Abstract
We report four human tachykinins, endokinins A, B, C, and D (EKA–D), encoded from a single tachykinin precursor 4 gene that generates four mRNAs (α, β, γ, and δ). Tachykinin 4 gene expression was detected primarily in adrenal gland and in the placenta, where, like neurokinin B, significant amounts of EKB-like immunoreactivity were detected. EKA/B 10-mers displayed equivalent affinity for the three tachykinin receptors as substance P (SP), whereas a 32-mer N-terminal extended form of EKB was significantly more potent than EKA/B or SP. EKC/D, which possess a previously uncharacterized tachykinin motif, FQGLL-NH2, displayed low potency. EKA/B displayed identical hemodynamic effects to SP in rats, causing short-lived falls in mean arterial blood pressure associated with tachycardia, mesenteric vasoconstriction, and marked hindquarter vasodilatation. Thus, EKA/B could be the endocrine/paracrine agonists at peripheral SP receptors and there may be as yet an unidentified receptor(s) for EKC/D.
The mammalian tachykinins, a family of peptides traditionally classified as neurotransmitters that includes substance P (SP) and neurokinins A (NKA) and B (NKB), have been implicated to have a wide variety of biological actions including smooth muscle contraction, vasodilatation, pain transmission, neurogenic inflammation, and the activation of the immune system (1). They all share a common C-terminal region: FXGLM-NH2 where X is hydrophobic. The N-terminal sequence is believed to convey receptor specificity to each of the three known mammalian tachykinin receptors, NK1, NK2, and NK3. SP shows preference for NK1, NKA for NK2, and NKB for NK3.
The SP precursor was first determined to be encoded on two complementary cDNA sequences (2). The second sequence contained a previously uncharacterized tachykinin, now known as NKA, produced by alternative RNA splicing of two distinct transcripts encoding SP alone (α tachykinin precursor 1, αTAC1) or with NKA (βTAC1) (3). Relative amounts of these mRNAs varied in different tissues, suggesting tissue-specific regulation. A third TAC1 transcript containing both SP and NKA but differing in its sequence organization was subsequently reported (4) and named γTAC1. The latter two transcripts encode biologically active N-terminally extended forms of NKA (5, 6). The total number of human TAC1 transcripts discovered is now four after the more recent discovery of δTAC1 (7). NKB, the third mammalian tachykinin, is encoded on a single TAC3 transcript (8). This peptide is secreted in significant amounts into the maternal circulation from the placenta, where its action has been suggested to cause the symptoms of preeclampsia (9). This notion has recently been reinforced by the ability of NKB to induce lung and liver edema in mice (10).
Evidence of a fourth mammalian tachykinin, hemokinin 1 (HK-1), encoded on TAC4 and expressed in the hematopoietic cells of mice has been demonstrated (11). This tachykinin is uniquely expressed outside of neuronal tissue in immune tissues (11). It has subsequently been found to be a full agonist at the NK1, NK2, and NK3 receptors, with highest selectivity for NK1 (12, 13). Here, we report the identification, pharmacology, and cardiovascular physiology of four previously uncharacterized members of the human tachykinin family encoded on the human TAC4 gene that we have named endokinins (EK) A–D. As their name suggests, they are mainly distributed in the periphery, and their presence in the placenta suggests that these tachykinins may be involved in controlling blood flow during pregnancy.
Materials and Methods
Materials.
Tachykinins for SP and NKA, mouse HK-1 (mHK-1), [N-Me-Phe7]NKB, and custom synthesized EKA/B, EKC, EKD, and γTAC4(32–50) (AETWEGAGPSIQLQLQEVK) were supplied by CN Biosciences (Nottingham, U.K.), Tocris (Bristol, U.K.), Bachem, and Cambridge Research Biochemicals, respectively. γTAC4(30–61)-NH2, TEAETWEGAGPSIQLQLQEVKTGKASQFFGLM-NH2, was kindly synthesized by H. P. J. Bennett (McGill University, Montreal). Neurokinin antagonists L-733060 (13), GR94800 (14), and SB 222200 (15) were obtained from Tocris, Neosystems (Strasbourg, France), and D. W. Hay (GlaxoSmithKline), respectively. [3H] SR140333 [specific activity 24.0 Ci/mmol (1 Ci = 37 GBq), Kd = 0.3 nM; ref. 17], [3H] SR48968 (specific activity 27.0 Ci/mmol, Kd = 0.5 nM; ref. 18), and [3H] SR142801 (specific activity 64.0 Ci/mmol, Kd = 0.13 nM; ref. 19) were purchased from Amersham Biosciences, as were membranes for NK1 (0.18 pmol/mg), NK2 (2.4 pmol/mg), and NK3 (5.1 pmol/mg) tachykinin receptors. All cell culture reagents and other reagents were purchased from Sigma-Aldrich unless otherwise stated.
Cloning of the Human TAC4 cDNA Splice Variants.
Primers were designed to match the region on human clone AC027801 (GenBank) homologous to the mouse TAC4 gene (GenBank accession no. AF235035; sense 5′-GCCAGTTCTTTGGGCTGATGGGGAAGCGAGT-3′, antisense 5′-CAGGATACAGAGTGTGCGAGTCTCCTCACT-3′) to perform 5′ and 3′ SMART RACE (BD Biosciences Clontech) cDNA amplification on human adrenal cDNA as described (20).
Gene Expression Studies.
