Abstract
Depression is a multifactorial illness and genetic factors play a role in its etiology. The understanding of its physiopathology relies on the availability of experimental models potentially mimicking the disease. Here we describe a model built up by selective breeding of mice with strikingly different responses in the tail suspension test, a stress paradigm aimed at screening potential antidepressants. Indeed, “helpless” mice are essentially immobile in the tail suspension test, as well as the Porsolt forced-swim test, and they show reduced consumption of a palatable 2% sucrose solution. In addition, helpless mice exhibit sleep–wakefulness alterations resembling those classically observed in depressed patients, notably a lighter and more fragmented sleep, with an increased pressure of rapid eye movement sleep. Compared with “nonhelpless” mice, they display higher basal seric corticosterone levels and lower serotonin metabolism index in the hippocampus. Remarkably, serotonin1A autoreceptor stimulation induces larger hypothermia and inhibition of serotoninergic neuronal firing in the nucleus raphe dorsalis in helpless than in nonhelpless mice. Thus, helpless mice exhibit a decrease in serotoninergic tone, which evokes that associated with endogenous depression in humans. Finally, both the behavioral impairments and the serotoninergic dysfunction can be improved by chronic treatment with the antidepressant fluoxetine. The helpless line of mice may provide an opportunity to approach genes influencing susceptibility to depression and to investigate neurophysiological and neurochemical substrates underlying antidepressant effects.
The prevalence of depression worldwide is such that this disorder represents a major health problem. It is estimated, for example, that ≈10% of men and 20% of women in Western Europe will suffer from a major depressive episode at some time in their life. The monoamine hypothesis of depression suggests that one of the biological bases of affective disorders is a deficiency in the neurotransmitter serotonin (5-HT). During the past 40 yr, this hypothesis has been refined, as more experimental and clinical evidence has emerged. The selective 5-HT reuptake inhibitors, in particular, allowed important progress in our understanding of the role of 5-HT in depression. To some extent, the mechanism of action of 5-HT reuptake inhibitors, which are the most widely prescribed antidepressant drugs today, can be anticipated from our knowledge of the anatomy and chemistry of the central serotoninergic system. However, it must be accepted that extensive investigations have so far failed to find convincing evidence of a primary dysfunction of the serotoninergic system in patients with depression. Disturbances of sleep are typical for most depressed patients and belong to the core symptoms of the disorder. Polysomnographic sleep research has demonstrated that, besides disturbances of sleep continuity, depression is associated with a reduction of slow-wave sleep and a shortening of rapid eye movement (REM) sleep latency. While the hunt for the biological basis of depression is intensifying, better therapies are also actively sought.
Animal models investigated in the past often dealt with a particular aspect of the syndrome (1). Because inherited interindividual variation in vulnerability to depression is a well known phenomenon (2, 3), selectively bred rat models have been developed recently (4, 5). Here we report on the behavioral, neurochemical, and electrophysiological characteristics of a potential mouse model of depression. We sought to investigate whether a selective breeding strategy for divergent magnitudes of immobility in the tail suspension test (TST) would result in the selection of two different lines of nonhelpless (NHL) versus helpless (HL) mice. We used the TST, which, besides the forced-swim test (FST), is a widely used screening method for antidepressants in mice (6, 7). During these tests, mice show alternate periods of agitation and immobility, the latter being considered to mimic a state of helplessness (4). Taken together, the data presented here give evidence that helpless mice fulfil the main criteria of a relevant animal model of depression: behavior in validated tests, sleep characteristics, and response to antidepressants. Moreover, the results point to an impairment of serotoninergic neurotransmission, notably through functional alterations of 5-HT1A autoreceptors. This genetic model of affective disorders may provide insights into the pathophysiology of depression and the effects of antidepressants.
Materials and Methods
Animals.
Mice selectively bred in our facilities (Unité Mixte de Recherche Centre National de la Recherche Scientifique 6036, Rouen, France) for high or low spontaneous helplessness in the TST were derived from an original stock of Swiss albino CD1 mice (Charles River Breeding Laboratories). They were kept on a 7 a.m.–7 p.m. light cycle with food and water ad libitum. For breeding, male and female mice were housed together in pairs. When not under experimentation, they were kept in same-sex groups. Testing was performed between 9 a.m. and 5 p.m. and was in accordance with the European Community Council Directive of November 24, 1986 (86/609/EEC).
Selection and Breeding.
