Abstract
Miltefosine (hexadecylphosphocholine) is the first orally active drug approved for the treatment of leishmaniasis. We have previously shown the involvement of LtrMDR1, a P-glycoprotein-like transporter belonging to the ATP-binding cassette superfamily, in miltefosine resistance in Leishmania. Here we show that overexpression of LtrMDR1 increases miltefosine efflux, leading to a decrease in drug accumulation in the parasites. Although LtrMDR1 modulation might be an efficient way to overcome this resistance, a main drawback associated with the use of P-glycoprotein inhibitors is related to their intrinsic toxicity. In order to diminish possible side effects, we have combined suboptimal doses of modulators targeting both the cytosolic and transmembrane domains of LtrMDR1. Preliminary structure-activity relationships have allowed us to design a new and potent flavonoid derivative with high affinity for the cytosolic nucleotide-binding domains. As modulators directed to the transmembrane domains, we have selected one of the most potent dihydro-β-agarofuran sesquiterpenes described, and we have also studied the effects of two of the most promising, latest-developed modulators of human P-glycoprotein, zosuquidar (LY335979) and elacridar (GF120918). The results show that this combinatorial strategy efficiently overcomes P-glycoprotein-mediated parasite miltefosine resistance by increasing intracellular miltefosine accumulation without any side effect in the parental, sensitive, Leishmania line and in different mammalian cell lines.
Leishmaniasis is one of the neglected diseases included in the World Health Organization's list of the top guns of antimicrobial resistance (www.who.int/infectious-disease-report/2000/ch4.htm). Fortunately, the current situation for the chemotherapy of leishmaniasis has been considerably improved with the development of miltefosine (hexadecylphosphocholine), the first highly effective oral drug approved against visceral (46) and cutaneous (44) leishmaniasis. However, a first case of in vitro Leishmania miltefosine resistance has already been described in a multidrug-resistant (MDR) line (38) and resistance can be very easily developed experimentally by either drug selection pressure (42) or mutagenesis (33). Miltefosine resistance in Leishmania is mainly due to a defect in drug internalization (31) as a consequence of either the overexpression of a P-glycoprotein (Pgp)-like transporter (LtrMDR1) (38), a drug efflux pump implicated in the MDR phenotype (5, 35), or to the malfunctioning of the recently discovered miltefosine transporter LdMT (33). Interestingly, LtrMDR1 inhibition sensitizes MDR parasites to miltefosine (38).
Pgps belong to the ATP-binding cassette (ABC) superfamily of transporters (19). They export a wide range of hydrophobic drugs from the cell, thus conferring an MDR phenotype on tumor cells (2) and protozoan parasites (6, 18, 36). Pgps consist of two homologous halves, each comprising a transmembrane domain (TMD) involved in drug efflux and a cytosolic nucleotide-binding domain (NBD) responsible for ATP binding and hydrolysis. Mammalian Pgp can be inhibited by reversal agents which compete with drug binding to the TMDs (14). However, these modulators only poorly sensitize the MDR phenotype in Leishmania parasites (35). In contrast, two different families of natural compounds, flavonoids and dihydro-β-agarofuran sesquiterpenes, are able to efficiently overcome the Leishmania MDR phenotype, probably by acting at different levels (35). Some flavonoid derivatives bind to a purified recombinant NBD from LtrMDR1 and interact with both the ATP-binding site and a vicinal hydrophobic region (7, 11, 34) with an affinity that correlates with their abilities to modulate drug accumulation and to reverse the resistance phenotype of a Leishmania tropica MDR line (34, 37). On the other hand, some sesquiterpenes efficiently overcome the Leishmania MDR phenotype (21, 38, 39) by increasing drug accumulation (21, 38); their binding to the TMDs of human Pgp has been suggested recently (27).
A main problem that has hampered the clinical use of many human Pgp inhibitors is related to their intrinsic cytotoxicity (14). To diminish such possible side effects, in the present study we have tested the ability of combined suboptimal doses of the above different modulators targeting both NBDs and TMDs within LtrMDR1 to increase drug accumulation and reversal of the parasite MDR phenotype while avoiding any toxic effect in mammalian cells. Preliminary structure-activity relationships have allowed us to design a new, potent flavonoid derivative with high affinity for the cytosolic NBDs. As modulators directed to the TMDs, we have used one of the most potent sesquiterpenes described, named C-3 (38), and we have also studied the effects of two of the most promising, latest-developed modulators of human Pgp, zosuquidar (LY335979) (8, 9) and elacridar (GF120918) (20, 40), currently used in clinical trials. The results show that this combinatorial strategy efficiently overcomes parasite miltefosine resistance by inhibiting drug efflux without any cytotoxicity in the parental nonresistant Leishmania line and in different mammalian cell lines.
MATERIALS AND METHODS
Chemical compounds.
Daunomycin (DNM) was purchased from Pfizer (Madrid, Spain), imidazole, N-acetyltryptophanamide, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and urea were from Sigma. IPTG (isopropyl-1-thio-β-d-galactopyranoside) was purchased from Roche. Edelfosine (1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine, ET-18-OCH3) was obtained from Bachem AG (Bubendorf, Switzerland). Miltefosine (hexadecylphosphocholine) and [14C]miltefosine were obtained from Zentaris (Frankfurt, Germany). 8-(1,1-Dimethylallyl)-dehydrosilybin [8-(1,1-DMA)-DHS] (see Fig. 2) was synthesized as described elsewhere (M.M. and D.B., unpublished data). Sesquiterpene C-3 (9α-benzoyloxy-8α,2-methylbutyroyloxy-1α,6β,15-triacetoxy-4β-hydroxydihydro-β-agarofuran) was isolated from Maytenus canariensis as previously described (17). Zosuquidar (LY335979) was kindly provided by Eli Lilly and Company (Indianapolis, IN) (to A.D.), and elacridar (GF120918) was kindly provided by GlaxoSmithKline (Madrid, Spain) (to F.G.). 2′-(3′)-N-Methylanthraniloyl-ATP (MANT-ATP) and 2′,3′-O-(2,4,6-trinitrophenyl)-ATP (TNP-ATP) were obtained as described previously (10). The pQE-30 plasmid, Escherichia coli M15/pREP4 cells, and Ni2+-nitrilotriacetic acid agarose gel were from QIAGEN.
FIG. 2.
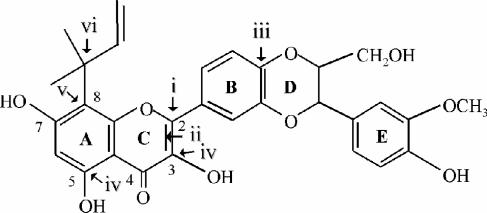
Rational design of 8-(1,1-DMA)-DHS. Chemical structure of the designed flavonoid with (i) ring B branched at position 2, (ii) an oxidized 2,3 bond, (iii) a monolignol unit adjacent to ring B, (iv) hydroxyl groups at positions 3 and 5, (v) a hydrophobic substitution at position 8, and (vi) 1,1-dimethylallyl as the hydrophobic group.