Twenty normalized cDNAs from different tissues were taken from the mouse MTC I and II panels (BD Biosciences Clontech). Normalization was confirmed by 18 cycles of PCR to amplify the mGAPDH gene (sense 5′-TGAAGGTCGGTGTGAACGGATTTGGC-3′, antisense 5′-CATGTAGGCCATGAGGTCCACCAC-3′) before 40 cycles of PCR to amplify the mTAC1 (sense 5′-CACCATGTTGCCTCTCCTTGCCCTGCTTCT-3′, antisense 5′-GGTGGCAGAGTGCTACACGTTGCTGGTGGTTA-3′) and mTAC4 (sense 5′-ACCTGCTCCACTCCTGCACCGCGGCCAAG-3′, antisense 5′-GAACTGCTGAGGCTTGGGTCTTCGGGCGAT-3′) genes, as described (20). Human total RNA was obtained from a human total RNA master panel (BD Biosciences Clontech) or extracted directly from tissues and cells by using Tri-reagent and converted to SMART 3′-RACE-ready cDNA as described (20). The cDNA levels were normalized and confirmed by PCR amplification of the hβ-actin (sense 5′-TCCATCATGAAGTGTGACGTGGACATC-3′, antisense 5′-CCAGACTCGTCATACTCCTGCTTGCTG-3′) and hGAPDH genes (sense 5′-AGGCTGAGAACGGGAAGCTTGTGATCA-3′, antisense 5′-TCCCGTTCAGCTCAGGGATGACCTTGC-3′) for 18 cycles. These cDNAs were then subjected to 32 cycles of PCR to amplify the hTAC1 (sense 5′-CGTGCGCTCGGAGGAACCAGAGAAACTC-3′, antisense 5′-TGAGGAATCAGCATCCCGTTTGCCCAT-3′), hTAC4 (antisense: 5′-CAGGATACAGAGTGTGCGAGTCTCCTCACT-3′ for all variants; sense: 5′-GCAGAGACCTGGGTAATTGTGGCCTTGGAG-3′ for α, 5′-GCTGATGGGGAAGCGAGTGGGAGCATATC-3′ for β, 5′-CCTCTGATCCA GCCAAGGAGAAAAAAAGGCAGAG-3′ for γ, and 5′-GCTGATGGGGAAGCGAGTGGGAGGCAGAGA-3′ for δ), and hNK1 (sense 5′-GGCTTTACCGCCTAGCTTCGAAATGGA-3′, antisense 5′-CTGGGCATGGTCTCTGTGGTTGAGTAG-3′) receptor genes. PCRs were performed in triplicate, and controls containing no reverse transcriptase and no template were included. PCR products were cloned and sequenced as described (20).
Developing Antibodies to Human TAC4.
Polyclonal antibodies to EKA1, GKASQFFGLM-NH2 (EKA/B), and the N-terminal precursor sequence of EKB (AETWEGAGPSIQLQLQEVK; residues 32–50 of γTAC4; EKA2) were raised in sheep. Each synthetic peptide (2 mg) was conjugated to purified protein derivative of tuberculin (Central Veterinary Laboratory, Addlestone, U.K.) at a ratio of 1:2 (wt/wt) in the presence of 1.2% (vol/vol) glutaraldehyde. Individual sheep were immunized with the peptide conjugates (100 μg) emulsified in 1 ml of Freund's complete adjuvant. Thereafter, sheep were immunized with 100 μg of the same peptide conjugate in 1 ml of Freund's incomplete adjuvant at four weekly intervals and bled 10 days after each immunization. Individual sheep sera were collected and filtered through a 5-μm cellulose nitrate filter (Sartorius). Affinity chromatography was used to purify peptide-specific antibodies from each antisera by the coupling of 0.5 mg of each peptide to 300 mg of CNBr-activated Sepharose 4B beads according to the manufacturer's instructions (Amersham Biosciences). The peptide coupled resin was packed into an Econo-Column (Bio-Rad). Each column was washed with 500 ml of 0.9% (wt/vol) NaCl, 0.1% (wt/vol) NaN3, and 5 mM EDTA (pH 7.5). Sera were diluted 1:10 in the same solution and then allowed to perfuse through the column overnight. After washing with 10 ml each 0.5 M CH3COONa and 20% acetonitrile and then 0.05 M CH3COONa and 20% acetonitrile (pH 6.0), high-affinity antibodies were eluted from the columns in 0.05 M CH3COONa/20% acetonitrile (pH 4.0) and 1-ml fractions were collected. Each fraction was neutralized by addition of 100 μl of saturated NaHCO3. EKA1 antibodies were further affinity purified by passing through an Econo-Column containing (Lys-0)-NKB coupled to CNBr-activated Sepharose to remove antibodies to the common C-terminal tachykinin motif, GLM-NH2, as described above. IgG content was estimated by Coomassie blue reagent and absorbance at 280 nm.
Preparation of Placental Extract.
Human placenta was homogenized in 1 M HCl/5% (vol/vol) formic acid/1% (wt/vol) NaCl/1% (vol/vol) trifluoroacetic acid by using 5 ml per gram of tissue, and, after centrifugation at 3,500 × g, peptides were extracted from the supernatant by using Sep-Pak Vac/1CC, C18 octadecasilyl-silica cartridges (Millipore) as described (9).
Two-Site Enzyme-Linked Immunoassay for Human TAC4.
