et al. (23, 25, 26) and Polonelli et al. (40-42, 44) demonstrated that several models of antibodies against the killer toxin of P. anomala were therapeutically active in murine models of invasive candidiasis. Moragues et al. (32) described how a MAb (MAb C7) directed against a protein epitope of a cell wall stress mannoprotein expressed in different agents exerted a direct in vitro candidacidal activity. Matthews et al. (28) reported that different antibodies against fungal heat shock protein 90 (HSP90) were also therapeutically active in mouse models. The preclinical assessment of a humanized recombinant antibody, Mycograb, against an epitope of HSP90 showed cytocidal activity against a wide range of yeast species. Mycograb is intrinsically fungicidal in vitro, with an MIC of 128 to 256 μg/ml and a mechanism of action that participates in the inhibition of HSP90 (29). In this study, we showed that scFv anti-idiotypic antibodies have potential candidacidal activity in vitro through inhibition of β-1,3-glucan synthase. We believe that the recombinant scFv antibodies obtained in this study are also excellent candidates for antimycotic drugs and should support the concept of a family of fungicidal antibodies such as the natural monoclonal as well as recombinant killer antibodies of P. anomala KT (23, 43, 44).
On the basis of our recent observations, peptides reproducing the complementarity-determining regions of both heavy and light chains of scFv antibodies were synthesized and their effectiveness for candidacidal activity in vitro was tested (unpublished observations). There is a possibility that scFv antibody-derived peptides could provide a unique approach for the development of a new class of antibiotic, which would be deliverable directly to the mucosal site. They could also be active against pathogenic microorganisms that are currently resistant to conventional drugs.
Acknowledgments
We thank Tadashi Mayumi and his colleagues at Jichi Medical School for kindly providing nMAb-KT-producing hybridoma clones. We also thank Koji Yokoyama of Chiba University for providing the C. albicans strain. Special thanks go to Toshinori Nakayama and Masaru Taniguchi of the Graduate School of Medicine, Chiba University, for valuable discussions.
This work was supported by a grant from the Ministry of Education, Science, Sports, and Culture of Japan.
REFERENCES
- 1.Antuch, W., P. Güntert, and K. Wüthrich. 1996. Ancestral beta gamma-crystallin precursor structure in a yeast killer toxin. Nat. Struct. Biol. 3:662-665. [DOI] [PubMed] [Google Scholar]
- 2.Bruck, C., M. S. Co, M. Slaoui, G. N. Gaulton, T. Smith, B. N. Fields, J. I. Mullins, and M. I. Greene. 1986. Nucleic acid sequence of an internal image-bearing monoclonal anti-idiotype and its comparison to the sequence of the external antigen. Proc. Natl. Acad. Sci. USA 83:6578-6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabib, E., and M. S. Kang. 1987. Fungal 1,3-beta-glucan synthase. Methods Enzymol. 138:637-642. [DOI] [PubMed] [Google Scholar]
- 4.Cabib, E., B. Bowers, A. Sburlati, and S. J. Silverman. 1988. Fungal cell wall synthesis: the construction of a biological structure. Microbiol. Sci. 5:370-375. [PubMed] [Google Scholar]
- 5.Clackson, T., H. R. Hoogenboom, A. D. Griffiths, and G. Winter. 1991. Making antibody fragments using phage display libraries. Nature 352:624-628. [DOI] [PubMed] [Google Scholar]
- 6.Colombo, A. L., M. Nucci, R. Salomao, M. L. Branchini, R. Richtmann, A. Derossi, and S. B. Wey. 1999. High rate of non-albicans candidemia in Brazilian tertiary care hospitals. Diagn. Microbiol. Infect. Dis. 34:281-286. [DOI] [PubMed] [Google Scholar]
