Abstract
Commensal bacteria in the intestine play an important role in the development of immune response. These bacteria interact with cells of the gut-associated lymphoid tissues (GALT). Among cells of the GALT, B-1 cells are of note. These cells are involved in the production of natural antibodies. In the present study, we determined whether manipulation of the intestinal microbiota by administration of probiotics, which we had previously shown to enhance specific systemic antibody response, could affect the development of natural antibodies in the intestines and sera of chickens. Our findings demonstrate that when 1-day-old chicks were treated with probiotics, serum and intestinal antibodies reactive to tetanus toxoid (TT) and Clostridium perfringens alpha-toxin in addition to intestinal immunoglobulin A (IgA) reactive to bovine serum albumin (BSA) were increased in unimmunized chickens. Moreover, IgG antibodies reactive to TT were increased in the intestines of probiotic-treated chickens compared to those of untreated controls. In serum, IgG and IgM reactive to TT and alpha-toxin were increased in probiotic-treated, unimmunized chickens compared to levels in untreated controls. However, no significant difference in serum levels of IgM or IgG response to BSA was observed. These results are suggestive of the induction of natural antibodies in probiotic-treated, unimmunized chickens. Elucidating the role of these antibodies in maintenance of the chicken immune system homeostasis and immune response to pathogens requires further investigation.
Commensal bacteria in the intestine are in close contact with cells of the gut-associated immune system. Interactions between host cells and the bacteria or their structural components may lead to modulation of T- or B-cell-mediated immune responses, either locally or systemically (19). Development and diversification of the preimmune antibody repertoire in some species, such as rabbits, are dependent on the presence of microbiota (31). As a part of the developmental defects in the gut-associated lymphoid tissues (GALT) of germ-free animals, the intestinal lamina propria of these animals either lacks or contains only a small number of immunoglobulin A (IgA)-producing plasma cells (14). The lamina propria plasma cells are involved in the production of T-cell-independent antibodies against commensal bacteria, and bacteria may employ these antibodies as an evasive mechanism (14, 16).
Some of the IgA-producing plasma cells in the intestinal lamina propria may originate from B-1 cells (19). B-1 cells are a subset of B lymphocytes that are distinct from B-2 cells, which constitute the predominant subset of B cells in mammals (7). While B-2 cells produce the majority of circulating specific antibodies possessing high binding affinities, antibodies secreted by B-1 cells typically have low binding affinities and broad specificities (7, 12). These antibodies may be called natural antibodies, because they are usually produced without prior exposure to immunogens (7, 11). In humans and mice, natural antibodies may be of isotype IgM, IgG, or IgA, but IgM is the predominant isotype (7, 11). However, the relative contributions of B-1 and B-2 cells to the production of intestinal IgA may be a matter of debate, because in a gnotobiotic mouse model, B-2 cells appear to produce most of the intestinal IgA and B-1 cells are responsible for production of the natural IgM antibodies in serum (35).
The presence of natural antibodies in chicken sera has been demonstrated previously (17, 21, 26, 31). These antibodies may be reactive to self or foreign antigens (5, 17, 24, 26, 32). The function of natural antibodies in the chicken is not known, but there is an association between high specific antibody responsiveness and high levels of natural antibodies in serum (26, 32). Importantly, some natural antibodies in the chicken bind to antigens in a specific manner and the affinity of these interactions increases with age, suggesting a role for external stimuli (17, 26).
Colonization of the chicken intestine by commensal bacteria is an ongoing process which begins immediately after hatch, and the microbiota of the small intestine is established by week 2 posthatch (1). Commensal bacteria belonging to the Lactobacillus spp. are present predominantly in the small intestines of young chickens (2 weeks of age), whereas obligate anaerobes, such as members of the Bifidobacterium spp., are present predominantly in the ceca of older chickens (25 days of age) (1). It is possible that commensal bacteria or their products, which interact closely with cells within the chicken GALT, play a role in the development of immune response. It has been demonstrated that the chicken GALT reaches its functional maturity by week 2 posthatch (4). By this time, the chicken GALT encompasses cells of the immune system, including T and B cells, macrophages, and natural killer (NK) cells (18, 23).