Parasite and cell culture.
Promastigote forms of a cloned L. tropica LRC strain (wild type [WT]) and a derivative MDR L. tropica DNM-R150 cloned line, maintained in the presence of 150 μM DNM to keep Pgp overexpression, were cultured and used as previously described (38). The modulation of alkyl-lysophospholipid (ALP) resistance and the sensitization to 150 μM DNM by reversal agents were monitored as described in reference 38 after a 72-h incubation period. Parasite viability after shorter miltefosine treatments was determined by the colorimetric MTT assay as previously described (21). Mammalian cell lines used in the cytotoxic assays were NIH 3T3, provided by I. Pastan (National Cancer Institute, National Institutes of Health, Bethesda, MD); epithelial MDCKII (25); epithelial-cell-like MCF-7 and MDA-MB-23 (4, 45); Vero (Cercopithecus aethiops ATCC CRL-1586); and mouse macrophage J774 (ATCC HB-197). All cell lines were cultured as previously described (27). Cytotoxic assays of combinations of inhibitors were performed by the MTT colorimetric assay as previously described (32) after a 72-h incubation period. Cell growth values are averages of two independent experiments done in quadruplicate with different batches of cells.
Overexpression and purification of the N-terminal NBD and binding assays. (i) Construction of expression vectors.
Amplification of the DNA encoding N-terminal NBD1 including the linker region (NBD1ext) was performed by PCR. The two primers specific for LtrMDR1 and corresponding to NBD1ext, stretching from Thr-417 to Lys-770, were 5′-GTCGACTCACCGAGTCTCGTGCTG-3′ and 5′-AAGCTTGTCCTTATTCATTTCCATCAG-3′, respectively. The PCR product was ligated into plasmid pQE-30 (QIAGEN), and the resulting plasmid, pQE30-NBD1ext, was restriction mapped and sequenced to confirm the expected sequence.
(ii) Overexpression, purification, and renaturation of NBD1ext.
E. coli M15/pREP4 cells were transformed with pQE30-NBD1ext and grown at 37°C in Terrific broth medium (41) containing 50 μg of ampicillin/ml and 25 μg of kanamycin/ml until the absorbance at 600 nm reached 0.7. Expression of NBD1ext was induced with 0.5 mM IPTG for 4 h at 37°C. Cells were harvested by centrifugation and resuspended (5 ml buffer/g pellet) in a buffer containing 10 mM potassium phosphate (pH 7.5), 10 mM β-mercaptoethanol, 1.3 mM benzamidine, 1 mM 1,10-phenanthroline, 57 μM phenylmethylsulfonyl fluoride, 48 μg/ml crude soybean trypsin inhibitor, 48 μg/ml aprotinin, and 20 μg/ml leupeptin. Cells were lysed with lysozyme (1 mg/ml) at room temperature for 20 min, and the solution was sonicated. NBD1ext was found as inclusion bodies that were solubilized in urea buffer (50 mM potassium phosphate [pH 8.0], 10 mM β-mercaptoethanol, 10 mM imidazole, 8 M urea). NBD1ext was purified by affinity chromatography in an Ni2+-nitriloacetic acid column equilibrated in urea buffer. The retained protein was eluted with an imidazole linear gradient of 0 to 100 mM in urea buffer. One-milliliter fractions were collected and analyzed by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. NBD1ext was renatured with 20 volumes of refolding buffer (50 mM potassium phosphate [pH 8.0], 10 mM β-mercaptoethanol, 10 mM EDTA) and concentrated with Centriprep Amicon 30 and dialyzed twice, first in refolding buffer without 10 mM β-mercaptoethanol and then in 10 mM potassium phosphate (pH 8.0)-1 mM EDTA. Dialyzed protein was aliquoted and stored at −80°C. Protein concentration was routinely determined by the method of Bradford with a Coomassie blue protein assay reagent kit from Bio-Rad.
(iii) Fluorescence emission measurements.
Experiments were performed at 25°C with an SLM-AMINCO series 2 spectrofluorimeter. The binding of the different compounds was monitored as previously described (34), except that 0.5 μM NBD1ext was used and the protein was excited at a wavelength of 295 nm and the emission wavelength was scanned in a range of 310 to 370 nm.
Western blot analysis.
Western blot analysis of crude Leishmania extracts was performed as previously detailed (30), with the polyclonal antibody against LtrMDR1 previously described by Chiquero et al. (5).
Electron microscopic analysis.
Log-phase cultures of wild-type and resistant L. tropica promastigotes were incubated at 28°C for 8 h in the absence or presence of 150 μM miltefosine. For electron microscopy, 2 × 108 cells of each sample were harvested by centrifugation at 2,000 × g for 15 min at 4°C, washed twofold by resuspension in ice-cold phosphate-buffered saline, and fixed with glutaraldehyde (2.5%) for 4 h at 4°C. After fixation, the cells were washed three times for 20 min at 4°C with 0.1 M cacodylate (pH 7.4). Postfixation was performed in 2% (wt/vol) osmium tetroxide (OsO4) for 2 h at room temperature. Subsequently, the cells were washed two times for 20 min; dehydrated in 50%, 70%, 90%, and 2 × 100% ethanol; and embedded in Epon 812. Ultrathin sections of 500 Å were cut on a Leica Ultracut S ultramicrotome, counterstained with uranyl acetate and lead citrate, and observed with a Zeiss 902 transmission electron microscope.
Intracellular [14C]miltefosine determination.
The internalization of [14C]miltefosine and the efflux of internalized [14C]miltefosine were measured as previously described (31). The effect of the cocktail of inhibitors on miltefosine accumulation was studied by incubating the parasites with [14C]miltefosine for 1 h with or without the modulators.
RESULTS
Radioactive miltefosine accumulation and efflux.
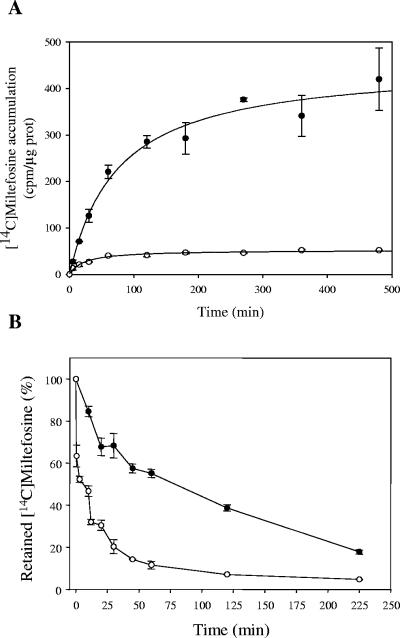
Pgps confer drug resistance by actively pumping drugs out of the cell, thus diminishing their intracellular concentration. Therefore, we determined the time-dependent accumulation of [14C]miltefosine in both wild-type and MDR Leishmania lines. Figure 1A shows that the level of miltefosine accumulation at saturating times was around 8.5-fold lower in the resistant parasites than in the wild-type line, thus explaining the resistance phenotype. In contrast to the results observed in a miltefosine-resistant L. donovani line with a defective inward translocation of the drug (31), the lower miltefosine accumulation described here was due to a higher efflux of the drug (Fig. 2B), probably as a result of the activity of LtrMDR1. In fact, when wild-type and MDR parasites were loaded under conditions that yielded similar amounts of intracellular drug and then incubated in drug-free culture medium, MDR parasites eliminated 80% of the accumulated [14C]miltefosine in 30 min, while wild-type parasites required around 7.5-fold more time to expulse the same amount of drug (Fig. 2B).