- 7.Ernst, E. J., M. E. Klepser, and M. A. Pfaller. 2000. Postantifungal effects of echinocandin, azole, and polyene antifungal agents against Candida albicans and Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:1108-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldmesser, M., Y. Kress, A. Mednick, and A. Casadevall. 2000. The effect of the echinocandin analogue caspofungin on cell wall glucan synthesis by Cryptococcus neoformans. J. Infect. Dis. 182:1791-1795. [DOI] [PubMed] [Google Scholar]
- 9.Fostel, J. M., and P. A. Lartey. 2000. Emerging novel antifungal agents. Drug Discov. Today 5:25-32. [DOI] [PubMed] [Google Scholar]
- 10.Gaulton, G. N., and M. I. Greene. 1986. Idiotypic mimicry of biological receptors. Annu. Rev. Immunol. 4:253-280. [DOI] [PubMed] [Google Scholar]
- 11.Georgopapadakou, N. H. 2001. Update on antifungals targeted to the cell wall: focus on beta-1,3-glucan synthase inhibitors. Expert Opin. Investig. Drugs 10:269-280. [DOI] [PubMed] [Google Scholar]
- 12.Georgopapadakou, N. H., and T. J. Walsh. 1996. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob. Agents Chemother. 40:279-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldman, R. C., D. J. Frost, J. O. Capobianco, S. Kadam, R. R. Rasmussen, and C. Abad-Zapatero. 1995. Antifungal drug targets: Candida secreted aspartyl protease and fungal wall beta-glucan synthesis. Infect. Agents Dis. 4:228-247. [PubMed] [Google Scholar]
- 14.Groll, A. H., and T. J. Walsh. 2001. Uncommon opportunistic fungi: new nosocomial threats. Clin. Microbiol. Infect. 7:8-24. [DOI] [PubMed] [Google Scholar]
- 15.Guyard, C., P. Evrard, A. M. Corbisier-Colson, H. Louvart, E. Dei-Cas, F. D. Menozzi, L. Polonelli, and J. Cailliez. 2001. Immuno-crossreactivity of an anti-Pichia anomala killer toxin monoclonal antibody with a Williopsis saturnus var. mrakii killer toxin. Med. Mycol. 39:395-400. [DOI] [PubMed] [Google Scholar]
- 16.Huston, J. S., J. McCartney, M. S. Tai, C. Mottola-Hartshorn, D. Jin, F. Warren, P. Keck, and H. Oppermann. 1993. Medical applications of single-chain antibodies. Int. Rev. Immunol. 10:195-217. [DOI] [PubMed] [Google Scholar]
- 17.Jerne, N. K. 1974. Towards a network theory of the immune system. Ann. Immunol. (Paris) 125C:373-389. [PubMed] [Google Scholar]
- 18.Karlowsky, J. A., G. G. Zhanel, K. A. Klym, D. J. Hoban, and A. M. Kabani. 1997. Candidemia in a Canadian tertiary care hospital from 1976 to 1996. Diagn. Microbiol. Infect. Dis. 29:5-9. [DOI] [PubMed] [Google Scholar]
- 19.Kasahara, S., S. Ben Inoue, T. Mio, T. Yamada, T. Nakajima, E. Ichishima, Y. Furuichi, and H. Yamada. 1994. Involvement of cell wall beta-glucan in the action of HM-1 killer toxin. FEBS Lett. 348:27-32. [DOI] [PubMed] [Google Scholar]
- 20.Kashiwagi, T., N. Kunishima, C. Suzuki, F. Tsuchiya, S. Nikkuni, Y. Arata, and K. Morikawa. 1997. The novel acidophilic structure of the killer toxin from halotolerant yeast demonstrates remarkable folding similarity with a fungal killer toxin. Structure 5:81-94. [DOI] [PubMed] [Google Scholar]
- 21.Komiyama, T., T. Ohta, H. Urakami, Y. Shiratori, T. Takasuka, M. Sato, T. Watanabe, and Y. Furuichi. 1996. Pore formation on proliferating yeast Saccharomyces cerevisiae cell buds by HM-1 killer toxin. J. Biochem. 119:731-736. [DOI] [PubMed] [Google Scholar]
- 22.Komiyama, T., Q.-Z. Zhang, M. Miyamoto, D. Selvakumar, and Y. Furuichi. 2004. Monoclonal antibodies and sandwich ELISA for quantitation of HM-1 killer toxin. Biol. Pharm. Bull. 27:691-693. [DOI] [PubMed] [Google Scholar]
- 23.Magliani, W., S. Conti, F. de Bernardis, M. Gerloni, D. Bertolotti, P. Mozzoni, A. Cassone, and L. Polonelli. 1997. Therapeutic potential of antiidiotypic single chain antibodies with yeast killer toxin activity. Nat. Biotechnol. 15:155-158. [DOI] [PubMed] [Google Scholar]
- 24.Magliani, W., S. Conti, M. Gerloni, D. Bertolotti, and L. Polonelli. 1997. Yeast killer systems. Clin. Microbiol. Rev. 10:369-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magliani, W., S. Conti, S. Arseni, R. Frazzi, A. Salati, and L. Polonelli. 2001. Killer anti-idiotypes in the control of fungal infections. Curr. Opin. Investig. Drugs 2:477-479. [PubMed] [Google Scholar]