In a recent study by our group, early colonization of intestines of 1-day-old chicks by a probiotic containing Lactobacillus acidophilus, Bifidobacterium bifidum, and Streptococcus faecalis resulted in a significant enhancement of systemic antibody response, mostly of the IgM isotype, to sheep red blood cells (13). The objective of the present study was to examine the effects of this probiotic on the enhancement of preimmune or natural antibodies in serum and intestinal contents (IC).
MATERIALS AND METHODS
Chickens and housing.
Newly hatched female broiler chicks were maintained in floor pens at an isolation unit (University of Guelph, Ontario, Canada). The chicks were provided with free access to water and feed. The research complied with University of Guelph Animal Care Committee guidelines.
Experimental design.
Fourteen female chicks were randomly divided into two treatment groups: group I was probiotic treated (n = 7) and group II was nontreated (n = 7). The chicks in group I were inoculated via oral gavage with 0.5 ml phosphate-buffered saline (PBS) containing 106 bacteria from a commercial probiotic, Interbac (Intervet, Whitby, Ontario, Canada), on the day of hatching. Interbac contains Lactobacillus acidophilus, Bifidobacterium bifidum, and Streptococcus faecalis. PBS was used as a placebo in group II, which did not receive probiotics. Blood and gut content samples were collected when the chickens were 14 days of age.
Sample collection.
Blood and intestinal content samples were collected as previously described (13).
Measurement of antibodies in serum and intestinal contents.
Total antibody in intestinal contents and serum was determined by a sandwich enzyme-linked immunosorbent assay (ELISA). Briefly, flat-bottomed 96-well plates (Nunc Maxisorp; VWR International, Mississauga, Ontario, Canada) were coated with unconjugated rabbit anti-chicken IgG heavy plus light chains (Cedarlane, Hornby, Ontario, Canada) (2 μg/ml) and incubated at 4°C overnight. Plates were then washed and blocked for 1 h at 37°C with PBS-Tween 20 containing 0.25% gelatin (HiPure liquid gelatin; Norland Products Inc., New Brunswick, N.J.). Serum or intestinal contents (diluted 1:100 and 1:20 in blocking buffer, respectively) were then added, and plates were incubated for 1 h at 37°C. Subsequently, rabbit anti-chicken IgG heavy plus light chains conjugated with horseradish peroxidase (Cedarlane, Hornby, Ontario, Canada) (diluted 1:20,000 in blocking buffer) was added and plates were incubated for 1 h at 37°C. The substrate, ABTS [2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid); Mandel Scientific, Guelph, Ontario, Canada], was then added and plates were incubated for 30 min at room temperature in the dark. The absorbance was measured at 405 nm using a microplate reader (Bio-Tek, Winooski, VT).
Detection of IgG and IgM antibody responses against tetanus toxoid (TT), bovine serum albumin (BSA), and alpha-toxin of Clostridium perfringens in sera was performed by an indirect ELISA. Briefly, 96-well microplates (Nunc Maxisorp) were coated overnight with BSA (Fisher, New Jersey) (30 μg/ml), alpha-toxin (Sigma) (2 μg/ml), or 100 μl TT solution (Intervet Inc., Millsboro, DE) (diluted 1:50 in coating buffer) at 4°C. After blocking, serum samples (diluted 1:100) were added and plates were incubated at 37°C for 1 h. Following washings, rabbit anti-chicken IgG or IgM conjugated with horseradish peroxidase (diluted 1:2,000) was added and plates were incubated for 1 h at 37°C. The addition of substrate and the measurement of the optical density values were performed as described above.
The evaluation of IgA and IgG in IC was also performed using an indirect ELISA. Plates were coated as described above. IC samples (diluted 1:20) were added, and incubation took place for 1 h at room temperature for those plates coated with TT or at 37°C for those plates coated with BSA and alpha-toxin. Subsequently, goat anti-chicken IgA or IgG conjugated with alkaline phosphatase (diluted 1:500 in blocking buffer) was added. Incubation conditions were the same as those for the last step. The substrate, p-nitrophenylphosphate solution (KPL, Gaithersburg, MD), was then added and plates were incubated for 1 h at room temperature in the dark. Measurement of the absorbance was performed as described above.
Statistical analysis.
Statistical analysis of data was performed using Student's t test to compare antibody responses between probiotic-treated and control groups. Statistical significance was assessed at a P value of ≤0.05.
RESULTS
Quantification of total antibody in IC and sera.