Selective breeding was initiated in April 1995 with the testing of 92 male and 58 female adult CD1 mice (8). The chosen selection criteria, which were the same at each generation, were a high immobility score (>115 sec) for HL and a low immobility score (<35 sec) for NHL in TST. Each mouse of any generation that entered the study was tested three times at weekly intervals. From the original CD1 mice, two pairs of HL and NHL mice were bred to produce the first generation (S1) of HL and NHL lines. To minimize inbreeding, animals that were least related to one another were mated to produce next generations but brother-sister mating was systematically done for each generation after S5. After S1 generation, there were 2–6 families in S2–S3 generations and 6–16 families in S4–S14 generations, with numbers of offspring per litter being ≈8.9 ± 0.5 and 9.4 ± 0.6 (means ± SEM.) for the 14 generations of HL and NHL mice, respectively. Pups were weaned at 21 ± 2 days, and animals were subjected to the first TST at age 35–50 days (middle adolescence). Mice did not go into combined experiments, except for correlation studies in the following order: TST, motor activity, FST. Unless otherwise stated, all experiments were performed with mice aged from 9 to 18 weeks.
Behavioral Studies.
The TST was performed with a computerized device (ITEM-LABO, Le Kremlin-Bicêtre, France) (9). Mice were suspended by the tail with adhesive tape to a hook connected to a strain gauge. The latter transmitted movements to a computer that calculated the total duration of immobility during a 6-min test. Mice that climbed up their tail during the test session (for example, at S10 generation HL mice: 7% of males, 11% of females; NHL mice: 1% of males, 2% of females) were withdrawn from the study. For the FST, mice were plunged individually into a vertical Plexiglas cylinder (25 cm high; 10 cm in diameter) filled with 9-cm-deep water (21–23°C). After the 6-min period of the test, they were removed and allowed to dry. Immobility (i.e., making only minimal movements to keep the head above water or floating) was measured for two 3-min periods by an observer blind to the condition of the mouse. Exploratory motor activity was monitored in the 20 × 20-cm arena of a Digiscan actimeter (Omnitech Electronics, Columbus, OH) during various periods, depending on the experimental design (10).
Sucrose Consumption Test.
Testing for sucrose consumption (single-bottle test) was performed with individually housed mice. Consumption of a 2% sucrose solution in water was measured during a 96-h period when a sweet bottle was given in place of water. Results are expressed as sucrose intake (g per kg of body weight).
Corticosterone Assay.
Corticosterone in serum from blood collected after decapitation was assayed as described (11).
Sleep and Wakefulness Analysis.
Electrodes for polygraphic sleep monitoring were implanted under general anesthesia as described in detail elsewhere (12). Recordings were performed in the home cage, after at least 10 days of habituation to the recording conditions. Animals were connected to the recording devices by means of a cable and a swivel allowing free displacements in the cage, and polygraphic recordings were performed by using an Embla system and the SOMNOLOGICA software (Flaga, Iceland). Spontaneous sleep–wakefulness cycles were recorded during 2 (for males) or 4 (for females) consecutive days (12-h light-dark cycle, light on at 7 a.m.). On the third (for males) or fifth (for females) day at 10 a.m., mice were gently awakened and left to sleep again to evaluate their REM sleep latency, which was defined as the time interval between sleep onset and the first REM sleep episode. Polygraphic tracings were scored every 15 sec as wakefulness, slow-wave sleep (light: SWS1, or deep: SWS2), and REM sleep (12).
8-Hydroxy-2-(di-n-propylamino)-tetralin (8-OH-DPAT)-Induced Hypothermia.
Core body temperature was measured at 23 ± 1°C ambient temperature by means of a thermocouple probe (Betatherm, Galway, Ireland, 1.5 mm in diameter) inserted 2 cm into the rectum while gently holding the animal (13). Temperature was stable within 5–7 sec, and the whole procedure lasted ≈10 sec. Data were obtained every 15 min, from 30 min before (for baseline), until 70 min after s.c. injection (at 11 a.m.) of 8-OH-DPAT (dissolved in 0.1 ml of saline), and then every hour until ≈6 h posttreatment. 8-OH-DPAT-induced hypothermia was calculated as the maximal decrease (from baseline) in body temperature during 70-min postinjection. Each animal received saline alone and all tested doses of 8-OH-DPAT with a minimal 8-day washout period between two successive treatments. The effects of pretreatment with the 5-HT1A antagonist WAY 100635 (0.1 mg/kg s.c. 30 min before 8-OH-DPAT) on the response were also examined. Finally, the hypothermia induced by the muscarinic agonist (5) arecoline (0.1–10 mg/kg i.p.) was assessed by using the same conditions.
Electrophysiological Study.
Electrophysiological recordings of spontaneously active dorsal raphe nucleus (DRN) neurons were performed as described (14). Baseline firing activity was evaluated for 5–10 min. Then, in intravenously cannulated animals, boli of increasing doses of 8-OH-DPAT (dissolved in 10 μl of saline) were injected every 2 min until complete cessation of firing. At that time, the serotoninergic nature of the recorded neuron was checked by means of an i.v. injection of WAY 100635 (expecting a restoration of the cell firing; see ref. 14). Only one neuron per animal contributed to this 5-HT1A challenge, and histological examination of the recording site was subsequently performed to ascertain that the recorded neuron belonged to the DRN. In a set of experiments, chronic treatment with fluoxetine (10 mg/kg i.p. daily for 21 days) followed by a 48-h washout period was performed before the electrophysiological testings.