Exiqon protein immobilizer plates were coated with 1 μg of the affinity-purified EKA1 antibody for capture and 100 ng of biotinylated EKA2 antibody for detection. Biotinylation of 0.5 mg of EKA2 antibody was performed in 1 ml of 0.1M NaHCO3 by adding 0.15 mg biotinamidocaproate N-hydroxysuccinimide dissolved in 0.1 ml DMSO. After 30 min, unreacted reagent was removed by passing through an Econo-Column containing Sephadex G-25. The derived IgG fraction was diluted in assay buffer [0.05 Na2HPO4/0.1% (wt/vol) NaN3/0.5% (wt/vol) BSA] such that 100-μl aliquots would contain 100 ng of biotinylated IgG. To carry out the immunoassay, placental extract and standards (γTAC4 30–61-NH2) were dissolved and diluted in assay buffer and 100 μl was added in duplicate to EKA1-coated wells and incubated overnight at 4°C before washing three times with 0.9% (wt/vol) NaCl/0.2% (vol/vol) Triton X-100. One hundred microliters of biotinylated EKA2 (100 ng) was added to each well, and the wells were left at room temperature for 4 h before washing again as above. One hundred microliters of streptavidin-peroxidase was added to each well and incubated for a further 30 min. After washing again, 200 μl of TMB liquid:Stable Stop (Europa Bioproducts, Cambridge, U.K.) per well was added and left for 10 min. Reactions were stopped by addition of 100 μl of 0.5 M HCl per well, and absorbance was read at 450 and 600 nm.
Radioligand Binding Assays.
Assays were performed with membranes from human glioblastoma astrocytoma UC11 cells (21) expressing the NK1 receptor and Chinese hamster ovary (CHO) cells expressing NK2 receptor and NK3 receptor. Competition assays with NK1, NK2, and NK3 receptors were conducted with 18 μg of membrane protein and 0.3 nM [3H] SR140333, 19 μg of membrane protein and 0.5 nM [3H] SR48968, and 5 μg of membrane protein and 0.25 nM [3H] SR142801, respectively. Nonspecific binding was assessed as binding in the presence of 10 μM L-733060, GR94800, and SB222200 for NK1, NK2, and NK3 receptors, respectively. NK1 receptor incubations were performed in 40 mM Hepes (pH 7.4)/5 mM MgCl2/0.5% BSA and NK2 and NK3 receptor incubations were performed in 20 mM Hepes (pH 7.4)/1 mM MnCl2/0.1% BSA, all containing 10 μM phosphoramidon with or without various concentrations of cold ligand, for 90 min at 27°C in 1-ml assays. Incubations were stopped by rapid filtration through Whatman GF/C filters, presoaked for 15 min in 0.5% BSA, with a Brandel tissue harvester (Gaithersburg, MD). Membranes were washed four times with 5 ml of 1× PBS before being placed in vials with 2 ml of optiphase HiSafe 3 (Fisher) and counted in a liquid scintillation counter. Competition binding assays at the NK1 receptor were also conducted in the presence or absence of 100 μM guanosine 5′-triphosphate. Each ligand binding experiment was performed in duplicate in three independent sets of experiments. Inhibition constants (Ki) were determined from the concentration–response curves by using prism (GraphPad, San Diego).
Cell Culture and Calcium Mobilization Assays.
Human glioblastoma astrocytoma U373 MG cells (expressing high levels of endogenous NK1 receptor, 68 fmol/mg; ref. 22) were obtained from the European Collection of Animal Cell Culture (Salisbury, U.K.) and maintained and split as instructed. Analysis of intracellular calcium ion flux was performed as described (23) by using 1 μM final concentrations of SP, EKA/B, and mHK-1, and the log(EC50) values were determined from concentration–response curves by using 10−6 to 10−9 M in half log points of EKA/B with prism.
Cardiovascular Effects of Tachykinins in Conscious Rats.
Under anesthesia [fentanyl (Martindale Pharmaceuticals, Essex, U.K.) and medetomidine (Pfizer), 300 μg⋅kg−1 each, i.p.], male Sprague–Dawley rats (Charles River Breeding Laboratories; 380–440 g) had miniature pulsed Doppler flow probes implanted around the left renal artery, the superior mesenteric artery, and the distal abdominal aorta (hindquarters). After surgery, anesthesia was reversed and analgesia provided with atipamezole (Pfizer) and nalbuphine (DuPont) (1 mg⋅kg−1 s.c. of each). At least 10 days after probe implantation, rats were reanesthetized (as above) and had catheters implanted in the right jugular vein for drug and peptide administration and in the abdominal aorta (via the caudal artery) for the measurement of arterial blood pressure and heart rate. Experiments began at least 24 h after catheter implantation. Cardiovascular variables were continuously monitored in conscious, freely moving animals by using a custom-designed data acquisition system (University of Maastricht, Maastricht, The Netherlands), which sampled the signals every 2 ms, averaged every cardiac cycle, and stored to disk at 5-sec intervals. After a period of baseline recording, rats (six in each group) were randomized to receive either SP, EKA/B, EKC, or EKD (0.1, 1, and 10 nmol⋅kg−1 i.v. in a volume of 0.1 ml) in ascending order, with at least 30 min between the first and second doses and 60 min between the second and third doses. After a further 60 min, EKC and EKD were given at a dose of 100 nmol⋅kg−1. Vehicle-treated controls received sterile, isotonic saline at a volume of 0.1 ml. Data were analyzed by using the Friedman's test for within-group comparisons and the Kruskal–Wallis test, applied to areas under or over the curves, for between-group comparisons. P values ≤0.05 were taken as significant.
Results
Discovery of the Human TAC4 Transcript.