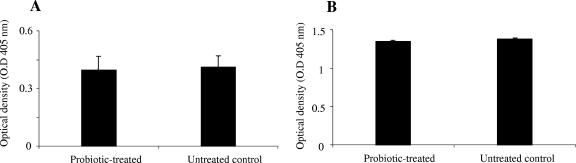
The levels of total antibodies (IgM, IgG, and IgA) present in sera and IC of probiotic-treated and untreated groups were measured. There was no significant difference in total antibodies between probiotic-treated and untreated control chickens either in IC or in sera (Fig. 1A and B).
FIG. 1.
Total antibody levels in sera and IC of two groups of chickens 2 weeks after oral gavage with probiotics or with PBS. The two groups were a probiotic-treated group and an untreated control (negative). The figure shows mean ELISA optical densities for total antibody in (A) IC and (B) sera. Error bars represent standard errors of the means of optical densities.
Gut antibody response to TT, alpha-toxin, and BSA.
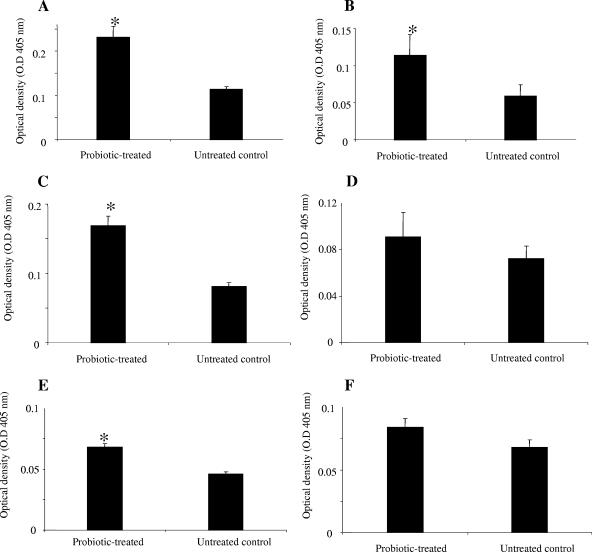
The effect of probiotics on the generation of natural antibodies in the gut was evaluated by measuring the amounts of antibodies reactive to TT, alpha-toxin, and BSA. There were significantly more IgA antibodies reactive to TT, alpha-toxin, and BSA in IC of probiotic-treated chickens than in IC of untreated control chickens (P ≤ 0.001) (Fig. 2A, C, and E). However, only IgG antibodies reactive to TT were significantly higher (P ≤ 0.001) in IC of probiotic-treated chickens than in IC of untreated chickens (Fig. 2B). The two groups did not differ significantly in the amounts of alpha-toxin- and BSA-reactive IgG antibodies in their IC (Fig. 2D and F).
FIG. 2.
IgA and IgG antibodies reactive to TT, alpha-toxin, and BSA in two groups of chickens 2 weeks after oral gavage with probiotics or with PBS. The two groups were a probiotic-treated group and an untreated control (negative). The figure shows mean ELISA optical densities for IgA and IgG antibodies. Error bars represent standard errors of the means of optical densities. *, statistically significant (P ≤ 0.05). (A) IgA reactive to TT. (B) IgG reactive to TT. (C) IgA reactive to alpha-toxin. (D) IgG reactive to alpha-toxin. (E) IgA reactive to BSA. (F) IgG reactive to BSA.
Systemic antibody response to TT, alpha-toxin, and BSA.