Autoradiography.
Autoradiographic labeling of 5-HT transporter (15) was achieved on frontal brain sections (10 μm) with 1 nM [3H]citalopram (Amersham Pharmacia; 85 Ci mmol−1) as radioligand without or with 10 μM fluoxetine for the determination of nonspecific labeling. Sections were applied onto Hyperfilm-3H (Amersham Pharmacia) for 4 weeks to generate autoradiographs. Quantification of radioactive labeling was made by means of an image analysis system (Alcatel TITN Answare, Samba Technologies, Meylan, France). Optical densities were converted into fmol/mg tissue according to a calibration curve obtained with tritiated standard strips (Amersham Pharmacia).
Measurement of Tissue Levels of 5-HT and Its Metabolite.
The prefrontal cortex and the hippocampus were dissected immediately after decapitation and frozen in isopentane (−32°C). The preparation of homogenates and HPLC determinations were made as described (16).
Drugs.
Solutions of imipramine and desipramine (Novartis, Rueil Malmaison, France), citalopram (Lundbeck, Copenhagen), fluoxetine (Lilly Research Laboratories, Indianapolis), paroxetine (SmithKline Beecham), 8-OH-DPAT (Research Biochemicals, Natick, MA), WAY 100635 (N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl) cyclohexane carboxamide, Wyeth), and arecoline (Sigma) were prepared daily. Doses always refer to the free bases.
Statistical Analyses.
Results are expressed as mean ± SEM. Statistical analysis of differences between groups was performed by using ANOVA (with one or two factors and with or without repeated measures where appropriate). Where F ratios were significant, statistical analyses were extended by using Newman–Keuls multiple comparison tests. Significance levels were set at P < 0.05.
Results
Behavioral Features of Selectively Bred HL and NHL Lines.
After 10 generations of selective breeding, a 40-fold average difference in immobility time in the TST was achieved between the selectively bred HL and NHL mice (Fig. 1A). Indeed, immobility scores in TST were extremely low with a tendency to decrease even further across the generations among mice of the NHL line. In contrast, immobility scores of HL mice increased moderately until the S10 generation (Fig. 1A). In addition, the percentages of animals showing scores corresponding to the criteria for selection rose gradually in both HL and NHL mice as breeding continued, and HL female mice were more immobile than HL male mice at all generations up to the 11th one (Fig. 1B). Realized heritability (17) over 10 generations of selection for HL and NHL behavior was 0.099 and 0.027, respectively. As illustrated in Fig. 1C, immobility scores of selectively bred HL and NHL mice determined in three trials at weekly intervals under drug-free conditions exhibited a great stability across trials. Middle adolescent and adult mice exhibited similar immobility scores (not shown).
Figure 1.
The behavioral traits of HL and NHL mice selectively bred move apart quickly and show a great stability. (A) Evolution of patterns of immobility shown by selectively bred male (M) and female (F) mice from S1 to S14 generation in the TST. Results are mean ± SEM of first trial data for mice (n = 6–44 at generations S0 to S3; n = 53–338 at generations S4–S14) reaching the selection criterion three times at weekly intervals. (B) Percentage of mice reaching the selection criteria for HL (Upper) or NHL (Lower) at each generation. (C) Test-retest reliability of the duration of immobility of HL and NHL mice (S2 and S14 generations are depicted) in the TST. Recordings were for 6 min at weekly intervals (W1, W2, and W3). Error bars (SEM) are smaller than the symbols in size.
The FST is another “behavioral despair” paradigm (18) used to identify antidepressants. Thus, we tested whether immobility scores in the FST would be in accord with those obtained in the TST (Fig. 2 A and B). Immobility times were ≈2.5-fold longer in HL than NHL mice during FST performed at S14 but not S2 generation. A strong correlation (r = 0.848; P < 0.001) was found between immobility times in both paradigms for animals at S14 generation; however, immobility times in the two “despair” paradigms were not correlated (P > 0.05) when the two substrains were analyzed separately (HL line: r = 0.200; NHL line: r = 0.286). The performance in these two behavioral tests was not influenced by motor activity, because locomotor activity in an environment was similar in both lines at S2 generation but diverged later on toward lower levels in the HL line or a tendency to higher levels in the NHL line (Fig. 2C). Differences in horizontal activity between lines were not counterbalanced by opposite differences in vertical activity (Fig. 2C). On the other hand, locomotor activity of mice measured in the open field was not correlated (P > 0.05) with their immobility times either in the TST (HL line: r = 0.190; NHL line: r = 0.165) or the FST (HL line: r = 0.100; NHL line: r = 0.325; see also ref. 19).
