Abstract
For over a century, purified protein derivatives (PPD) have been used to detect mycobacterial infections in humans and livestock. Among these, reagents to detect infections by Mycobacterium avium complex organisms have been produced, but the utility of these reagents has not been clearly established due in part to limited biologic and immunologic standardization. Because there is little information about the strains used to produce these reagents (avian PPD, intracellulare PPD, scrofulaceum PPD, and Johnin), we have performed genetic characterizations of strains used to produce these products. Sequence analysis of 16S rRNA and the hsp65 gene provided results concordant with species designations provided for M. avium, Mycobacterium intracellulare, and Mycobacterium scrofulaceum organisms. For M. avium strains, comparative genomic hybridization was performed on a whole-genome DNA microarray, revealing one novel 7.9-kilobase genomic deletion in certain Johnin-producing strains, in addition to genomic variability inherent to the particular M. avium subspecies. Our findings indicate that considerable genomic differences exist between organisms used for reagents and the infecting organism being studied. These results serve as a baseline for potency studies of different preparations and should aid in comparative studies of newly discovered antigens for the diagnosis of infection and disease by M. avium complex organisms.
The successful control of mycobacterial diseases is contingent on early diagnosis of infection and/or the successful application of treatment to contagious cases. In the case of tuberculosis, both strategies are applied, with immunologic testing being used to inform about infection and microbiologic diagnosis guiding the detection of active disease. The cornerstone of immunologic diagnosis of mycobacterial infection has been skin testing to detect cell-mediated immune responses to constituents of the bacillus. Several types of products were used for skin testing during the past century, with Koch's “old tuberculin” being replaced first by heat-concentrated synthetic medium tuberculins and later by purified protein derivatives (PPDs) (13, 30). Interpretable skin test results may be ensured, at least in part, by careful standardization of the active reagent and the application of potency assays for quality control. For tuberculin PPD, a WHO International Standard termed PPD-M is widely used throughout the world (10).
Infection and disease due to organisms of the Mycobacterium avium complex (MAC) are recognized to be health concerns for humans and livestock; however, testing for MAC infection has been less standardized than tuberculin testing. Traditionally, MAC responsiveness was studied to guide the interpretation of tuberculin skin testing by assaying to distinguish sensitization to environmental mycobacteria from infection with members of the Mycobacterium tuberculosis complex. This could be accomplished by comparative testing using PPD-Battey, a reagent derived from the Battey bacillus now known as Mycobacterium intracellulare, in parallel with tuberculin skin testing (23). The same approach was later used in comparative skin testing using M. avium PPD RS10 (Statens Serum Institut, Copenhagen, Denmark) (43-46) and, more recently, in the first generation of in vitro assays (QuantiFERON-TB; Cellestis Limited, Victoria, Australia), wherein one determines the ratio of gamma interferon responses to tuberculin PPD versus those to avian PPD (20).
There has also been a more direct interest in determining the prevalence of infection with members of the MAC in humans, not only in the context of AIDS but also in understanding the epidemiology of pulmonary MAC disease and cervical adenitis in children (18, 44, 46). To address this interest, investigators have applied different PPD reagents in epidemiologic surveys, although none of these have been rigorously standardized or extensively tested. In livestock, M. avium reagents have also been used for discriminating between tuberculous and nontuberculous exposure in cattle (as in the commercial gamma interferon assay Bovigam; Prionics AG, Switzerland) (28) but their greater utility may be in direct detection of disease due to M. avium organisms themselves. In the case of M. avium subsp. paratuberculosis, the etiological agent of a chronic inflammatory bowel disease in ruminants called Johne's disease, early infection is characterized by a cell-mediated immune response, so PPD-based skin tests (or interferon-based assays) promise the greatest likelihood of detecting subclinically infected hosts (3, 35). Unlike bovine or human tuberculosis, however, such tests are not currently in wide use, having been abandoned as a result of reported problems with the sensitivity and specificity of skin-based testing (4, 5, 11, 16).
A critical concern in the use of these tests has been the availability of standardized PPDs to be used towards diagnostic tests for these organisms. A number of different MAC antigen preparations have been used, including the use of avian PPD for Johne's surveillance by some groups (11, 12, 36) and the use of different Johnin preparations by others. For example, in Canada, Johnin has been produced by six strains that have been continuously passaged in the laboratory for more than 70 years (II, III, IV, C286, C300, and III.V) (6, 47). Little information is available about the history of strains used elsewhere, although one of the strains used in The Netherlands to produce Johnin is thought to have originated in Canada (strain C), while another was provided by the Veterinary Laboratory Agency in the United Kingdom (strain 3+5). Because of this confusing history, we have employed genetic and genomic modalities to genotype the organisms used to produce the M. avium complex PPD reagents as a first step towards an improved understanding of these reagents.