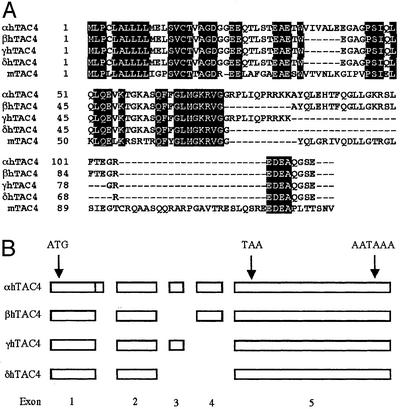
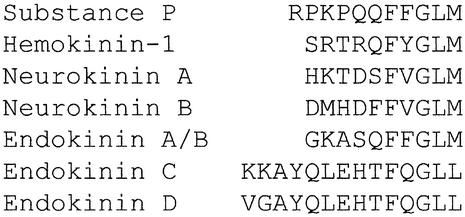
We used the published mouse TAC4 transcript (GenBank accession no. AF235035) to search for homologous regions in the human genome and found a short match on genomic clone AC027801 from chromosome 17 within the high-throughput genomic sequence database (GenBank). By alignment of the conserved synteny maps of human chromosome 17 with mouse chromosome 11 (GenBank clone AL627222 containing the mouse TAC4 gene) we determined that these two regions were evolutionarily conserved. PCR primers were designed to the human sequence to amplify the corresponding TAC4 cDNA by 5′- and 3′-RACE from the human thymus and spleen. A cDNA transcript was amplified and cloned from the spleen containing a translational ORF of 342 bp encoding a 113-aa protein with a predicted molecular mass of 12.3 kDa that was 60% homologous to the mouse TAC4 (Fig. 1A). The human precursor was found to contain a HK-1-like decapeptide sequence (GKASQFFGLM) with a dibasic cleavage site adjacent to a glycine at the C terminus having the potential to liberate an amidated peptide with the tachykinin signature motif (Fig. 1A). Unlike the other mammalian tachykinins, which are identical across the species and flanked by dibasic residues, this human peptide sequence was found not to share complete homology with mHK-1, especially at the N terminus. In particular, there is a single-base-pair substitution adjacent to the N terminus of the HK-1-like peptide that changes R to T, destroying the dibasic processing site at the N terminus (Fig. 1A). The N-terminal of this precursor also lacks additional dibasic residues. Cleavage of VAG-DG between positions 20 and 21 (deduced by using Signal P; ref. 24) would therefore predict a 47-aa peptide (Fig. 1A). The C terminus substitution in the X position of the QFXGLM-NH2 motif from Y in the mouse to F in humans produces a peptide with the last six residues identical to SP (Fig. 2). The mouse TAC4 encodes only mHK-1, whereas the human TAC4 transcript predicts a second tachykinin-like peptide located C-terminal to the position of the first. This second 14-aa peptide (KKAYQLEHTFQGLL) is flanked by dibasic residues and contains a C-terminal G that converts to the amide, but unusually it contains both a glutamine residue in the hydrophobic X position and a C-terminal leucine amide in the tachykinin motif (Fig. 2). The alignment of the exon map of the human and mouse transcript provided further evidence for the evolution of the second human tachykinin, with the inclusion of an additional unique exon (exon 3) that provides the N-terminal dibasic residues for cleavage of this peptide from its precursor (Fig. 1B).
Figure 1.
(A) Amino acid sequence alignment and homologies of human α, β, γ, and δTAC4 and mouse TAC4. (B) Intron/exon organization of the human TAC4 gene transcripts.
Figure 2.
Alignment of the amino acid sequences of the mammalian tachykinins.
Determination of Four Splice Variants of the Human TAC4 Gene.
Semiquantitative PCR was performed to determine the expression pattern of the human TAC4 transcript. Multiple bands were observed initially that were cloned, sequenced, and aligned with the genomic clone AC027801 (GenBank). Alignments were compared with predicted donor and acceptor splice sites by using a neural network recognizer (25) to identify four splice variants. The splice variants are encoded on a combination of five different exons (Fig. 1B). We named these transcripts α, β, γ, and δ TAC4 and predicted that they are capable of encoding four previously uncharacterized tachykinins classified as EKA–D. Our original transcript (αTAC4) predicts a 47-aa N-terminally extended HK-1-like peptide (EKA) and the shorter 14-aa peptide KKAYQLEHTFQGLL-NH2 (EKC). βTAC4 was also found to predict two tachykinins, EKB and EKD. EKB is predicted to be a 41-aa truncated form of EKA with the sequence VIVALE deleted from position 16–21 and EKD to be an N-terminally modified 14-aa (VGAYQLEHTFQGLL-NH2) version of EKC. γ and δTAC4 were found to predict EKB only (Fig. 2).
The TAC4 Gene Is Expressed Predominantly in the Periphery.
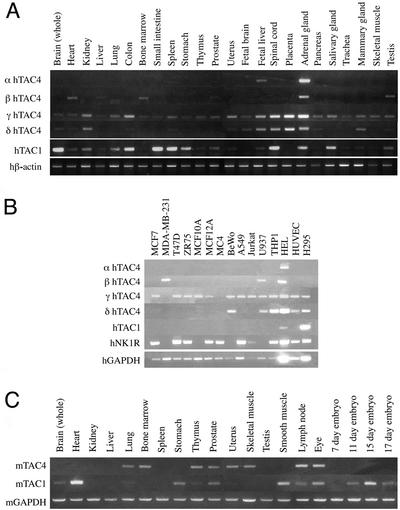
αTAC4 (uniquely encoding EKA and EKC) has a very restricted expression pattern found only in the adrenal gland and fetal liver, and very weakly in the spleen (Fig. 3A). βTAC4 expression (uniquely encoding EKB and EKD) was found in the heart, liver, bone marrow, prostate, adrenal gland, and testis only. γTAC4 expression (encoding only EKB) was predominant in the adrenal gland and placenta. The shorter δTAC4 (encoding only EKB) had a similar expression pattern to γTAC4 (Fig. 3A). By using the two-site immunometric assay, significant amounts of immunoreactive human TAC4 (7.52 fmol/g) were found probably representing the 21–61 fragment of either the γ or δTAC4 present in human term placental extracts. The adrenal gland was the only tissue found to express all four TAC4 transcripts and expected to produce all four previously uncharacterized tachykinins. Comparison of human TAC4 and TAC1 expression demonstrated distinct expression differences, especially in the whole brain, spinal cord and the organs of the gastrointestinal tract where TAC1 expression is strong but TAC4 expression is weak (Fig. 3A). In the mouse, TAC1 was expressed in brain, heart, stomach, and smooth muscle, whereas TAC4 expression was found in bone marrow, uterus, and skeletal muscle but not in the brain (Fig. 3C), with no evidence of splice variants.