Figure 2.
Differences in responses to behavioral tests amplify between HL and NHL mouse lines when pressure selection continues and differing patterns of sleep–wakefulness rhythms are revealed. Duration of NHL and HL mouse immobility recorded for 6 min in the TST (A) and FST (B) for the same animals (n = 8–13) at S2 and S14 generations. (C) Locomotor activity of NHL and HL mice in the open field. Horizontal (exploratory) and vertical activities (expressed as the number of beam crossings) were recorded for 45 min (n = 8–14) at S2 and S14 generations. (D) Amounts of the different states of vigilance, wakefulness, slow-wave sleep (light: SWS1, or deep: SWS2), and REM sleep (REMS). Data are expressed as min per 24 h. Error bars represent SEM. Statistical significance was determined by two-way ANOVAs. Because there were no interactions between factors, asterisks (***, P < 0.001) depict main effect of substrain and sharps (#, P < 0.05) depict main effect of sex.
To validate the immobility models that may not reflect only helplessness, we next addressed the issue of anhedonia (20) by examining whether consumption of a sucrose solution would differ between HL and NHL mice. At S13 generation, average daily sucrose intake (g per kg of body weight) during a 96-h period was significantly lower in HL mice: males, 199 ± 11.9 vs. 250 ± 13.1 in NHL mice; P = 0.05; Females: 291 ± 19.5 vs. 419± 22.6 in NHL mice, P < 0.001, n = 24 mice per group). The difference was larger between females, as revealed by the significant gender × substrain interaction [F (1, 92) = 4.2, P = 0.044].
Altered Patterns of Circadian Sleep–Wakefulness Rhythms in HL Mice.
Sleep disorders are cardinal symptoms of depression and alterations of sleep architecture in depressed patients have been well described (21). Polygraphic recordings of males and females indicated that both lines of mice exhibited a classical circadian rhythm of sleep and wakefulness (not shown). However, substantial differences were observed between the two lines with two-way ANOVAs revealing no interaction between factors in conjunction with no gender effect. Thus, HL mice spent less time in wakefulness and more in SWS1 and REM sleep than NHL mice (Fig. 2D), and exhibited a significant [F (1, 27) = 35.9, P < 0.001] decrease in REM sleep latency (in min, HL: 14.0 ± 1.7 and 15.4 ± 1.8 in males and females, respectively; NHL: 30.1 ± 1.3 and 31.5 ± 2.8 in males and females, respectively, n = 5–12 in each group). In addition, sleep was more fragmented in HL females as illustrated by significantly more frequent episodes of wakefulness (approximately +50%, P < 0.001) than in NHL females.
Because in behavioral tests females of the HL line were generally more affected than males, the following studies were performed mainly with females.
Hormonal and Neurochemical Studies in HL and NHL Mice.
We examined whether HL mice would exhibit abnormalities of the hypothalamic-pituitary-adrenal axis, a hormonal system that is often dysregulated in depressed patients (22). Under basal conditions, seric corticosterone concentrations (μg/100 ml) were higher in HL mice (males: 9.1 ± 2.0 vs. 6.0 ± 1.0 in NHL; females: 19.1 ± 3.3 vs. 12.2 ± 1.8 in NHL, n = 20–22 in each group). A two-way ANOVA revealed a significant main effect of substrain on corticosterone levels [F (1, 78) = 4.9, P = 0.029], along with a significant overall gender effect [F (1, 78) = 13.3, P < 0.001], with females displaying higher levels.
We also characterized some of the neurochemical features of the 5-HT system that could be associated with the differential behavior of HL vs. NHL female mice (23). 5-HT levels in prefrontal cortex (PCx) and hippocampus (Hipp) were significantly higher (P < 0.05) in HL mice (in ng/mg protein: PCx: HL, 5.9 ± 0.4 vs. NHL, 4.3 ± 0.5; Hipp: HL, 8.5 ± 0.8 vs. NHL, 5.8 ± 0.6; n = 7–10 mice per group). This was associated with a lower 5-HT metabolism index (estimated by the 5-HIAA/5-HT ratio) notably in the Hipp (HL, 0.29 ± 0.02 vs. NHL, 0.36 ± 0.03, P < 0.05). In addition, densities (expressed as fmol/mg tissue equivalent) of the 5-HT transporter were higher in the motor cortex of HL mice (HL, 54 ± 2 vs. NHL, 44 ± 3; P < 0.05, n = 7–8).
Functional Characteristics of 5-HT1A Autoreceptors.
Somatodendritic 5-HT1A autoreceptors play a critical role in 5-HT neurotransmission and in the physiopathology of depression (24, 25). Thus, we investigated their functional status in female mice of both lines, by examining the effects of 5-HT1A receptor activation on body temperature (26) and on serotoninergic neuronal firing in the DRN (14, 27).
