Abstract
Most individuals exposed to Mycobacterium tuberculosis become infected but hinder the infectious process in dormant foci, known as latent tuberculosis. This limited infection usually stimulates strong T-cell responses, which provide lifelong resistance to tuberculosis. However, latent tuberculosis is still poorly understood, particularly because of the lack of a reliable animal model of dormant infection. Here we show that inoculation of mice with a unique streptomycin-auxotrophic mutant of Mycobacterium tuberculosis recapitulates dormant infection. The mutant grows unimpaired in the presence of streptomycin and no longer grows but remains viable for long periods of time after substrate removal, shifting from the log growth phase to the latent stage, as indicated by augmented production of α-crystallin. Mice challenged with the mutant and inoculated with streptomycin for ∼3 weeks developed a limited infection characterized by a low bacteriological burden and the presence of typical granulomas. After substrate withdrawal, the infection was hindered but few microorganisms remained viable (dormant) in the animals' tissues for at least 6 months. In addition, the animals developed both potent T-cell responses to M. tuberculosis antigens, such as early culture filtrate, Ag85B, and ESAT-6, and resistance to reinfection with virulent M. tuberculosis. Therefore, infection of mice or other animals (e.g., guinea pigs) with M. tuberculosis strain 18b constitutes a simple and attractive animal model for evaluation of antituberculosis vaccines in the context of an M. tuberculosis-presensitized host, a prevailing condition among humans in need of a vaccine.
Infection with Mycobacterium tuberculosis usually results in so-called primary tuberculosis or Ghon's complex in approximately 90% of the individuals. This usually limited infection comprises a focal multiplication of the Mycobacterium in the lung tissue in association with lymphangitis, infection, and massive enlargement of the corresponding hilar draining lymph node. In approximately 95% of these individuals, the inflammatory reaction is spontaneously contained and in many cases calcifies and persists for the remainder of the person's life (26). Notwithstanding the clinical resolution of Ghon's complex, several M. tuberculosis organisms remain viable for life, a condition known as latent or dormant tuberculosis (21, 26). Primary tuberculosis usually stimulates strong and long-lasting cellular immune response to M. tuberculosis antigens, which can be detected even years later by the delayed-type hypersensitivity skin test (the purified protein derivative [PPD] or Mantoux test). Reactivation of latent tuberculosis leading to chronic pulmonary tuberculosis or adult tuberculosis may occur afterwards, even decades later, due to intervening events such as malnutrition or immunosuppression. However, clinical and molecular evidence indicates that adult tuberculosis occurs most often due to exogenous reinfection with M. tuberculosis in geographical areas where the rate of contagion is high, such as in many developing countries (8, 17, 27, 34, 36, 40). Although the general consensus is that adult tuberculosis caused by reactivation of latent infection is associated with some predisposing immunodeficiency, the need for such an intervening condition for disease development due to exogenous reinfection of PPD-positive healthy immunocompetent individuals is still a controversial issue. Unfortunately, experiments designed to study this controversy have been hampered by the lack of a reliable animal model of dormant infection. Moreover, such an animal model is in high demand for validation of vaccine candidates in the context of presensitization with M. tuberculosis antigens, a condition highly prevalent among the human population in need of an antituberculosis vaccine.
Interestingly, early studies carried out approximately two decades ago suggested that a unique streptomycin-auxotrophic mutant of M. tuberculosis (strain 18b) could be used to study persistent or dormant tuberculosis (10, 18, 19). We implemented these original studies and present evidence indicating that mice inoculated with M. tuberculosis strain 18b develop an infectious process that recapitulates the dormant infection. The infection is characterized by focal and typical granuloma formation and the persistent presence of low numbers of viable nonreplicating organisms in the animals' tissues for long periods of time. In addition, this infection, similar to the case in humans with dormant tuberculosis, induces potent T-cell responses to native and purified recombinant proteins of M. tuberculosis. Moreover, the animals develop resistance to exogenous reinfection with virulent M. tuberculosis strains.
MATERIALS AND METHODS
Mice.
BALB/c mice were obtained from Charles River Laboratories (Wilmington, MA). All mice were maintained under specific-pathogen-free conditions and were used at 8 to 12 weeks of age.
Bacteria and mouse infections.
A virulent M. tuberculosis H37Rv strain (strain ATCC 35718) was obtained from the American Type Culture Collection, Manassas, VA. Streptomycin-auxotrophic M. tuberculosis strain 18b was provided by Stewart Cole, Institute Pasteur, Paris, France. Mycobacterium bovis BCG was obtained from Aventis Pasteur (Swiftwater, PA). Bacteria were cultured either in BBL Middlebrook 7H9 medium plus albumin-dextrose-catalase enrichment or in Middlebrook 7H10 Bacto agar plates plus oleic acid-albumin-dextrose-catalase enrichment (Becton Dickinson Microbiology Systems, Cockeysville, MD) containing or not containing streptomycin sulfate (Sigma, St. Louis, MO). For infections, the mycobacteria were suspended in phosphate-buffered saline (PBS)-Tween 80 (0.05%), pushed through a 26-gauge needle six times, and delivered intravenously (i.v.) at 1 × 106 to 2 × 106 (strain 18b) per mouse. Alternatively, infection with virulent strain M. tuberculosis H37Rv was done by the respiratory route with approximately 100 to 200 CFU per mouse by using an aerosol generation device (Inhalation Exposure System; Glas-Col, Terre Haute, IN). Frozen glycerol stocks of both mycobacterial strains used in these studies were prepared from organisms isolated from the lungs of mice after two cycles of infection.
Specific reagents.