FIG. 1.
[14C]miltefosine accumulation and efflux in Leishmania lines. (A) Time-dependent accumulation of [14C]miltefosine. Labeling of wild-type (solid circles) and MDR (open circles) parasites was measured as described in Materials and Methods, and the [14C]miltefosine concentration, expressed in counts per minute per microgram of protein, was monitored at different times. All values represent the means ± the standard errors of two independent experiments, each of which was performed in duplicate. (B) Time-dependent [14C]miltefosine efflux. The outward transport of [14C]miltefosine was measured after preincubation of wild-type (solid circles) and MDR (open circles) parasites with [14C]miltefosine as described in Materials and Methods, and the decay in radioactivity was monitored at different times. The data are expressed as the percentage of the initial amount of [14C]miltefosine incorporated and represent the means ± the standard errors of two independent experiments, each of which was performed in duplicate.
Rational design and effect of a compound directed to the cytosolic domains of LtrMDR1.
Preliminary structure-activity relationships with the Leishmania MDR line have allowed the rational design of a flavonoid derivative meeting all of the requirements reported to increase interaction with the cytosolic NBDs of LtrMDR1, especially (i) ring B connected to position 2 of ring C (7, 34), (ii) oxidized 2,3 bond of ring C (34, 37) (interestingly, reduction of this 2,3 double bond of similar flavonoids also resulted in a decreased competitive inhibition of H+,K+-ATPase with respect to ATP [28]), (iii) a monolignol unit adjacent to ring B (37), (iv) hydroxyl groups at position 3 of ring C and position 5 of ring A (34) (this hydroxyl group also favored ATP mimetism [12, 43] and competitive inhibition of H+,K+-ATPase with respect to ATP [28]), and (v) a hydrophobic substitution at position 8 of ring A with 1,1-dimethylallyl, as deduced when comparing different prenyl substitutions (1,1-dimethylallyl > prenylation > geranylation) at different positions of ring A (position 8 > position 6) (37). The resulting compound, 8-(1,1-DMA)-DHS, was hemisynthesized starting from the therapeutic agent silybin (M.M., D.B., et al., unpublished data), which explains the additional OH at position 7 of ring A, known not to affect the interaction with the NBDs (7, 34), and its structure is show in Fig. 2.
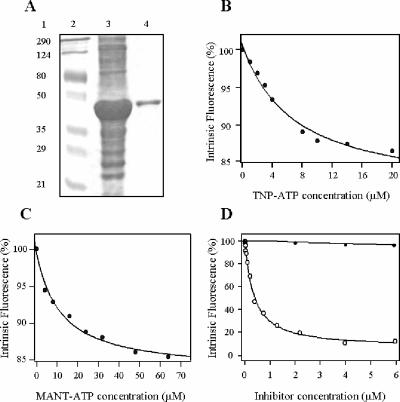
In order to study the interaction of this new compound with the cytosolic domains of LtrMDR1, the N-terminal NBD (NBD1ext) of the transporter was purified as a hexahistidine-tagged recombinant protein. As shown in Fig. 3A, the recombinant protein was highly overexpressed in E. coli upon induction of the bacteria with IPTG and mainly recovered as inclusion bodies. A protocol including urea denaturation and renaturation by quick dilution after affinity chromatography allowed the purification of 10 mg of protein per liter of bacterial culture. The binding of different compounds to renatured and purified NBD1ext was monitored by quenching of the protein's intrinsic fluorescence. NBD1ext bound the ATP analogues TNP-ATP (Fig. 3B) and MANT-ATP (Fig. 3C) with respective Kd values of 6.75 ± 1.80 μM and 11.48 ± 2.66 μM, similar to those previously described for LtrMDR1 NBD2 and NBDs isolated from other ABC transporters (35). Finally, the flavonoid derivative 8-(1,1-DMA)-DHS bound with high affinity to NBD1ext (Fig. 3D), with a Kd in the nanomolar range (0.109 ± 0.038 μM) and high maximal quenching (84.2%).
FIG. 3.
Interaction of purified recombinant NBD1ext with ATP analogues and LtrMDR1 inhibitors. (A) Overexpression and purification of recombinant NBD1ext. Coomassie-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis of inclusion bodies after IPTG induction, cell lysis, and recovery of the insoluble fraction (lane 3) and of purified and refolded NBD1ext (lane 4). Lane 2 corresponds to molecular mass markers (Bio-Rad) with the values (in kDa) indicated on the left (lane 1). (B, C) Interaction of recombinant NBD1ext with ATP analogues. The binding of TNP-ATP (B) or MANT-ATP (C) to 0.5 mM purified recombinant NBD1ext was determined by quenching of the protein's intrinsic fluorescence as described in Materials and Methods. (D) Concentration-dependent binding of the flavonoid 8-(1,1-DMA)-DHS (open circles) and the sesquiterpene C-3 (closed circles) to purified NBD1ext under the same conditions as described for panels B and C.
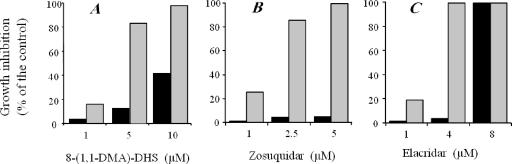
Flavonoid reversal effect on the MDR phenotype in Leishmania was studied by incubating resistant parasites with 150 μM DNM, the concentration routinely used to maintain this cell line (38), in the presence of increasing concentrations of 8-(1,1-DMA)-DHS (Fig. 4A). The flavonoid completely reversed the DNM resistance at 10 μM, although its intrinsic toxicity in the control parental wild-type line was also significantly high (40%). At 5 μM, its reversal effect was already high (more than 80% growth inhibition), while the side effect in the parental line was much lower (around 15%).
FIG. 4.
Reversal of DNM resistance by inhibitors in an MDR L. tropica line. Cell growth of either wild-type or resistant parasites was determined after incubation at 28°C for 72 h. Wild-type parasites (black bars) were incubated in the presence of different concentrations of inhibitors. Resistant parasites (gray bars) were incubated with the same concentrations of inhibitors in the presence of 150 μM DNM. The results are expressed as the percent growth inhibition observed in each cell line compared to the absence of a modulator (control cells). Data are the mean of three independent experiments performed in duplicate, with standard deviations below 10%.