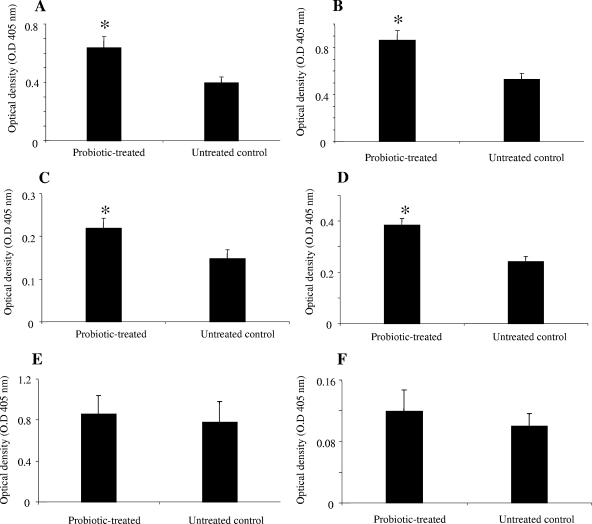
To evaluate the effects of probiotics on serum antibodies, the presence of natural antibodies to TT, alpha-toxin, and BSA in sera of probiotic-treated and untreated birds was assessed. Sera of birds in the probiotic-treated group contained significantly higher (P ≤ 0.05) levels of IgG antibodies reactive to TT and alpha-toxin (P ≤ 0.05) than sera of untreated controls (Fig. 3A and C). However, there was no significant difference between the two groups when the IgG antibodies reactive to BSA were examined (Fig. 3E). Evaluation of IgM response to the antigens revealed that probiotic-treated chickens had significantly higher IgM antibodies (P ≤ 0.01) reactive to TT and alpha-toxin than untreated chickens did (Fig. 3B and D). However, there was no significant difference between the two groups in terms of serum IgM antibodies reactive to BSA (Fig. 3F).
FIG. 3.
Serum IgG and IgM antibodies reactive to TT, alpha-toxin, and BSA in IC of two groups of chickens 2 weeks after oral gavage with probiotics or with PBS. The two groups were a probiotic-treated group and an untreated control (negative). The figure shows mean ELISA optical densities for IgG and IgM antibodies. Error bars represent standard errors of the means of optical densities. *, statistically significant (P ≤ 0.05). (A) IgG reactive to TT. (B) IgM reactive to TT. (C) IgG reactive to alpha-toxin. (D) IgM reactive to alpha-toxin. (E) IgG reactive to BSA. (F) IgM reactive to BSA.
DISCUSSION
Commensal bacteria in the intestine, which are in close contact with GALT cells, play a role in the development of natural antibodies. In the present study, we determined whether a probiotic, which had previously been shown to enhance specific systemic antibody response (13), could affect the development of natural antibodies in the intestines and sera of chickens. Our findings demonstrate that when 1-day-old chicks were treated with this probiotic, serum and intestinal antibodies reactive to TT and alpha-toxin in addition to intestinal IgA reactive to BSA were increased. Moreover, IgG antibodies reactive to TT were increased in the intestines of probiotic-treated chickens compared to those of untreated controls. These results are suggestive of the induction of natural antibodies in probiotic-treated, unimmunized chickens.
In our study, the increase in IgA and IgG antibodies was not associated with an increase in the total antibodies in the intestines of probiotic-treated birds. The IgA isotype is the predominant immunoglobulin in intestinal secretions and is synthesized by plasma cells in the lamina propria (8). The presence of commensal bacteria of the microbiota is essential for generation of intestinal IgA in some mammals, because this immunoglobulin is absent in the intestines of germ-free animals and colonization of germ-free animals results in emergence of intestinal IgA antibodies (8, 33). The specificity of intestinal IgA antibodies may be a matter of debate. A fraction of these IgA antibodies is generated in a T-cell-independent manner and may be specific for commensal bacterial antigens (19), but some of the intestinal IgA antibodies are polyspecific (8). It has been demonstrated that lipopolysaccharides (LPS) of gram-negative bacteria and unknown structural components of gram-positive bacteria are responsible for elicitation of intestinal IgA antibodies (8). Irrespective of the specificity of these antibodies, it appears that these IgA antibodies, at least in mammals, are involved in providing an immune evasion mechanism for commensal bacteria (14, 16). The reason is that commensal bacteria coated with IgA are prevented from mucosal penetration and, as a result, may not be able to elicit a protective immune response in the host (20).