MATERIALS AND METHODS
Bacterial isolates and their genetic identification.
We collected killed bacterial cells from 15 strains in total (Table 1); 6 were used for the production of Johnin in Canada (Canadian Food Inspection Agency, Nepean, Ontario), 2 were used in The Netherlands (CIDC-Lelystad, The Netherlands), 2 were used in Norway (National Veterinary Institute, Norway), 2 were used in the United States (strain 18, a gift from M. E. Hines III, Georgia, and strain ATCC19698, purchased from the American Tissue Culture Collection), and 3 were used at the Statens Serum Institut (SSI, Copenhagen, Denmark) for the production of PPDs from M. avium, M. intracellulare, and Mycobacterium scrofulaceum. DNA extraction was performed according to a standard protocol (42). Each strain was characterized by PCR and sequencing of 16S rRNA and the 3′ end of hsp65, a typing scheme that we recently proposed to classify members of the M. avium complex (40). Additionally, the characterization into subspecies and distinct types was determined using three-primer PCR assays testing for the presence or absence of large sequence polymorphisms LSPA 8 (specifically missing from M. avium subsp. paratuberculosis), LSPA 20 (missing from M. avium subsp. paratuberculosis of the sheep type but present in the cattle type) and LSPA 17 (missing from M. avium subsp. avium of the bird type), according to a previously described diagnostic algorithm (33).
TABLE 1.
Characteristics of strains used to produce M. avium PPD reagentsa
| Reagent | Reference(s) | Strain name | Provider | 16S rRNA designation | hsp65 designation | ML-SSR genotypeb | LSP-based identification | Microarray-based genomic profile |
|---|---|---|---|---|---|---|---|---|
| Avian PPD | 11, 20, 25, 36, 46 | 10 | SSI | M. avium | Code 4 | NA | M. avium subsp. avium bird type | Similar to prototype strain (M. avium subsp. avium strain R13)c |
| Intracellulare PPD | 9 | 23 | SSI | M. intracellulare | NA | NA | NA | ND |
| Scrofulaceum PPD | 9 | 95 | SSI | M. scrofulaceum | NA | NA | NA | ND |
| Johnin | 15, 16 | 18 | M. Hines | M. avium | Code 4 | NA | M. avium subsp. avium bird type | ND |
| Johnin (NL) | 12 | C | CIDC-Lelystad | M. avium | Code 5 | ggt5 tgc5 | M. avium subsp. paratuberculosis cattle type | Deletion of LSPjn |
| 3+5 | VLA-Weybridge | M. avium | Code 5 | ggt5 tgc5 | M. avium subsp. paratuberculosis cattle type | Deletion of LSPjn | ||
| Johnin (NVI) | 29 | 316 | NVI | M. avium | Code 5 | ggt5 tgc5 | M. avium subsp. paratuberculosis cattle type | Similar to prototype strain (M. avium subsp. paratuberculosis strain K10) |
| 2E | NVI | M. avium | Code 5 | ggt5 tgc5 | M. avium subsp. paratuberculosis cattle type | Similar to prototype strain (M. avium subsp. paratuberculosis strain K10) | ||
| Johnin (USDA) | 26, 27, 37 | 19698 | ATCC | M. avium | Code 5 | ggt5 tgc5 | M. avium subsp. paratuberculosis cattle type | Similar to prototype strain (M. avium subsp. paratuberculosis strain K10) |
| Johnin (CFIA) | 5, 21, 47 | II | CFIA | M. avium | Code 5 | ggt4 tgc5 | M. avium subsp. paratuberculosis cattle type | Similar to prototype strain (M. avium subsp. paratuberculosis strain K10) |
| III | CFIA | M. avium | Code 5 | ggt5 tgc5 | M. avium subsp. paratuberculosis cattle type | Deletion of LSPjn | ||
| IV | CFIA | M. avium | Code 5 | ggt5 tgc5 | M. avium subsp. paratuberculosis cattle type | Deletion of LSPjn | ||
| C286 | CFIA | M. avium | Code 5 | ggt5 tgc5 | M. avium subsp. paratuberculosis cattle type | Deletion of LSPjn | ||
| C300 | CFIA | M. avium | Code 5 | ggt5 tgc5 | M. avium subsp. paratuberculosis cattle type | Deletion of LSPjn | ||
| III.V | CFIA | M. avium | Code 5 | ggt5 tgc5 | M. avium subsp. paratuberculosis cattle type | Deletion of LSPjn |
SSI, Statens Serum Institut, Copenhagen, Denmark; CIDC-Lelystad, Central Institute for Animal Disease Control, Lelystad, The Netherlands; VLA-Weybridge, Veterinary Laboratory Agency, Weybridge, United Kingdom; NVI, National Veterinary Institute, Oslo, Norway; CFIA, Canadian Food Inspection Agency, Ontario, Canada; NA, not applicable; ND, not done.