The following monoclonal antibodies (MAbs) and polyclonal antibodies were used in the current studies: purified rat anti-mouse CD4 (RM4-5), CD8 (53-6.7), CD11b (M1/70), purified rat immunoglobulin G2a (IgG2a; Pharmingen, San Diego, CA), and goat anti-rat Ig horseradish peroxidase (HRP) conjugate (BioSource International, Camarillo, CA); purified rat anti-mouse gamma interferon (IFN-γ; R4-6A2); biotinylated rat anti-mouse IFN-γ (XMG1.2); biotinylated rat anti-mouse IL-4 (BVD6-24G2) (Pharmingen); anti-α-crystallin (Colorado State University, NIAID/NIH Tuberculosis Research Materials contract N01-AI-25147); and goat anti-mouse Ig HRP conjugate (Pharmingen).
Western blotting.
Purified recombinant α-crystallin protein (Colorado State University, NIAID/NIH Tuberculosis Research Materials contract N01-AI-25147) and whole bacterial lysate were electrophoresed on a sodium dodecyl sulfate-polyacrylamide gel (4 to 20% gradient acrylamide gel; Bio-Rad, Hercules, CA) under reducing conditions and transferred to a nitrocellulose membrane (Bio-Rad). The blot was blocked overnight at 4°C with PBS-0.1% Tween 20 containing 5% nonfat dry milk and subsequently incubated for 1 h with an anti-α-crystallin monoclonal antibody. The membrane was rinsed several times with PBS plus 0.1% Tween 20, followed by incubation (30 min) with peroxidase-labeled purified goat anti-mouse IgG. After additional washings, the bound conjugate was detected by using an enhanced chemiluminescence system (Amersham Biosciences), and the proteins were visualized by exposing the blot to autoradiography film (Kodak BioMax, Rochester, NY).
Histology.
Tissue sections were fixed in 10% formalin and embedded in paraffin. Sections (5 μm) were stained with hematoxylin-eosin or by the Ziehl-Neelsen method for acid-fast bacilli.
Immunohistochemistry.
The lungs of the infected mice were infused with 30% of the embedding medium “Optimal Cutting Temperature” (OCT; Tissue-Tek, Inc., Torrance, CA) in PBS through the trachea, removed, embedded in OCT, frozen in liquid nitrogen, and stored at −20°C. Serial sections, 5 μm thick, were cut on a cryostat (CM 1850; Leica), fixed in cold acetone for 10 min, and air dried. Purified primary antibodies, at the appropriate concentration, were incubated overnight at 4°C. Control sections were incubated with the isotype control IgG2a or IgG2b. Following several washings with PBS, endogenous peroxidase was blocked for 20 min at room temperature with 0.3% peroxide hydrogen in methanol (Sigma). Sections were washed in PBS and incubated with the detection antibody goat anti-rat Ig conjugated to HRP. The reaction was then developed by using diaminobenzidine and hydrogen peroxide (Pharmingen) as the substrate. Sections were counterstained with Mayer's hematoxylin (Sigma) and covered with Permount (Fisher Chemicals).
CFU.
After infection, organ tissue (spleen, liver, and lung) homogenates in PBS-Tween 80 (0.05%) were prepared and plated at a 5- or 10-fold serial dilution on Middlebrook 7H10 Bacto agar with or without 50 μg/ml of streptomycin sulfate. The CFU were enumerated 3 to 4 weeks later.
Assay for T-cell response.
Spleens cells were obtained by conventional procedures and were then centrifuged over Ficoll-Hypaque to remove the dead cells, granulocytes, and red cells. Mononuclear cells (2 × 105/well of 96-well microtiter culture plates) were stimulated at 37°C in CO2 either with medium or with the following M. tuberculosis antigens: mycobacterial culture filtrate (CF) antigens, Ag85, and recombinant ESAT-6 (all provided by John Belisle, Colorado State University, through NIAID/NIH Tuberculosis Research Materials contract N01-AI-25147). Production of the T-cell cytokines IFN-γ and IL-4 in the supernatants of 72-h cultures was measured by a double-sandwich enzyme-linked immunosorbent assay (ELISA) with specific MAb (Pharmingen), as described previously (41).
RESULTS
In vitro growth and survival of M. tuberculosis strain 18b.
Unique strain 18b was isolated in 1955 by Hashimoto (10) from a patient with pulmonary tuberculosis caused by a streptomycin-“resistant” strain of M. tuberculosis. The drug resistance was subsequently found to be a mutation that gave rise to an auxotrophic phenotype that made the organism incapable of growing in Lowenstein-Jensen medium containing less than 40 μg/ml of streptomycin. In addition, these organisms could maintain their viability in vitro for several weeks in the absence of streptomycin. Moreover, strain 18b could multiply in mouse tissues if the animals were inoculated intravenously with the mycobacterium and injected daily with the substrate streptomycin. Therefore, this auxotrophic mutant of M. tuberculosis constitutes a useful strain for studies related to both immunopathogenesis and vaccine development. More recently, the molecular characterization of the strain 18b streptomycin auxotrophism was investigated (12). Those studies found that a mutation in the rrs gene encoding the 16S rRNA is associated with the streptomycin dependency. Because the original studies were carried out over 50 years ago and the mutant was preserved frozen for a long period of time, it became important to reevaluate its level of streptomycin dependence to achieve maximal in vitro growth. Therefore, a dose-response assay was performed. The mycobacteria were washed three times with PBS, suspended to approximately 105 CFU/ml, and inoculated 1/100 in BBL Middlebrook 7H9 medium plus enrichment in the presence of various concentrations of streptomycin sulfate. Growth was monitored daily by turbidimetry. The results confirmed the original observation that M. tuberculosis strain 18b is highly dependent on streptomycin, requiring a minimum of 25 μg/ml of the antibiotic to achieve maximal growth after 3 weeks of incubation at 37°C (data not shown).