Figure 3.
(A) Semiquantitative PCR analysis of the tissue distribution of the human α, β, γ, δTAC4, and TAC1 transcripts in 24 human tissues. Expression is normalized to that of the human β-actin transcript. (B) Semiquantitative PCR analysis of the distribution of the human α, β, γ, δTAC4, and TAC1 transcripts, and that of the NK1 receptor, in 15 human peripheral cell lines. Expression is normalized to that of the hGAPDH transcript. (C) Semiquantitative PCR analysis of the tissue distribution of the mouse TAC4 and TAC1 transcripts in 20 mouse tissues. Expression is normalized to that of the mGAPDH transcript.
In human peripheral cell lines, the γTAC4 transcript was widely expressed compared with that of TAC1, which was restricted to the adrenal cortical cell line, H295, and the acute myeloid leukemia cell line, HEL. The βTAC4 transcript was expressed in the monocyte cell line, U937, and HEL cells, as well as the breast cancer cell line MDA-MB-231. The αTAC4 transcript was found only in the HEL cell line. The lack of α and βTAC4 expression in the H295 adrenal cortical cell line may indicate that EKA/B, EKC, and EKD (detected in whole adrenal; Fig. 3A) are expressed in the adrenal medulla. Most cell lines were found to express the NK1 receptor in the absence of TAC1 (Fig. 3B).
EKA/B Is a Competitive Agonist at the Tachykinin Receptors.
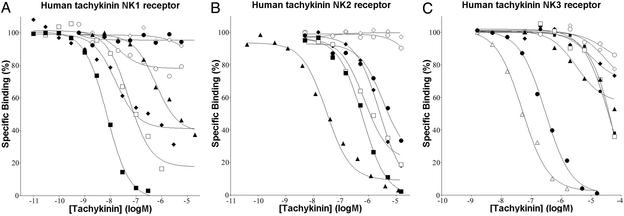
Competition binding assays were performed by using SP, mHK-1, NKA, [N-Me-Phe7]NKB, EKA/B (using the common C-terminal decapeptide GKASQFFGLM-NH2), EKC, and EKD. SP displayed the highest affinity for the NK1 receptor, with Ki values 137-fold lower than NKA. Binding of EKA/B at NK1 was similar to SP, but with Ki values 6.6-fold higher than SP and 20-fold lower than NKA. Interestingly the N-terminal-extended form of EKB [γTAC4 (30–61)-NH2] had a higher affinity again at the NK1 receptor (Ki = 4.4 ± 2.6 nM). EKC exhibited only partial inhibition even at high concentrations. [N-Me-Phe7]NKB and EKD did not inhibit in a concentration-dependent manner the binding of [3H] SR140333 to NK1 (Fig. 4A). NKA displayed the highest affinity for the NK2 receptor, with Ki values ≈42-fold lower than SP, which had identical affinity to EKA/B. SP and EKA/B were >7-fold more potent than [N-Me-Phe7]NKB in binding NK2. EKC and EKD failed to inhibit in a concentration-dependent manner the binding of [3H] SR48968 to NK2 (Fig. 4B). The NK3 antagonist SB 222200 displayed the highest affinity for NK3, with Ki values 3-fold lower than those for [N-Me-Phe7]NKB, whereas SP and EKA/B showed similar Ki values at NK3: ≈380-fold higher than [N-Me-Phe7]NKB. EKC and EKD were shown to be very weak competitors, inhibiting [3H] SR142801 at NK3 with affinities ≈2.7- and 5.5-fold lower than SP and ≈950- and 2,100-fold lower than [N-Me-Phe7]NKB, respectively (Fig. 4C, Table 1). The affinity of mHK-1 was significantly lower than SP and EKA/B at the NK2 and NK3 receptor: ≈4- and 3-fold, respectively. The addition of GTP to the competition binding assays for SP, mHK-1, and EKA/B at the NK1 receptor produced rightward shifts in the competition curves for these agonists, suggesting uncoupling of the receptor from their G proteins (Table 2). In vitro functional assays were performed on U373 MG cells by measuring changes in intracellular calcium ions to evaluate the agonist activity of SP, EKA/B, mHK-1, EKC, and EKD at the NK1 receptor and in the presence of the selective NK1 antagonist L-733060. SP, EKA/B, and mHK-1 at 1 μM induced increases in intracellular calcium ions of 610.7 ± 72.6, 576.8 ± 71.3, and 369.0 ± 81.0 nM, respectively. These were not significantly different based on ANOVA (P < 0.05). No increases in intracellular calcium ions were seen in the presence of L-733060. EKC and EKD did not induce any increase in intracellular calcium ions, even when used at a concentration of 600 μM. When a concentration–response curve was constructed for EKA/B, the data fit well into a sigmoidal curve with a Hill coefficient of 1. The pEC50 (7.64) similar to the pKi for this agonist (7.62) at the NK1 receptor indicated little amplification of response.
Figure 4.