ML-SSR, multilocus short sequence repeats. The genotypes given are based on numbers of repeats for locus 8 (ggt repeat) and locus 9 (tgc repeat).
See reference 33 for details.
Microarray-based genomic comparisons.
The test isolates were compared to M. avium 104 (the reference sequenced strain, a clinical isolate from a human AIDS patient in the United States) in cohybridization experiments on a whole-genome DNA microarray representative of 98% of the open reading frames (ORFs) for the genome of M. avium subsp. paratuberculosis strain K10 and 93% of the predicted ORFs for the genome of M. avium strain 104 (Table 1 shows a list of the strains tested by microarray). Fluorescence labeling of the DNA samples, hybridizations, and scanning were performed according to previously described methods (33, 34). Sequences that were identified by microarray analysis as potentially divergent and not representative of previously described polymorphisms were flagged for further study using a PCR approach. In this study, we opted to analyze only sequences that were greater than three ORFs, as these are more likely to represent truly divergent sequences rather than experimental artifacts. We first verified that these sequences were missing from test isolates with primers designed towards a sequence internal to these regions. In a second step, we performed PCR using primers designed towards the flanking regions, such that an amplicon would be obtained only if the region was missing. Primers were designed using Primer 3 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). Primer sequences are given in Table 2. The PCR products were sequenced in a core sequencing facility (McGill University and Génome Québec Innovation Centre) on a 3730XL DNA analyzer system using ABI dye terminator chemistry. The resulting sequences were aligned to completed genome sequences (M. avium subsp. paratuberculosis K10 [AE016598]) and M. avium 104 (www.tigr.org) to identify the exact site at which the sequence polymorphism occurs.
TABLE 2.
Primers used to genotype M. avium strains
| Target sequencea | Primer 1 (forward) | Primer 2 (reverse) | Primer 3 (reverse) |
|---|---|---|---|
| LSPjn | ACACACCGACCAGAGAGGAG | CGATCCAGAGGCTGAAGATG | ACAACTTCGCCAAGGTCATC |
| SSR locus 1 | GTGTTCGGCAAAGTCGTTGT | TCAGACTGTGCGGTATGGAA | |
| SSR locus 2 | GTGACCAGTGTTTCCGTGTG | TGCACTTGCACGACTCTAGG | |
| SSR locus 8 | AGATGTCGACCATCCTGACC | AAGTAGGCGTAACCCCGTTC | |
| SSR locus 9 | GACAAGTTCGGGTTGACCAC | AGTTCCTCGACCCAGTCGT | |
| 16S rRNA | AGAGTTTGATCCTGGCTCAG | GTATTACCGCGGCTGCTG | |
| Hsp65 (3′ end) | CGGTTCGACAAGGGTTACAT | ACGGACTCAGAAGTCCATGC |
Testing for the presence or absence of LSPjn was performed using three primers in a multiplex PCR reaction, such that a 233-bp product is expected if the sequence is present (primers 1 and 3) and a 396-bp product is expected if the sequence is missing (primers 1 and 2). SSR, short sequence repeat. Locus 1 and locus 2 are mononucleotide (G) repeats; locus 8 and locus 9 are variable-number trinucleotide repeats.
Molecular subtyping of isolates.
Molecular subtyping of the M. avium subsp. paratuberculosis strains tested was done by multilocus short sequence repeat analysis, a tool that is expected to generate a greater degree of genetic diversity than genotyping methods based on insertion elements (IS900 restriction fragment length polymorphism and multiplex PCR of IS900 integration loci). We selected four loci suggested in a previous study to be the most discriminatory: locus 1, locus 2, locus 8, and locus 9 (1). The first two loci consist of mononucleotide (G) repeats, while the last two are variable-number trinucleotide (GGT and TGC) repeats. We performed PCR amplification and sequencing of these loci using previously described primers (1). The resulting chromatograms were edited manually for accuracy and added to the alignment component of MEGA3 (14). For each strain tested, the number of nucleotide repeats was analyzed and an allele number was assigned to reflect the number of copies per locus.
PCRs.
PCRs were performed in 50-μl volumes, using 5 μl (equivalent to about 20 ng) of DNA, 1 U Taq polymerase (MBI Fermentas), 5 μl of 10× PCR buffer (MBI Fermentas), 2.5 mM MgCl2, 5 μl acetamide 50% (wt/vol), 0.2 mM deoxynucleoside triphosphates (dNTPs), and 0.5 μM of each primer. PCR amplification consisted of an initial denaturation step at 94°C for 5 min, followed by 35 cycles of denaturation at 94°C for 45 s, with annealing at 55°C (for amplification across large sequence polymorphisms) or 60°C (for amplification across short sequence repeats) for 45 s, elongation at 72°C for 2 min, and a final elongation step at 72°C for 10 min. PCR products were separated by electrophoresis in 1.5% (wt/vol) agarose gels containing ethidium bromide.