To determine if substrate starvation interferes with the viability of the auxotrophic mutant, the mycobacterium was grown in Middlebrook 7H9 medium plus enrichment for 3 weeks in the presence of 50 μg/ml of streptomycin. Approximately 106 CFU were retrieved from the culture, washed three times with PBS to achieve substrate starvation, and then incubated in streptomycin-free Middlebrook 7H9 medium for up to 4 weeks at 37°C. At different time points (immediately after the inoculation and at 7, 14, 21, and 28 days postinoculation), starvation was interrupted by plating the organisms on streptomycin (50 μg/ml) containing Middlebrook 7H10 Bacto agar plus oleic acid-albumin-dextrose-catalase enrichment, followed by 3 weeks of incubation at 37°C and CFU enumeration. Regardless of the duration of substrate starvation, no differences in the numbers of CFU (∼100,000 ± 10,000) recovered from these cultures at each time point were observed (data not shown). These results indicate that the auxotrophic mutant does not lose viability in the absence of a substrate for at least 4 weeks.
Expression of α-crystallin by M. tuberculosis strain 18b after substrate withdrawal.
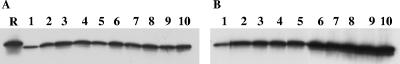
α-Crystallin is encoded by a gene that, among the members of the genus Mycobacterium, is present only in the slowly growing M. tuberculosis complex organisms (42). Expression of this molecule is greatly enhanced during the stationary growth phase of the mycobacterium or in cultures following a shift to oxygen-limiting conditions (5, 14). In addition, overexpression of α-crystallin in wild-type M. tuberculosis isolates results in a slower decline in viability following the end of the log-phase growth of these mutated organisms (14). These and other observations (6, 30, 37, 38) have led to the proposal that during latency M. tuberculosis produces α-crystallin at enhanced concentrations. Therefore, to investigate if the growth arrest of M. tuberculosis strain 18b caused by withdrawal of the substrate streptomycin would result in the enhanced production of α-crystallin, Western blot analysis was performed with samples obtained daily from the mycobacterium cultured in the presence or the absence of streptomycin. The mycobacterium was grown for 3 weeks in Middlebrook 7H9 medium plus enrichment in the presence of 50 μg/ml of streptomycin, followed by three washes with PBS to achieve substrate starvation. The culture was then divided in two equal volumes and dispensed in two separate flasks. One of the flasks was reinoculated with streptomycin sulfate (50 μg/ml), and the second flask was inoculated with an equal volume of PBS. Samples were collected from each flask for 10 consecutive days, washed three times with PBS, lysed, normalized so that they had the same protein concentrations, submitted to sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions, blotted, and tested for the presence of α-crystallin with a specific monoclonal antibody. Figure 1 shows the results, which point to steady basal production of α-crystallin in the cultures of M. tuberculosis strain 18b growing in the presence of streptomycin. In contrast, samples obtained from cultures reincubated under substrate starvation clearly show a steady augmentation of α-crystallin production after substrate removal. These results indicate that in the absence of streptomycin the mutant recapitulates the dormant growth phase of the mycobacterium, as indicated by growth arrest and augmented production of α-crystallin, a molecular marker of latency.
FIG. 1.
Production of α-crystallin by Mycobacterium tuberculosis strain 18b. Approximately 108 CFU of the mycobacterium was incubated for 10 days in Middlebrook 7H9 medium either in the presence of 50 μg/ml of streptomycin (A) or in the absence of the antibiotic (B). Bacterial samples were harvested daily, lysed, normalized for the same protein concentration, and analyzed for the presence of α-crystallin by Western blotting with the specific MAb 04.CS49. Lane R, purified recombinant α-crystallin; lanes 1 to 10, days of Mycobacterium growth in culture. Note the steady augmentation of α-crystallin in the samples obtained from the Mycobacterium incubated in streptomycin-free medium.
Multiplication and growth arrest of M. tuberculosis strain 18b in tissues of infected mice.
The in vivo growth and survival of M. tuberculosis strain 18b were next tested in BALB/c mice. Two hours before infection, one group of mice was inoculated subcutaneously (s.c.) with 2 mg of streptomycin sulfate. A second group was injected with PBS. The mice were then infected via the lateral tail vein with 106 CFU of M. tuberculosis strain 18b. For the next 4 weeks, the mice initially inoculated with streptomycin continued to receive daily s.c. injections of the antibiotic. The second group of mice was injected with PBS for the same length of time. Groups of five animals were periodically euthanized, on days 7, 14, 21, and 28 after the challenge, and their spleens and lungs were removed, homogenized, and plated on Middlebrook 7H10 Bacto agar plates containing or not containing 50 μg/ml of streptomycin sulfate. The numbers of CFU were determined after incubation for 3 to 4 weeks at 37°C. Figure 2 shows the results and clearly point to the in vivo replication of the mutant in both the spleens and the lungs of the infected and streptomycin-treated mice. Replication peaked at approximately 3 to 4 weeks after infection, which basically follows the same kinetics of infection observed in mice infected with virulent M. tuberculosis isolates (4, 28). In addition, Fig. 2A shows that in the absence of substrate the mutant did not replicate and several microorganisms retained their viability in vivo for the entire duration of the experiment.
FIG. 2.