Reversal effects of compounds targeting the transmembrane domains of LtrMDR1.
We recently described the ability of the sesquiterpene C-3 to increase drug accumulation in the resistant line, reversing the MDR phenotype (38). Here, fluorescence quenching studies showed that this compound did not interact significantly with recombinant NBD1ext (Fig. 3D), suggesting that its reversal effect was due to direct binding to the TMDs of the transporter.
We also studied the reversal effects of two of the latest-developed potent inhibitors of human Pgp known to interact with its TMDs, namely, zosuquidar (LY335979) (8, 9) and elacridar (GF120918) (20, 40). In contrast to other modulators of mammalian Pgp, both compounds were quite active in reversing DNM resistance in the MDR Leishmania line, elacridar being more toxic for the parental wild-type line (Fig. 4B and C).
Many human Pgp modulators which bind to its TMD are themselves also transported by the pump, requiring high concentrations for efficient inhibition, which can produce toxic effects in cells not overexpressing the transporter (14). However, the above compounds are probably not transported by LtrMDR1, as indirectly deduced from the absence of cross-resistance in the MDR line (data not shown): all four inhibitors were similar in toxicity in both MDR and parental wild-type Leishmania lines, elacridar being the more toxic compound (with a 50% inhibitory concentration of around 6.5 μM) and the sesquiterpene being the less toxic one (50% inhibitory concentration of about 150 μM).
Effects of combining suboptimal doses of inhibitors on the MDR phenotype.
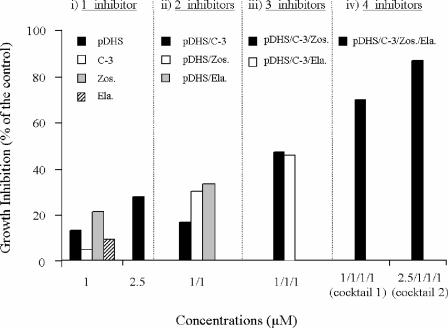
One of the main drawbacks of human Pgp modulators is their relative intrinsic cytotoxicity in the patients. Besides, these kinds of flavonoids and sesquiterpenes usually are more cytotoxic to mammalian cells than to Leishmania cells (unpublished results). In order to minimize such a problem, we have studied the reversal effect produced by combining concentrations of modulators that alone produced less than 30% reversal, but without any side effect in the parental wild-type line, as a control of intrinsic cytotoxic effects. The DNM-reversing ability of this drug combination is shown in Fig. 5. When 1 μM flavonoid, the compound directed against the NBDs, was combined with one of the three compounds targeting the TMDs (C-3, elacridar, or zosuquidar) at 1 μM, growth inhibition of 16 to 31% was observed. This reversal effect was increased to up to around 50% when 1 μM flavonoid was combined with two of the TMD-directed inhibitors at 1 μM. Finally, when all of the inhibitors were combined at 1 μM (cocktail 1) or the flavonoid concentration was increased to 2.5 μM and the TMD-directed inhibitors were kept at 1 μM (cocktail 2), additive reversal effects in the Leishmania MDR line were observed, leading to almost complete reversal of DNM resistance. This combination of suboptimal modulator doses was not cytotoxic at all for the parental wild-type line (less than 4% growth inhibition; data not shown), suggesting that the effect is really due to Pgp inhibition. Furthermore, only slight toxicity was produced by these inhibitor combinations in five different mammalian cell lines (Table 1).
FIG. 5.
Reversal of DNM resistance by combination of suboptimal doses of inhibitors in the MDR L. tropica line. Cell growth of resistant parasites was determined under the conditions described in the legend to Fig. 4, in the presence of different combinations of inhibitors. Data are the means of three independent experiments performed in duplicate, with standard deviations below 15%. pDHS, 8-(1,1-DMA)-DHS; C-3, sesquiterpene C-3; Zos., zosuquidar (LY335979); Ela., elacridar (GF120918).
TABLE 1.
Effects of inhibitor cocktails on different mammalian cell lines
| Cell line | Growth (% of control)a
|
|
|---|---|---|
| Cocktail 1 | Cocktail 2 | |
| MDCKII | 106.6 ± 2.3 | 110.1 ± 3.5 |
| MCF7 | 100.0 ± 5.2 | 93.9 ± 1.8 |
| MDA-MD231 | 103.0 ± 3.4 | 95.4 ± 2.5 |
| Vero | 90.0 ± 6.7 | 83.0 ± 0.6 |
| J774 | 87.2 ± 5.3 | 96.3 ± 1.2 |
| NIH 3T3 | 87.6 ± 2.1 | 78.5 ± 0.2 |
The results are expressed as percent growth relative to that of the control in the absence of inhibitors. The data are the average of three independent experiments ± the standard deviation.
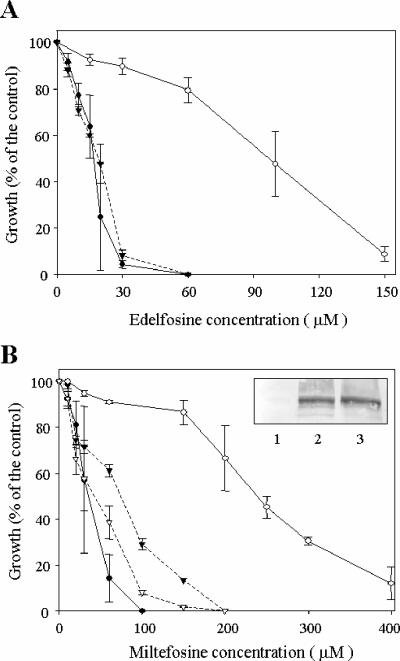
We then analyzed the ability of these cocktails of inhibitors to overcome miltefosine resistance. Seventy-two-hour growth inhibition experiments showed that the MDR Leishmania line has a significant profile of resistance to miltefosine and the related compound edelfosine (Fig. 6), as previously described. Coadministration of each modulator at 1 μM completely reversed its edelfosine resistance and efficiently reversed its miltefosine resistance. Cocktail 2, as previously shown with DNM, almost completely reversed its miltefosine resistance. A number of mammalian Pgp modulators, including the flavonoid quercetin, were found to decrease the expression of the transporter (22). In contrast, the miltefosine reversal effect observed with the cocktail of inhibitors was not related to any decrease in LtrMDR1 expression levels, as demonstrated by Western blot analysis with specific polyclonal antibodies against the transporter, in either the absence or the presence of the inhibitors (insert, Fig. 6B).
FIG. 6.
Reversal of ALP resistance in the MDR L. tropica line by cocktails of inhibitors. Cell growth of either wild-type parasites (solid circles) or resistant parasites deprived of DNM for 96 h (open circles) was determined after 72 h of incubation with different concentrations of the ALPs edelfosine (A) and miltefosine (B) and in the absence (circles) or in the presence (triangles) of cocktail 1 (solid triangles) or cocktail 2 (open triangles). Data are means of three independent experiments performed in duplicate, and standard deviations are represented by error bars. Inset in panel B, LtrMDR1 expression level in wild-type parasites (lane 1), resistant parasites (lane 2), and resistant parasites treated for 72 h with the cocktail 2 (lane 3), determined by Western blot assay.