- 26.Magliani, W., S. Conti, A. Salati, S. Arseni, L. Ravanetti, R. Frazzi, and L. Polonelli. 2003. Biotechnological approaches to the production of idiotypic vaccines and antiidiotypic antibiotics. Curr. Pharm. Biotechnol. 4:91-97. [DOI] [PubMed] [Google Scholar]
- 27.Marquina, D., A. Santos, and J. M. Peinado. 2002. Biology of killer yeasts. Int. Microbiol. 5:65-71. [DOI] [PubMed] [Google Scholar]
- 28.Matthews, R. C., J. P. Burnie, D. Howat, T. Rowland, and F. Walton. 1991. Autoantibody to heat-shock protein 90 can mediate protection against systemic candidiasis. Immunology 74:20-24. [PMC free article] [PubMed] [Google Scholar]
- 29.Matthews, R. C., G. Rigg, S. Hodgetts, T. Carter, C. Chapman, C. Gregory, C. Illidge, and J. Burnie. 2003. Preclinical assessment of the efficacy of Mycograb, a human recombinant antibody against fungal HSP90. Antimicrob. Agents Chemother. 47:2208-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monroe, J. G., and M. I. Greene. 1986. Anti-idiotypic antibodies and disease. Immunol. Investig. 15:263-286. [DOI] [PubMed] [Google Scholar]
- 31.Morace, G., C. Archibusacci, M. Sestito, and L. Polonelli. 1984. Strain differentiation of pathogenic yeasts by the killer system. Mycopathologia 84:81-85. [DOI] [PubMed] [Google Scholar]
- 32.Moragues, M. D., M. J. Omaetxebarria, N. Elguezabal, M. J. Sevilla, S. Conti, L. Polonelli, and J. Pontón. 2003. A monoclonal antibody directed against a Candida albicans cell wall mannoprotein exerts three anti-C. albicans activities. Infect. Immun. 71:5273-5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myskowski, P. L., M. H. White, and R. Ahkami. 1997. Fungal disease in the immunocompromised host. Dermatol. Clin. 15:295-305. [DOI] [PubMed] [Google Scholar]
- 34.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 35.Nguyen, M. H., J. E. Peacock, A. J. Morns, D. C. Tanner, M. L. Nguyen, D. R. Snydman, M. M. Wagener, M. G. Rinaldi, and V. L. Yu. 1996. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am. J. Med. 100:617-623. [DOI] [PubMed] [Google Scholar]
- 36.Nisonoff, A., and M. F. Gorish. 1984. From network theory toward vaccination by monoclonal antibodies, p. 119-128. In A. Sanna and G. Morace (ed.), New horizons in microbiology. Elsevier Science Publishers, Amsterdam, The Netherlands.
- 37.Onishi, J., M. Meinz, J. Thompson, J. Curotto, S. Dreikorn, M. Rosenbach, C. Douglas, G. Abruzzo, A. Flattery, L. Kong, A. Cabello, F. Vicente, F. Pelaez, M. T. Diez, I. Martin, G. Bills, R. Giacobbe, A. Dombrowski, R. Schwartz, S. Morris, G. Harris, A. Tsipouras, K. Wilson, and M. B. Kurtz. 2000. Discovery of novel antifungal (1,3)-beta-d-glucan synthase inhibitors. Antimicrob. Agents Chemother. 44:368-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poljak, R. J. 1994. An idiotope-anti-idiotope complex and the structural basis of molecular mimicking. Proc. Natl. Acad. Sci. USA 91:1599-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polonelli, L., and G. Morace. 1986. Reevaluation of the yeast killer phenomenon. J. Clin. Microbiol. 24:866-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polonelli, L., S. Conti, M. Gerloni, W. Magliani, M. Castagnola, G. Morace, and C. Chezzi. 1991. ‘Antibiobodies’: antibiotic-like anti-idiotypic antibodies. J. Med. Vet. Mycol. 29:235-242. [DOI] [PubMed] [Google Scholar]
- 41.Polonelli, L., R. Lorenzini, F. De Bernardis, M. Gerloni, S. Conti, G. Morace, W. Magliani, and C. Chezzi. 1993. Idiotypic vaccination: immunoprotection mediated by anti-idiotypic antibodies with antibiotic activity. Scand. J. Immunol. 37:105-110. [DOI] [PubMed] [Google Scholar]
