Abstract
Squalene is a naturally occurring oil which has been used in the development of vaccine adjuvants, such as the oil-in-water emulsion MF59. In past years, by use of noncontrolled and nonvalidated assays, a claim was made that antisqualene antibodies were detectable in the sera of individuals with the so-called Gulf War syndrome. Using a validated enzyme-linked immunosorbent assay for the quantitation of immunoglobulin G (IgG) and IgM antibodies against squalene, we demonstrated that antisqualene antibodies are frequently detectable at very low titers in the sera of subjects who were never immunized with vaccines containing squalene. More importantly, vaccination with a subunit influenza vaccine with the MF59 adjuvant neither induced antisqualene antibodies nor enhanced preexisting antisqualene antibody titers. In conclusion, antisqualene antibodies are not increased by immunization with vaccines with the MF59 adjuvant. These data extend the safety profile of the MF59 emulsion adjuvant.
Squalene is a triterpenoid hydrocarbon oil (C30H50) produced by plants and is present in many foods. Squalene is also produced abundantly by human beings, for whom it serves as a precursor of cholesterol and steroid hormones (8) It is synthesized in the liver and the skin, transported in the blood by very-low-density lipoproteins (VLDL) and low-density lipoproteins (LDL), and secreted in large amounts by sebaceous glands (10, 17).
Since it is a natural component of the human body and is biodegradable, squalene has been used as a component of vaccine adjuvants. One of these adjuvants is MF59, an oil-in-water emulsion developed by Chiron (14). MF59 has been shown in various preclinical and clinical studies to significantly enhance the immune response to a wide variety of vaccine antigens (15). MF59 is a part of an influenza subunit vaccine which has been licensed in various European countries since 1997. More than 20 million doses of this vaccine have been given, and it has been shown to have an excellent safety profile. The safety of vaccines with the MF59 adjuvant has also been shown by various investigational clinical studies using recombinant antigens from hepatitis B virus, hepatitis C virus, cytomegalovirus, herpes simplex virus, human immunodeficiency virus, uropathogenic Escherichia coli, etc., with various age groups, including 1- to 3-day-old newborns (16).
In 2000, antisqualene antibodies were reported to be present in the sera of veterans returning from the first Persian Gulf War with a series of symptoms diagnosed by the authors of the report as representing the so-called Gulf War syndrome (4). The conclusions of this work, based on Western blot assays, were severely criticized on technical grounds (1) and were considered inconclusive by the Institute of Medicine (7).
Despite the fact that vaccines given to veterans returning with Gulf War syndrome did not contain squalene (6) and despite that fact that symptoms similar to those of the so-called Gulf War syndrome have been reported after several wars, including the American Civil War (9), we decided to undertake a study to determine whether immunization with the influenza vaccine with the MF59 adjuvant stimulated antibody responses against squalene. To this end, we set up and validated an enzyme-linked immunosorbent assay (ELISA), originally developed by Matyas et al. (11), and tested serum samples from adults never immunized with vaccines with the MF59 adjuvant and serum samples from individuals vaccinated with the influenza vaccine with the MF59 adjuvant. We conclude that antisqualene antibodies are found very frequently at low titers in sera from healthy, unvaccinated adults and that vaccination with influenza vaccine with the MF59 adjuvant does not result in any measurable impact on these antibody titers.
MATERIALS AND METHODS
Study population.
Serum samples were taken from the following cohorts of subjects. (i) Forty-three samples were selected from healthy individuals (age range, 26 to 63 years) who had participated in various clinical trials with Chiron vaccines in the United States since 1995. None of these vaccines contained the MF59 adjuvant. (ii) Preimmunization serum samples were taken from 50 healthy adults (age range, 50 to 64 years) before the initiation of a trial carried out in western Europe with Chiron subunit influenza vaccines, either with or without adjuvant. (iii) Serum samples were taken before, 1 month after, and 6 months after vaccination with the Chiron influenza vaccine with the MF59 adjuvant (n = 48; 65 years of age or older) or with a conventional influenza split vaccine without adjuvant (n = 52; 65 years of age or older) in a clinical trial conducted in eastern Europe. All clinical trials had received the approval of the respective local ethics committees.