Body Temperature.
Baseline values were slightly lower in HL (36.6 ± 0.1°C) than in NHL (37.2 ± 0.1°C) mice (P < 0.05, n = 15 in each group). Acute administration of 8-OH-DPAT (0.4–2 mg/kg s.c.) induced a dose-dependent temperature decrease during the first 30–60 min, which was prevented by pretreatment with WAY 100635 (0.1 mg/kg s.c.; data not shown). The 8-OH-DPAT-induced hypothermia was of larger (P < 0.01) amplitude (Fig. 3A) and lasted longer (2 h 30 min vs. 1 h after 0.4 mg/kg, not shown) in HL compared with NHL mice. In contrast to 8-OH-DPAT, arecoline (0.1–10 mg/kg i.p.; ref. 28) induced the same hypothermia in both strains (data not shown).
Figure 3.
Functional status of 5-HT1A autoreceptors in HL and NHL female mice. (A) Dose-dependent hypothermia induced by s.c. injection of 8-OH-DPAT. Hypothermia (°C) is expressed as the difference from baseline (n = 5–6). (B) 5-HT1A autoreceptor-mediated inhibition by 8-OH-DPAT (0–48 μg/kg i.v.) of DRN 5-HT neuron firing in naive mice. Firing inhibition is expressed as percent of baseline frequency (n = 6–7 animals in each group). *, P < 0.05; ***, P < 0.001 compared with NHL mice, by using two-way ANOVAs.
Electrophysiological Recordings.
The spontaneous firing rate of serotoninergic neurons in the DRN was identical in HL and NHL mice (in spikes per second: 1.5 ± 0.1, n = 29, and 1.3 ± 0.1, n = 24, respectively). However, the inhibitory response to i.v. injection of 8-OH-DPAT was significantly different between the two lines, with a shift to the left of the dose-response curve in HL compared with NHL mice (in μg/kg i.v.: IC50 = 3.5 ± 0.6, and 9.4 ± 1.0, respectively, P < 0.01, Fig. 3B).
Effects of Antidepressants.
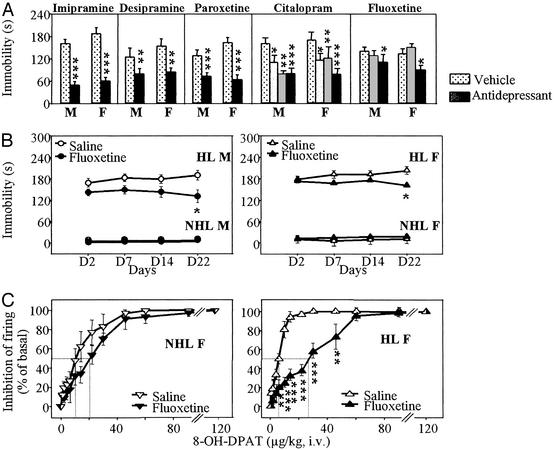
Acute administration of tricyclic antidepressants (imipramine, desipramine) as well as 5-HT reuptake inhibitors (citalopram, paroxetine, fluoxetine) reduced immobility of HL mice in the TST. However, fluoxetine was active only at the highest dose tested, 40 mg/kg i.p. (Fig. 4A). In contrast, under chronic conditions, 10 mg/kg fluoxetine i.p. (daily for 21 days) was enough to reduce significantly the immobility of HL mice (but not that of NHL mice, Fig. 4B). In addition, this treatment significantly decreased the potency of 8-OH-DPAT to inhibit the firing of DRN serotoninergic neurons in HL (in μg/kg i.v.: IC50 = 23.7 ± 5.9 after chronic fluoxetine, vs. 6.4 ± 0.8 in saline-treated controls, P < 0.01), but not NHL mice (Fig. 4C).
Figure 4.
Both male and female HL mice respond to acute and chronic antidepressant treatments. (A) Acute treatments (30 min before test) with antidepressants reduce immobility times of HL mice in the TST. Mice received i.p. vehicle, imipramine (30 mg/kg), desipramine (30 mg/kg), paroxetine (10 mg/kg), citalopram (10, 20, and 40 mg/kg), or fluoxetine (20 and 40 mg/kg). Multiple doses of citalopram and fluoxetine were used because these drugs were chosen as candidates for chronic studies. Statistics used two-way ANOVAs. Because, in any case, neither interactions between factors drug and sex nor significant main effect of sex occurred, asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001) show main effect of drug treatments. (B) Chronic treatment (once daily for 21 days, followed by a 24-h washout period before test) with fluoxetine (10 mg/kg) reduces immobility times of male and female HL mice (n = 9–13) in TST. Statistics used one-way ANOVA: No symbol, P > 0.05; *, P < 0.05 compared with vehicle. (C) 5-HT1A autoreceptor-mediated inhibition of DRN 5-HT neuron firing occurring 2 min after 8-OH-DPAT (cumulative i.v. doses) in female mice that had been treated with saline or fluoxetine (10 mg/kg/day i.p.) for 3 weeks. A significant effect of treatment occurred in HL (n = 6 in each group) but not in NHL (n = 6–7) mice. **, P < 0.01; ***, P < 0.001 compared with saline treatment, by using one-way ANOVA.