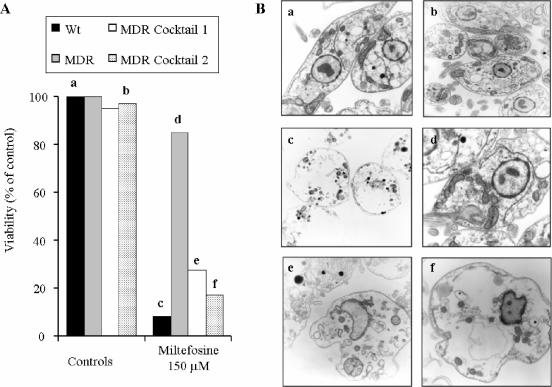
The reversal of miltefosine resistance was further studied by assaying parasite survival after shorter drug incubation times as determined by the parasites' ability to reduce MTT after the treatment (Fig. 7A) and by electron microscopic analysis of their ultrastructure (Fig. 7B). A miltefosine incubation time of 8 h was chosen because we previously showed that this was the time required to reach its steady-state accumulation in the parasites (Fig. 1A). The presence of both inhibitor cocktails did not significantly change the viability or structure of the parasites. Incubation with 150 μM miltefosine for 8 h almost completely killed control wild-type parasites, producing a cytotoxicity associated with loss of cellular content but maintaining the apparent membrane integrity. In contrast, the same drug concentrations only slightly decreased the ability to reduce the MTT in the MDR line, which correlated with a normal parasite ultrastructure. Finally, when both miltefosine and the inhibitor cocktail were assayed together, the effects were similar to those observed in the wild-type line, with the only exception that nuclei were more easily distinguished in theses parasites.
FIG. 7.
Effects of inhibitor cocktails on parasite survival after short miltefosine incubation times. (A) Cell viability of either WT parasites or resistant parasites deprived of DNM as described in the legend to Fig. 2D (MDR) and incubated in the absence or presence of either cocktail 1 or cocktail 2 was determined by their ability to reduce MTT after 8 h of incubation in the presence or absence of 150 μM miltefosine. Data are expressed as percent cell viability with respect to the viability measured for the controls (WT or MDR parasites without any treatment). (B) Before addition of MTT, 5 × 108 million parasites were separated and observed by electron microscopy as described in Materials and Methods. Each lowercase letter (a to f) in panel A corresponds to a part of panel B, as follows: (a) wild-type parasites without treatment (magnification, ×10,000), (b) resistant parasites incubated with cocktail 2 (magnification, ×10,000), (c) wild-type parasites treated with 150 μM miltefosine (magnification, ×10,000), (d) resistant parasites treated with 150 μM miltefosine (magnification, ×12,500), (e) resistant parasites incubated with cocktail 1 and 150 μM miltefosine (magnification, ×20,000), and (f) resistant parasites incubated with cocktail 2 and 150 μM miltefosine (magnification, ×20,000).
Effects of combining suboptimal doses of inhibitors on miltefosine accumulation.
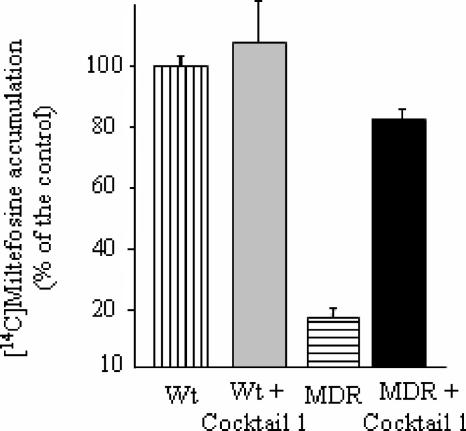
We finally analyzed the effect of the inhibitors on the intracellular accumulation of [14C]miltefosine. Wild-type and MDR parasites were therefore incubated with [14C]miltefosine for 1 h in the absence or presence of a cocktail containing each modulator at 1 μM. As shown in Fig. 8, the level of miltefosine accumulation in the resistant line after 1 h of incubation with the drug was only 20% of that measured for the wild-type line. In the presence of the combination of inhibitors, the level of miltefosine accumulation was increased around fourfold in the resistant line, reaching 82.3% of that observed for the wild-type controls. In contrast, coincubation with the modulator cocktail increased miltefosine uptake only 1.1-fold in the WT line, indicating that the reversal effect was specific for LtrMDR1 inhibition. Each of the four modulators produced only a partial effect when incubated alone at 1 μM in the resistant line, increasing miltefosine uptake between 1.3- and 1.5-fold (data not shown).
FIG. 8.
Effect of a cocktail of inhibitors on [14C]miltefosine accumulation. The uptake of [14C]miltefosine in wild-type and MDR parasites was measured for 1 h in the absence (vertical and horizontal shaded bars, respectively) or presence (gray and black filled bars, respectively) of cocktail 1, as described in Materials and Methods. The data shown are the means and standard errors of three independent experiments, each performed in duplicate, and are expressed as percent [14C]miltefosine accumulation with respect to the accumulation measured for the control.
DISCUSSION
The recent approval of miltefosine to treat visceral leishmaniasis in India led to the goal of eliminating the disease in a few years (16). However, two points suggest that this prevision might have been too optimistic; i.e., (i) miltefosine has a long terminal half-life which makes subtherapeutic levels remain for several weeks after a recommended 4-week course (3), and (ii) miltefosine resistance is easily developed experimentally through different mechanisms (33, 38, 42), and therefore its extensive and inappropriate use as a single agent in India might lead to the rapid emergence of widespread resistance (3). Specific inhibition of proteins involved in such a resistance, like LtrMDR1 (38, this paper), might help to overcome this problem.
We have previously demonstrated the involvement of LtrMDR1 overexpression in the miltefosine resistance of an MDR Leishmania line (38). In this paper, we show the direct involvement of this transporter in the level of miltefosine accumulation in L. tropica, as the resistant line presents a higher miltefosine efflux rate that leads to a reduced level of drug accumulation, and the specific inhibition of LtrMDR1 by the cocktail of inhibitors restores the uptake of [14C]miltefosine to levels close to that of the wild-type line. To our knowledge, this is the first report showing outward transport of the drug as a mechanism of miltefosine resistance in any cell type.