Quantitation of IgM and IgG antibodies against squalene.
Assays for quantitation of serum antisqualene immunoglobulin G (IgG) and IgM antibodies were carried out according to a method described by Matyas et al. (11), with minor modifications. Briefly, for the detection of IgG antibodies, 96-well microtiter plates were coated with 10 μM of squalene (Sigma Chemical Co., St. Louis, MO) dissolved in isopropanol. As a control, some wells were left uncoated (i.e., treated with isopropanol alone). After blocking of the uncoated sites with phosphate-buffered saline containing casein (0.5%, wt/vol) and 0.002% chlorhexidine, test samples and assay controls were assayed starting from a dilution of 1:10 followed by various twofold dilutions. IgG antibodies were detected by a horseradish peroxidase-conjugated goat anti-human IgG Fc fragment-specific antibody. A best-fit curve of dilution versus optical density was generated for each dilution series by using a four-parameter curve fitting routine to determine an endpoint dilution titer. IgG values of ≥20 were considered positive. Specificity was determined by comparing titers in squalene-coated wells with titers in uncoated wells. A decrease of titer by more than 50% in uncoated wells was used to classify the reactivity as specific.
The method for the detection of serum antisqualene IgM antibodies was similar to that used for the detection of IgG except for the use of bovine serum albumin instead of casein for the blocking of the uncoated sites, the appropriate assay controls, and horseradish peroxidase-conjugated goat anti-human IgM Fc fragment-specific antibody. Due to slightly higher nonspecific signals for IgM than for IgG, IgM values were considered positive from titers of ≥40.
Both assays were validated. Validation parameters included specificity, dilutional linearity, precision, plate homogeneity, analyte stability, plate coating stability, and determination of baseline serum level. The range of detection was between 10 and 1,280 for both assays. The coefficient of variation was 6.7% for the IgG assay and 12.1% for the IgM assay.
Statistical analysis.
Geometric mean titers (GMT) of serum IgG and IgM antisqualene antibodies, with 95% confidence intervals (CIs), were calculated by taking the exponents (base 10) of the least-squares means and of the lower and upper limits of associated 95% CI of the log10-transformed titers. Least-squares means, 95% CI, and P values were calculated by a general linear model with the vaccine type as a factor. To evaluate IgG and IgM antibody changes from baseline, time was also included in the general linear model with repeated measures for subjects. The proportions of subjects who had IgG titers of >20 and IgM titers of >40 were compared by χ2 test and by logistic regression.
RESULTS
Low titers of antisqualene antibodies are frequently detectable in the sera of healthy subjects.
The first question we asked was whether or not IgM and IgG antibodies against squalene were present in serum samples of healthy adult individuals who had never received vaccines containing MF59 emulsion adjuvant. To this end, we used the validated ELISA to test serum samples derived from adults from the United States and from western Europe. As shown in Table 1, serum antisqualene antibodies were very frequently detectable in healthy adults. Indeed, the frequency of IgG antibodies ranged from 26% found in the European cohort to 79% found in the U.S. cohort; the frequency of IgM antibodies varied from 64% (European cohort) to 100% (U.S. cohort). In the eastern European cohort used to study the effects of vaccine with the MF59 adjuvant (Table 2), 100% of subjects were positive for antisqualene IgG at study entry, versus 52 to 58% positive for antisqualene IgM. Thus, prevalence of antisqualene antibodies may vary in different populations. As a matter of fact, for IgG, all pairwise comparisons among the three cohorts resulted in statistically significant differences, whereas for IgM only differences between the U.S. and the two European cohorts were statistically significant. It does not appear that the frequency of antisqualene antibodies was linked to the age of the subjects, since these antibodies were found more frequently in the sera from the American cohort, which had an average age lower than that of the European cohorts.
TABLE 1.
Frequencies and GMT of serum IgG and IgM antibodies against squalene from healthy adults never immunized with vaccines with the MF59 adjuvant
| Antibody | Cohort | No. of serum samples | Frequency (%) of detectable antibodiesa | GMT (range) |
|---|---|---|---|---|
| IgG | U.S. | 43 | 79 | 36.9 (<10-616) |
| European | 50 | 26* | 20 (<10-154) | |
| IgM | U.S. | 43 | 100 | 51.8 (14.9-412) |
| European | 50 | 64* | 31 (<10-334) |
*, P of <0.001 for IgG and IgM antibody titers compared to titers for U.S. cohort.