Discussion
The results reported herein strongly substantiate the hypothesis that the HL line of mice should be a useful tool to provide insights into the neurobiological mechanisms that underlie depression and to assess the antidepressant potentialities of treatments. Indeed, the great stability across trials observed in both HL and NHL mice indicates that they may be helpful in drug-screening procedures. In addition, congruent with the well known gender differences in vulnerability to depression, HL females were always found to be more immobile than HL males in the TST across generations. Strikingly, immobility scores were extremely low at early points in the selection process for the NHL line, suggesting that a limited number of genes are responsible for the NHL trait. In all probability, more genes likely contribute to the HL trait (see ref. 29). However, it should be noted that some of the phenotypic differences observed between the HL and NHL might be attributed to a genetic drift phenomenon during the selection step (30), rather than being related to the trait of selection. Further segregating cross-breeding studies are necessary to determine whether phenotypic differences between the two lines have a genetic relationship to the trait of selection.
Susceptibility to helplessness in HL and NHL mice was also found different in another behavioral despair paradigm, the FST. This widely accepted test cannot be considered as a simple variant of the TST (31), and the present results give further support to this belief. Indeed, the distinction between the two lines in the FST resulted from the evolution of the score of NHL mice along generations. This finding confirms the growing evidence that overlapping but not similar behavioral strategies are at work in the two tests (32, 33). Interestingly, selection pressure had also an effect on motor activity in the open field, suggesting that some aspects of exploratory activity and coping strategies in an aversive situation may be under the influence of a common set of genes (32). The results obtained for the HL line are in accordance with a decrease in motor activity already reported in other animal models of depression (5). Nevertheless, opposite changes in the FST scores on one hand and in locomotor activity on the other hand from S2 to S14 generation substantiate the view that motor activity contributes only moderately, if any, to the helpless and non helpless phenotypes in HL and NHL mice, respectively.
In a further attempt to validate the immobility tests as screening methods for our model, the inability to experience pleasure was assessed in HL and NHL mice by using a sweet reward. A defective hedonic behavior appeared in HL mice, which was more obvious in females than in males. In this respect, HL mice behaved like rodents subjected to chronic mild stress, which is considered today as a well validated model of depression (20).
HL and NHL mice exhibited different sleep structure and stability. The lighter and more fragmented sleep, and the decreased REM sleep latency in HL mice are in harmony with those of studies where helplessness was induced experimentally in rats (34, 35). Moreover, alterations of sleep–wakefulness patterns in HL mice mimic to some extent those observed in depressed patients such as the enhanced REM sleep “pressure” and the sleep fragmentation (21). In addition, hyperactivity of the hypothalamic-pituitary-adrenal axis has been reliably observed in patients with major depression (22). Elevated serum corticosterone levels in HL mice points to a disturbance in the hypothalamic-pituitary-adrenal axis, which may have pathogenic importance.
We next investigated whether these behavioral characteristics may be partly linked to alterations in neurobiological indices related to central serotoninergic transmission in the DRN and limbic regions of the brain. Serotonin has been considered for decades now as key candidate in the physiopathology of depression (23), and may contribute to the different behavior of HL vs. NHL mice. Results reported herein regarding neurochemical indices of 5-HT system show that differences between HL and NHL mice concerned limbic-related structures known to be involved in affective disorders (36).
Previous investigations have shown that the brain density of 5-HT1A receptors, especially in the DRN, is significantly higher in HL than in NHL mice (37). Functional consequences of this difference were assessed in the present study. We found that acute administration of 8-OH-DPAT dose-dependently reduced body temperature to a greater extent in HL than in NHL mice, and that this effect was prevented by selective blockade of 5-HT1A receptors. Indeed, in mice, 8-OH-DPAT-induced hypothermia is specific of 5-HT1A autoreceptors (26), and the observed differences support the idea that these receptors are supersensitive in HL mice. In line with this interpretation, 8-OH-DPAT was more potent to inhibit the firing of DRN serotoninergic neurons in HL versus NHL mice. Through the action of endogenous 5-HT, such a hypersensitivity might also account for the (slightly) lower body temperature in naive HL compared with NHL mice. Supersensitivity of 5-HT1A autoreceptors has already been reported in other animal models of depression (5, 29, 38) as well as in depressed patients (39). The resulting increase in 5-HT1A-mediated inhibitory feedback on serotoninergic neuronal firing would lead to a reduction in central 5-HT neurotransmission, as actually supported by lower 5-HIAA/5-HT ratio in the brain of HL mice. In contrast, no differences between the two lines were found with regard to the hypothermic effect of arecoline (5, 29), thereby suggesting unaltered muscarinic-mediated processes in HL versus NHL mice. However, further studies are needed for a more complete analysis of neurotransmitter systems underlying behavioral differences between the two lines.