Our previous results concerning LtrMDR1 modulation suggested the presence of two different main targets for the binding of inhibitors to this ABC transporter: the drug-binding site(s) within the TMDs and the cytosolic NBDs (35). In addition, there will probably be different specific binding sites within these TMDs able to interact with drugs and/or modulators, as described for mammalian Pgps (29). The NBDs also contain, in addition to the ATP site, a vicinal hydrophobic binding region able to interact with nontransported hydrophobic steroids, protein kinase C inhibitor derivatives, and hydrophobic flavonoids (as reviewed in references 14 and 35). We therefore decided to combine suboptimal doses of different modulators targeting both NBDs and TMDs of LtrMDR1, in order to increase drug accumulation and induce reversal of the MDR phenotype, especially related to miltefosine resistance, while avoiding potential toxic effects in mammalian cells, an important drawback associated to Pgp inhibitors. To explore this possibility, we have rationally designed, as a modulator directed to the NBDs, a new compound meeting all of the requirements that had been shown to increase flavonoid interaction with the cytosolic NBDs of LtrMDR1, and therefore the reversal activity on the MDR of the parasite (Fig. 2). This new flavonoid showed the highest affinity ever described for a cytosolic domain of LtrMDR1 and the best reversal effect on DNM resistance in the MDR Leishmania line. Indeed, the Kd was around threefold lower than that observed with the same NBD1ext for 8-(3,3-DMA)-DHS, the previously most potent flavonoid derivative, also correlating with a twofold higher reversal of DNM resistance (data not shown). All of these data also support the ideas that the flavonoid reversal effect is correlated with a direct interaction with the cytosolic domains of LtrMDR1 and that both NBD1 and NBD2 can be used as drug targets for inhibitor design. As expected for an NBD-targeted compound, this flavonoid derivative does not seem to be transported by LtrMDR1, an interesting property for any inhibitor of these proteins (14). The structure-activity relationships shown here are clearly different from those reported for the interaction of flavonoids with other ABC transporters involved in mammalian MDR such as BCRP/ABCG2 (1) and MRP1 (47), where flavonoid inhibitory effects are probably due to binding to the TMDs. As modulators directed to the TMDs of the transporter, we have chosen first the sesquiterpene C-3. This compound efficiently overcame the MDR phenotype of the Leishmania line by modulating drug accumulation (38). Although this compound does not contain some of the general chemical features described for many MDR-reversing agents, such as a conjugated planar ring or a substituted tertiary amino group (15), its low binding to NBD1ext (Fig. 3D), together with its efficient competition with [3H]azidopine photolabeling of human Pgp (27), strongly supported an interaction with the TMDs of the transporter. This interaction at the TMDs, however, does not seem to lead to transport of the compound. We also analyzed the reversing effect of some new modulators of human Pgp that are known to interact with its TMDs and not to be transported (8, 9, 20, 40). While conventional Pgp inhibitors such as verapamil, cyclosporine, and quinidine were not very efficient at reversing the resistance phenotype in Leishmania (35), we show here that the latest-developed modulators zosuquidar (LY335979) and elacridar (GF120918) constitute new classes of promising reversal agents in these parasites.
Finally, we have shown that combining the flavonoid with the other three selected compounds, either separately or together, led to additivity of their reversing effects in the Leishmania MDR line, reaching complete sensitization to miltefosine, without producing any cytotoxicity in either the parental wild-type line or various mammalian cell lines. These results agree with the studies of Stein et al., who combined low, nontoxic, concentrations of up to 18 known human Pgp modulators, with cumulative effects on MDR reversal (26). The authors also detected cooperative, competitive, and uncompetitive interactions between the modulators (13, 23), probably due to the presence of different interacting sites for these agents within Pgp. A more detailed analysis of the mechanism of LtrMDR1 inhibition produced here by each of the inhibitors developed, alone and in combination, will require LtrMDR1 overexpression and purification, which is in progress. The use of combinations of chemosensitizers at nontoxic levels has also been efficiently used to overcome chloroquine resistance in Plasmodium falciparum and proposed to be a viable treatment to restore the efficacy of this drug in patients with malaria (48). Although the use of modulators to chemosensitize drug-resistant parasites is a very promising therapeutic strategy (recently reviewed in reference 24), their effect on the pharmacokinetic parameters of concomitantly administered antiparasitic drugs have to be investigated before they can be clinically applied.
In conclusion, we have shown that it is possible to overcome LtrMDR1-mediated miltefosine resistance in Leishmania, characterized by a high miltefosine efflux rate that leads to diminished drug accumulation in the parasite, by targeting different domains of the transporter with suboptimal doses of inhibitors, avoiding any toxic effect in the parental wild-type line and in different mammalian cell lines.
Acknowledgments
This work was supported by EU grant QLRT-2000-01404 to F.G., by Spanish grants SAF2001-1039 to S.C., SAF-2003-04200-CO2-01 and SAF2001-4562-E to F.G., and FIS Network RICET C03-04, by EU Marie Curie Research Training Network grant MRTN-CT-2004-005330 to F.G., by European reintegration grant MERG-CT-2004-513607 to F.G., by Plan Andaluz de Investigación (Cod. CVI-130), by French-Spanish PAI-Picasso grant 05149ZA (interministerial agreements between A.D. and F.G.), and by the Association pour la Recherche sur le Cancer (ARC3519 to A.D.). F.C.S was the recipient of a fellowship from the Fondo de Investigaciones Sanitarias (FIS), A.P.-T. was supported by the Agencia Española de Cooperación Internacional (Becas MUTIS), F.J.P.-V. and F.M.M. were supported by the Ministerio de Educación y Cultura, M.M. was supported by the French Ministry of National Education and Technological Research, and B.I.B. was supported by EU Marie Curie Host Fellowships (QLK2-CT-2001-60091).
We acknowledge Pharmacia-Spain (Barcelona, Spain), GlaxoSmithKline (Madrid, Spain), and Eli Lilly and Company (Indianapolis, IN) for the kind gift of DNM, elacridar (GF120918), and zosuquidar (LY335979), respectively. Also, we are grateful to Zentaris (Frankfurt, Germany) for providing the miltefosine used in this study.