TABLE 2.
Percentages of individuals with antisqualene IgG and IgM antibodies after vaccination with influenza vaccine with or without the MF59 adjuvant
| Antibody | Time point | No. of subjects positive/total no. of subjects (%)
|
P value
|
||
|---|---|---|---|---|---|
| Vaccine with MF59 adjuvant | Vaccine without adjuvant | χ2 test | Logistic regressiona | ||
| IgG | Prevaccination | 48/48 (100) | 52/52 (100) | 1.000 | 0.236* |
| 1 mo postvaccination | 47/48 (97.9) | 51/52 (98.1) | 0.954 | 0.901† | |
| 6 mo postvaccination | 48/48 (100) | 49/52 (94.2) | 0.091 | ||
| IgM | Prevaccination | 28/48 (58.3) | 27/52 (51.9) | 0.520 | 0.446* |
| 1 mo postvaccination | 26/48 (54.2) | 30/52 (57.7) | 0.722 | 0.910† | |
| 6 mo postvaccination | 28/48 (58.3) | 25/52 (48.1) | 0.305 | ||
P values in the last column were generated by bivariate logistic regression with vaccine type (*) and time (†) as cofactors.
Table 1 also shows that the GMT of serum antisqualene IgG and IgM antibodies were consistently very low, ranging from 20 to 36 for IgG antibodies and from 31 to 51 for IgM antibodies (quantitation limits of ≥10 and ≥40, respectively).
It should be added that when more than one serum sample was available from the same subject, no trend towards an increase or a decrease of antisqualene antibody titers was evident (not shown).
Antisqualene antibody titers are not influenced by immunization with vaccines with the MF59 adjuvant.
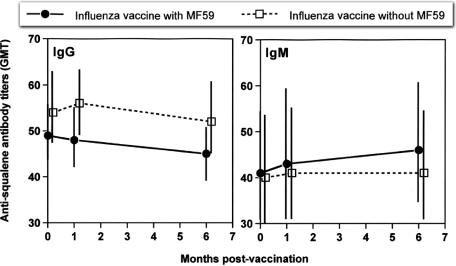
Having shown that antisqualene antibodies are found frequently in the sera of healthy adults, we asked whether immunization with vaccines with the MF59 emulsion adjuvant (which contains squalene) would enhance antibody titers over time. To this end, we tested IgM and IgG antibodies in the sera of 48 individuals immunized with the influenza subunit vaccine with the MF59 adjuvant and 52 individuals immunized with the control, split vaccine without adjuvant. As shown in Fig. 1, prevaccination serum antisqualene IgG and IgM antibody titers were not affected by vaccination with influenza vaccine with the MF59 adjuvant either 1 month or 6 months after vaccination. In addition, the serum IgG and IgM antisqualene antibody titers for subjects immunized with the influenza vaccine with the MF59 adjuvant did not differ significantly from those measured for subjects immunized with the vaccine without adjuvant at any of the time points studied (before, 1 month after, or 6 months after vaccination).
FIG. 1.
Antisqualene IgG and IgM antibodies in serum samples from individuals vaccinated with subunit influenza vaccine with the MF59 adjuvant (n = 48) or with a plain, split influenza vaccine without the MF59 adjuvant (n = 52). Vertical lines represent 95% CI. None of the differences (either between vaccines or between time points with one vaccine) were statistically significant (P values ranged between 0.130 [vaccine with the MF59 adjuvant versus vaccine without, IgG titers 1 month after vaccination] and 0.863). There were no trends over time detected as significant for either vaccine or either antibody (P ≥ 0.6212).
As expected from the results obtained with the two younger cohorts, we also observed in a controlled clinical trial with elderly subjects that serum antisqualene IgG and IgM antibodies were extremely frequent before vaccination (52% to 58% for IgM and 100% for IgG). These frequencies remained unchanged over time (1 and 6 months postvaccination) (P = 0.9011 and 0.9100 for IgG and IgM, respectively) (Table 2). Finally, antisqualene antibodies (IgG and IgM) were detected at similar frequencies in the cohort immunized with the influenza vaccine with the MF59 adjuvant and in the cohort immunized with the plain influenza vaccine without adjuvant (P = 0.2360 and 0.4464 for IgG and IgM, respectively). These P values were obtained when the analyses were repeated considering time and vaccine time as cofactors in the logistic regression model (Table 2).