Growth of M. tuberculosis strain 18b in tissues of infected mice. BALB/c mice were infected via the lateral tail vein with 106 CFU of M. tuberculosis strain 18b. One group of mice (filled symbols) received daily s.c. injections of streptomycin sulfate (2 mg/mouse) for 4 weeks. A second group of mice (open symbols) was injected with saline only. The animals were euthanized on days 7, 14, 21, and 28 (A) or at 6 months (B) after the challenge; and their spleens and lungs were removed, homogenized, and plated on Middlebrook 7H10 Bacto agar plates containing 50 μg/ml of streptomycin sulfate. The numbers of CFU were determined after incubation for 3 to 4 weeks at 37°C. Bars represent the standard errors of the means of the results for five mice per group. This figure shows the results of one representative experiment of three experiments performed, with essentially the same results obtained in each experiment.
The duration of survival of M. tuberculosis strain 18b under substrate starvation in vivo was next verified. The duration of survival is a crucial issue for studies involving dormant infection and the immunopathogenesis of M. tuberculosis. BALB/c mice (Charles River Laboratories) were infected i.v. with 106 CFU and were treated daily either with s.c. injections of streptomycin sulfate (2 mg/mouse) or with PBS for 2 weeks. The mice were euthanized 6 months after the interruption of the streptomycin injections, and the numbers of viable bacteria in the spleen and lungs were counted after growth on streptomycin-containing 7H10 agar plates. Figure 2B shows that viable mycobacteria could be recovered from the mouse tissues 6 months after substrate starvation. No bacteria could be recovered from the tissues of mice inoculated with the mutant but not treated with streptomycin. Not shown in Fig. 2 was the observation that no bacteria could be recovered from streptomycin-free agar plates. These results suggest that, in the absence of its substrate, the auxotrophic mutant develops a dormant infection in mice that lasts for at least 6 months.
Granulomatous inflammatory reaction in tissues of mice infected with M. tuberculosis strain 18b.
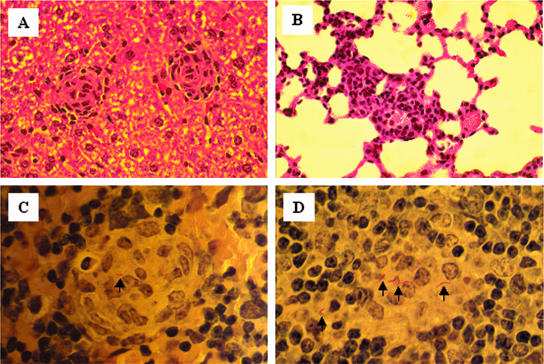
An organized granuloma has traditionally been considered a hallmark of a specific cellular immune response against mycobacterial infection. Therefore, to evaluate the ability of strain 18b of M. tuberculosis to stimulate the formation of granulomas, histopathological studies were performed with the lungs, the spleens, and the livers of mice infected with this organism. Mice were infected i.v. with 2 × 106 CFU of M. tuberculosis strain 18b and supplemented daily for 2 weeks with s.c. injections of 2 mg of streptomycin sulfate to permit full maturation of the granulomas. Two weeks after the withdrawal of the streptomycin, mice were euthanized and the lungs, spleens, and livers were removed and fixed with formaldehyde. Tissue sections were embedded in paraffin and stained with hematoxylin-eosin or by the Ziehl-Neelsen method for acid-fast bacilli. This evaluation confirmed the presence of well-organized granulomas in the infected animals (Fig. 3A). In addition, the histopathology showed moderate interstitial granulomatous pneumonia and perivascular lymphocytic infiltration in the lungs (Fig. 3B), a feature commonly seen in mice vaccinated with BCG and challenged with virulent M. tuberculosis (11). Acid-fast staining confirmed the presence of M. tuberculosis organisms within the histiocytic and epithelioid cells of the granulomas (Fig. 3C and D).
FIG. 3.
Histopathology of liver, lung, and spleen tissue from BALB/c mice infected with M. tuberculosis strain 18b. The mice were infected as described in the legend to Fig. 2, followed by daily injections (s.c.) of 2 mg of streptomycin sulfate for 2 weeks. The mice were euthanized, and the lungs, spleen, and liver were removed. Tissue sections were stained with hematoxylin-eosin (A and B) or by the Ziehl-Neelsen method (C and D) for acid-fast bacilli. Typical epithelioid granulomas are seen in the liver and spleen sections (A, C, and D). Perivascular infiltration of mononuclear cells is clearly seen in the lung tissue (B). Arrows point to several acid-fast M. tuberculosis organisms inside the spleen granulomas (C and D). Magnifications, ×200 (A and B) and ×1,000 (C and D).
In addition, immunohistochemistry studies with anti-CD4-, anti-CD8-, and anti-CD11b-specific antibodies were performed to further characterize the granulomas. The results are illustrated in Fig. 4 and indicated that the CD11b-positive cells (macrophages) are found in the center of the granuloma and are surrounded by CD4+ T cells as well by as sparse CD8+ T cells. These results indicate that M. tuberculosis strain 18b induces organized granulomas with the same spatial distribution of cells found in the granulomas formed in humans infected with M. tuberculosis (26).
FIG. 4.
Immunohistochemical staining of CD11b+ cells and CD4+ and CD8+ lymphocytes in frozen sections of lung tissue from BALB/c mice infected with M. tuberculosis strain 18b. The mice were infected as described in the legend to Fig. 2, followed by daily injections (s.c.) of 2 mg of streptomycin sulfate for 3 weeks. The mice were euthanized, and the lungs were removed and processed as described in Material and Methods. Note the characteristic granuloma organization with the central accumulation of CD11b+ cells (macrophages) surrounded by a dense mantle of CD4+ lymphocytes and the sparse distribution of CD8+ lymphocytes. These are serial sections (magnification, ×200) 5 μm thick and are representative of the findings obtained in three experiments.