REFERENCES
- 1.Ahmed-Belkacem, A., A. Pozza, F. Munoz-Martinez, S. E. Bates, S. Castanys, F. Gamarro, A. Di Pietro, and J. M. Pérez-Victoria. 2005. Flavonoid structure-activity studies identify 6-prenylchrysin and tectochrysin as potent and specific inhibitors of breast cancer resistance protein ABCG2. Cancer Res. 65:4852-4860. [DOI] [PubMed] [Google Scholar]
- 2.Ambudkar, S. V., S. Dey, C. A. Hrycyna, M. Ramachandra, I. Pastan, and M. M. Gottesman. 1999. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu. Rev. Pharmacol. Toxicol. 39:361-398. [DOI] [PubMed] [Google Scholar]
- 3.Bryceson, A. 2001. A policy for leishmaniasis with respect to the prevention and control of drug resistance. Trop. Med. Int. Health 6:928-934. [DOI] [PubMed] [Google Scholar]
- 4.Cailleau, R., R. Young, M. Olive, and W. J. Reeves, Jr. 1974. Breast tumor cell lines from pleural effusions. J. Natl. Cancer Inst. 53:661-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiquero, M. J., J. M. Pérez-Victoria, F. O'Valle, J. M. Gonzalez-Ros, R. G. del Moral, J. A. Ferragut, S. Castanys, and F. Gamarro. 1998. Altered drug membrane permeability in a multidrug-resistant Leishmania tropica line. Biochem. Pharmacol. 55:131-139. [DOI] [PubMed] [Google Scholar]
- 6.Chow, L. M., and S. K. Volkman. 1998. Plasmodium and Leishmania: the role of mdr genes in mediating drug resistance. Exp. Parasitol. 90:135-141. [DOI] [PubMed] [Google Scholar]
- 7.Conseil, G., H. Baubichon-Cortay, G. Dayan, J. M. Jault, D. Barron, and A. Di Pietro. 1998. Flavonoids: a class of modulators with bifunctional interactions at vicinal ATP- and steroid-binding sites on mouse P-glycoprotein. Proc. Natl. Acad. Sci. USA 95:9831-9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dantzig, A. H., K. L. Law, J. Cao, and J. J. Starling. 2001. Reversal of multidrug resistance by the P-glycoprotein modulator, LY335979, from the bench to the clinic. Curr. Med. Chem. 8:39-50. [DOI] [PubMed] [Google Scholar]
- 9.Dantzig, A. H., R. L. Shepard, J. Cao, K. L. Law, W. J. Ehlhardt, T. M. Baughman, T. F. Bumol, and J. J. Starling. 1996. Reversal of P-glycoprotein-mediated multidrug resistance by a potent cyclopropyldibenzosuberane modulator, LY335979. Cancer Res. 56:4171-4179. [PubMed] [Google Scholar]
- 10.Dayan, G., H. Baubichon-Cortay, J. M. Jault, J. C. Cortay, G. Deleage, and A. Di Pietro. 1996. Recombinant N-terminal nucleotide-binding domain from mouse P-glycoprotein. Overexpression, purification, and role of cysteine 430. J. Biol. Chem. 271:11652-11658. [DOI] [PubMed] [Google Scholar]
- 11.Dayan, G., J. M. Jault, H. Baubichon-Cortay, L. G. Baggetto, J. M. Renoir, E. E. Baulieu, P. Gros, and A. Di Pietro. 1997. Binding of steroid modulators to recombinant cytosolic domain from mouse P-glycoprotein in close proximity to the ATP site. Biochemistry 36:15208-15215. [DOI] [PubMed] [Google Scholar]
- 12.De Azevedo, W. F., Jr., H. J. Mueller-Dieckmann, U. Schulze-Gahmen, P. J. Worland, E. Sausville, and S. H. Kim. 1996. Structural basis for specificity and potency of a flavonoid inhibitor of human CDK2, a cell cycle kinase. Proc. Natl. Acad. Sci. USA 93:2735-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiDiodato, G., and F. J. Sharom. 1997. Interaction of combinations of drugs, chemosensitizers, and peptides with the P-glycoprotein multidrug transporter. Biochem. Pharmacol. 53:1789-1797. [DOI] [PubMed] [Google Scholar]
- 14.Di Pietro, A., G. Dayan, G. Conseil, E. Steinfels, T. Krell, D. Trompier, H. Baubichon-Cortay, and J. Jault. 1999. P-glycoprotein-mediated resistance to chemotherapy in cancer cells: using recombinant cytosolic domains to establish structure-function relationships. Braz. J. Med. Biol. Res. 32:925-939. [DOI] [PubMed] [Google Scholar]
- 15.Ford, J. M., and W. N. Hait. 1990. Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol. Rev. 42:155-199. [PubMed] [Google Scholar]
- 16.Ganguly, N. K. 2002. Oral miltefosine may revolutionize treatment of visceral leishmaniasis. TDR News 68.
- 17.González, A. G., I. A. Jiménez, A. G. Ravelo, and I. L. Bazzocchi. 1990. β-Agarofuran sesquiterpenes from Maytenus canariensis. Phytochemistry 29:2577-2579. [Google Scholar]
- 18.Henderson, D. M., C. D. Sifri, M. Rodgers, D. F. Wirth, N. Hendrickson, and B. Ullman. 1992. Multidrug resistance in Leishmania donovani is conferred by amplification of a gene homologous to the mammalian mdr1 gene. Mol. Cell. Biol. 12:2855-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins, C. F. 1992. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8:67-113. [DOI] [PubMed] [Google Scholar]
- 20.Hyafil, F., C. Vergely, P. Du Vignaud, and T. Grand-Perret. 1993. In vitro and in vivo reversal of multidrug resistance by GF120918, an acridonecarboxamide derivative. Cancer Res. 53:4595-4602. [PubMed] [Google Scholar]
- 21.Kennedy, M. L., F. Cortes-Selva, J. M. Pérez-Victoria, I. A. Jimenez, A. G. Gonzalez, O. M. Munoz, F. Gamarro, S. Castanys, and A. G. Ravelo. 2001. Chemosensitization of a multidrug-resistant Leishmania tropica line by new sesquiterpenes from Maytenus magellanica and Maytenus chubutensis. J. Med. Chem. 44:4668-4676. [DOI] [PubMed] [Google Scholar]
- 22.Kioka, N., N. Hosokawa, T. Komano, K. Hirayoshi, K. Nagata, and K. Ueda. 1992. Quercetin, a bioflavonoid, inhibits the increase of human multidrug resistance gene (MDR1) expression caused by arsenite. FEBS Lett. 301:307-309. [DOI] [PubMed] [Google Scholar]
- 23.Litman, T., T. Zeuthen, T. Skovsgaard, and W. D. Stein. 1997. Competitive, non-competitive and cooperative interactions between substrates of P-glycoprotein as measured by its ATPase activity. Biochim. Biophys. Acta 1361:169-176. [DOI] [PubMed] [Google Scholar]
- 24.Loiseau, P. M., and C. Bories. 2006. Mechanisms of drug action and drug resistance in leishmania as basis for therapeutic target identification and design of antileishmanial modulators. Curr. Top. Med. Chem. 6:539-550. [DOI] [PubMed] [Google Scholar]
- 25.Louvard, D. 1980. Apical membrane aminopeptidase appears at site of cell-cell contact in cultured kidney epithelial cells. Proc. Natl. Acad. Sci. USA 77:4132-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyubimov, E., L. B. Lan, I. Pashinsky, S. Ayesh, and W. D. Stein. 1995. Saturation reversal of the multidrug pump using many reversers in low-dose combinations. Anticancer Drugs 6:727-735. [DOI] [PubMed] [Google Scholar]