RESULTS
Genetic characterization of strains.
From 16S rRNA sequencing, all but two of the strains studied were identified as M. avium: strains 23 and 95 were identified as M. intracellulare and M. scrofulaceum, respectively, consistent with their previous designations. Since both of these species exhibit a high level of sequence difference from M. avium proper, these two isolates were not submitted to further comparative genomic studies. Strain 10, used to produce avian PPD, and strain 18, previously used to produce both an antigenic reagent and a vaccine for Johne's disease, were both identified as M. avium subsp. avium (bird type) based on hsp65 sequencing (code 4) and PCR for LSPs (LSPA 17 missing). The strains used in Canada, Norway, and The Netherlands and one strain recently used in the United States for the production of Johnin were all determined to be M. avium subsp. paratuberculosis of the cattle type (hsp65 code 5 and LSPA 20 present) (Table 1).
Genomic characterization of M. avium strains.
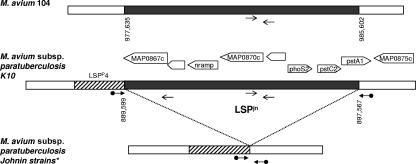
Using M. avium strain 104 as the referent, microarray-based study revealed that the strains tested had genomic profiles consistent with their subspecies and type designations (33, 34) (Table 1). Additionally, we identified one novel large sequence that was deleted in the following seven strains: III, IV, C286, C300, III.V, 3+5, and C. This sequence, which we called large sequence polymorphism Johnin (LSPjn), is a 7,968-bp sequence spanning MAP0867c to MAP0874 on the genome of M. avium subsp. paratuberculosis strain K10 (AE016598). This sequence is adjacent to a previously described large sequence, LSPP4, that is specific to M. avium subsp. paratuberculosis (missing from all nonparatuberculosis strains of M. avium). The sequence LSPjn is conserved and syntenic in M. avium 104 and the M. tuberculosis complex and encompasses an operon predicted to carry a membrane-associated phosphate transport complex called the Pst system and a gene predicted to be involved in manganese transport (mntH or bacterial NRAMP). Sequence-based analysis revealed that the polymorphism occurs at the same locus in all seven strains in which LSPjn is missing (corresponding to positions 889599 to 897567 in the M. avium subsp. paratuberculosis K10 genome) and truncates MAP0874 (Fig. 1). Testing for this sequence using a multiplex PCR approach in a panel of 20 M. avium subsp. paratuberculosis isolates (cattle- and sheep-type strains) characterized in a recent study (32) showed this region to be present and intact in a diverse collection of field isolates.
FIG. 1.
Schematic representation of large sequence polymorphism Johnin. Coordinates on the genome are given as base pairs starting from the first nucleotide of the start codon of dnaA in M. avium 104 and M. avium subsp. paratuberculosis K10, respectively. White boxes represent homologous sequences across M. avium 104, M. avium subsp. paratuberculosis K10, and M. avium subsp. paratuberculosis strains used to produce Johnin. The striped box represents a large sequence (LSPp4) that is present in M. avium subsp. paratuberculosis but missing in M. avium 104. LSPjn is depicted by the gray box and is missing from M. avium subsp. paratuberculosis strains III, IV, C286, C300, III.V, 3+5, and C. Thick arrows represent primers flanking LSPjn (bridging primers); a PCR product is obtained if the region is missing. Thin arrows represent primers targeting a sequence within LSPjn; a PCR product is obtained if the sequence is present.
Molecular subtyping of strains.
From analysis of two of the short sequence repeat loci (locus 8 and locus 9), we identified two genotypes among the isolates tested. All but one of the M. avium subsp. paratuberculosis strains tested clustered into a predominant genotype (ggt5, tgc5), while strain II (a strain in which LSPjn was intact) was determined to be of a different genotype (ggt4 tgc5).
The other two loci (mononucleotide repeats) could not be analyzed due to inconsistent and illegible sequences immediately following the mononucleotide stretch. This is consistent with previous reports of higher error rates in PCR amplification and sequencing of long mononucleotide repeats due to polymerase “slippage,” presumably caused by slipped-strand mispairing (2).
DISCUSSION
In this study, we formally analyzed the genetic and genomic characteristics of strains used to produce M. avium complex PPD reagents. Although these reagents have frequently been used to distinguish M. tuberculosis complex infection from exposure to nontuberculous mycobacteria, they are also used to detect MAC infection both in humans and in livestock (8, 23, 46), with recent reports suggesting that their utility has previously been underappreciated (12, 26, 27, 35, 36). Furthermore, while the postgenomic identification of specific antigens offers an opportunity for improved diagnostic assays, the utility of novel antigens will likely benefit from direct comparison against cocktails of antigens, as has been done in the case of ESAT-6 and tuberculin testing (41).