Finally, we examined how HL mice respond to antidepressant drugs. Acute administration of tricyclic antidepressants and 5-HT reuptake inhibitors shortened immobility of these mice in the TST, as expected from a relevant model for screening potential antidepressants (9). Furthermore, chronic treatment with fluoxetine significantly reduced both the immobility in the TST and the sensitivity of somatodendritic 5-HT1A autoreceptors in HL mice. These results are in accordance with previous data obtained in helpless rats (38) and represent another support to the hypothesis of a role for 5-HT1A autoreceptors in the mode of action of antidepressants (24).
In summary, these data suggest that HL mice might be of particular interest for investigating mechanisms underlying high susceptibility to helplessness. They should also facilitate the study of genetic aspects of vulnerability to despair, and beyond, should help in the identification of genes causally related to depression in humans. In addition, the HL line should be useful for assessing the potential antidepressant action of therapeutic strategies targeting psychoaffective disorders.
Acknowledgments
We thank the companies mentioned in Materials and Methods for their generous supply of drugs. This research was supported by grants from Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique, and Bristol–Myers Squibb Foundation (unrestricted biochemical research grant program). M.E.Y. was supported by grants from La Fondation pour la Recherche Médicale and from Sanofi-Synthélabo; D.P. was supported by a grant from La Fondation pour la Recherche Médicale, and S.B. was supported by a Ministère de l'Education Nationale de la Recherche et de la Technologie fellowship.
Abbreviations
- 5-HT
serotonin
- REM
rapid eye movement
- TST
tail suspension test
- NHL
nonhelpless
- HL
helpless
- FST
forced-swim test
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino)-tetralin
- DRN
dorsal raphe nucleus
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Henn F A, McKinney T. In: Psychopharmacology: The Third Generation of Progress. Meltzer H Y, editor. New York: Raven; 1987. pp. 687–695. [Google Scholar]
- 2.Kendler K S, Walter E E, Truett K T, Heath A C, Neale M C, Martin N G, Eaves L J. Am J Psychiatry. 1994;151:1605–1614. doi: 10.1176/ajp.151.11.1605. [DOI] [PubMed] [Google Scholar]
- 3.Sham P C, Sterne A, Purcell S, Cherny S, Webster M, Rijsdijk F, Asherson P, Ball D, Craig I, Eley T, et al. Twin Res. 2000;3:316–322. doi: 10.1375/136905200320565292. [DOI] [PubMed] [Google Scholar]
- 4.Willner P. Pharmacol Ther. 1990;45:425–455. doi: 10.1016/0163-7258(90)90076-e. [DOI] [PubMed] [Google Scholar]
- 5.Overstreet D H. Behav Genet. 2002;32:335–348. doi: 10.1023/a:1020262205227. [DOI] [PubMed] [Google Scholar]
- 6.Stéru L, Chermat R, Thierry B, Simon P. Psychopharmacology. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 7.Cryan J F, Markou A, Lucki I. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- 8.Vaugeois J-M, Odièvre C, Loisel L, Costentin J. Eur J Pharmacol. 1996;316:R1–R2. doi: 10.1016/s0014-2999(96)00800-x. [DOI] [PubMed] [Google Scholar]
- 9.Stéru L, Chermat R, Thierry B, Mico J-A, Lenègre A, Stéru M, Simon P, Porsolt R D. Prog Neuropsychopharmacol Biol Psychiatry. 1987;11:659–671. doi: 10.1016/0278-5846(87)90002-9. [DOI] [PubMed] [Google Scholar]
- 10.El Yacoubi M, Ledent C, Ménard J-F, Parmentier M, Costentin J, Vaugeois J-M. Br J Pharmacol. 2000;129:1465–1473. doi: 10.1038/sj.bjp.0703170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Cudennec C, Naudin B, Do Rego J C, Costentin J. Life Sci. 2002;72:163–171. doi: 10.1016/s0024-3205(02)02218-x. [DOI] [PubMed] [Google Scholar]