Recognition of CF antigens and purified recombinant antigens of M. tuberculosis by mononuclear spleen cells of mice infected with M. tuberculosis strain 18b.
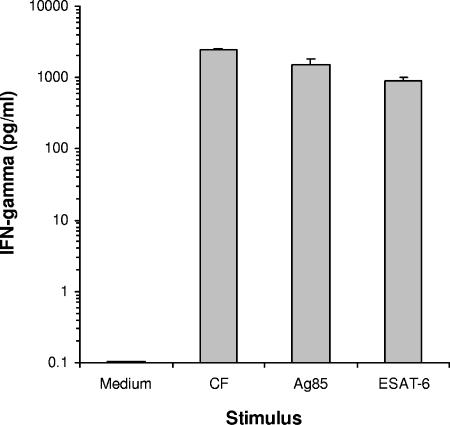
The induction of an immune response in M. tuberculosis strain 18b-infected mice was next investigated. Several findings support the concept that induction of protection against tuberculosis can be achieved with the mycobacterial CF antigens, which are proteins produced and released in vitro during the active multiplication phase of the microorganisms (1, 13, 15, 31, 33). Therefore, whole CF antigen as well as some individual purified proteins found in this antigenic mixture was used to evaluate the immune response generated in mice infected with the auxotrophic mutant. This evaluation was carried out with mice infected with strain 18b and supplemented daily with streptomycin for 2 weeks. The mice were euthanized 6 weeks after infection (4 weeks after the withdrawal of streptomycin); their spleen cells were cultured and stimulated for 72 h with CF, Ag85, and ESAT-6. The culture supernatants were assayed for IFN-γ production by a sandwich ELISA with specific MAb (Pharmingen). The results are presented in Fig. 5 and indicate that spleen cells obtained from mice infected with strain 18b produced large amounts of IFN-γ when they were stimulated with both native and recombinant antigens, which is a pattern of response usually observed in mice infected with virulent M. tuberculosis isolates. These results suggest that mice with a dormant infection caused by M. tuberculosis strain 18b develop T-cell responses to “protective” antigens of M. tuberculosis.
FIG. 5.
Cytokine production by spleen cells of M. tuberculosis-infected mice. BALB/c mice were infected i.v. with M. tuberculosis strain 18b and supplemented daily (s.c. injections) with streptomycin (2 mg/mouse) for 2 weeks. The animals were euthanized 4 weeks after the withdrawal of streptomycin. Mononuclear spleen cells were obtained and stimulated for 3 days with CF antigens of M. tuberculosis H37Rv, Ag85 complex, and ESAT-6. Supernatants were harvested and assayed for the presence of IFN-γ by ELISA. Bars are the standard deviations of the means obtained from triplicate cultures. This figure shows the results of one representative experiment of three experiments performed, with essentially the same results obtained in each experiment.
Fate of exogenous reinfection of mice with virulent M. tuberculosis.
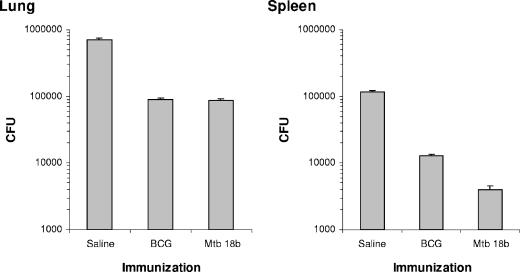
Finally, the outcome of exogenous secondary infection with a virulent M. tuberculosis strain of mice that had developed a dormant infection by previous exposure to M. tuberculosis strain 18b was tested. For this experiment the mice were initially infected i.v. with 2 × 106 CFU of M. tuberculosis strain 18b, followed by daily injections of streptomycin for 2 weeks to ascertain in vivo replication of the mutant. As controls, a second group of mice was vaccinated intradermally with 5 × 104 CFU of BCG and a third group was treated with saline only. Ten weeks after infection with the auxotrophic mutant (8 weeks after streptomycin withdrawal) all mice were challenged with virulent M. tuberculosis by the aerosol route. The induction of protection was evaluated 4 weeks later by enumerating the bacterial burden in the lungs of the mice. Figure 6 shows the results and indicates that the dormant infection established in mice conferred a level of protection against challenge of the animals with virulent M. tuberculosis comparable to the level of protection induced by immunization with BCG.
FIG. 6.
Growth of M. tuberculosis H37Rv in the tissues of mice with established latent infection with M. tuberculosis strain 18b (Mtb 18b). Dormant infection was induced by infecting five mice i.v. with 106 CFU of M. tuberculosis strain 18b, followed by daily s.c. injections of streptomycin (2 mg/mouse) for 2 weeks (preinfection was with M. tuberculosis strain 18b). Control groups of mice were either treated with saline only (saline) or vaccinated with BCG. Ten weeks after infection with the auxotrophic mutant, the animals were challenged by the respiratory route with 200 aerosolized CFU of M. tuberculosis H37Rv. The animals were euthanized 4 weeks later, and the CFU in the spleens and lungs were enumerated on 7H10 agar plates. Bars represent the standard errors of the means of the results for five mice. This figure shows the results of one representative experiment of two experiments performed, with essentially the same results obtained in each experiment.