We discovered that administration of probiotics resulted in a significant increase in serum IgM and IgG antibodies reactive to TT and alpha-toxin but not to BSA. Serum natural antibodies are predominantly of the IgM isotype, although some may be of the IgG and IgA isotypes (24). Natural IgM antibodies in the serum are produced by B-1 cells (7, 16). These antibodies usually bind to self or bacterial antigens with low affinity (16). The bacterial antigens include LPS, capsular polysaccharide, and unknown determinants of Salmonella enterica serovar Typhimurium and Escherichia coli (16). Natural IgM antibodies possess a wide range of activities, including regulation of immune response, induction of specific IgG antibodies, and protection against bacterial and viral infections (3, 16, 25). Natural antibodies reactive to self and foreign antigens have been detected in chickens (5, 17, 24, 26, 32). Foreign antigens that bind natural antibodies in the sera of unimmunized chickens include LPS, lipoteichoic acid, keyhole limpet hemocyanin, and BSA (25, 31). In accordance with previous findings, the present study revealed that serum antibodies reactive to BSA are present in unimmunized birds. However, administration of probiotics did not significantly enhance serum IgM and IgG antibodies reactive to this antigen (Fig. 3E and F). In contrast, probiotic treatment resulted in the enhancement of TT- and C. perfringens alpha-toxin-reactive antibodies in serum. Both of these antigens are produced by anaerobic Clostridium spp., and it is possible that due to a degree of similarity between these exotoxins, natural antibodies elicited by probiotic bacteria could bind to them.
It is not clear how commensal bacteria might influence the preimmune antibody repertoire. It is possible that resident dendritic cells (DCs) in the lamina propria, which directly sample the intestinal lumen and engulf commensal bacteria, could play a role (20, 30). DCs express a repertoire of Toll-like receptors (TLRs) (29), and binding of structural components of commensal bacteria to TLRs expressed on the surface of DCs may lead to activation and maturation of these cells (22, 27). Upon activation, DCs promote the activation and differentiation of different subsets of immune system cells, leading to the production of cytokines, such as interleukin 4 (IL-4), IL-10, and transforming growth factor β, that are important for antibody production and isotype switching (10, 22). Furthermore, the uptake of commensal bacteria by resident DCs results in elicitation of IgA antibodies, which may in turn prevent commensals from eliciting an immune response which could lead to their clearance from the intestine (20).
B-1 cells may mediate some of the effects of commensals on generation of the preimmune repertoire. These cells usually operate in a T-cell-independent manner, but production of some of the IgM antibodies by mammalian B-1 cells requires the production of IL-4 by LPS-stimulated T cells (2). Also, it is possible that B-1 cells may become directly activated after engagement of their TLRs with bacterial ligands, such as LPS and CpG motifs (34, 36). In mammals, TLR-9 acts as a receptor for CpG DNA; CD5+ B-1 cells express higher levels of TLR-9 than CD5− B cells, and the former cells produce IL-10 in response to CpG stimulation (9, 34). However, due to the paucity of information on chicken B-1 cells, the potential role of these cells in our study remains to be addressed. Importantly, all chicken B cells express the CD5 marker, which is usually expressed by mammalian B-1 cells (15); it is unclear whether chicken B cells share functional characteristics with mammalian B-1 cells, although it appears that these cells are developmentally similar (15, 28). Overall, these findings raise the possibility that cytokines produced by immune system cells contribute to the production of natural antibodies after exposure to commensal bacteria.
In summary, the results of this study demonstrate that administration of probiotics enhances serum and intestinal natural antibodies to several foreign antigens. Based on the finding for other species that natural antibodies are important for defense against pathogens (6, 25), it is possible that the effects of probiotic bacteria in reducing colonization of intestinal pathogens may be due partly to the stimulation of natural antibodies. Further studies are required to determine the effects of these antibodies on the intestinal immunity of the chicken.
Acknowledgments
This study was funded by the Ontario Ministry of Agriculture and Food (Food Safety Research and Poultry Research Programs), the Saskatchewan Chicken Industry Development, and the Poultry Industry Council.
We acknowledge Bruce Wilkie for stimulating discussions about natural antibodies.