Binding of tachykinin ligands to the human NK1 (A), NK2 (B), and NK3 (C) receptors. The binding of tachykinin ligands (■, SP; ⧫, mHK-1; ▴, NKA; ●, [N-Me-Phe7]NKB; □, EKA/B; ○, EKC; ◊, EKD; ▵, SB 222200) as determined and described in Materials and Methods by using [3H] SR140333 at the human NK1 receptor, [3H] SR48968 at the human NK2 receptor, and [3H] SR142801 at the human NK3 receptor. The data are from representative experiments previously replicated in Table 1.
Table 1.
Binding of the tachykinin ligands at each of the human NK receptors
| hNK1 | hNK2 | hNK3 | |
|---|---|---|---|
| SP | 3.6 ± 1.2 | 188.2 ± 16.0 | 10,139 ± 908.3 |
| HK-1 | 6.0 ± 2.4 | 782.5 ± 24.5 | 36,230 ± 2,530 |
| NKA | 495.1 ± 75.3 | 4.4 ± 0.1 | 176.3 ± 93.1 |
| [N-Me-Phe7]NKB | * | 1,379.5 ± 287.5 | 29.3 ± 9.7 |
| EKA/B | 23.8 ± 3.4 | 185.4 ± 21.7 | 12,130 ± 221.8 |
| EKC | * | * | 55,526.6 ± 5,788.3 |
| EKD | * | * | 28,080 ± 1,700 |
The results are presented as Ki values (nM) and are the mean ± SE of three to five separate experiments, each performed in duplicate.
No significant displacement at 10−5 M.
Table 2.
Binding of the tachykinin ligands at the human NK1 receptor in the absence or presence of GTP (100 μM)
| −GTP | +GTP | Ki + GTP/Ki − GTP | |
|---|---|---|---|
| SP | 3.6 ± 1.2 | 6.7 ± 2.2 | 1.86 |
| HK-1 | 6.0 ± 2.4 | 21.5 ± 9.1 | 3.58 |
| EKA/B | 23.8 ± 3.4 | 459.2 ± 173.2 | 19.3 |
The results are presented as Ki values (nM) and are the mean ± SE of three to five separate experiments, each performed in duplicate.
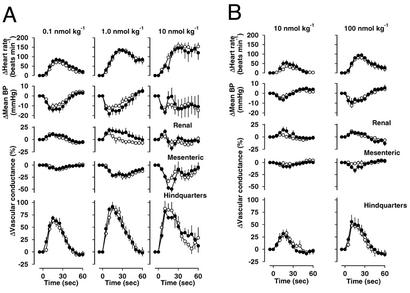
Regional Hemodynamic Effects of SP, EKA/B, EKC, and EKD.
At all doses tested, both SP and EKA/B caused falls in mean arterial blood pressure associated with dose-dependent tachycardia, mesenteric vasoconstriction, and marked hindquarters vasodilatation (Fig. 5). At the lower doses there was modest renal vasodilatation, whereas at the highest dose, particularly for SP, the renal vasodilatation gave way to vasoconstriction and there was an associated, transient recovery in blood pressure. There were no significant differences between the integrated hemodynamic effects of SP and EKA/B. The lower doses (0.1 and 1 nmol⋅kg−1) of EKC and EKD had no hemodynamic effects (data not shown). At doses of 10 and 100 nmol⋅kg−1, both peptides caused dose-dependent falls in mean arterial blood pressure and tachycardia, associated with hindquarters vasodilatation. At the highest dose tested (100 nmol⋅kg−1), EKC but not EKD caused transient mesenteric vasoconstriction (Fig. 5).
Figure 5.
Regional hemodynamic effects of SP and EKA/B (A, ● and ○) and EKC and EKD (B, ● and ○) in conscious male rats (six in each group). There were no significant differences between the integrated effects of equimolar doses of SP and EKA/B with no measurable hemodynamic effects in the vehicle-treated controls.
Discussion
We have identified a human tachykinin gene transcript that is related to that of the mouse TAC4 gene. Although this gene encodes only a single transcript in the mouse, in humans it results in four splice variants that lead to the prediction of four unique tachykinin peptides. We have named these peptides EKA–D, in line with their proposed peripheral endocrine roles rather than any neuronal role (i.e., neurokinin). In mice and humans, the TAC4 gene is widely distributed with expression levels and sites distinct from that of the TAC1 gene, particularly within the peripheral tissues. The peripheral expression of TAC4 is therefore particularly intriguing because mHK-1 has been shown to be a full agonist at each of the three NK receptors, with a remarkable selectivity for the NK1 receptor that is similar to that of SP (11, 12). This leads us to propose that mHK-1 and EKA/B are the peripheral SP-like endocrine/paracrine agonists where SP is not expressed.