One of the strains most commonly used to produce the M. avium sensitin PPD or avian PPD, the SSI strain 10, was determined by genomic analysis to be M. avium subsp. avium of the bird type. There is extensive evidence that M. avium subsp. avium bird-type strains are the cause of tuberculosis in birds and are associated with severe disease in many other animals including livestock. However, these strains have rarely been encountered in human disease, although the SSI strain 10 was isolated from a Danish child in 1944 (19). This suggests either that human exposure is quite rare or that humans are inherently more resistant to these strains (7, 22, 39). Given that SSI strains 23 and 95 are indeed environmental organisms occasionally associated with human disease (M. intracellulare and M. subsp. scrofulaceum, respectively), it is surprising that reagents based on these two strains are rarely used, while avian PPD based on a strain seldom encountered in humans is used more widely. Nevertheless, studies conducted in different geographical regions using avian PPD produced from SSI strain 10 have indicated that certain individuals do mount a skin reaction to this sensitin (43-46). In a recent skin survey, skin test positivity to this reagent was not more frequent in those at risk of disseminated MAC disease (such as human immunodeficiency virus-infected persons) or pulmonary MAC disease but rather was associated with other, less obvious risk factors, such as black race and exposure to soil, the epidemiological relevance of which is not entirely clear (25).
The strain that was extensively used in earlier studies of skin testing for Johne's disease in livestock, strain 18, is now well documented to be M. avium subsp. avium of the bird type. Results from these skin test studies were disappointing (15, 16), but the use of avian PPD made from M. avium subsp. avium bird-type strains (such as SSI strain 10) to test for paratuberculosis continues with, perhaps predictably, variable results (11, 12, 36). Because of the complexity of PPD products, in which production methods and other factors are critical, it is difficult to relate the biological effect of a PPD reagent to the strain used to produce it. However, there are large regions of genomic differences between M. avium subsp. avium and M. avium subsp. paratuberculosis, with some large sequences present in the former but absent in the latter and others uniquely present in M. avium subsp. paratuberculosis (31, 33). So, while such reagents undoubtedly help to discriminate between tuberculous and nontuberculous infection in cattle (as is done through the commercial gamma interferon assay Bovigam [Prionics A.G. Switzerland]), genomic considerations predict that reagents based on M. avium subsp. avium may suffer poor specificity in detecting infection with M. avium subsp. paratuberculosis.
Among the M. avium subsp. paratuberculosis strains used to produce Johnin PPD by several different laboratories, we identified a large sequence, LSPjn, missing from seven of the Johnin production strains. Since the precise coordinates of this deletion are shared among these seven strains and the locus is otherwise intact in field isolates, this most probably represents a genomic deletion. In a historical context of collaborations and strain sharing between different veterinary laboratories producing diagnostic reagents during the 20th century, we conclude that the LSPjn-deleted strains share a common origin, with the deletion likely having occurred during prolonged in vitro passage. The sequence encodes proteins putatively involved in the transport of phosphate. The homologous genes in M. tuberculosis encode proteins that are present at the mycobacterial surface as well as in the culture filtrate, have been associated with virulence in an experimental infection model, and are potentially immunostimulatory antigens of M. tuberculosis (17, 24, 38). The occurrence of this polymorphism in M. avium subsp. paratuberculosis may have occurred as a result of continuous passaging in synthetic medium rich in inorganic phosphate. Of more practical interest is the observational report that Johnin reagents produced from these strains may suffer from poor sensitivity (5), in contrast with studies using M. avium subsp. paratuberculosis strains genomically intact for this locus (26, 27).
In conclusion, in this study we show that the strains used to produce sensitins used for diagnosis of M. avium infections, both in humans and in livestock, are not always based on strains more commonly associated with disease in these hosts. We also show that some M. avium subsp. paratuberculosis strains used to produce Johnin PPD have suffered a genomic deletion, the biological impact of which needs further study. Genetically and genomically well-characterized strains should be utilized to produce standardized M. avium PPD reagents, and strict care should be taken in maintaining these production strains to prevent the creation of genetically divergent strains.
Acknowledgments
This work was supported by a grant from the Natural Science and Engineering Research Council (grant number GEN2282399). M.S. is funded by the Fonds de la Recherche en Sante du Quebec (FRSQ), and M.A.B. is a New Investigator of the Canadian Institutes of Health Research.
None of the authors have a conflict of interest or any commercial association that may pose a conflict of interest.
We thank Peter Hubrechts for providing us with strains used to produce avian PPD and Fiona McIntosh for technical assistance with the microarray experiments.