DISCUSSION
In the present study we have employed a validated ELISA for the detection and quantitation of serum antisqualene antibodies to show that immunization with vaccines containing the MF59 emulsion adjuvant does not induce antisqualene IgM or IgG antibodies. Instead, antisqualene antibodies were detected very frequently at low titers in sera from healthy individuals that had never received any vaccine containing squalene.
Our study confirms that squalene is very poorly immunogenic. Indeed, in studies carried out by Matyas et al. (12), antibodies against squalene were elicited in mice only when squalene was formulated within liposomes containing endotoxin (lipid A). These data are in agreement with those previously reported showing that antibodies against cholesterol could be induced after immunization of mice with cholesterol-loaded liposomes containing lipid A (18).
A clear finding of our study is that the vast majority of healthy adults have antibodies to squalene circulating in their sera. It is of note that these antibodies were found in individuals from various geographical areas, such as the United States, western Europe, and eastern Europe, which to our knowledge had never received vaccines or other pharmacological treatments containing squalene. The statistically significant difference found among the three cohorts studied here cannot be ascribed to vaccinations, since these people were never vaccinated previously with vaccines containing MF59. In some cohorts, the frequency of individuals with detectable levels of antisqualene antibodies was as high as 100%. This finding is reminiscent of similar findings reported by Alving et al. (2), who showed that virtually all normal human sera contain naturally occurring antibodies against cholesterol. One may speculate that these naturally occurring antisqualene antibodies could behave as an immunomodulating mechanism for regulation of LDL and VLDL metabolism, as proposed for anticholesterol antibodies (3), since LDL and VLDL transport both squalene and cholesterol in the bloodstream (13). The hypothetical role of naturally occurring antisqualene antibodies still needs formal demonstration.
Due to its natural occurrence, squalene was used as the oil phase in the design of new adjuvant preparations, such as MF59. MF59 emulsion is used as an adjuvant for an influenza subunit vaccine licensed since 1997 in various countries in and outside Europe (16). More than 20 million doses of this influenza vaccine with the MF59 adjuvant have been distributed with an excellent safety profile. In addition, MF59 has been tested and is being tested in clinical trials as an adjuvant for several new vaccines for various age groups and has always been associated with a good clinical tolerability (16).
The interest in antisqualene antibodies was raised by reports claiming that these antibodies were detected in the sera of military personnel with the so-called Gulf War syndrome (4, 5). These reports were criticized on technical grounds, since the assay employed (Western blotting) was not validated and lacked any controls (1). In addition, these claims were considered inconclusive by the Institute of Medicine (7). Using a validated ELISA, we have shown formally that no increases in antisqualene antibodies over and above preexisting levels are observed following vaccination with influenza vaccine with the MF59 adjuvant, either at 1 month or at 6 months postvaccination. Interestingly enough, antisqualene antibodies were found at identical titers in the sera of control subjects that had been immunized with an influenza split vaccine without adjuvant.
In conclusion, we have shown that antisqualene antibodies of both IgG and IgM isotypes are detected very frequently in the sera of healthy adult individuals of different ages and from various geographical areas. Additionally, we have shown that vaccines with the squalene-containing MF59 adjuvant emulsion do not induce any increase either in the titer or in the proportion of subjects with antibodies against squalene. These data add to the safety profile of the MF59 adjuvant emulsion, already shown by extensive clinical trials and with the regular use of the licensed vaccine with the MF59 adjuvant in routine influenza immunization practices.