There is significant divergence between SP, mHK-1, and the human HK-1 peptides, particularly at their N termini, and an unusual species difference between the HK peptides (i.e., the structures of SP, NKA, and NKB are identical throughout the mammalia). We found that all of the tachykinins except EKC and EKD possessed a predominantly hydrophilic N terminus and a hydrophobic C terminus. The C-terminal decapeptide sequence of EKA/B shares the FXGLM-NH2, where X is an aromatic residue (F and Y) in the NK1-preferring ligands or an aliphatic residue (V or I) in the NK2/NK3-preferring ligands. Furthermore, we have demonstrated pharmacologically that the C-terminal decapeptide sequence of EKA/B maintains similar binding characteristics to SP and mHK-1 at each of the NK receptors. We have additionally tested an extended form of the C-terminal decapeptide sequence of EKB, TEAETWEGAGPSIQLQLQEVKTGKASQFFGLM-NH2, that showed higher affinity for the NK1 receptor. At the NK1 receptor EKA/B, 10-mers also maintain similar agonist profiles as demonstrated by their ability to initiate intracellular calcium flux in cells that can be blocked by a NK1 antagonist. Interestingly, the uncoupled state of the NK1 receptor has ≈10-fold lower affinity for EKA/B 10-mers than SP, indicating that the two peptides may bind to a slightly different site on the receptor. Diversity in the amino acid sequence between these naturally occurring NK1 ligands appears moderately flexible, and this is especially significant with regard to their role and functions. In bioassays, mHK-1 produces hypotension in guinea pigs and salivary secretion in rats in the same way as SP (12). In rats, we have shown that the complex, but characteristic, regional hemodynamic response to SP (26) is closely mimicked by EKA/B. The striking similarity between the regional hemodynamic effects of the peptides might indicate that EKA/B and SP operate through a common receptor pathway in vivo. Intriguing are the sites of expression of EKA/B compared with SP. In the absence of nerves, the local production of EKA/B by the placenta and endothelial cells devoid of SP could have particular importance in determining regional blood flow such as that of the placental/uterine circulation, as previously suggested for NKB secreted from the placenta (9). EKA/B released into the bloodstream could target NK receptors in the local or systemic vasculature. In the adrenal gland, it is noteworthy that SP treatment enhances aldosterone production by eliciting catecholamine secretion. Although there are indications that SP stimulates the maintenance of normal growth and steroidogenic capacity, playing an important role in the stimulation of adrenal growth during fetal life (27), many of these functions, in reality, could be performed by the endokinins. It is highly possible that some earlier reports determining the presence of SP-like peptides in endothelial cells (28) and the placenta (29) were caused by the cross-reactivity of anti-SP antibodies with EKA/B.
The most marked hemodynamic effect of SP and EKA/B was a short-lived vasodilatation in the hindquarters vascular bed. Others have reported particularly marked vasodilator actions of SP in skeletal muscle in several species, including man, which may or may not depend on endothelial-derived relaxing factors (see ref. 1 for review). Because we have detected the NK1 receptor on a variety of peripheral cell lines that also express EKA/B and not SP, we propose a more fundamental role for EKA/B in cellular physiology whereby, via NK1 receptors, they can interact in an autocrine or paracrine manner. In this regard it is interesting that SP has been shown to be mitogenic and a potent stimulator of fibroblast proliferation (30). SP may be limited to the central and peripheral nervous system by the constraints of its promoter, explaining the evolution of the second, peripheral NK1 ligand. Further work is necessary to determine the precise processing of TAC4s that do not possess the classical dibasic motif N-terminal to the EKA/B 10-mer sequence. This would suggest that the natural biologically active peptide has an extended N-terminal that starts at the end of the leader/signal sequence. We cannot at this stage define the molecular form of the placental peptide that reacted in the two-site immunometric assay apart from the fact that it should contain both epitopes (γ and δTAC4 32–50 and 52–61). Full characterization of all of these naturally occurring peptide(s) is required to resolve this issue.
Exceptional to the mammalian tachykinin signature motif of EKC and EKD (which at the moment appear to be unique to humans) is the introduction in the X position of a hydrophilic glutamine residue, although some examples of polar residues in the X position have been shown to exist in a few invertebrate tachykinins (1). This would affect the hydrophobic nature of the tachykinin signature motif and its interaction with the hydrophobic pocket of each of the NK receptors. A more conservative substitution is also found in this motif, causing a transition from the C-terminal methionine to a leucine. These substitutions led to very weak activity at the known NK receptors and indicate that a fourth NK or related receptor remains to be elucidated. In this context it is interesting to note that the ability of NKB to induce lung and liver edema (two complications of preeclampsia in human pregnancy) in a NK1 receptor knockout mouse could not be blocked by either NK2 or NK3 antagonists (10). Further work is necessary before we can conclude whether the inappropriate placental secretion of, e.g., EKA/B along with NKB may also be contributory to the multisystem disorder of preeclampsia, but their potential damaging activity particularly at peripheral NK1 receptors in this condition is a distinct possibility.
In summary, EKA/B may provide new insights into many of the actions formerly attributed to SP in the periphery, such as rheumatoid arthritis, vasodilatation, inflammation, and asthma, and may be involved in controlling placental/uterine blood flow. The discovery of EKC and EKD may also uncover novel regulatory mechanisms for these peptides particularly in human adrenal physiology, but their species-specific nature and the lack of a candidate receptor limits their further characterization at the present time.
Acknowledgments
This work was supported by the Medical Research Council.