- 42.Polonelli, L., F. De Bernardis, S. Conti, M. Boccanera, M. Gerloni, G. Morace, W. Magliani, C. Chezzi, and A. Cassone. 1994. Idiotypic intravaginal vaccination to protect against candidal vaginitis by secretory, yeast killer toxin-like anti-idiotypic antibodies. J. Immunol. 152:3175-3182. [PubMed] [Google Scholar]
- 43.Polonelli, L., F. De Bernardis, S. Conti, M. Boccanera, W. Magliani, M. Gerloni, C. Cantelli, and A. Cassone. 1996. Human natural yeast killer toxin-like candidacidal antibodies. J. Immunol. 156:1880-1885. [PubMed] [Google Scholar]
- 44.Polonelli, L., N. Seguy, S. Conti, M. Gerloni, D. Bertolotti, C. Cantelli, W. Magliani, and J. C. Cailliez. 1997. Monoclonal yeast killer toxin-like candidacidal anti-idiotypic antibodies. Clin. Diagn. Lab. Immunol. 4:142-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rocco, T. R., S. E. Reinert, and H. H. Simms. 2000. Effects of fluconazole administration in critically ill patients: analysis of bacterial and fungal resistance. Arch. Surg. 135:160-165. [DOI] [PubMed] [Google Scholar]
- 46.Ruiz-Herrera, J. 1991. Biosynthesis of beta-glucans in fungi. Antonie Leeuwenhoek 60:72-81. [DOI] [PubMed] [Google Scholar]
- 47.Saxen, H., M. Virtanen, P. Carlson, K. Hoppu, M. Pohjavuori, M. Vaara, J. Vuopio-Varkila, and H. Peltola. 1995. Neonatal Candida parapsilosis outbreak with a high case fatality rate. Pediatr. Infect. Dis. J. 14:776-781. [DOI] [PubMed] [Google Scholar]
- 48.Selitrennikoff, C. P., and M. Nakata. 2003. New cell wall targets for antifungal drugs. Curr. Opin. Investig. Drugs 4:200-205. [PubMed] [Google Scholar]
- 49.Selvakumar, D., Q. Z. Zhang, M. Miyamoto, Y. Furuichi, and T. Komiyama. 2006. Identification and characterization of a neutralizing monoclonal antibody for the epitope on HM-1 killer toxin. J. Biochem. 139:399-406. [DOI] [PubMed] [Google Scholar]
- 50.Selvakumar, D., M. Miyamoto, Y. Furuichi, and T. Komiyama. 2006. Inhibition of β-1,3-glucan synthase and cell growth of Cryptococcus species by recombinant single-chain anti-idiotypic antibodies. J. Antibiot. 59:73-79. [DOI] [PubMed] [Google Scholar]
- 51.Serena, C., B. Fernández-Torres, F. J. Pastor, L. Trilles, M. dos Santos Lazéra, N. Nolard, and J. Guarro. 2005. In vitro interactions of micafungin with other antifungal drugs against clinical isolates of four species of Cryptococcus. Antimicrob. Agents Chemother. 49:2994-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takasuka, T., T. Komiyama, Y. Furuichi, and T. Watanabe. 1995. Cell wall synthesis specific cytocidal effect of Hansenula mrakii toxin-1 on Saccharomyces cerevisiae. Cell. Mol. Biol. Res. 41:575-581. [PubMed] [Google Scholar]
- 53.Vazquez, J. A. 2005. Anidulafungin: a new echinocandin with a novel profile. Clin. Ther. 27:657-673. [DOI] [PubMed] [Google Scholar]
- 54.Winter, G., and C. Milstein. 1991. Man-made antibodies. Nature 349:293-299. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto, T., T. Hiratani, H. Hirata, M. Imai, and H. Yamaguchi. 1986. Killer toxin from Hansenula mrakii selectively inhibits cell wall synthesis in a sensitive yeast. FEBS Lett. 197:50-54. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto, T., M. Imai, K. Tachibana, and M. Mayumi. 1986. Application of monoclonal antibodies to the isolation and characterization of a killer toxin secreted by Hansenula mrakii. FEBS Lett. 195:253-257. [DOI] [PubMed] [Google Scholar]
- 57.Yamamoto, T., K. Uchida, T. Hiratani, T. Miyazaki, J. Yagiu, and H. Yamaguchi. 1988. In vitro activity of the killer toxin from yeast Hansenula mrakii against yeasts and molds. J. Antibiot. 41:398-403. [DOI] [PubMed] [Google Scholar]
- 58.Yamamura, D. L., C. Rotstein, L. E. Nicolle, S. Ioannou, et al. 1999. Candidemia at selected Canadian sites: results from the Fungal Disease Registry 1992-1994. Can. Med. Assoc. J. 160:493-499. [PMC free article] [PubMed] [Google Scholar]