REFERENCES
- 1.Amonsin, A., L. L. Li, Q. Zhang, J. P. Bannantine, A. S. Motiwala, S. Sreevatsan, and V. Kapur. 2004. Multilocus short sequence repeat sequencing approach for differentiating among Mycobacterium avium subsp. paratuberculosis strains. J. Clin. Microbiol. 42:1694-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke, L. A., C. S. Rebelo, J. Goncalves, M. G. Boavida, and P. Jordan. 2001. PCR amplification introduces errors into mononucleotide and dinucleotide repeat sequences. Mol. Pathol. 54:351-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coussens, P. M. 2001. Mycobacterium paratuberculosis and the bovine immune system. Anim. Health Res. Rev. 2:141-161. [PubMed] [Google Scholar]
- 4.de Lisle, G. W., and J. R. Duncan. 1981. Bovine paratuberculosis III. An evaluation of a whole blood lymphocyte transformation test. Can. J. Comp. Med. 45:304-309. [PMC free article] [PubMed] [Google Scholar]
- 5.de Lisle, G. W., P. Seguin, B. S. Samagh, A. H. Corner, and J. R. Duncan. 1980. Bovine paratuberculosis I. A herd study using complement fixation and intradermal tests. Can. J. Comp. Med. 44:177-182. [PMC free article] [PubMed] [Google Scholar]
- 6.Dunkin, G. W. 1928. A diagnostic agent for the detection of Johne's disease and its method of preparation. J. Comp. Pathol. 41:94-108. [Google Scholar]
- 7.Dvorska, L., T. J. Bull, M. Bartos, L. Matlova, P. Svastova, R. T. Weston, J. Kintr, I. Parmova, D. van Soolingen, and I. Pavlik. 2003. A standardised restriction fragment length polymorphism (RFLP) method for typing Mycobacterium avium isolates links IS901 with virulence for birds. J. Microbiol. Methods 55:11-27. [DOI] [PubMed] [Google Scholar]
- 8.Edwards, L. B., F. A. Acquaviva, V. T. Livesay, F. W. Cross, and C. E. Palmer. 1969. An atlas of sensitivity to tuberculin, PPD-B, and histoplasmin in the United States. Am. Rev. Respir. Dis. 99(Suppl.):1-132. [PubMed] [Google Scholar]
- 9.Fine, P. E., S. Floyd, J. L. Stanford, P. Nkhosa, A. Kasunga, S. Chaguluka, D. K. Warndorff, P. A. Jenkins, M. Yates, and J. M. Ponnighaus. 2001. Environmental mycobacteria in northern Malawi: implications for the epidemiology of tuberculosis and leprosy. Epidemiol. Infect. 126:379-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guld, J., M. W. Bentzon, M. A. Bleiker, W. A. Griep, M. Magnusson, and H. Waaler. 1958. Standardization of a new batch of purified tuberculin (PPD) intended for international use. Bull. W. H. O. 19:845-951. [PMC free article] [PubMed] [Google Scholar]
- 11.Jungersen, G., A. Huda, J. J. Hansen, and P. Lind. 2002. Interpretation of the gamma interferon test for diagnosis of subclinical paratuberculosis in cattle. Clin. Diagn. Lab. Immunol. 9:453-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalis, C. H., M. T. Collins, J. W. Hesselink, and H. W. Barkema. 2003. Specificity of two tests for the early diagnosis of bovine paratuberculosis based on cell-mediated immunity: the Johnin skin test and the gamma interferon assay. Vet. Microbiol. 97:73-86. [DOI] [PubMed] [Google Scholar]
- 13.Koch, R. 1882. Die Aetiologic der Tuberkulose. Berl. Klin. Wochenschr. 15:221-230. [Google Scholar]
- 14.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 15.Larsen, A. B., and K. E. Kopecky. 1965. Studies on the intravenous administration of Johnin to diagnose Johne's disease. Am. J. Vet. Res. 26:673-675. [PubMed] [Google Scholar]
- 16.Larsen, A. B., T. H. Vardaman, and R. S. Merkal. 1963. An extended study of a herd of cattle naturally infected with Johne's disease. II. The significance of the intradermic Johnin test. Am. J. Vet. Res. 24:91-93. [PubMed] [Google Scholar]
- 17.Lefevre, P., M. Braibant, L. de Wit, M. Kalai, D. Roeper, J. Grotzinger, J. P. Delville, P. Peirs, J. Ooms, K. Huygen, and J. Content. 1997. Three different putative phosphate transport receptors are encoded by the Mycobacterium tuberculosis genome and are present at the surface of Mycobacterium bovis BCG. J. Bacteriol. 179:2900-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lind, A., L. O. Larsson, M. W. Bentzon, M. Magnusson, J. Olofson, I. Sjogren, I. L. Strannegard, and B. E. Skoogh. 1991. Sensitivity to sensitins and tuberculin in Swedish children. I. A study of schoolchildren in an urban area. Tubercle 72:29-36. [DOI] [PubMed] [Google Scholar]