DISCUSSION
Two murine models of latent or dormant M. tuberculosis infection have been used the most frequently for over 50 years. In one of these models (29), mice are infected (by the aerosol route) with a low dose of M. tuberculosis (5 to 10 CFU). Approximately 3 months later the bacillary burden reaches ∼104 CFU/lung and remains at these levels for 15 to 18 months, after which time the infection takes off and the mice succumb to tuberculosis. This model has the advantage of keeping the bacterial burden under control for an extended period of time, but it has the disadvantage of a high bacillary burden that is unlikely to reproduce the actual low rate of bacterial multiplication that is found in human latent tuberculosis. The second model, also called the Cornell model, was first described in the late 1950s (22-25). In this model mice are inoculated i.v. with ∼2 × 106 viable bacilli of virulent M. tuberculosis, and the resultant infection is treated for 12 weeks with the antimycobacterial drugs isoniazid and pyrazinamide beginning within 20 min after infection. From this point on, no tubercle bacilli can be cultured from the animals' organs for many months. However, administration of cortisone (at immunosuppressive doses) at 2 to 3 months after the interruption of the antibiotic therapy causes this condition to revert, and M. tuberculosis can be cultured from the lungs and the spleens of ∼50% of the animals. Alternatively, administration of amino guanidine (a nitric oxide synthase inhibitor) has been shown to also favor the reactivation of the mycobacterial reproduction in vivo (9). Even though it has the advantage of achieving and maintaining very low numbers of the tubercle bacilli within the tissues of infected mice for many weeks, this model has three major limitations: (i) dormancy is difficult to standardize because the optimal antibiotic concentration and the optimal duration of treatment required to achieve low numbers of bacilli vary from experiment to experiment; (ii) only 50% of the animals successfully treated with antibiotic develop dormant infection, which imposes a major complication in the interpretation of the results of the experiments (20); and (iii) most variants of the Cornell model use high doses of immunosuppressive reagents to achieve reactivation, which by definition constitutes a complication for studies designed to evaluate the host immune response during the reactivation of the disease.
The strain 18b model can circumvent the limitations of a high bacillary burden during dormancy (low-dose infection model) and has an advantage over the Cornell model because it is easy to standardize and dormant infection is reproducibly achieved. Indeed, the current model offers a unique opportunity to verify the immune response during two distinct phases of the infectious processes caused by M. tuberculosis: the initial multiplication of the bacteria in the host tissues and the latency characterized by the presence of few viable nonreplicating organisms in the tissues of infected mice for months. In other words, infection of mice with M. tuberculosis strain 18b results in a limited infectious process that mimics the focal dormant foci that develop in humans after primary tuberculosis, which is followed by the persistence of few viable organisms in the individual's tissues for decades.
The general consensus is that during dormancy the bacteria keep viability without detectable growth through minimal metabolism. The experiments described here indicate that the streptomycin-auxotrophic mutant grows normally both in vitro and in vivo in the presence of the substrate and that its viability is preserved for months under conditions of substrate starvation. More importantly, these experiments also showed that during the growth arrest of the mutant caused by substrate starvation, the microorganisms produce increased amounts of α-crystallin, thus confirming that the bacteria remain metabolically active in the absence of the substrate. Because augmented production of this molecule by M. tuberculosis has been used as a molecular marker of latency (5, 6, 14, 30, 37, 38, 42), these findings also indicate that during substrate starvation M. tuberculosis strain 18b shifts its growth from logarithmic phase to a dormant stage.
From the pathological viewpoint, the infection of mice with the mutant results in the development of focal granulomas in the lungs as well as in the spleen and liver. This localized pathology is in sharp contrast to the severe and extensive granulomatous pneumonia observed in mice infected with virulent M. tuberculosis (32). Moreover, the granulomas formed in the tissues of mice infected with the mutant are well organized and are constituted of many macrophages (CD11b+ cells) surrounded by a mantle of CD4+ T cells and by sparse CD8+ T cells, a morphology consistent with that of the granulomas seen in humans with primary tuberculosis.
In addition, T cells obtained from strain 18b-infected mice several weeks after substrate starvation recognize a broad range of M. tuberculosis antigens. These antigens included a mixture of native antigens (culture filtrate) and highly purified proteins such as Ag85b and ESAT-6 (an antigen not present in BCG). Therefore, the limited and focal infection caused by the mutant growing in vivo in the presence of streptomycin followed by a state of growth arrest after substrate withdrawal (dormant infection) stimulates T-cell responses that recapitulate the PPD sensitivity and the antigenic recognition repertoire of the T cells of humans with either latent tuberculosis or tuberculosis disease (2, 3, 7).
Similar to the results of the original studies (18, 19), the results of the present study show that strain 18b induces protection in mice against challenge with virulent M. tuberculosis. Interestingly, recent studies show that several other auxotrophic mutants of M. tuberculosis obtained by mutating genes encoding essential biosynthetic pathways of either purines (16), amino acids (11, 39), or vitamins (35) are highly attenuated and, like strain 18b, induce immunity against challenge with virulent organisms. However, in contrast to the infection caused by the streptomycin auxotrophic mutant, a high bacterial burden is maintained for long periods of time in the tissues of mice infected with those organisms. Indeed, this situation is particularly evident with the proline- and tryptophan-auxotrophic mutants (39). It is possible that the high bacterial burden maintained by these mutants in vivo is sustained by the presence of low concentrations of the substrates (purines, amino acids, or vitamins) in the milieu of the host infected tissues. In contrast, because of the strict dependency on a totally foreign substrate, a low bacterial burden and the dormancy of strain 18b can easily be achieved, controlled, and maintained at minimal levels in infected animals.