REFERENCES
- 1.Amit-Romach, E., D. Sklan, and Z. Uni. 2004. Microflora ecology of the chicken intestine using 16S ribosomal DNA primers. Poult. Sci. 83:1093-1098. [DOI] [PubMed] [Google Scholar]
- 2.Askenase, P. W., A. Itakura, M. C. Leite-de-Moraes, M. Lisbonne, S. Roongapinun, D. R. Goldstein, and M. Szczepanik. 2005. TLR-dependent IL-4 production by invariant Valpha14+Jalpha18+ NKT cells to initiate contact sensitivity in vivo. J. Immunol. 175:6390-6401. [DOI] [PubMed] [Google Scholar]
- 3.Avrameas, S. 1991. Natural autoantibodies: from ‘horror autotoxicus’ to ‘gnothi seauton’. Immunol. Today 12:154-159. [DOI] [PubMed] [Google Scholar]
- 4.Bar-Shira, E., D. Sklan, and A. Friedman. 2003. Establishment of immune competence in the avian GALT during the immediate post-hatch period. Dev. Comp. Immunol. 27:147-157. [DOI] [PubMed] [Google Scholar]
- 5.Barua, A., and Y. Yoshimura. 2001. Ovarian autoimmunity in relation to egg production in laying hens. Reproduction 121:117-122. [PubMed] [Google Scholar]
- 6.Baumgarth, N., J. W. Tung, and L. A. Herzenberg. 2005. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin. Immunopathol. 26:347-362. [DOI] [PubMed] [Google Scholar]
- 7.Berland, R., and H. H. Wortis. 2002. Origins and functions of B-1 cells with notes on the role of CD5. Annu. Rev. Immunol. 20:253-300. [DOI] [PubMed] [Google Scholar]
- 8.Bos, N. A., H. Q. Jiang, and J. J. Cebra. 2001. T cell control of the gut IgA response against commensal bacteria. Gut 48:762-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dasari, P., I. C. Nicholson, G. Hodge, G. W. Dandie, and H. Zola. 2005. Expression of Toll-like receptors on B lymphocytes. Cell. Immunol. 236:140-145. [DOI] [PubMed] [Google Scholar]
- 10.Di Giacinto, C., M. Marinaro, M. Sanchez, W. Strober, and M. Boirivant. 2005. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. J. Immunol. 174:3237-3246. [DOI] [PubMed] [Google Scholar]
- 11.Dono, M., G. Cerruti, and S. Zupo. 2004. The CD5+ B-cell. Int. J. Biochem. Cell Biol. 36:2105-2111. [DOI] [PubMed] [Google Scholar]
- 12.Haas, K. M., J. C. Poe, D. A. Steeber, and T. F. Tedder. 2005. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity 23:7-18. [DOI] [PubMed] [Google Scholar]
- 13.Haghighi, H. R., J. Gong, C. L. Gyles, M. A. Hayes, B. Sanei, P. Parvizi, H. Gisavi, J. R. Chambers, and S. Sharif. 2005. Modulation of antibody-mediated immune response by probiotics in chickens. Clin. Diagn. Lab. Immunol. 12:1387-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang, H. Q., M. C. Thurnheer, A. W. Zuercher, N. V. Boiko, N. A. Bos, and J. J. Cebra. 2004. Interactions of commensal gut microbes with subsets of B- and T-cells in the murine host. Vaccine 22:805-811. [DOI] [PubMed] [Google Scholar]
- 15.Koskinen, R., T. W. Gobel, C. A. Tregaskes, and J. R. Young. 1998. The structure of avian CD5 implies a conserved function. J. Immunol. 160:4943-4950. [PubMed] [Google Scholar]
- 16.Kroese, F. G., R. de Waard, and N. A. Bos. 1996. B-1 cells and their reactivity with the murine intestinal microflora. Semin. Immunol. 8:11-18. [DOI] [PubMed] [Google Scholar]
- 17.Lammers, A., M. E. V. Klomp, M. G. B. Nieuwland, H. F. J. Savelkoul, and H. K. Parmentier. 2004. Adoptive transfer of natural antibodies to non-immunized chickens affects subsequent antigen-specific humoral and cellular immune responses. Dev. Comp. Immunol. 28:51-60. [DOI] [PubMed] [Google Scholar]
- 18.Lillehoj, H. S., and J. M. Trout. 1996. Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clin. Microbiol. Rev. 9:349-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macpherson, A. J., D. Gatto, E. Sainsbury, G. R. Harriman, H. Hengartner, and R. M. Zinkernagel. 2000. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 288:2222-2226. [DOI] [PubMed] [Google Scholar]