- 19.Magnusson, M. 1961. Specificity of mycobacterial sensitins. Am. Rev. Respir. Dis. 83:57-68. [DOI] [PubMed] [Google Scholar]
- 20.Mazurek, G. H., P. A. LoBue, C. L. Daley, J. Bernardo, A. A. Lardizabal, W. R. Bishai, M. F. Iademarco, and J. S. Rothel. 2001. Comparison of a whole-blood interferon gamma assay with tuberculin skin testing for detecting latent Mycobacterium tuberculosis infection. JAMA 286:1740-1747. [DOI] [PubMed] [Google Scholar]
- 21.McIntosh, C. W., and H. Konst. 1943. Preparation and standardization of Johnin purified protein derivative. Can. J. Public Health 35:557-563. [Google Scholar]
- 22.Mijs, W., P. de Haas, R. Rossau, L. T. Van der, L. Rigouts, F. Portaels, and D. van Soolingen. 2002. Molecular evidence to support a proposal to reserve the designation Mycobacterium avium subsp. avium for bird-type isolates and ‘M. avium subsp. hominissuis’ for the human/porcine type of M. avium. Int. J. Syst. Evol. Microbiol. 52:1505-1518. [DOI] [PubMed] [Google Scholar]
- 23.Palmer, C. E., and L. B. Edwards. 1968. Identifying the tuberculous infected. The dual-test technique. JAMA 205:167-169. [PubMed] [Google Scholar]
- 24.Peirs, P., P. Lefevre, S. Boarbi, X. M. Wang, O. Denis, M. Braibant, K. Pethe, C. Locht, K. Huygen, and J. Content. 2005. Mycobacterium tuberculosis with disruption in genes encoding the phosphate binding proteins PstS1 and PstS2 is deficient in phosphate uptake and demonstrates reduced in vivo virulence. Infect. Immun. 73:1898-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reed, C., C. F. von Reyn, S. Chamblee, T. V. Ellerbrock, J. W. Johnson, B. J. Marsh, L. S. Johnson, R. J. Trenschel, and C. R. Horsburgh, Jr. 2006. Environmental risk factors for infection with Mycobacterium avium complex. Am. J. Epidemiol. 164:32-40. [DOI] [PubMed] [Google Scholar]
- 26.Robbe-Austerman, S., I. A. Gardner, B. V. Thomsen, D. G. Morrical, B. M. Martin, M. V. Palmer, C. O. Thoen, and C. Ewing. 2006. Sensitivity and specificity of the agar-gel-immunodiffusion test, ELISA and the skin test for detection of paratuberculosis in United States midwest sheep populations. Vet. Res. 37:553-564. [DOI] [PubMed] [Google Scholar]
- 27.Robbe-Austerman, S., J. R. Stabel, and M. V. Palmer. 2006. Evaluation of the gamma interferon ELISA in sheep subclinically infected with Mycobacterium avium subspecies paratuberculosis using a whole-cell sonicate or a Johnin purified-protein derivative. J. Vet. Diagn. Investig. 18:189-194. [DOI] [PubMed] [Google Scholar]
- 28.Rothel, J. S., S. L. Jones, L. A. Corner, J. C. Cox, and P. R. Wood. 1990. A sandwich enzyme immunoassay for bovine interferon-gamma and its use for the detection of tuberculosis in cattle. Aust. Vet. J. 67:134-137. [DOI] [PubMed] [Google Scholar]
- 29.Saxegaard, F., and F. H. Fodstad. 1985. Control of paratuberculosis (Johne's disease) in goats by vaccination. Vet. Rec. 116:439-441. [DOI] [PubMed] [Google Scholar]
- 30.Seibert, F. B., and E. H. Dufour. 1954. Comparison between the international standard tuberculins, PPD-S and old tuberculin. Am. Rev. Tuberc. 69:585-594. [DOI] [PubMed] [Google Scholar]
- 31.Semret, M., D. C. Alexander, C. Y. Turenne, P. de Haas, P. Overduin, D. van Soolingen, D. Cousins, and M. A. Behr. 2005. Genomic polymorphisms for Mycobacterium avium subsp. paratuberculosis diagnostics. J. Clin. Microbiol. 43:3704-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semret, M., C. Y. Turenne, and M. A. Behr. 2006. Insertion sequence IS900 revisited. J. Clin. Microbiol. 44:1081-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semret, M., C. Y. Turenne, P. de Haas, D. M. Collins, and M. A. Behr. 2006. Differentiating host-associated variants of Mycobacterium avium by PCR for detection of large sequence polymorphisms. J. Clin. Microbiol. 