REFERENCES
- 1.Alving, C. R., and J. D. Grabenstein. 2000. Letter to the editor. Exp. Mol. Pathol. 68:196-197. [DOI] [PubMed] [Google Scholar]
- 2.Alving, C. R., G. M. Swartz, Jr., and N. M. Wassef. 1989. Naturally occurring autoantibodies to cholesterol in humans. Biochem. Soc. Trans. 17:637-639. [DOI] [PubMed] [Google Scholar]
- 3.Alving, C. R., and N. M. Wassef. 1999. Naturally occurring antibodies to cholesterol: a new theory of LDL cholesterol metabolism. Immunol. Today 20:362-366. [DOI] [PubMed] [Google Scholar]
- 4.Asa, P. B., Y. Cao, and R. F. Garry. 2000. Antibodies to squalene in Gulf War syndrome. Exp. Mol. Pathol. 68:55-64. [DOI] [PubMed] [Google Scholar]
- 5.Asa, P. B., R. B. Wilson, and R. F. Garry. 2002. Antibodies to squalene in recipients of anthrax vaccine. Exp. Mol. Pathol. 73:19-27. [DOI] [PubMed] [Google Scholar]
- 6.Federal Register. 2005. Biological products; bacterial vaccines and toxoids; implementation of efficacy review; anthrax vaccine adsorbed; final order. Fed. Regist. 70:75180-75198. [PubMed] [Google Scholar]
- 7.Fulco, C. E., C. T. Liverman, and H. C. Sox. 2000. Gulf War and health: depleted uranium, pyridostigmine bromide, sarin, vaccines, p. 267-324. In C. E. Fulco, C. T. Liverman, and H. C. Sox (ed.), Vaccines, vol. 1. National Academy Press, Washington, D.C. [PubMed] [Google Scholar]
- 8.Granner, D. K. 2000. Hormones of the adrenal cortex, p. 575-587. In R. K. Murray, D. K. Granner, P. A. Mayes, and V. W. Rodwell (ed.), Harper's biochemistry, 25th ed. Appleton and Lang, Stanford, Conn.
- 9.Hyams, K. C., F. S. Wignall, and R. Roswell. 1996. War syndromes and their evaluation: from the U.S. Civil War to the Persian Gulf War. Ann. Intern. Med. 125:398-405. [DOI] [PubMed] [Google Scholar]
- 10.Koivisto, P. V. I., and T. A. Miettinen. 1988. Increased amount of cholesterol precursors in lipoproteins after ileal exclusion. Lipids 23:993-996. [DOI] [PubMed] [Google Scholar]
- 11.Matyas, G. R., M. Rao, and P. R. Pittman. 2004. Detection of antibodies to squalene. III. Naturally occurring antibodies to squalene in humans and mice. J. Immunol. Methods 286:47-67. [DOI] [PubMed] [Google Scholar]
- 12.Matyas, G. R., N. M. Wassef, M. Rao, and C. R. Alving. 2000. Induction and detection of antibodies to squalene. J. Immunol. Methods 245:1-14. [DOI] [PubMed] [Google Scholar]
- 13.Miettinen, T. A. 1982. Diurnal variation of cholesterol precursors squalene and methyl sterols in human plasma lipoproteins. J. Lipid Res. 23:466-473. [PubMed] [Google Scholar]
- 14.Ott, G., R. Radhakrishnan, J. Fant, and M. Hora. 2000. The adjuvant MF59: a perspective, p. 211-228. In D. T. O'Hagan (ed.), Methods in molecular medicine, vol. 42. Vaccine adjuvants: preparation methods and research protocols. Humana Press, Totowa, N.J. [Google Scholar]
- 15.Podda, A., and G. Del Giudice. 2003. MF59-adjuvanted vaccines: increased immunogenicity with an optimal safety profile. Expert Rev. Vaccines 2:197-203. [DOI] [PubMed] [Google Scholar]
- 16.Podda, A., and G. Del Giudice. 2004. MF59 adjuvant emulsion, p. 225-235. In M. M. Levine, J. B. Kaper, R. Rappuoli, M. A. Liu, and M. F. Good (ed.), New generation vaccines, 3rd ed. Marcel Dekker, New York, N.Y.
- 17.Stewart, M. E. 1992. Sebaceous gland lipids. Semin. Dermatol. 11:100-105. [PubMed] [Google Scholar]
- 18.Swartz, G. M., Jr., M. K. Gentry, L. M. Amende, E. J. Blanchette-Mackie, and C. R. Alving. 1988. Antibodies to cholesterol. Proc. Natl. Acad. Sci. USA 85:1902-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]