Abbreviations
- EK
endokinin
- HK-1
hemokinin
- mHK-1
mouse HK-1
- NK
neurokinin
- SP
substance P
- TAC
tachykinin precursor
Footnotes
References
- 1.Severini C, Improta G, Falconieri-Erspamer G, Salvadori S, Erspamer V. Pharmacol Rev. 2002;54:285–322. doi: 10.1124/pr.54.2.285. [DOI] [PubMed] [Google Scholar]
- 2.Nawa H, Hirose T, Takashima H, Inayama S, Nakanishi S. Nature. 1983;306:32–36. doi: 10.1038/306032a0. [DOI] [PubMed] [Google Scholar]
- 3.Nawa H, Kotani H, Nakanishi S. Nature. 1984;312:729–734. doi: 10.1038/312729a0. [DOI] [PubMed] [Google Scholar]
- 4.Kawaguchi Y, Hoshimaru M, Nawa H, Nakanishi S. Biochem Biophys Res Commun. 1986;139:1040–1060. doi: 10.1016/s0006-291x(86)80282-0. [DOI] [PubMed] [Google Scholar]
- 5.Tatemoto K, Lundberg J M, Jornvall H, Mutt V. Biochem Biophys Res Commun. 1985;128:947–953. doi: 10.1016/0006-291x(85)90138-x. [DOI] [PubMed] [Google Scholar]
- 6.Kage R, McGregor G P, Thim L, Conlon J M. J Neurochem. 1988;50:1412–1417. doi: 10.1111/j.1471-4159.1988.tb03024.x. [DOI] [PubMed] [Google Scholar]
- 7.Lai J P, Douglas S D, Rappaport E, Wu J M, Ho W Z. J Neuroimmunol. 1998;91:121–128. doi: 10.1016/s0165-5728(98)00170-2. [DOI] [PubMed] [Google Scholar]
- 8.Kotani H, Hoshimaru M, Nawa H, Nakanishi S. Proc Natl Acad Sci USA. 1986;83:7074–7078. doi: 10.1073/pnas.83.18.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page N M, Woods R J, Gardiner S M, Lomthaisong K, Gladwell R T, Butlin D J, Manyonda I T, Lowry P J. Nature. 2000;405:797–800. doi: 10.1038/35015579. [DOI] [PubMed] [Google Scholar]
- 10.Grant A D, Akhtar R, Gerard N P, Brain S D. J Physiol. 2002;543:1007–1014. doi: 10.1113/jphysiol.2002.018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Lu L, Furlonger C, Wu G E, Paige C J. Nat Immunol. 2000;1:392–397. doi: 10.1038/80826. [DOI] [PubMed] [Google Scholar]
- 12.Bellucci F, Carini F, Catalani C, Cucchi P, Lecci A, Meini S, Patacchini R, Quartara L, Ricci R, Tramontana M, et al. Br J Pharmacol. 2002;135:266–274. doi: 10.1038/sj.bjp.0704443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camarda V, Rizzi A, Calo G, Guerrini R, Salvadori S, Regoli D. Life Sci. 2002;71:363–370. doi: 10.1016/s0024-3205(02)01682-x. [DOI] [PubMed] [Google Scholar]
- 14.Seabrook G R, Shepheard S L, Williamson D J, Tyrer P, Rigby M, Cascieri M A, Harrison T, Hargreaves R J, Hill R G. Eur J Pharmacol. 1996;317:129–135. doi: 10.1016/s0014-2999(96)00706-6. [DOI] [PubMed] [Google Scholar]
- 15.Lepre M, Olpe H R, Evans R H, Brugger F. Eur J Pharmacol. 1994;258:23–31. doi: 10.1016/0014-2999(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 16.Medhurst A D, Hay D W, Parsons A A, Martin L D, Griswold D E. Br J Pharmacol. 1997;122:469–476. doi: 10.1038/sj.bjp.0701406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emonds-Alt X, Doutremepuich J D, Heaulme M, Neliat G, Santucci V, Steinberg R, Vilain P, Bichon D, Ducoux J P, Proietto V. Eur J Pharmacol. 1993;250:403–413. doi: 10.1016/0014-2999(93)90027-f. [DOI] [PubMed] [Google Scholar]
- 18.Emonds-Alt X, Vilain P, Goulaouic P, Proietto V, Van Broeck D, Advenier C, Naline E, Neliat G, Le Fur G, Breliere J C. Life Sci. 1992;50:101–106. doi: 10.1016/0024-3205(92)90352-p. [DOI] [PubMed] [Google Scholar]
- 19.Oury-Donat F, Carayon P, Thurneyssen O, Pailhon V, Emonds-Alt X, Soubrie P, Le Fur G. J Pharmacol Exp Ther. 1995;274:148–154. [PubMed] [Google Scholar]
- 20.Page N M, Butlin D J, Lomthaisong K, Lowry P J. Genomics. 2001;74:71–78. doi: 10.1006/geno.2001.6534. [DOI] [PubMed] [Google Scholar]
- 21.Johnson C L, Johnson C G. J Neurochem. 1992;58:471–477. doi: 10.1111/j.1471-4159.1992.tb09745.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee C M, Kum W, Cockram C S, Teoh R, Young J D. Brain Res. 1989;488:328–331. doi: 10.1016/0006-8993(89)90724-5. [DOI] [PubMed] [Google Scholar]
- 23.Mueller A, Mahmoud N G, Goedecke M C, McKeating J A, Strange P G. Br J Pharmacol. 2002;135:1033–1043. doi: 10.1038/sj.bjp.0704540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen H, Engelbrecht J, Søren Brunak S, von Heijne G. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Brunak S, Engelbrecht J, Knudsen S. J Mol Biol. 1991;220:49–65. doi: 10.1016/0022-2836(91)90380-o. [DOI] [PubMed] [Google Scholar]
- 26.Bachelard H, Gardiner S M, Kemp P A, Bennett T. Br J Pharmacol. 1992;105:202–210. doi: 10.1111/j.1476-5381.1992.tb14235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nussdorfer, G. G. & Malendowicz, L. K. (1998) 19, 949–968.
- 28.Cai W Q, Dikranian K, Bodin P, Turmaine M, Burnstock G. Cell Tissue Res. 1993;274:533–538. doi: 10.1007/BF00314550. [DOI] [PubMed] [Google Scholar]
- 29.Sastry B V, Tayeb O S, Barnwell S L, Janson V E, Owens L K. Placenta Suppl. 1981;3:327–337. [PubMed] [Google Scholar]
- 30.Kahler C M, Herold M, Wiedermann C J. J Cell Physiol. 1993;156:579–587. doi: 10.1002/jcp.1041560318. [DOI] [PubMed] [Google Scholar]
- 31.Severini C, Salvadori S, Guerrini R, Falconieri-Erspamer G, Mignogna G, Erspamer V. Peptides. 2000;21:1587–1595. doi: 10.1016/s0196-9781(00)00290-4. [DOI] [PubMed] [Google Scholar]