44:881-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Semret, M., G. Zhai, S. Mostowy, C. Cleto, D. Alexander, G. Cangelosi, D. Cousins, D. M. Collins, D. van Soolingen, and M. A. Behr. 2004. Extensive genomic polymorphism within Mycobacterium avium. J. Bacteriol. 186:6332-6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stabel, J. R. 1996. Production of gamma-interferon by peripheral blood mononuclear cells: an important diagnostic tool for detection of subclinical paratuberculosis. J. Vet. Diagn. Investig. 8:345-350. [DOI] [PubMed] [Google Scholar]
- 36.Stabel, J. R., and R. H. Whitlock. 2001. An evaluation of a modified interferon-gamma assay for the detection of paratuberculosis in dairy herds. Vet. Immunol. Immunopathol. 79:69-81. [DOI] [PubMed] [Google Scholar]
- 37.Steadham, E. M., B. M. Martin, and C. O. Thoen. 2002. Production of a Mycobacterium avium ssp. paratuberculosis purified protein derivative (PPD) and evaluation of potency in guinea pigs. Biologicals 30:93-95. [DOI] [PubMed] [Google Scholar]
- 38.Tanghe, A., P. Lefevre, O. Denis, S. D'Souza, M. Braibant, E. Lozes, M. Singh, D. Montgomery, J. Content, and K. Huygen. 1999. Immunogenicity and protective efficacy of tuberculosis DNA vaccines encoding putative phosphate transport receptors. J. Immunol. 162:1113-1119. [PubMed] [Google Scholar]
- 39.Thegerstrom, J., B. I. Marklund, S. Hoffner, D. Axelsson-Olsson, J. Kauppinen, and B. Olsen. 2005. Mycobacterium avium with the bird type IS1245 RFLP profile is commonly found in wild and domestic animals, but rarely in humans. Scand. J. Infect. Dis. 37:15-20. [DOI] [PubMed] [Google Scholar]
- 40.Turenne, C. Y., M. Semret, D. V. Cousins, D. M. Collins, and M. A. Behr. 2006. Sequencing of hsp65 distinguishes among subsets of the Mycobacterium avium complex. J. Clin. Microbiol. 44:433-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Pinxteren, L. A., P. Ravn, E. M. Agger, J. Pollock, and P. Andersen. 2000. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin. Diagn. Lab. Immunol. 7:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Soolingen, D., P. E. de Haas, P. W. Hermans, and J. D. Van Embden. 1994. DNA fingerprinting of Mycobacterium tuberculosis. Methods Enzymol. 235:196-205. [DOI] [PubMed] [Google Scholar]
- 43.von Reyn, C. F., T. W. Barber, R. D. Arbeit, C. H. Sox, G. T. O'Connor, R. J. Brindle, C. F. Gilks, K. Hakkarainen, A. Ranki, C. Bartholomew, et al. 1993. Evidence of previous infection with Mycobacterium avium-Mycobacterium intracellulare complex among healthy subjects: an international study of dominant mycobacterial skin test reactions. J. Infect. Dis. 168:1553-1558. [DOI] [PubMed] [Google Scholar]
- 44.von Reyn, C. F., P. A. Green, D. McCormick, G. A. Huitt, B. J. Marsh, M. Magnusson, and T. W. Barber. 1994. Dual skin testing with Mycobacterium avium sensitin and purified protein derivative: an open study of patients with M. avium complex infection or tuberculosis. Clin. Infect. Dis. 19:15-20. [DOI] [PubMed] [Google Scholar]
- 45.von Reyn, C. F., C. R. Horsburgh, K. N. Olivier, P. F. Barnes, R. Waddell, C. Warren, S. Tvaroha, A. S. Jaeger, A. D. Lein, L. N. Alexander, D. J. Weber, and A. N. Tosteson. 2001. Skin test reactions to Mycobacterium tuberculosis purified protein derivative and Mycobacterium avium sensitin among health care workers and medical students in the United States. Int. J. Tuberc. Lung Dis. 5:1122-1128. [PubMed] [Google Scholar]
- 46.von Reyn, C. F., D. E. Williams, C. R. Horsburgh, Jr., A. S. Jaeger, B. J. Marsh, K. Haslov, and M. Magnusson. 1998. Dual skin testing with Mycobacterium avium sensitin and purified protein derivative to discriminate pulmonary disease due to M. avium complex from pulmonary disease due to Mycobacterium tuberculosis. J. Infect. Dis. 177:730-736. [DOI] [PubMed] [Google Scholar]
- 47.Watson, E. A. 1935. Tuberculin, Johnin and Mallein derived from nonprotein media. Can. J. Public Health 26:268-275. [Google Scholar]