In conclusion, the dormant infection that can be established in vivo with the streptomycin-auxotrophic mutant constitutes an attractive and simple animal model for the evaluation of antituberculosis vaccines in the context of an M. tuberculosis-presensitized host, a prevailing condition in the majority of the human population in need of a vaccine.
Acknowledgments
This investigation was conducted in a Forsyth Institute facility renovated with support from Research Facilities Improvement grant C06RR11244 from the National Center for Research Resources, National Institutes of Health. This work was supported by grant AI 43528 from the National Institutes of Health to A. Campos-Neto.
We thank Stewart Cole for providing the streptomycin auxotroph M. tuberculosis strain 18b; John Beslile and Karen Dobos, Colorado State University (NIAID/NIH Tuberculosis Research Materials contract no. 1-A125174), for kindly supplying M. tuberculosis-related reagents; and Maryellen Feeney for reviewing the manuscript.
REFERENCES
- 1.Andersen, P. 1994. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect. Immun. 62:2536-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brock, I., M. E. Munk, A. Kok-Jensen, and P. Andersen. 2001. Performance of whole blood IFN-gamma test for tuberculosis diagnosis based on PPD or the specific antigens ESAT-6 and CFP-10. Int. J. Tuberc. Lung Dis. 5:462-467. [PubMed] [Google Scholar]
- 3.Cardoso, F. L., P. R. Antas, A. S. Milagres, A. Geluk, K. L. Franken, E. B. Oliveira, H. C. Teixeira, S. A. Nogueira, E. N. Sarno, P. Klatser, T. H. Ottenhoff, and E. P. Sampaio. 2002. T-cell responses to the Mycobacterium tuberculosis-specific antigen ESAT-6 in Brazilian tuberculosis patients. Infect. Immun. 70:6707-6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins, F. M., and V. Montalbine. 1976. Distribution of mycobacteria grown in vivo in the organs of intravenously infected mice. Am. Rev. Respir. Dis. 113:281-286. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham, A. F., and C. L. Spreadbury. 1998. Mycobacterial stationary phase induced by low oxygen tension: cell wall thickening and localization of the 16-kilodalton alpha-crystallin homolog. J. Bacteriol. 180:801-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desjardin, L. E., L. G. Hayes, C. D. Sohaskey, L. G. Wayne, and K. D. Eisenach. 2001. Microaerophilic induction of the alpha-crystallin chaperone protein homologue (HspX) mRNA of Mycobacterium tuberculosis. J. Bacteriol. 183:5311-5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dillon, D. C., M. R. Alderson, C. H. Day, T. Bement, A. Campos-Neto, Y. A. Skeiky, T. Vedvick, R. Badaro, S. G. Reed, and R. Houghton. 2000. Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis BCG. J. Clin. Microbiol. 38:3285-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzpatrick, L. K., A. Okwera, R. Mugerwa, R. Ridzon, J. Ehiner, and I. Onorato. 2002. An investigation of suspected exogenous reinfection in tuberculosis patients in Kampala, Uganda. Int. J. Tuberc. Lung Dis. 6:550-552. [DOI] [PubMed] [Google Scholar]
- 9.Flynn, J. L., C. A. Scanga, K. E. Tanaka, and J. Chan 1998. Effects of aminoguanidine on latent murine tuberculosis. J. Immunol. 160:1796-1803. [PubMed] [Google Scholar]
- 10.Hashimoto, T. 1955. Experimental studies on the mechanism of infection and immunity in tuberculosis from the standpoint of streptomycin-dependent tubercle bacilli. I. Isolation and biological characteristics of a streptomycin-dependent mutant, and effect of streptomycin administration on its pathogenicity in guinea pigs. Kekkaku 30:4-8. [PubMed] [Google Scholar]
- 11.Hondalus, M. K., S. Bardarov, R. Russell, J. Chan, W. R. Jacobs, Jr., and B. R. Bloom. 2000. Attenuation of and protection induced by a leucine auxotroph of Mycobacterium tuberculosis. Infect. Immun. 68:2888-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honore, N., G. Marchal, and S. T. Cole. 1995. Novel mutation in 16S rRNA associated with streptomycin dependence in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 39:769-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horwitz, M. A., B. E. Lee, B. J. Dillon, and G. Harth. 1995. Protective immunity against tuberculosis by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 92:1530-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu, Y., and A. R. Coates. 1999. Transcription of the stationary-phase-associated hspX gene of Mycobacterium tuberculosis is inversely related to synthesis of the 16-kilodalton protein. J. Bacteriol. 181:1380-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hubbard, R. D., C. M. Flory, and F. M. Collins. 1992. Immunization of mice with mycobacterial culture filtrate proteins. Clin. Exp. Immunol. 87:94-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson, M., S. W. Phalen, M. Lagranderie, D. Ensergueix, P. Chavarot, G. Marchal, D. N. McMurray, B. Gicquel, and C. Guilhot. 1999. Persistence and protective efficacy of a Mycobacterium tuberculosis auxotroph vaccine. Infect. Immun. 67:2867-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jagirdar, J., and D. Zagzag. 1996. Pathology and insights into pathogenesis of tuberculosis, p. 467-482. In W. N. Rom and S. M. Garay (ed.), Tuberculosis. Little, Brown & Company, Boston, Mass.