- 27.Muñoz-Martinez, F., P. Lu, F. Cortes-Selva, J. M. Pérez-Victoria, I. A. Jimenez, A. G. Ravelo, F. J. Sharom, F. Gamarro, and S. Castanys. 2004. Celastraceae sesquiterpenes as a new class of modulators that bind specifically to human P-glycoprotein and reverse cellular multidrug resistance. Cancer Res. 64:7130-7138. [DOI] [PubMed] [Google Scholar]
- 28.Murakami, S., M. Muramatsu, and K. Tomisawa. 1999. Inhibition of gastric H+, K+-ATPase by flavonoids: a structure-activity study. J. Enzyme Inhibition 14:151-166. [DOI] [PubMed] [Google Scholar]
- 29.Orlowski, S., and M. Garrigos. 1999. Multiple recognition of various amphiphilic molecules by the multidrug resistance P-glycoprotein: molecular mechanisms and pharmacological consequences coming from functional interactions between various drugs. Anticancer Res. 19:3109-3123. [PubMed] [Google Scholar]
- 30.Parodi-Talice, A., J. M. Araujo, C. Torres, J. M. Pérez-Victoria, F. Gamarro, and S. Castanys. 2003. The overexpression of a new ABC transporter in Leishmania is related to phospholipid trafficking and reduced infectivity. Biochim. Biophys. Acta 1612:195-207. [DOI] [PubMed] [Google Scholar]
- 31.Pérez-Victoria, F. J., S. Castanys, and F. Gamarro. 2003. Leishmania donovani resistance to miltefosine involves a defective inward translocation of the drug. Antimicrob. Agents Chemother. 47:2397-2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pérez-Victoria, F. J., G. Conseil, F. Munoz-Martinez, J. M. Pérez-Victoria, G. Dayan, V. Marsaud, S. Castanys, F. Gamarro, J. M. Renoir, and A. Di Pietro. 2003. RU49953: a non-hormonal steroid derivative that potently inhibits P-glycoprotein and reverts cellular multidrug resistance. Cell. Mol. Life Sci. 60:526-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pérez-Victoria, F. J., F. Gamarro, M. Ouellette, and S. Castanys. 2003. Functional cloning of the miltefosine transporter. A novel P-type phospholipid translocase from Leishmania involved in drug resistance. J. Biol. Chem. 278:49965-49971. [DOI] [PubMed] [Google Scholar]
- 34.Pérez-Victoria, J. M., M. J. Chiquero, G. Conseil, G. Dayan, A. Di Pietro, D. Barron, S. Castanys, and F. Gamarro. 1999. Correlation between the affinity of flavonoids binding to the cytosolic site of Leishmania tropica multidrug transporter and their efficiency to revert parasite resistance to daunomycin. Biochemistry 38:1736-1743. [DOI] [PubMed] [Google Scholar]
- 35.Pérez-Victoria, J. M., A. Di Pietro, D. Barron, A. G. Ravelo, S. Castanys, and F. Gamarro. 2002. Multidrug resistance phenotype mediated by the P-glycoprotein-like transporter in Leishmania: a search for reversal agents. Curr. Drug Targets 3:311-333. [DOI] [PubMed] [Google Scholar]
- 36.Pérez-Victoria, J. M., A. Parodi-Talice, C. Torres, F. Gamarro, and S. Castanys. 2001. ABC transporters in the protozoan parasite Leishmania. Int. Microbiol. 4:159-166. [DOI] [PubMed] [Google Scholar]
- 37.Pérez-Victoria, J. M., F. J. Pérez-Victoria, G. Conseil, M. Maitrejean, G. Comte, D. Barron, A. Di Pietro, S. Castanys, and F. Gamarro. 2001. High-affinity binding of silybin derivatives to the nucleotide-binding domain of a Leishmania tropica P-glycoprotein-like transporter and chemosensitization of a multidrug-resistant parasite to daunomycin. Antimicrob. Agents Chemother. 45:439-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pérez-Victoria, J. M., F. J. Pérez-Victoria, A. Parodi-Talice, I. A. Jimenez, A. G. Ravelo, S. Castanys, and F. Gamarro. 2001. Alkyl-lysophospholipid resistance in multidrug-resistant Leishmania tropica and chemosensitization by a novel P-glycoprotein-like transporter modulator. Antimicrob. Agents Chemother. 45:2468-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pérez-Victoria, J. M., B. M. Tincusi, I. A. Jimenez, I. L. Bazzocchi, M. P. Gupta, S. Castanys, F. Gamarro, and A. G. Ravelo. 1999. New natural sesquiterpenes as modulators of daunomycin resistance in a multidrug-resistant Leishmania tropica line. J. Med. Chem. 42:4388-4393. [DOI] [PubMed] [Google Scholar]
- 40.Planting, A. S., P. Sonneveld, A. van der Gaast, A. Sparreboom, M. E. van der Burg, G. P. Luyten, K. de Leeuw, M. de Boer-Dennert, P. S. Wissel, R. C. Jewell, E. M. Paul, N. B. Purvis, Jr., and J. Verweij. 2005. A phase I and pharmacologic study of the MDR converter GF120918 in combination with doxorubicin in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 55:91-99. [DOI] [PubMed] [Google Scholar]
- 41.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 42.Seifert, K., S. Matu, F. J. Pérez-Victoria, S. Castanys, F. Gamarro, and S. L. Croft. 2003. Characterisation of Leishmania donovani promastigotes resistant to hexadecylphosphocholine (miltefosine). Int. J. Antimicrob. Agents 22:380-387. [DOI] [PubMed] [Google Scholar]
- 43.Sicheri, F., I. Moarefi, and J. Kuriyan. 1997. Crystal structure of the Src family tyrosine kinase Hck. Nature 385:602-609. [DOI] [PubMed] [Google Scholar]
- 44.Soto, J., J. Toledo, P. Gutierrez, R. S. Nicholls, J. Padilla, J. Engel, C. Fischer, A. Voss, and J. Berman. 2001. Treatment of American cutaneous leishmaniasis with miltefosine, an oral agent. Clin. Infect. Dis. 33:E57-E61. [DOI] [PubMed] [Google Scholar]
- 45.Soule, H. D., J. Vazguez, A. Long, S. Albert, and M. Brennan. 1973. A human cell line from a pleural effusion derived from a breast carcinoma. J. Natl. Cancer Inst. 51:1409-1416. [DOI] [PubMed] [Google Scholar]
- 46.Sundar, S., T. K. Jha, C. P. Thakur, J. Engel, H. Sindermann, C. Fischer, K. Junge, A. Bryceson, and J. Berman. 2002. Oral miltefosine for Indian visceral leishmaniasis. N. Engl. J. Med. 347:1739-1746. [DOI] [PubMed] [Google Scholar]
- 47.Trompier, D., H. Baubichon-Cortay, X. B. Chang, M. Maitrejean, D. Barron, J. R. Riordon, and A. Di Pietro. 2003. Multiple flavonoid-binding sites within multidrug resistance protein MRP1. Cell. Mol. Life Sci. 60:2164-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Schalkwyk, D. A., J. C. Walden, and P. J. Smith. 2001. Reversal of chloroquine resistance in Plasmodium falciparum using combinations of chemosensitizers. Antimicrob. Agents Chemother. 45:3171-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]