- 12.Boutrel B, Franc B, Hen R, Hamon M, Adrien J. J Neurosci. 1999;19:3204–3212. doi: 10.1523/JNEUROSCI.19-08-03204.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Q, Wichems C, Heils A, Lesch K P, Murphy D L. J Neurosci. 2000;20:7888–7895. doi: 10.1523/JNEUROSCI.20-21-07888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evrard A, Laporte A M, Chastanet M, Hen R, Hamon M, Adrien J. Eur J Neurosci. 1999;11:3823–3831. doi: 10.1046/j.1460-9568.1999.00800.x. [DOI] [PubMed] [Google Scholar]
- 15.D'Amato R J, Largent B L, Snowman A M, Snyder S H. J Pharmacol Exp Ther. 1987;242:364–371. [PubMed] [Google Scholar]
- 16.Naudon L, Leroux-Nicollet I, Raisman-Vozari R, Botton D, Costentin J. Synapse. 1995;21:29–36. doi: 10.1002/syn.890210105. [DOI] [PubMed] [Google Scholar]
- 17.Falconer D S, Mackay T F C. Introduction to Quantitative Genetics. Harlow, U.K.: Longman; 1996. [Google Scholar]
- 18.Porsolt R D, Le Pichon M, Jalfre M. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 19.Trullas R, Jackson B, Skolnick P. Psychopharmacology. 1989;99:287–288. doi: 10.1007/BF00442824. [DOI] [PubMed] [Google Scholar]
- 20.Willner P. Psychopharmacology. 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 21.Kupfer D J. Biol Psychiatry. 1975;11:159–174. [PubMed] [Google Scholar]
- 22.Arborelius L, Owens M J, Plotsky P M, Nemeroff C B. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 23.Hirschfeld R M. J Clin Psychiatry. 2000;61, Suppl. 6:4–6. [PubMed] [Google Scholar]
- 24.Pineyro G, Blier P. Pharmacol Rev. 1999;51:533–591. [PubMed] [Google Scholar]
- 25.Mann J J. Neuropsychopharmacology. 1999;21, Suppl. 2:99S–105S. doi: 10.1016/S0893-133X(99)00040-8. [DOI] [PubMed] [Google Scholar]
- 26.Goodwin G M, De Souza R J, Green A R. Nature. 1985;317:531–533. doi: 10.1038/317531a0. [DOI] [PubMed] [Google Scholar]
- 27.Sprouse J S, Aghajanian G K. Synapse. 1987;1:3–9. doi: 10.1002/syn.890010103. [DOI] [PubMed] [Google Scholar]
- 28.Overstreet D H, Daws L C, Schiller G D, Orbach J, Janowsky D S. Pharmacol Biochem Behav. 1998;59:777–785. doi: 10.1016/s0091-3057(97)00514-5. [DOI] [PubMed] [Google Scholar]
- 29.Eley T C, Plomin R. Curr Opin Neurobiol. 1997;7:279–284. doi: 10.1016/s0959-4388(97)80017-7. [DOI] [PubMed] [Google Scholar]
- 30.Henderson N D. Behav Genet. 1989;19:473–502. doi: 10.1007/BF01066250. [DOI] [PubMed] [Google Scholar]
- 31.Porsolt R D, Lenègre A. In: Experimental Approaches to Anxiety and Depression. Elliott J, Heal D J, Marsden C A, editors. London: Wiley; 1992. pp. 73–85. [Google Scholar]
- 32.Turri M G, Datta S R, DeFries J, Henderson N D, Flint J. Curr Biol. 2001;11:725–734. doi: 10.1016/s0960-9822(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 33.Yoshikawa T, Watanabe A, Ishitsuka Y, Nakaya A, Nakatani N. Genome Res. 2002;12:357–366. doi: 10.1101/gr.222602. [DOI] [PubMed] [Google Scholar]
- 34.Adrien J, Dugovic C, Martin P. Physiol Behav. 1991;49:257–262. doi: 10.1016/0031-9384(91)90041-l. [DOI] [PubMed] [Google Scholar]
- 35.Dugovic C, Maccari S, Weibel L, Turek F W, Van Reeth O. J Neurosci. 1999;19:8656–8664. doi: 10.1523/JNEUROSCI.19-19-08656.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drevets W C, Raichle M E. Psychopharmacol Bull. 1992;28:261–274. [PubMed] [Google Scholar]
- 37.Naudon L, El Yacoubi M, Vaugeois J-M, Leroux-Nicollet I, Costentin J. Brain Res. 2002;936:68–75. doi: 10.1016/s0006-8993(02)02548-9. [DOI] [PubMed] [Google Scholar]
- 38.Maudhuit C, Prévot E, Dangoumau L, Martin P, Hamon M, Adrien J. Psychopharmacology. 1997;130:269–275. doi: 10.1007/s002130050239. [DOI] [PubMed] [Google Scholar]
- 39.Stockmeier C A, Shapiro L A, Dilley G E, Kolli T N, Friedman L, Rajkowska G. J Neurosci. 1998;18:7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]