- 18.Kanai, K. 1966. Experimental studies on host-parasite equilibrium in tuberculosis infection, in relation to vaccination and chemotherapy. Jpn. J. Med. Sci. Biol. 19:181-199. [DOI] [PubMed] [Google Scholar]
- 19.Kondo, E., and K. Kanai. 1988. An experimental model of chemotherapy on dormant tuberculous infection, with particular reference to rifampicin. Jpn. J. Med. Sci. Biol. 41:37-47. [DOI] [PubMed] [Google Scholar]
- 20.Lenaerts, A. J., P. L. Chapman, and I. M. Orme. 2004. Statistical limitations to the Cornell model of latent tuberculosis infection for the study of relapse rates. Tuberculosis (Edinburgh) 84:361-364. [DOI] [PubMed] [Google Scholar]
- 21.Lurie, M. B. 1950. Native and acquired resistance to tuberculosis. Am. J. Med. 9:591-610. [DOI] [PubMed] [Google Scholar]
- 22.McCune, R. M., F. M. Feldmann, H. P. Lambert, and W. McDermott. 1966. Microbial persistence. I. The capacity of tubercle bacilli to survive sterilization in mouse tissues. J. Exp. Med. 123:445-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCune, R. M., F. M. Feldmann, and W. McDermott. 1966. Microbial persistence. II. Characteristics of the sterile state of tubercle bacilli. J. Exp. Med. 123:469-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCune, R. M., W. McDermott, and R. Tompsett. 1956. The fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. II. The conversion of tuberculous infection to the latent state by the administration of pyrazinamide and a companion drug. J. Exp. Med. 104:763-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCune, R. M., Jr., and R. Tompsett. 1956. Fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. I. The persistence of drug-susceptible tubercle bacilli in the tissues despite prolonged antimicrobial therapy. J. Exp. Med. 104:737-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medlar E. M. 1950. Pathogenetic concepts of tuberculosis. Am. J. Med. 9:611-622. [DOI] [PubMed] [Google Scholar]
- 27.Murray, M., and E. Nardell. 2002. Molecular epidemiology of tuberculosis: achievements and challenges to current knowledge. Bull. W. H. O. 80:477-482. [PMC free article] [PubMed] [Google Scholar]
- 28.North, R. J., and A. A. Izzo. 1993. Mycobacterial virulence. Virulent strains of Mycobacteria tuberculosis have faster in vivo doubling times and are better equipped to resist growth-inhibiting functions of macrophages in the presence and absence of specific immunity. J. Exp. Med. 177:1723-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orme, I. M. 1988. A mouse model of the recrudescence of latent tuberculosis in the elderly. Am. Rev. Respir. Dis. 137:716-718. [DOI] [PubMed] [Google Scholar]
- 30.O'Toole, R., M. J. Smeulders, M. C. Blokpoel, E. J. Kay, K. Lougheed, and H. D. Williams. 2003. A two-component regulator of universal stress protein expression and adaptation to oxygen starvation in Mycobacterium smegmatis. J. Bacteriol. 185:1543-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pal, P. G., and M. A. Horwitz. 1992. Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect. Immun. 60:4781-4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhoades, E. R., A. A. Frank, and I. M. Orme. 1997. Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tuber. Lung Dis. 78:57-66. [DOI] [PubMed] [Google Scholar]
- 33.Roberts, A. D., M. G. Sonnenberg, D. J. Ordway, S. K. Furney, P. J. Brennan, J. T. Belisle, and I. M. Orme. 1995. Characteristics of protective immunity engendered by vaccination of mice with purified culture filtrate antigens. Immunology 85:502-508. [PMC free article] [PubMed] [Google Scholar]
- 34.Romeyn, J. A. 1970. Exogenous reinfection in tuberculosis. Am. Rev. Respir. Dis. 101:923-927. [DOI] [PubMed] [Google Scholar]
- 35.Sambandamurthy, V. K., X. Wang, B. Chen, R. G. Russell, S. Derrick, F. M. Collins, S. L. Morris, and W. R. Jacobs, Jr. 2002. A pantothenate auxotroph of Mycobacterium tuberculosis is highly attenuated and protects mice against tuberculosis. Nat. Med. 8:1171-1174. [DOI] [PubMed] [Google Scholar]
- 36.Shafer, R. W., S. P. Singh, C. Larkin, and P. M. Small. 1995. Exogenous reinfection with multidrug-resistant Mycobacterium tuberculosis in an immunocompetent patient. Tuber. Lung Dis. 76:575-577. [DOI] [PubMed] [Google Scholar]
- 37.Sherman, D. R., M. Voskuil, D. Schnappinger, R. Liao, M. I. Harrell, and G. K. Schoolnik. 2001. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding alpha-crystallin. Proc. Natl. Acad. Sci. USA 98:7534-7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi, L., Y. J. Jung, S. Tyagi, M. L. Gennaro, and R. J. North. 2003. Expression of Th1-mediated immunity in mouse lungs induces a Mycobacterium tuberculosis transcription pattern characteristic of nonreplicating persistence. Proc. Natl. Acad. Sci. USA 100:241-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith, D. A., T. Parish, N. G. Stoker, and G. J. Bancroft. 2001. Characterization of auxotrophic mutants of Mycobacterium tuberculosis and their potential as vaccine candidates. Infect. Immun. 69:1142-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Styblo, K. 1980. Recent advances in epidemiological research in tuberculosis. Adv. Tuberc. Res. 20:1-63. [PubMed] [Google Scholar]
- 41.Webb, J. R., A. Campos-Neto, P. J. Ovendale, T. I. Martin, E. J. Stromberg, R. Badaro, and S. G. Reed. 1998. Human and murine immune responses to a novel Leishmania major recombinant protein encoded by members of a multicopy gene family. Infect. Immun. 66:3279-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan, Y., D. D. Crane, and C. E. Barry III. 1996. Stationary phase-associated protein expression in Mycobacterium tuberculosis: function of the mycobacterial alpha-crystallin homolog. J. Bacteriol. 178:4484-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]