- 20.Macpherson, A. J., and T. Uhr. 2004. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303:1662-1665. [DOI] [PubMed] [Google Scholar]
- 21.Matson, K. D., R. E. Ricklefs, and K. C. Klasing. 2005. A hemolysis-hemagglutination assay for characterizing constitutive innate humoral immunity in wild and domestic birds. Dev. Comp. Immunol. 29:275-286. [DOI] [PubMed] [Google Scholar]
- 22.Mohamadzadeh, M., S. Olson, W. V. Kalina, G. Ruthel, G. L. Demmin, K. L. Warfield, S. Bavari, and T. R. Klaenhammer. 2005. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc. Natl. Acad. Sci. USA 102:2880-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muir, W. I., W. L. Bryden, and A. J. Husband. 2000. Immunity, vaccination and the avian intestinal tract. Dev. Comp. Immunol. 24:325-342. [DOI] [PubMed] [Google Scholar]
- 24.Neu, N., K. Hala, and G. Wick. 1984. “Natural” chicken antibodies to red blood cells are mainly directed against the B-G antigen, and their occurrence is independent of spontaneous autoimmune thyroiditis. Immunogenetics 19:269-277. [DOI] [PubMed] [Google Scholar]
- 25.Ochsenbein, A. F., T. Fehr, C. Lutz, M. Suter, F. Brombacher, H. Hengartner, and R. M. Zinkernagel. 1999. Control of early viral and bacterial distribution and disease by natural antibodies. Science 286:2156-2159. [DOI] [PubMed] [Google Scholar]
- 26.Parmentier, H. K., A. Lammers, J. J. Hoekman, G. De Vries Reilingh, I. T. Zaanen, and H. F. Savelkoul. 2004. Different levels of natural antibodies in chickens divergently selected for specific antibody responses. Dev. Comp. Immunol. 28:39-49. [DOI] [PubMed] [Google Scholar]
- 27.Rakoff-Nahoum, S., J. Paglino, F. Eslami-Varzaneh, S. Edberg, and R. Medzhitov. 2004. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118:229-241. [DOI] [PubMed] [Google Scholar]
- 28.Raman, C., and K. L. Knight. 1992. CD5+ B cells predominate in peripheral tissues of rabbit. J. Immunol. 149:3858-3864. [PubMed] [Google Scholar]
- 29.Reis e Sousa, C. 2004. Toll-like receptors and dendritic cells: for whom the bug tolls. Semin. Immunol. 16:27-34. [DOI] [PubMed] [Google Scholar]
- 30.Rescigno, M., M. Urbano, B. Valzasina, M. Francolini, G. Rotta, R. Bonasio, F. Granucci, J. P. Kraehenbuhl, and P. Ricciardi-Castagnoli. 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 4:361-367. [DOI] [PubMed] [Google Scholar]
- 31.Rhee, K. J., P. Sethupathi, A. Driks, D. K. Lanning, and K. L. Knight. 2004. Role of commensal bacteria in development of gut-associated lymphoid tissues and preimmune antibody repertoire. J. Immunol. 172:1118-1124. [DOI] [PubMed] [Google Scholar]
- 32.Siwek, M., B. Buitenhuis, S. Cornelissen, M. Nieuwland, E. F. Knol, R. Crooijamans, M. Groenen, H. Parmentier, and J. van der Poel. 2006. Detection of QTL for innate: non-specific antibody levels binding LPS and LTA in two independent populations of laying hens. Dev. Comp. Immunol. 30:659-666. [DOI] [PubMed]
- 33.Stoel, M., H. Q. Jiang, C. C. van Diemen, J. C. Bun, P. M. Dammers, M. C. Thurnheer, F. G. Kroese, J. J. Cebra, and N. A. Bos. 2005. Restricted IgA repertoire in both B-1 and B-2 cell-derived gut plasmablasts. J. Immunol. 174:1046-1054. [DOI] [PubMed] [Google Scholar]
- 34.Sun, C. M., E. Deriaud, C. Leclerc, and R. Lo-Man. 2005. Upon TLR9 signaling, CD5+ B cells control the IL-12-dependent Th1-priming capacity of neonatal DCs. Immunity 22:467-477. [DOI] [PubMed] [Google Scholar]
- 35.Thurnheer, M. C., A. W. Zuercher, J. J. Cebra, and N. A. Bos. 2003. B1 cells contribute to serum IgM, but not to intestinal IgA, production in gnotobiotic Ig allotype chimeric mice. J. Immunol. 170:4564-4571. [DOI] [PubMed] [Google Scholar]
- 36.Viau, M., and M. Zouali. 2005. B-lymphocytes, innate immunity, and autoimmunity. Clin. Immunol. 114:17-26. [DOI] [PubMed] [Google Scholar]